A Review on the Biocompatibility of PMMA-Based Dental Materials for Interim Prosthetic Restorations with a Glimpse into Their Modern Manufacturing Techniques
Abstract
:1. Introduction
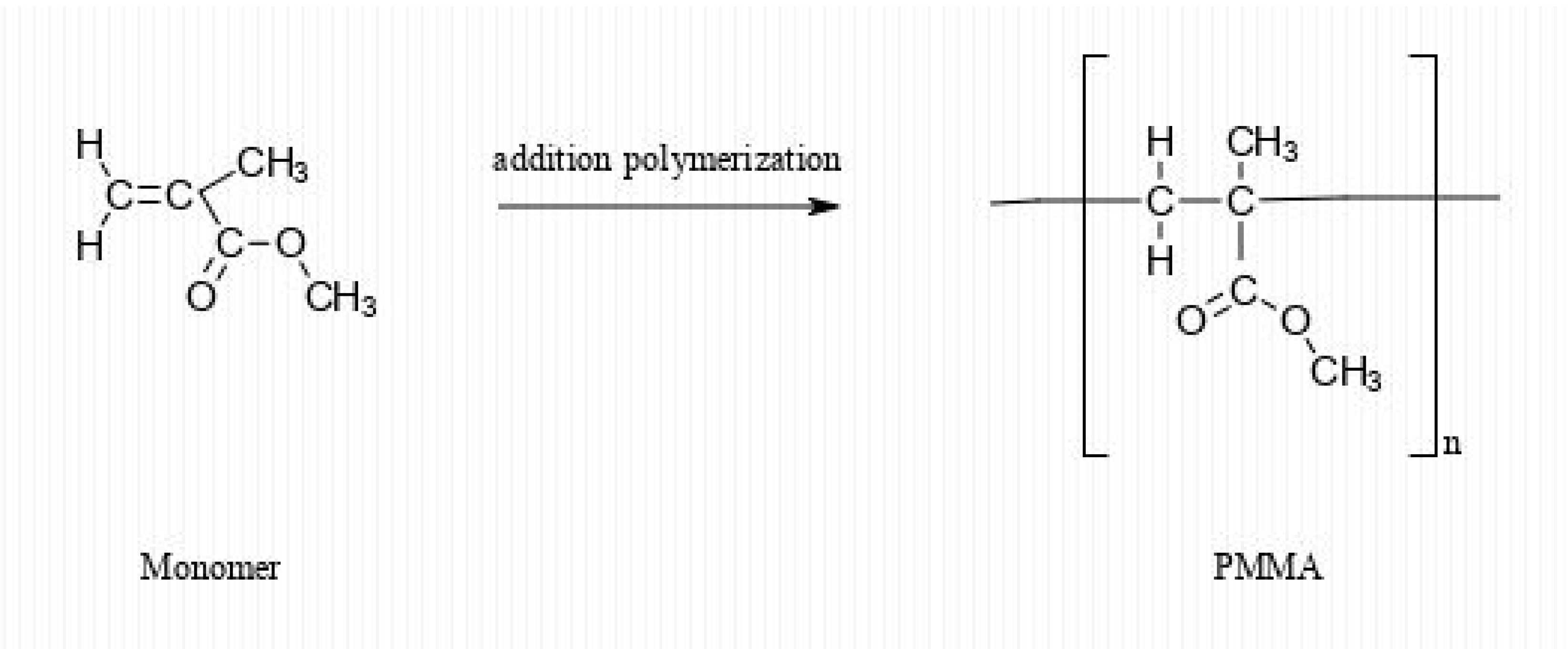
2. PMMA Chemistry
3. PMMA-Based Dental Materials’ Interaction with Oral Cells and Tissues
4. Possible Toxic Effects of PMMA-Based Dental Materials on Oral Cells
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, Y.; Xiao, J.F.; Yang, H.F.; Jiao, Y.; Cao, W.W.; Shi, H.M.; Cun, J.F.; Tay, F.R.; Ping, J.; Xiao, Y.H. N-Acetyl cysteine as a novel polymethyl methacrylate resin component: Protection against cell apoptosis and genotoxicity. Oxidative Med. Cell. Longev. 2019. [Google Scholar] [CrossRef] [PubMed]
- Rosenstiel, S.F.; Land, M.F.; Fujimoto, J. Contemporary Fixed Prosthodontics, 5th ed.; Mosby: St. Louis, MI, USA, 2015; pp. 401–404. [Google Scholar]
- Burns, D.R.; Beck, D.A.; Nelson, S.K. A review of selected dental literature on contemporary provisional fixed prosthodontic treatment: Report of the committee on research in fixed prosthodontics of the academy of fixed prosthodontics. J. Prosthet. Dent. 2003, 90, 474–497. [Google Scholar] [CrossRef]
- Gough, M. A review of temporary crowns and bridges. Dent. Update 1994, 21, 203–207. [Google Scholar] [PubMed]
- Lee, J.; Lee, S. Evaluation of add-on methods for bis-acryl composite resin interim restorations. J. Prosthet. Dent. 2015, 114, 594–601. [Google Scholar] [CrossRef]
- Zhang, J.; Liao, J.; Mo, A.; Li, Y.; Li, J.; Wang, X. Characterization and human gingival fibroblasts biocompatibility of hydroxyapatite/PMMA nanocomposites for provisional dental implant restoration. Appl. Surf. Sci. 2008, 255, 328–330. [Google Scholar] [CrossRef]
- Balkenhol, M.; Mautner, M.C.; Ferger, P.; Wöstmann, B. Mechanical properties of provisional crown and bridge materials: Chemical-curing versus dual-curing systems. J. Dent. 2008, 36, 15–20. [Google Scholar] [CrossRef]
- Gracis, S.; Fradeani, M.; Celletti, R.; Bracchetti, G. Biological integration of aesthetic restorations: Factors influencing appearance and long-term success. Periodontology 2000 2001, 27, 29–44. [Google Scholar] [CrossRef]
- Krishna Prasad, D.; Shetty, M.; Alva, H.; Anupama Prasad, D. Provisional restorations in prosthodontic rehabilitations—Concepts, materials and techniques. J. Health Allied Sci. 2012, 2, 72–77. [Google Scholar] [CrossRef] [Green Version]
- Tahayeri, A.; Morgan, M.; Fugolin, A.P.; Bompolaki, D.; Athirasala, A.; Pfeifer, C.S.; Ferracane, J.L.; Bertassoni, L.E. 3D printed versus conventionally cured provisional crown and bridge dental materials. Dent. Mater. 2018, 34, 192–200. [Google Scholar] [CrossRef]
- Totu, E.E.; Cristache, C.M. Could the old poly (methylmethacrylate) face arrising challanges of new advanced technologies for dental prosthesis manufacturing. Rev. Chim. 2017, 68, 2102–2107. [Google Scholar] [CrossRef]
- Organisation For Economic Co-Operation and Development (OECD). Series on Testing and Assessment No. 80. Guidance on Grouping of Chemicals; OECD: Paris, France, 2007. [Google Scholar]
- Albertini, R.J. The lower alkyl methacrylates: Genotoxic profile of non-carcinogenic compounds. Regul. Toxicol. Pharmacol. 2017, 84, 77–93. [Google Scholar] [CrossRef] [PubMed]
- Osman, R.; Clark, W.J.; Mazurek, A.P.; Weinstein, H. Theoretical studies of molecular mechanisms of DNA damage induced by hydroxyl radicals. Free Radic. Res. Commun. 1989, 6, 131–132. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.M.; Amtower, A.; Doerr, C.L.; Brock, K.H.; Dearfield, K.L. Genotoxicity of acrylic acid, methyl acrylate, ethyl acrylate, methyl methacrylate, and ethyl methacrylate in L5178Y mouse lymphoma cells. Environ. Mol. Mutagen. 1988, 11, 49–63. [Google Scholar] [CrossRef]
- Yoshii, E. Cytotoxic effects of acrylates and methacrylates: Relationships of monomer structures and cytotoxicity. J. Biomed. Mater Res. 1997, 37, 517–524. [Google Scholar] [CrossRef]
- Dearfield, K.L.; Millis, C.S.; Harrington-Brock, K.; Doerr, C.L.; Moore, M.M. Analysis of the genotoxicity of nine acrylate/methacrylate compounds in L5178Y mouse lymphoma cells. Mutagenesis 1989, 4, 381–393. [Google Scholar] [CrossRef] [PubMed]
- Jun, S.K.; Mahapatra, C.; Lee, H.H.; Kim, H.W.; Lee, J.H. Biological effects of provisional resin materials on human dental pulp stem cells. Oper. Dent. 2017, 42, E81–E92. [Google Scholar] [CrossRef]
- Gonçalves, F.P.; Alves, G.; Guimarães, V.O.; Gallito, M.A.; Oliveira, F.; Scelza, M.Z. Cytotoxicity evaluation of two bis-acryl composite resins using human gingival fibroblasts. Braz. Dent. J. 2016, 27, 492–496. [Google Scholar] [CrossRef] [Green Version]
- Borzangy, S.; Labban, N.; Windsor, L.J. Effects of interim acrylic resins on the expression of cytokines from epithelial cells and on collagen degradation. J. Prosthet. Dent. 2013, 110, 296–302. [Google Scholar] [CrossRef]
- Dubiel, E.A.; Martin, Y.; Vermette, P. Bridging the gap between physicochemistry and interpretation prevalent in cell-surface interactions. Chem. Rev. 2011, 111, 2900–2936. [Google Scholar] [CrossRef]
- Allen, L.T.; Tosetto, M.; Miller, I.S.; O’Connor, D.P.; Penney, S.C.; Lynch, I.; Keenan, A.K.; Pennington, S.R.; Dawson, K.A.; Gallagher, W.M. Surface-induced changes in protein adsorption and implications for cellular phenotypic responses to surface interaction. Biomaterials 2006, 27, 3096–3108. [Google Scholar] [CrossRef]
- Unadkat, H.V.; Groen, N.; Doorn, J.; Fischer, B.; Barradas, A.M.; Hulsman, M.; van de Peppel, J.; Moroni, L.; van Leeuwen, J.P.; Reinders, M.J.; et al. High content imaging in the screening of biomaterial-induced MSC behavior. Biomaterials 2013, 34, 1498–1505. [Google Scholar] [CrossRef] [PubMed]
- Unadkat, H.V.; Hulsman, M.; Cornelissen, K.; Papenburg, B.J.; Truckenmüller, R.K.; Carpenter, A.E.; Wessling, M.; Post, G.F.; Uetz, M.; Reinders, M.J.T.; et al. An algorithm-based topographical biomaterials library to instruct cell fate. Proc. Natl. Acad. Sci. USA 2011, 108, 16565–16570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeung, T.; Georges, P.C.; Flanagan, L.A.; Marg, B.; Ortiz, M.; Funaki, M.; Zahir, N.; Ming, W.; Weaver, V.; Janmey, P.A. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil. Cytoskelet. 2005, 60, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Mao, A.S.; Shin, J.W.; Mooney, D.J. Effects of substrate stiffness and cell-cell contact on mesenchymal stem cell differentiation. Biomaterials 2016, 98, 184–191. [Google Scholar] [CrossRef] [Green Version]
- Abdallah, M.N.; Tran, S.D.; Abughanam, G.; Laurenti, M.; Zuanazzi, D.; Mohamed, A.; Mezour, M.A.; Xiao, Y.; Cerruti, M.; Siqueira, W.L.; et al. Biomaterial surface proteomic signature determines interaction with epithelial cells. Acta Biomater. 2017, 54, 150–163. [Google Scholar] [CrossRef]
- Roach, P.; Eglin, D.; Rohde, K.; Perry, C.C. Modern biomaterials: A review—Bulk properties and implications of surface modifications. J. Mater. Sci. Mater. Med. 2007, 18, 1263–1277. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.J.; Clegg, R.E.; Leavesley, D.I.; Pearcy, M.J. Mediation of biomaterial-cell interactions by adsorbed proteins: A review. Tissue Eng. 2005, 11, 1–18. [Google Scholar] [CrossRef]
- Bosshardt, D.D.; Lang, N.P. The junctional epithelium: From health to disease. J. Dent. Res. 2005, 84, 9–20. [Google Scholar] [CrossRef]
- de Berker, D.A.; Andre, J.; Baran, R. Nail biology and nail science. Int. J. Cosmet. Sci. 2007, 29, 241–275. [Google Scholar] [CrossRef]
- Shimono, M.; Ishikawa, T.; Enokiya, Y.; Muramatsu, T.; Matsuzaka, K.; Inoue, T.; Abiko, Y.; Yamaza, T.; Kido, M.A.; Tanaka, T.; et al. Biological characteristics of the junctional epithelium. J. Electron Microsc. 2003, 52, 627–639. [Google Scholar] [CrossRef]
- Saito, M.; Ohyama, M.; Amagai, M. Exploring the biology of the nail: An intriguing but less-investigated skin appendage. J. Dermatol. Sci. 2015, 79, 187–193. [Google Scholar] [CrossRef] [PubMed]
- LeBleu, V.S.; Macdonald, B.; Kalluri, R. Structure and function of basement membranes. Exp. Biol. Med. 2007, 232, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, T.; Amano, S.; Tsunenaga, M.; Kadoya, K.; Takeda, A.; Adachi, E.; Burgeson, R.E. The importance of laminin 5 in the dermal-epidermal basement membrane. J. Dermatol. Sci. 2000, 24, S51–S59. [Google Scholar] [CrossRef]
- Atsuta, I.; Ayukawa, Y.; Ogino, Y.; Moriyama, Y.; Jinno, Y.; Koyano, K. Evaluations of epithelial sealing and peri-implant epithelial down-growth around ‘‘steptype” implants. Clin. Oral Implants Res. 2012, 23, 459–466. [Google Scholar] [CrossRef]
- Atsuta, I.; Yamaza, T.; Yoshinari, M.; Mino, S.; Goto, T.; Kido, M.A.; Terada, Y.; Tanaka, T. Changes in the distribution of laminin-5 during peri-implant epithelium formation after immediate titanium implantation in rats. Biomaterials 2005, 26, 1751–1760. [Google Scholar] [CrossRef]
- Gordon, D.J.; Bhagawati, D.D.; Pendegrass, C.J.; Middleton, C.A.; Blunn, G.W. Modification of titanium alloy surfaces for percutaneous implants by covalently attaching laminin. J. Biomed. Mater. Res. A 2010, 94, 586–593. [Google Scholar] [CrossRef]
- Alam, H.; Sehgal, L.; Kundu, S.T.; Dalal, S.N.; Vaidya, M.M. Novel function of keratins 5 and 14 in proliferation and differentiation of stratified epithelial cells. Mol. Biol. Cell 2011, 22, 4068–4078. [Google Scholar] [CrossRef]
- Gu, L.-H.; Coulombe, P.A. Keratin function in skin epithelia: A broadening palette with surprising shades. Curr. Opin. Cell Biol. 2007, 19, 13–23. [Google Scholar] [CrossRef]
- Mokkapati, S.; Baranowsky, A.; Mirancea, N.; Smyth, N.; Breitkreutz, D.; Nischt, R. Basement membranes in skin are differently affected by lack of nidogen 1 and 2. J. Investig. Dermatol. 2008, 128, 2259–2267. [Google Scholar] [CrossRef]
- Vanea, E.; Simon, V. XPS study of protein adsorption onto nanocrystalline aluminosilicate microparticles. Appl. Surf. Sci. 2011, 257, 2346–2352. [Google Scholar] [CrossRef]
- Abdallah, M.N.; Light, N.; Amin, W.M.; Retrouvey, J.M.; Cerruti, M.; Tamimi, F. Development of a composite resin disclosing agent based on the understanding of tooth staining mechanisms. J. Dent. 2014, 42, 697–708. [Google Scholar] [CrossRef]
- Mahjoubi, H.; Kinsella, J.M.; Murshed, M.; Cerruti, M. Surface modification of poly(D, L-lactic acid) scaffolds for orthopedic applications: A biocompatible nondestructive route via diazonium chemistry. ACS Appl. Mater. Interfaces 2014, 6, 9975–9987. [Google Scholar] [CrossRef] [PubMed]
- Wegehaupt, F.J.; Lunghi, N.; Belibasakis, G.N.; Attin, T. Influence of light-curing distance on degree of conversion and cytotoxicity of etch-and-rinse and self-etch adhesives. BMC Oral Health 2016, 17, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michelsen, V.B.; Kopperud, H.B.; Lygre, G.B.; Bjorkman, L.; Jensen, E.; Kleven, I.S.; Svahn, J.; Lygre, H. Detection and quantification of monomers in unstimulated whole saliva after treatment with resin-based composite fillings in vivo. Eur. J. Oral Sci. 2012, 120, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Cebe, M.A.; Cebe, F.; Cengiz, M.F.; Cetin, A.R.; Arpag, O.F.; Ozturk, B. Elution of monomer from different bulk fill dental composite resins. Dent. Mater. 2015, 31, e141–e149. [Google Scholar] [CrossRef]
- Reichl, F.X.; Löhle, J.; Seiss, M.; Furche, S.; Shehata, M.M.; Hickel, R.; Muller, M.; Dranert, M.; Durner, J. Elution of TEGDMA and HEMA from polymerized resin-based bonding systems. Dent. Mater. 2012, 28, 1120–1125. [Google Scholar] [CrossRef]
- Singh, R.D.; Gautam, R.; Siddhartha, R.; Singh, B.P.; Chand, P.; Sharma, V.P.; Jurel, S.K. High performance liquid chromatographic determination of residual monomer released from heat-cured acrylic resin: An in vivo study. J. Prosthodont. 2013, 22, 358–361. [Google Scholar] [CrossRef]
- Jiao, Y.; Ma, S.; Wang, Y.; Li, J.; Shan, L.; Chen, J. Epigallocatechin-3-gallate reduces cytotoxic effects caused by dental monomers: A hypothesis. Med. Sci. Monit. 2015, 21, 3197–3202. [Google Scholar] [CrossRef] [Green Version]
- Gautam, R.; Singh, R.D.; Sharma, V.P.; Siddhartha, R.; Chand, P.; Kumar, R. Biocompatibility of polymethylmethacrylate resins used in dentistry. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 1444–1450. [Google Scholar] [CrossRef]
- Leggat, P.A.; Kedjarune, U. Toxicity of methyl methacrylate in dentistry. Int. Dent. J. 2003, 53, 126–131. [Google Scholar] [CrossRef]
- Mittermüller, P.; Hiller, K.A.; Schmalz, G.; Buchalla, W. Five hundred patients reporting on adverse effects from dental materials: Frequencies, complaints, symptoms, allergies. Dent. Mater. 2018, 34, 1756–1768. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, S.; Atsumi, T. Cytotoxicities of a 4-META/MMA-TBBO resin against human pulp fibroblasts. Dent. Mater. J. 2011, 23, 106–108. [Google Scholar] [CrossRef] [Green Version]
- Shimada, Y.; Seki, Y.; Uzzaman, M.A.; Sattabanasuk, V.; Sasafuchi, Y.; Foxton, R.M.; Otsuki, M.; Tagami, J. Monkey pulpal response to an MMA-based resin cement as adhesive luting for indirect restorations. J. Adhes. Dent. 2005, 7, 247–251. [Google Scholar]
- Krifka, S.; Spagnuolo, G.; Schmalz, G.; Schweikl, H. A review of adaptive mechanisms in cell responses towards oxidative stress caused by dental resin monomers. Biomaterials 2013, 34, 4555–4563. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Niu, L.N.; Ma, S.; Li, J.; Tay, F.R.; Chen, J.H. Quaternary ammonium-based biomedical materials: State-of-theart, toxicological aspects and antimicrobial resistance. Prog. Polym. Sci. 2017, 71, 53–90. [Google Scholar] [CrossRef] [PubMed]
- Schweikl, H.; Spagnuolo, G.; Schmalz, G. Genetic and cellular toxicology of dental resin monomers. J. Dent. Res. 2006, 85, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Kojima, N.; Yamada, M.; Paranjpe, A.; Tsukimura, N.; Kubo, K.; Jewett, A.; Ogawa, T. Restored viability and function of dental pulp cells on poly-methylmethacrylate (PMMA)-based dental resin supplemented with N-acetyl cysteine (NAC). Dent. Mater. 2008, 24, 1686–1693. [Google Scholar] [CrossRef]
- Miricescu, D.; Totan, A.; Calenic, B.; Mocanu, B.; Didilescu, A.; Mohora, M.; Spinu, T.; Greabu, M. Salivary biomarkers: Relationship between oxidative stress and alveolar bone loss in chronic periodontitis. Acta Odontol. Scand. 2014, 72, 42–47. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative stress and inflammation: What polyphenols can do for us? Oxidative Med. Cell. Longev. 2016. [Google Scholar] [CrossRef] [Green Version]
- Salzano, S.; Checconi, P.; Hanschmann, E.M.; Lillig, C.H.; Bowler, L.D.; Chan, P.; Vaudry, D.; Mengozzi, M.; Coppo, L.; Sacre, S.; et al. Linkage of inflammation and oxidative stress via release of glutathionylated peroxiredoxin-2, which acts as a danger signal. Proc. Natl. Acad. Sci. USA 2014, 111, 12157–12162. [Google Scholar] [CrossRef] [Green Version]
- Ilea, A.; Băbţan, A.M.; Boşca, B.A.; Crișan, M.; Petrescu, N.B.; Collino, M.; Sainz, R.M.; Gerlach, J.Q.; Câmpian, R.S. Advanced glycation end products (AGEs) in oral pathology. Arch. Oral Biol. 2018, 93, 22–30. [Google Scholar] [CrossRef]
- Jiao, Y.; Ma, S.; Wang, Y.; Li, J.; Shan, L.; Liu, Q.; Liu, Y.; Song, Q.; Yu, F.; Yu, H.; et al. N-Acetyl cysteine depletes reactive oxygen species and prevents dental monomer-induced intrinsic mitochondrial apoptosis in vitro in human dental pulp cells. PLoS ONE 2016, 11, e0147858. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Wang, Y.; Guo, S.; Wang, G. Glutathione peroxidases as oncotargets. Oncotarget 2017, 8, 80093–80102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallorini, M.; Petzel, C.; Bolay, C.; Hiller, K.A.; Cataldi, A.; Buchalla, W.; Krifka, S.; Schweikl, H. Activation of the Nrf2-regulated antioxidant cell response inhibits HEMA-induced oxidative stress and supports cell viability. Biomaterials 2015, 56, 114–128. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Niu, T.; Liu, H.; Tay, F.R.; Chen, J. Protection against HEMA-induced mitochondrial injury in vitro by Nrf2 activation. Oxidative Med. Cell. Longev. 2019. [Google Scholar] [CrossRef]
- Szczepanska, J.; Poplawski, T.; Synowiec, E.; Pawlowska, E.; Chojnacki, C.J.; Chojnacki, J.; Blasiak, J. 2-hydroxylethyl methacrylate (HEMA), a tooth restoration component, exerts its genotoxic effects in human gingival fibroblasts trough methacrylic acid, an immediate product of its degradation. Mol. Biol. Rep. 2012, 39, 1561–1574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleinsasser, N.H.; Schmid, K.; Sasse, A.W.; Harreus, U.A.; Staudenmaier, R.; Folwaczny, M.; Glas, J.; Reichl, F.-X. Cytotoxic and genotoxic effects of resin monomers in human salivary gland tissue and lymphocytes as assessed by the single cell microgel electrophoresis (comet) assay. Biomaterials 2005, 27, 1762–1770. [Google Scholar] [CrossRef] [PubMed]
- Urcan, E.; Scherthan, H.; Styllou, M.; Haertel, U.; Hickel, R.; Reichl, F.-X. Induction of DNA double-strand breaks in primary gingival fibroblasts by exposure to dental resin composites. Biomaterials 2009, 31, 2010–2014. [Google Scholar] [CrossRef]
- Eckhardt, A.; Gerstmayr, N.; Hiller, K.A.; Bolay, C.; Waha, C.; Spagnuolo, G.; Camargo, C.; Schmalz, G.; Schweikl, H. TEGDMA-induced oxidative DNA damage and activation of ATM and MAP kinases. Biomaterials 2009, 30, 2006–2014. [Google Scholar] [CrossRef]
- Cline, D.; Hanawalt, P.C. Who’s on first in the cellular response to DNA damage? Nat. Rev. Mol. Biol. Cell 2003, 4, 361–373. [Google Scholar] [CrossRef]
- Slee, E.A.; O’Connor, D.J.; Lu, X. To die or not to die: How does p53 decide? Oncogene 2004, 23, 2809–2818. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, R.; Meuth, M. Chk1 and p21 cooperate to prevent apoptosis during DNA replication fork stress. Mol. Biol. Cell 2005, 17, 402–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schweikl, H.; Petzel, C.; Bolay, C.; Hiller, K.-A.; Buchalla, W.; Krifka, S. 2-Hydroxyethyl methacrylate-induced apoptosis through the ATM- and p53-dependent intrinsic mitochondrial pathway. Biomaterials 2014, 35, 2890–2904. [Google Scholar] [CrossRef] [PubMed]
- Fridman, J.S.; Lowe, S.W. Control of apoptosis by p53. Oncogene 2003, 22, 9030–9040. [Google Scholar] [CrossRef] [Green Version]
- Francipane, M.G.; Lagasse, E. mTOR pathway in colorectal cancer: An update. Oncotarget 2014, 1, 49–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, S.; Banerjee, S.; Chakraborty, T. Vanadium quercetin complex attenuates mammary cancer by regulating the P53, Akt/mTOR pathway and downregulates cellular proliferation correlated with increased apoptotic events. Biometals 2018, 31, 647–667. [Google Scholar] [CrossRef] [PubMed]
- Lavorgna, L.; Cervino, G.; Fiorillo, L.; Di Leo, G.; Troiano, G.; Ortensi, M.; Galantucci, L.; Cicciù, M. Reliability of a virtual prosthodontic project realized through a 2d and 3d photographic acquisition: An experimental study on the accuracy of different digital systems. Int. J. Environ. Res. Public Health 2019, 16, 5139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Xu, Z.; Wu, D.; Bai, J. Current Status and Prospects of Polymer Powder 3D Printing Technologies. Materials 2020, 13, 2406. [Google Scholar] [CrossRef]
- Campaner, M.; Takamiya, A.S.; Bitencourt, S.B.; Mazzaa, L.C.; Penha de Oliveira, S.H.; Shibayama, R.; Barãof, V.A.R.; Sukotjoe, C.; Alves Pesqueira, A. Cytotoxicity and inflammatory response of different types of provisional restorative materials. Arch. Oral. Biol. 2020, 111, 104643. [Google Scholar] [CrossRef]
- Shim, J.S.; Kim, H.C.; Park, S.I.; Yun, H.J.; Ryu, J.J. Comparison of various implant provisional resin materials for cytotoxicity and attachment to human gingival fibroblasts. Int. J. Oral Maxillofac. Implants 2019, 34, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Engler, M.L.P.D.; Güth, J.; Keul, C.; Erdelt, K.; Edelhoff, D.; Liebermann, A. Residual monomer elution from different conventional and CAD/CAM dental polymers during artificial aging. Clin. Oral Invest. 2020, 24, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Atay, A.; Gürdal, I.; Bozok Çetıntas, V.; Üşümez, A.; Cal, E. Effects of new generation all-ceramic and provisional materials on fibroblast cells. J. Prosthodont. 2019, 28, e383–e394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herráez-Galindo, C.; Rizo-Gorrita, M.; Luna-Oliva, I.; Serrera-Figallo, M.Á.; Castillo-Oyagüe, R.; Torres-Lagares, D. In vitro comparative study of fibroblastic behaviour on polymethacrylate (PMMA) and Lithium disilicate polymer surfaces. Polymers 2019, 11, 744. [Google Scholar] [CrossRef] [Green Version]
- Barui, S.; Panda, A.K.; Naskar, S.; Kuppuraj, R.; Basu, S.; Basu, B. 3D inkjet printing of biomaterials with strength reliability and cytocompatibility: Quantitative process strategy for Ti-6Al-4V. Biomaterials 2019, 213, 119212. [Google Scholar] [CrossRef] [PubMed]
- Herzberger, J.; Sirrine, J.M.; Williams, C.B.; Long, T.E. Polymer design for 3D printing elastomers: Recent advances in structure, properties, and printing. Prog. Polym. Sci. 2019. [Google Scholar] [CrossRef]
- Szymczyk-Ziółkowska, P.; Łabowska, M.B.; Detyna, J.; Michalak, I.; Gruber, P. A review of fabrication polymer scaffolds for biomedical applications using additive manufacturing techniques. Probl. Biocybern. Biomed. Eng. 2020, 40, 624–638. [Google Scholar] [CrossRef]
- Di Giacomo, G.D.A.; Cury, P.R.; da Silva, A.M.; da Silva, J.V.; Ajzen, S.A. A selective laser sintering prototype guide used to fabricate immediate interim fixed complete arch prostheses in flapless dental implant surgery: Technique description and clinical results. J. Prosthet. Dent. 2016, 116, 874–879. [Google Scholar] [CrossRef]
- Nayar, S.; Bhuminathan, S.; Bhat, W.M. Rapid prototyping and stereolithography in dentistry. J. Pharm. Bioallied Sci. 2015, 7, S216. [Google Scholar] [CrossRef]
- Alharbi, N.; Osman, R.; Wismeijer, D. Effects of build direction on the mechanical properties of 3D-printed complete coverage interim dental restorations. J. Prosthet. Dent. 2016, 115, 760–767. [Google Scholar] [CrossRef]
- Zhang, A.P.; Qu, X.; Soman, P.; Hribar, K.C.; Lee, J.W.; Chen, S.; He, S. Rapid fabrication of complex 3D extracellular microenvironments by dynamic optical projection stereolithography. Adv. Mater. 2012, 24, 4266–4270. [Google Scholar] [CrossRef] [Green Version]
- Revilla-León, M.; Meyers, M.J.; Zandinejad, A.; Özcan, M. A review on chemical composition, mechanical properties, and manufacturing workflow of additively manufactured current polymers for interim dental restorations. J. Esthet. Restor. Dent. 2019, 31, 51–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jockusch, J.; Özcan, M. Additive manufacturing of dental polymers: An overview on processes, materials and applications. Dent. Mater. J. 2020, 39, 345–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ide, Y.; Nayar, S.; Logan, H.; Gallagher, B.; Wolfaardt, J. The effect of the angle of acuteness of additive manufactured models and the direction of printing on the dimensional fidelity: Clinical implications. Odontology 2017, 105, 108–115. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pituru, S.M.; Greabu, M.; Totan, A.; Imre, M.; Pantea, M.; Spinu, T.; Tancu, A.M.C.; Popoviciu, N.O.; Stanescu, I.-I.; Ionescu, E. A Review on the Biocompatibility of PMMA-Based Dental Materials for Interim Prosthetic Restorations with a Glimpse into Their Modern Manufacturing Techniques. Materials 2020, 13, 2894. https://doi.org/10.3390/ma13132894
Pituru SM, Greabu M, Totan A, Imre M, Pantea M, Spinu T, Tancu AMC, Popoviciu NO, Stanescu I-I, Ionescu E. A Review on the Biocompatibility of PMMA-Based Dental Materials for Interim Prosthetic Restorations with a Glimpse into Their Modern Manufacturing Techniques. Materials. 2020; 13(13):2894. https://doi.org/10.3390/ma13132894
Chicago/Turabian StylePituru, Silviu Mirel, Maria Greabu, Alexandra Totan, Marina Imre, Mihaela Pantea, Tudor Spinu, Ana Maria Cristina Tancu, Nicoleta Olivia Popoviciu, Iulia-Ioana Stanescu, and Ecaterina Ionescu. 2020. "A Review on the Biocompatibility of PMMA-Based Dental Materials for Interim Prosthetic Restorations with a Glimpse into Their Modern Manufacturing Techniques" Materials 13, no. 13: 2894. https://doi.org/10.3390/ma13132894
APA StylePituru, S. M., Greabu, M., Totan, A., Imre, M., Pantea, M., Spinu, T., Tancu, A. M. C., Popoviciu, N. O., Stanescu, I.-I., & Ionescu, E. (2020). A Review on the Biocompatibility of PMMA-Based Dental Materials for Interim Prosthetic Restorations with a Glimpse into Their Modern Manufacturing Techniques. Materials, 13(13), 2894. https://doi.org/10.3390/ma13132894








