Wet Synthesis of Elongated Hexagonal ZnO Microstructures for Applications as Photo-Piezoelectric Catalysts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of ZnO Crystals
2.2. Morphological and Spectroscopic Characterization of ZnO Samples
2.3. Piezoelectric Measurements
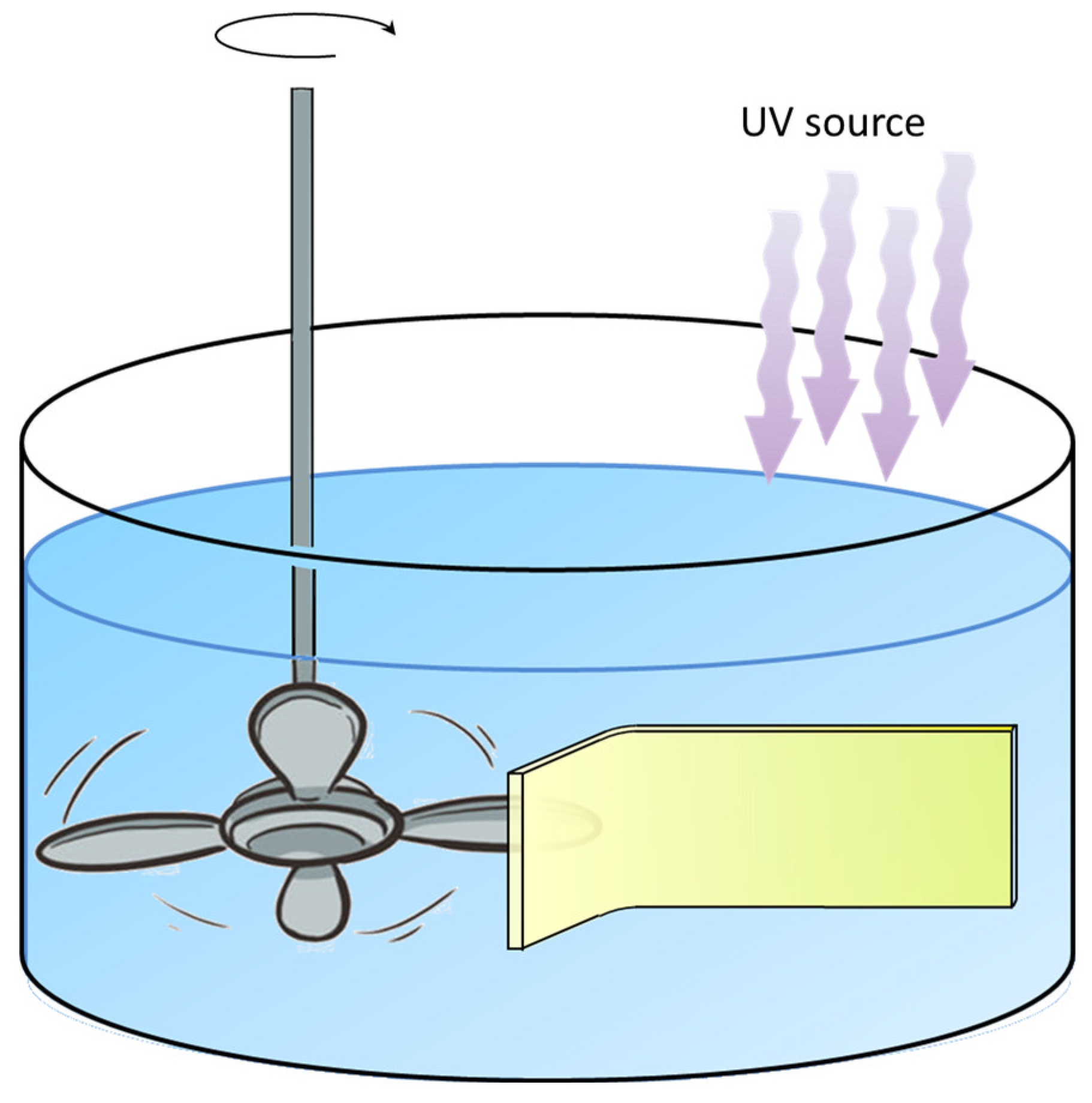
2.4. Photo-Degradation Experiment in Aqueous Solution
2.5. Piezo-Photo Degradation Measurements
3. Discussion
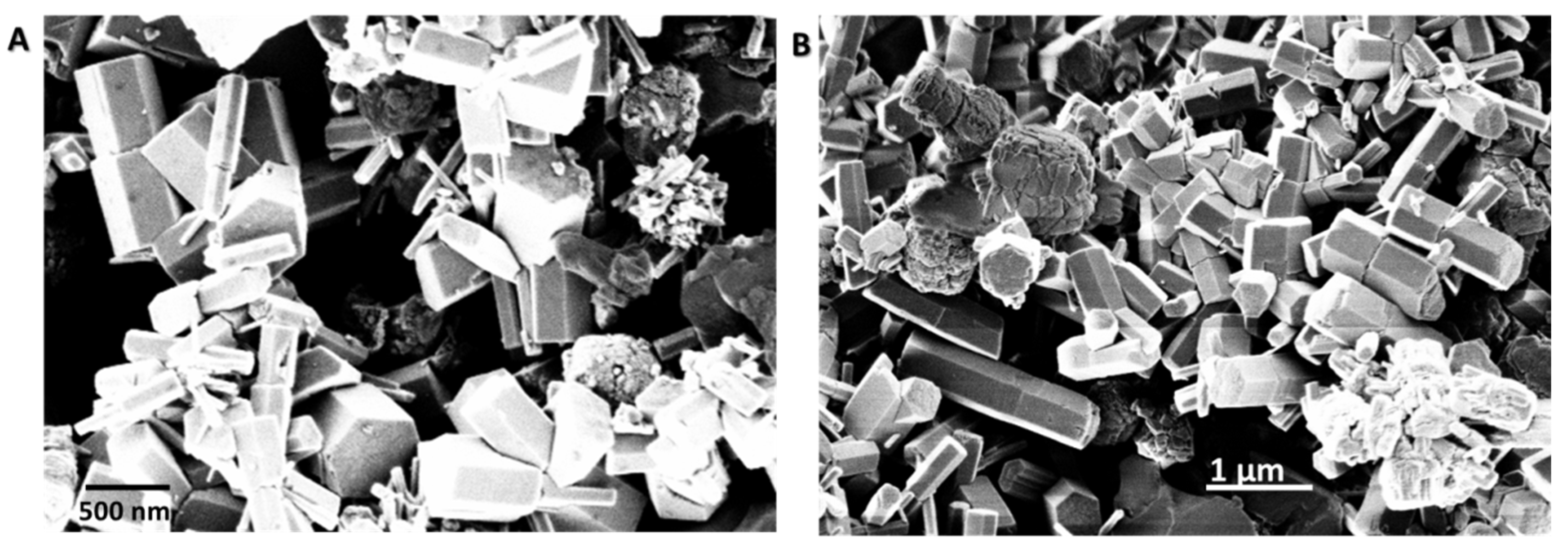
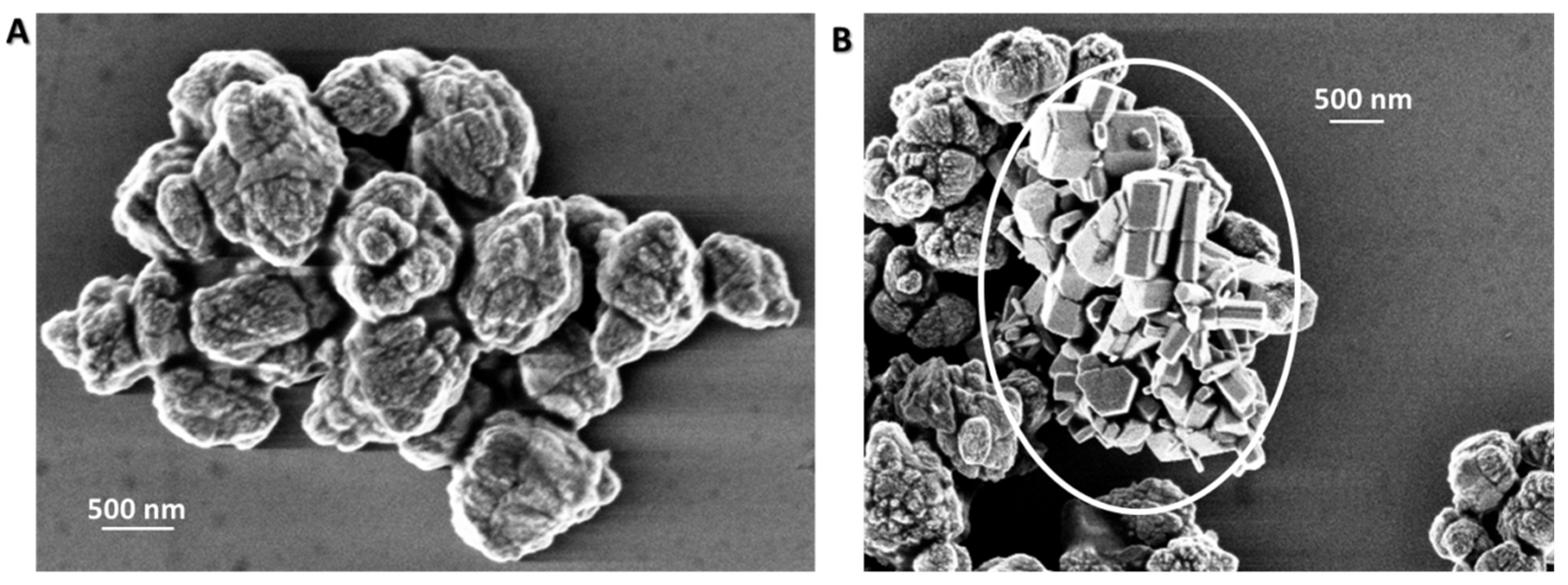
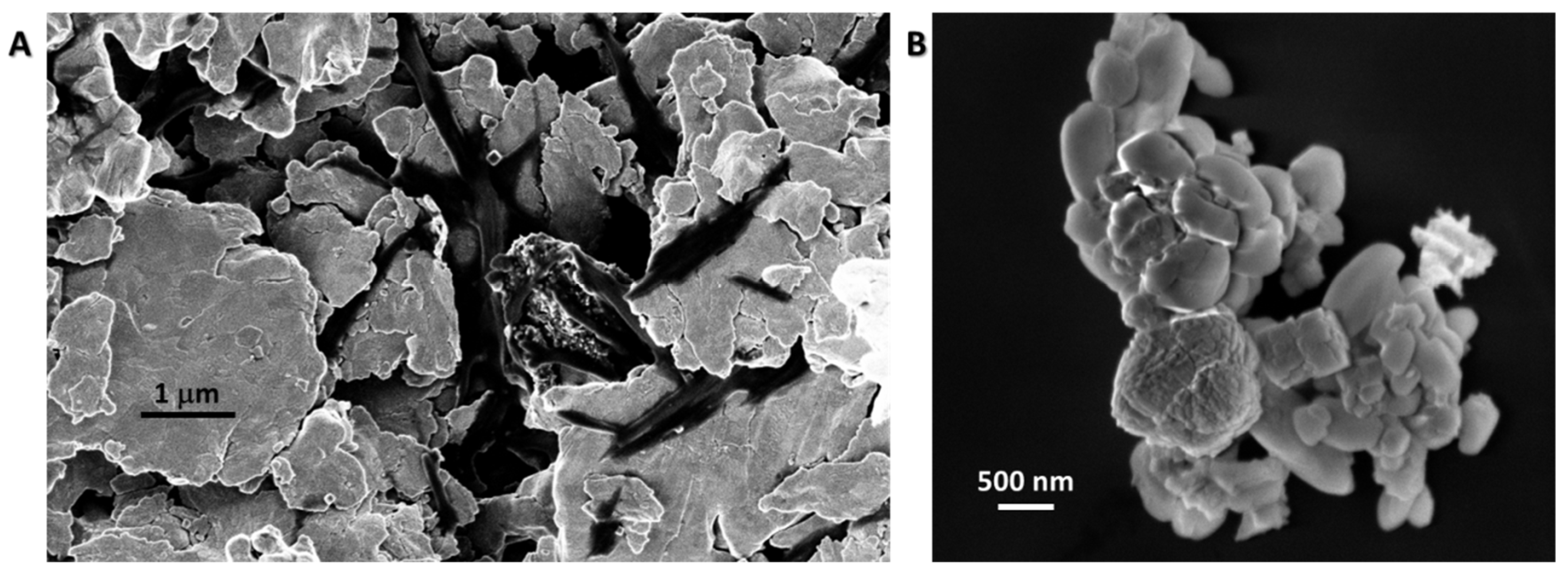
3.1. Synthesis and Morphological Characterization of ZnO Samples
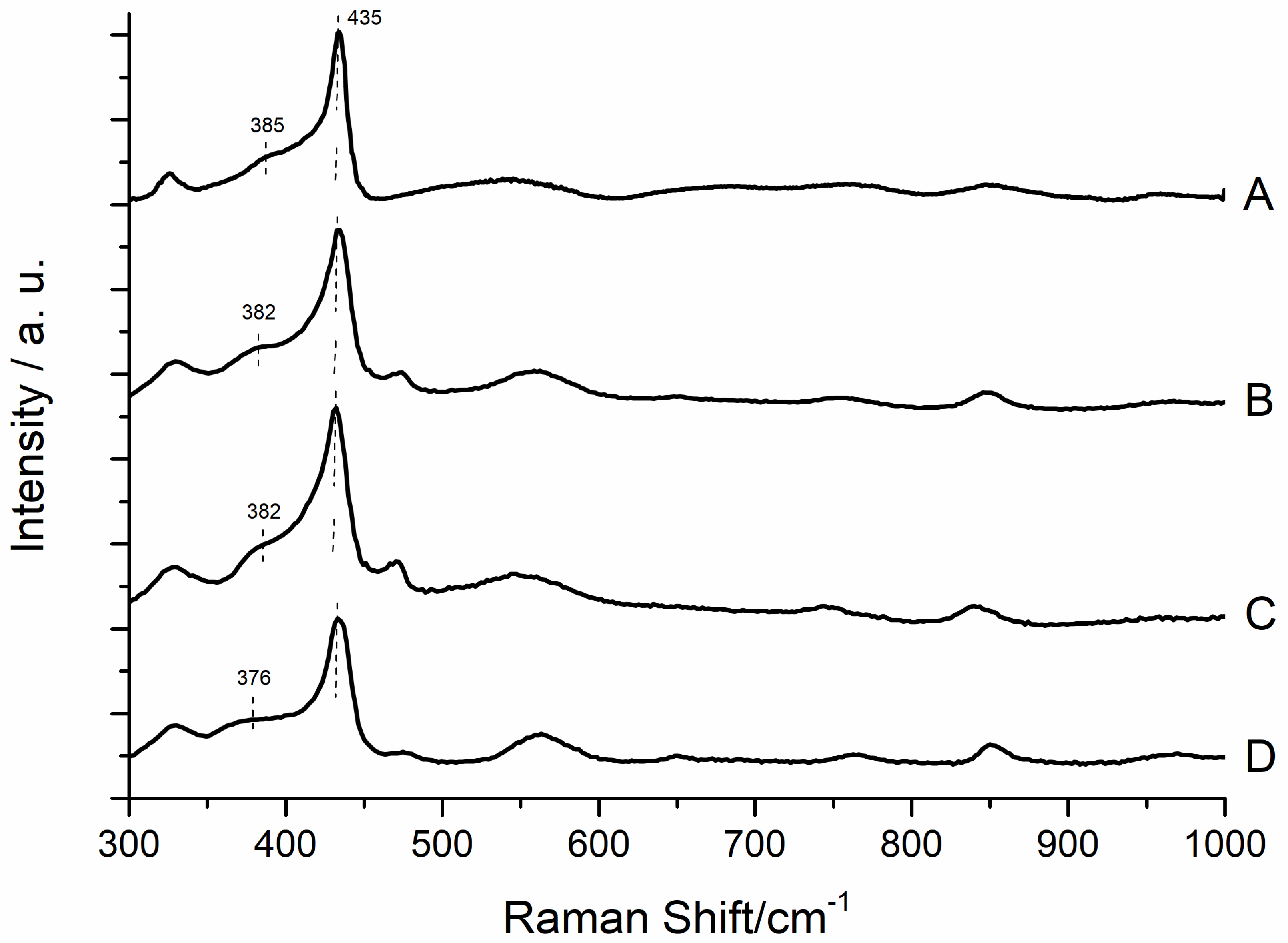
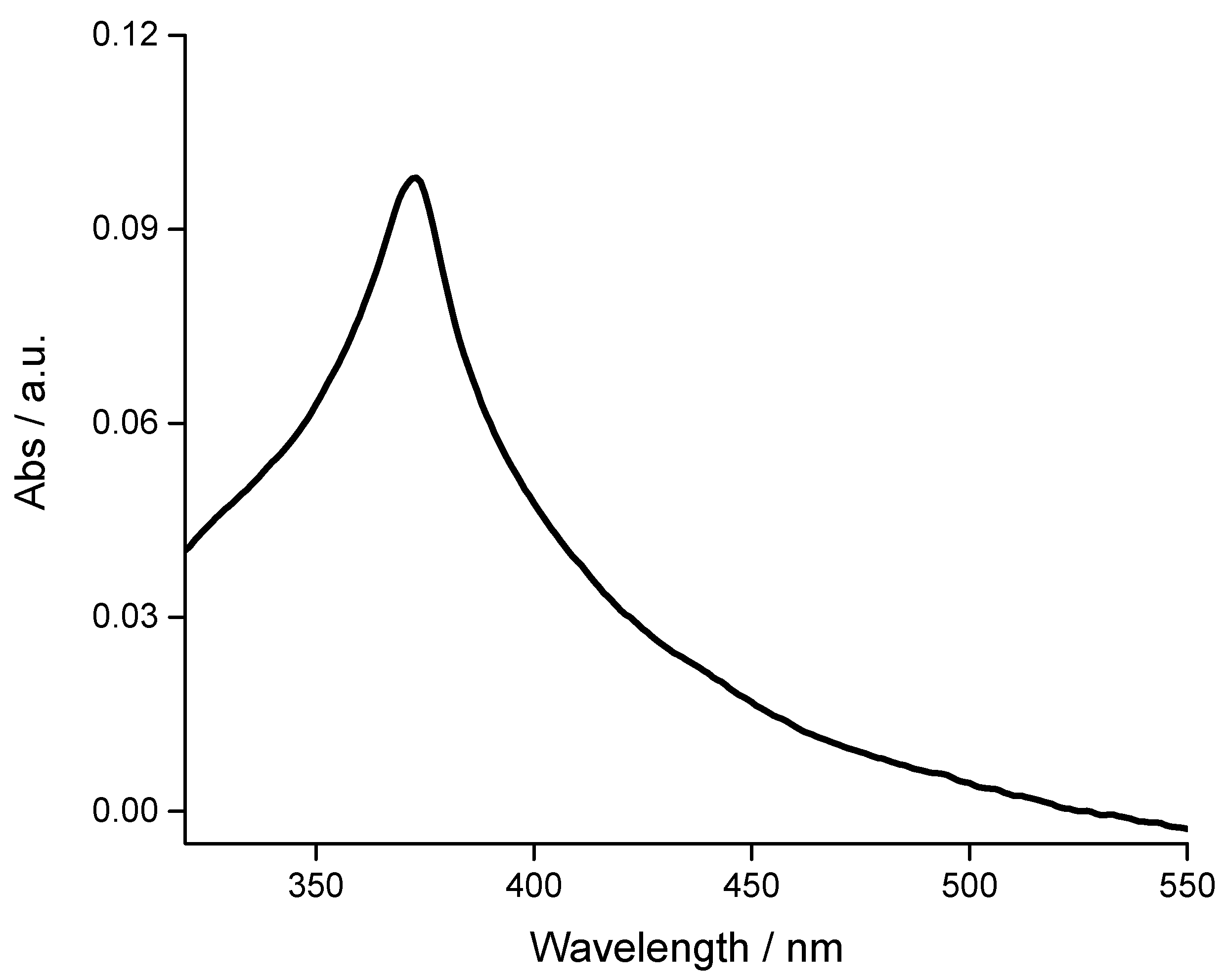
3.2. Spectroscopic Characterization of ZnO Samples
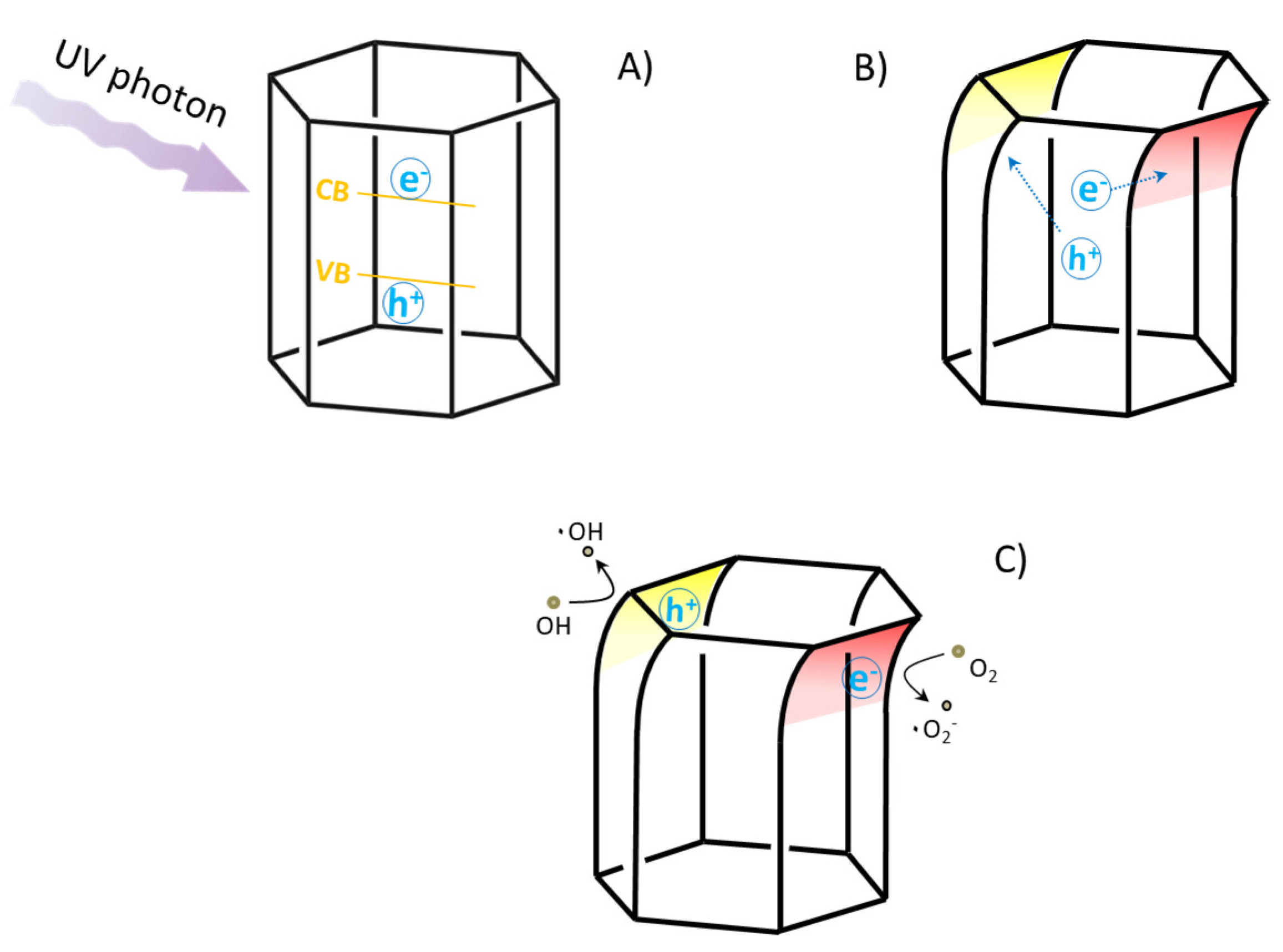
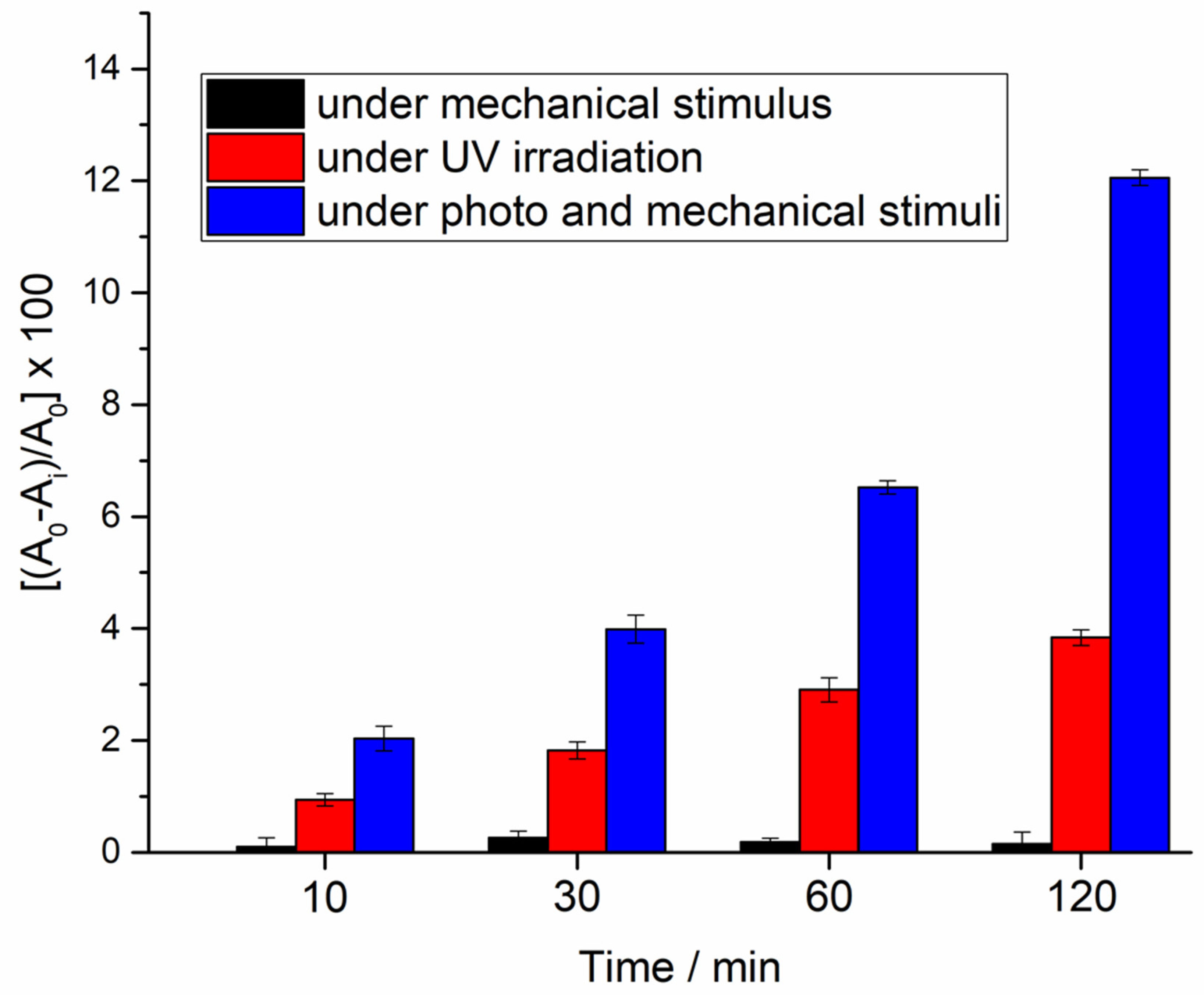
3.3. Piezoelectric and Piezo-Photocatalytic Responses of the ZnO Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Liu, Z. Global Energy Interconnection; Elsevier: Amsterdam, The Netherlands, 2015; ISBN 9780128044063. [Google Scholar]
- Verma, D.; Fortunati, E.; Jain, S.; Zhang, X. Biomass, Biopolymer-Based Materials, and Bioenergy: Construction, Biomedical, and Other Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780081024263. [Google Scholar]
- Yang, Z.; Zhou, S.; Zu, J.; Inman, D. High-Performance Piezoelectric Energy Harvesters and Their Applications. Joule 2018, 2, 642–697. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; He, J.; Wen, T.; Zhai, C.; Han, J.; Mu, J.; Jia, W.; Zhang, B.; Zhang, W.; Chou, X.; et al. Magnetically levitated-triboelectric nanogenerator as a self-powered vibration monitoring sensor. Nano Energy 2017, 33, 88–97. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.-K.; Kim, M.-S.; Lee, S.; Kim, C.; Kim, Y.-J. Wearable thermoelectric generator for harvesting human body heat energy. Smart Mater. Struct. 2014, 23, 105002. [Google Scholar] [CrossRef]
- Amar, A.B.; Kouki, A.B.; Cao, H. Power approaches for implantable medical devices. Sensors (Switzerland) 2015, 15, 28889–28914. [Google Scholar] [CrossRef]
- Li, J.; Wang, X. Research Update: Materials design of implantable nanogenerators for biomechanical energy harvesting. APL Mater. 2017, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Q.; Zou, Y.; Zhang, Y.; Liu, Z.; Shi, B.; Wang, X.; Jin, Y.; Ouyang, H.; Li, Z.; Wang, Z.L. Biodegradable triboelectric nanogenerator as a life-time designed implantable power source. Sci. Adv. 2016, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.L. ZnO nanowire and nanobelt platform for nanotechnology. Mater. Sci. Eng. R Rep. 2009, 64, 33–71. [Google Scholar] [CrossRef]
- Williams, W.S.; Breger, L. Piezoelectricity in tendon and bone. J. Biomech. 1975, 8, 407–413. [Google Scholar] [CrossRef]
- Mangayil, R.; Rajala, S.; Pammo, A.; Sarlin, E.; Luo, J.; Santala, V.; Karp, M.; Tuukkanen, S. Engineering and Characterization of Bacterial Nanocellulose Films as Low Cost and Flexible Sensor Material. ACS Appl. Mater. Interfaces 2017, 9, 19048–19056. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kang, L.; Yu, Y.; Long, Y.; Jeffery, J.J.; Cai, W.; Wang, X. Study of long-term biocompatibility and bio-safety of implantable nanogenerators. Nano Energy 2018, 51, 728–735. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, J.; Song, J.; Liu, J.; Xu, N.; Wang, Z.L. Piezoelectric field effect transistor and nanoforce sensor based on a single ZnO nanowire. Nano Lett. 2006, 6, 2768–2772. [Google Scholar] [CrossRef]
- Curie, J.; Curie, P. Développement par compression de l’électricité polaire dans les cristaux hémièdres à faces inclinées. Bull. la Société minéralogique Fr. 1880, 3, 90–93. [Google Scholar] [CrossRef]
- Bettini, S.; Syrgiannis, Z.; Pagano, R.; Dordević, L.; Salvatore, L.; Prato, M.; Giancane, G.; Valli, L. Perylene Bisimide Aggregates as Probes for Subnanomolar Discrimination of Aromatic Biogenic Amines. ACS Appl. Mater. Interfaces 2019, 11, 17079–17089. [Google Scholar] [CrossRef]
- Zhang, C.; Xing, D.; Li, Y. Micropumps, microvalves, and micromixers within PCR microfluidic chips: Advances and trends. Biotechnol. Adv. 2007, 25, 483–514. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, K.A.; Osman, M.K.; Hussain, Z.; Abdullah, M.F.; Manaf, A.A.; Abdullah, N. Design and characterization piezoelectric acoustic transducer for sonar application. In Proceedings of the 8th IEEE International Conference on Control System, Computing and Engineering, ICCSCE, Penang, Malaysia, 23–25 November 2018; pp. 233–237. [Google Scholar]
- Xu, S.; Qin, Y.; Xu, C.; Wei, Y.; Yang, R.; Wang, Z.L. Self-powered nanowire devices. Nat. Nanotechnol. 2010, 5, 366–373. [Google Scholar] [CrossRef]
- Meller, C.; Havas, T.E. Piezoelectric technology in otolaryngology, and head and neck surgery: A review. J. Laryngol. Otol. 2017, 131, S12–S18. [Google Scholar] [CrossRef]
- Hamlehdar, M.; Kasaeian, A.; Safaei, M.R. Energy harvesting from fluid flow using piezoelectrics: A critical review. Renew. Energy 2019, 143, 1826–1838. [Google Scholar] [CrossRef]
- Gholikhani, M.; Roshani, H.; Dessouky, S.; Papagiannakis, A.T. A critical review of roadway energy harvesting technologies. Appl. Energy 2020, 261, 114388. [Google Scholar] [CrossRef]
- Shin, D.M.; Hong, S.W.; Hwang, Y.H. Recent advances in organic piezoelectric biomaterials for energy and biomedical applications. Nanomaterials 2020, 10. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, S.S.; Bhatt, U.M.; Gautam, P.; Thote, S.; Joglekar, M.M.; Manhas, S.K. Fabrication and modeling of β-phase PVDF-TrFE based flexible piezoelectric energy harvester. Sens. Actuators A Phys. 2020, 304, 111879. [Google Scholar] [CrossRef]
- Rodrigues, C.; Gomes, A.; Ghosh, A.; Pereira, A.; Ventura, J. Power-generating footwear based on a triboelectric-electromagnetic-piezoelectric hybrid nanogenerator. Nano Energy 2019, 62, 660–666. [Google Scholar] [CrossRef]
- Dagdeviren, C.; Joe, P.; Tuzman, O.L.; Park, K.I.; Lee, K.J.; Shi, Y.; Huang, Y.; Rogers, J.A. Recent progress in flexible and stretchable piezoelectric devices for mechanical energy harvesting, sensing and actuation. Extrem. Mech. Lett. 2016, 9, 269–281. [Google Scholar] [CrossRef] [Green Version]
- Fan, F.R.; Tang, W.; Wang, Z.L. Flexible Nanogenerators for Energy Harvesting and Self-Powered Electronics. Adv. Mater. 2016, 28, 4283–4305. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-I.; Lee, M.; Liu, Y.; Moon, S.; Hwang, G.-T.; Zhu, G.; Kim, J.E.; Kim, S.O.; Kim, D.K.; Wang, Z.L.; et al. Flexible Nanocomposite Generator Made of BaTiO3 Nanoparticles and Graphitic Carbons. Adv. Mater. 2012, 24, 2999–3004. [Google Scholar] [CrossRef]
- Giancane, G.; Guascito, M.R.; Malitesta, C.; Mazzotta, E.; Picca, R.A.; Valli, L. QCM sensors for aqueous phenols based on active layers constituted by tetrapyrrolic macrocycle Langmuir films. J. Porphyr. Phthalocyanines 2009, 13, 1129–1139. [Google Scholar] [CrossRef]
- Zhou, J.; Gu, Y.; Fei, P.; Mai, W.; Gao, Y.; Yang, R.; Bao, G.; Wang, Z.L. Flexible piezotronic strain sensor. Nano Lett. 2008, 8, 3035–3040. [Google Scholar] [CrossRef] [Green Version]
- Bettini, S.; Pagano, R.; Bonfrate, V.; Maglie, E.; Manno, D.; Serra, A.; Valli, L.; Giancane, G. Promising Piezoelectric Properties of New ZnO@Octadecylamine Adduct. J. Phys. Chem. C 2015, 119, 20143–20149. [Google Scholar] [CrossRef]
- Bettini, S.; Pagano, R.; Semeraro, P.; Ottolini, M.; Salvatore, L.; Marzo, F.; Lovergine, N.; Giancane, G.; Valli, L. SiO 2 -Coated ZnO Nanoflakes Decorated with Ag Nanoparticles for Photocatalytic Water Oxidation. Chem. A Eur. J. 2019, 25, 14123–14132. [Google Scholar] [CrossRef] [PubMed]
- Pagano, R.; Quarta, A.; Pal, S.; Licciulli, A.; Valli, L.; Bettini, S. Enhanced Solar-Driven Applications of ZnO@Ag Patchy Nanoparticles. J. Phys. Chem. C 2017, 121, 27199–27206. [Google Scholar] [CrossRef]
- Xue, X.; Zang, W.; Deng, P.; Wang, Q.; Xing, L.; Zhang, Y.; Wang, Z.L. Piezo-potential enhanced photocatalytic degradation of organic dye using ZnO nanowires. Nano Energy 2015, 13, 414–422. [Google Scholar] [CrossRef]
- Xu, X.; Jia, Y.; Xiao, L.; Wu, Z. Strong vibration-catalysis of ZnO nanorods for dye wastewater decolorization via piezo-electro-chemical coupling. Chemosphere 2018, 193, 1143–1148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, X.; Yeom, J. A Floatable Piezo-Photocatalytic Platform Based on Semi-Embedded ZnO Nanowire Array for High-Performance Water Decontamination. Nano-Micro Lett. 2019, 11, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Tian, Z.R.; Voigt, J.A.; Liu, J.; Mckenzie, B.; Mcdermott, M.J.; Rodriguez, M.A.; Konishi, H.; Xu, H. Complex and oriented ZnO nanostructures. Nat. Mater. 2003, 2, 821–826. [Google Scholar] [CrossRef]
- Bettini, S.; Pagano, R.; Valli, L.; Giancane, G. Enhancement of Open Circuit Voltage of a ZnO-Based Dye-Sensitized Solar Cell by Means of Piezotronic Effect. Chem. An Asian J. 2016, 11, 1240–1245. [Google Scholar] [CrossRef]
- Amin, G.; Asif, M.H.; Zainelabdin, A.; Zaman, S.; Nur, O.; Willander, M. Influence of pH, Precursor Concentration, Growth Time, and Temperature on the Morphology of ZnO Nanostructures Grown by the Hydrothermal Method. J. Nanomater. 2011, 2011, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Venu Gopal, V.R.; Kamila, S. Effect of temperature on the morphology of ZnO nanoparticles: a comparative study. Appl. Nanosci. 2017, 7, 75–82. [Google Scholar] [CrossRef] [Green Version]
- Araújo, E.A.; Nobre, F.X.; Sousa, G.D.S.; Cavalcante, L.S.; Rita De Morais Chaves Santos, M.; Souza, F.L.; Elias De Matos, J.M. Synthesis, growth mechanism, optical properties and catalytic activity of ZnO microcrystals obtained via hydrothermal processing. RSC Adv. 2017, 7, 24263–24281. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Li, X.; Yu, W. Flowerlike ZnO nanostructures via hexamethylenetetramine-assisted thermolysis of zinc-ethylenediamine complex. J. Phys. Chem. B 2005, 109, 1155–1161. [Google Scholar] [CrossRef]
- Kuo, C.L.; Kuo, T.J.; Huang, M.H. Hydrothermal synthesis of ZnO microspheres and hexagonal microrods with sheetlike and platelike nanostructures. J. Phys. Chem. B 2005, 109, 20115–20121. [Google Scholar] [CrossRef]
- Dem’yanets, L.N.; Kostomarov, D.V.; Kuz’mina, I.P. Chemistry and kinetics of ZnO growth from alkaline hydrothermal solutions. Inorg. Mater. 2002, 38, 124–131. [Google Scholar] [CrossRef]
- Zhang, S.C.; Li, X.G. Preparation of ZnO particles by precipitation transformation method and its inherent formation mechanisms. Colloids Surfaces A Physicochem. Eng. Asp. 2003, 226, 35–44. [Google Scholar] [CrossRef]
- Wang, Y.X.; Sun, J.; Fan, X.; Yu, X. A CTAB-assisted hydrothermal and solvothermal synthesis of ZnO nanopowders. Ceram. Int. 2011, 37, 3431–3436. [Google Scholar] [CrossRef]
- Liu, Y.; Lv, H.; Li, S.; Xi, G.; Xing, X. Synthesis and characterization of ZnO with hexagonal dumbbell-like bipods microstructures. Adv. Powder Technol. 2011, 22, 784–788. [Google Scholar] [CrossRef]
- Cho, S.; Jang, J.W.; Jung, S.H.; Lee, B.R.; Oh, E.; Lee, K.H. Precursor effects of citric acid and citrates on ZnO crystal formation. Langmuir 2009, 25, 3825–3831. [Google Scholar] [CrossRef]
- Wang, B.G.; Shi, E.W.; Zhong, W.Z. Twinning Morphologies and Mechanisms of ZnO Crystallites under Hydrothermal Conditions. Cryst. Res. Technol. 1998, 33, 937–941. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, S.; Zhang, C.; Yang, Y.; Lv, K. Raman Spectra and Microstructure of Zinc Oxide irradiated with Swift Heavy Ion. Crystals 2019, 9, 395. [Google Scholar] [CrossRef] [Green Version]
- Sánchez Zeferino, R.; Barboza Flores, M.; Pal, U. Photoluminescence and raman scattering in ag-doped zno nanoparticles. J. Appl. Phys. 2011, 109, 014308. [Google Scholar] [CrossRef]
- Vinogradov, V.S.; Dzhagan, V.N.; Zavaritskaya, T.N.; Kucherenko, I.V.; Mel’nik, N.N.; Novikova, N.N.; Janik, E.; Wojtowicz, T.; Plyashechnik, O.S.; Zahn, D.R.T. Optical phonons in the bulk and on the surface of ZnO and ZnTe/ZnO nanowires in Raman spectra. Phys. Solid State 2012, 54, 2083–2090. [Google Scholar] [CrossRef]
- Jusserand, B.; Cardona, M. Raman spectroscopy of vibrations in superlattices. In Light Scattering in Solids V; Springer-Verlag: Berlin/Heidelberg, Germany, 2006; pp. 49–152. [Google Scholar]
- Yu, J.L.; Lai, Y.F.; Wang, Y.Z.; Cheng, S.Y.; Chen, Y.H. Polarized Raman scattering of single ZnO nanorod. J. Appl. Phys. 2014, 115, 033505. [Google Scholar] [CrossRef]
- Palani, I.A.; Nakamura, D.; Okazaki, K.; Shimogaki, T.; Higashihata, M.; Okada, T. Influence of ZnO buffer layer on growth of Sb doped ZnO nano wires using nano particle assisted pulsed laser deposition (NAPLD) using Sb as catalyst. Adv. Mater. Lett. 2012, 3, 66–70. [Google Scholar] [CrossRef]
- Lupan, O.; Chow, L.; Ono, L.K.; Cuenya, B.R.; Chai, G.; Khallaf, H.; Park, S.; Schulte, A. Synthesis and characterization of ag- or sb-doped zno nanorods by a facile hydrothermal route. J. Phys. Chem. C 2010, 114, 12401–12408. [Google Scholar] [CrossRef]
- Sinha, N.; Goel, S.; Joseph, A.J.; Yadav, H.; Batra, K.; Gupta, M.K.; Kumar, B. Y-doped ZnO nanosheets: Gigantic piezoelectric response for an ultra-sensitive flexible piezoelectric nanogenerator. Ceram. Int. 2018, 44, 8582–8590. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, W.; Zhu, J.; Jiang, G.; Sheng, Q.; Wang, B.; Zheng, Y. A comprehensive picture in the view of atomic scale on piezoelectricity of ZnO tunnel junctions: The first principles simulation. AIP Adv. 2016, 6, 065217. [Google Scholar] [CrossRef] [Green Version]
- Jadhav, R.W.; La, D.D.; More, V.G.; Tung Vo, H.; Nguyen, D.A.; Tran, D.L.; Bhosale, S.V. Self-assembled kanamycin antibiotic-inorganic microflowers and their application as a photocatalyst for the removal of organic dyes. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Zhong, C.; Cao, J.; Hao, Y.; Lei, M.; Bi, K.; Sun, Q.; Wang, Z.L. Enhanced photocatalytic H2 evolution by plasmonic and piezotronic effects based on periodic Al/BaTiO3 heterostructures. Nano Energy 2019, 62, 513–520. [Google Scholar] [CrossRef]
- Lei, H.; Zhang, H.; Zou, Y.; Dong, X.; Jia, Y.; Wang, F. Synergetic photocatalysis/piezocatalysis of bismuth oxybromide for degradation of organic pollutants. J. Alloys Compd. 2019, 809, 151840. [Google Scholar] [CrossRef]
- Chen, Y.; Deng, X.; Wen, J.; Zhu, J.; Bian, Z. Piezo-promoted the generation of reactive oxygen species and the photodegradation of organic pollutants. Appl. Catal. B Environ. 2019, 258, 118024. [Google Scholar] [CrossRef]


| ZnSO4. 7H2O/g | Citric Acid/g | NaOH (1st add)/g | Milli-Q Water/mL | Stirring @R.T. | NaOH (2nd add)/g | Stirring @R.T. | Thermal Treatment @100 °C | |
|---|---|---|---|---|---|---|---|---|
| A | 0.84 | 1.5 | 0.6 | 60 | 2 h | 0.4 | overnight | 8 h |
| B | 0.84 | 1.5 | 0.6 | 60 | 2 h | 0.4 | 2 h | 8 h |
| C | 0.84 | 1.5 | 1 | 60 | 2 h | / | / | 8 h |
| D | 0.84 | 1.5 | 1 | 60 | overnight | / | / | 8 h |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pagano, R.; Ingrosso, C.; Giancane, G.; Valli, L.; Bettini, S. Wet Synthesis of Elongated Hexagonal ZnO Microstructures for Applications as Photo-Piezoelectric Catalysts. Materials 2020, 13, 2938. https://doi.org/10.3390/ma13132938
Pagano R, Ingrosso C, Giancane G, Valli L, Bettini S. Wet Synthesis of Elongated Hexagonal ZnO Microstructures for Applications as Photo-Piezoelectric Catalysts. Materials. 2020; 13(13):2938. https://doi.org/10.3390/ma13132938
Chicago/Turabian StylePagano, Rosanna, Chiara Ingrosso, Gabriele Giancane, Ludovico Valli, and Simona Bettini. 2020. "Wet Synthesis of Elongated Hexagonal ZnO Microstructures for Applications as Photo-Piezoelectric Catalysts" Materials 13, no. 13: 2938. https://doi.org/10.3390/ma13132938
APA StylePagano, R., Ingrosso, C., Giancane, G., Valli, L., & Bettini, S. (2020). Wet Synthesis of Elongated Hexagonal ZnO Microstructures for Applications as Photo-Piezoelectric Catalysts. Materials, 13(13), 2938. https://doi.org/10.3390/ma13132938









