Improving Sodium Alginate Films Properties by Phenolic Acid Addition
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples Preparation
2.2. Infrared Spectroscopy (FTIR-ATR)
2.3. Mechanical Parameters
2.4. Wettability
2.5. DPPH Radical Scavenging Assay
2.6. Color Change
2.7. Water Vapor Permeation Rate (WVPR)
2.8. Oxygen Permeability
3. Results
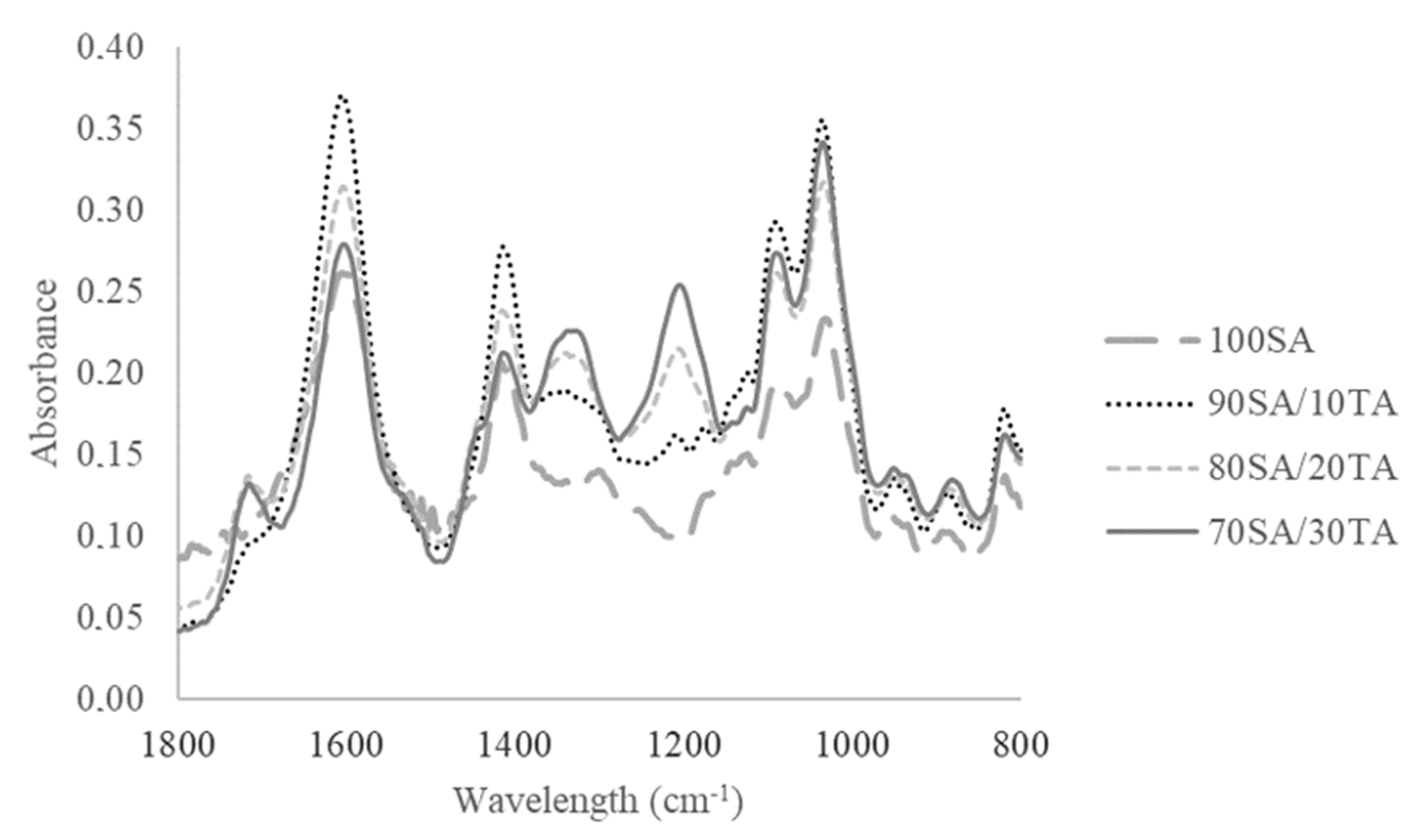
3.1. Infrared Spectroscopy (FTIR-ATR)
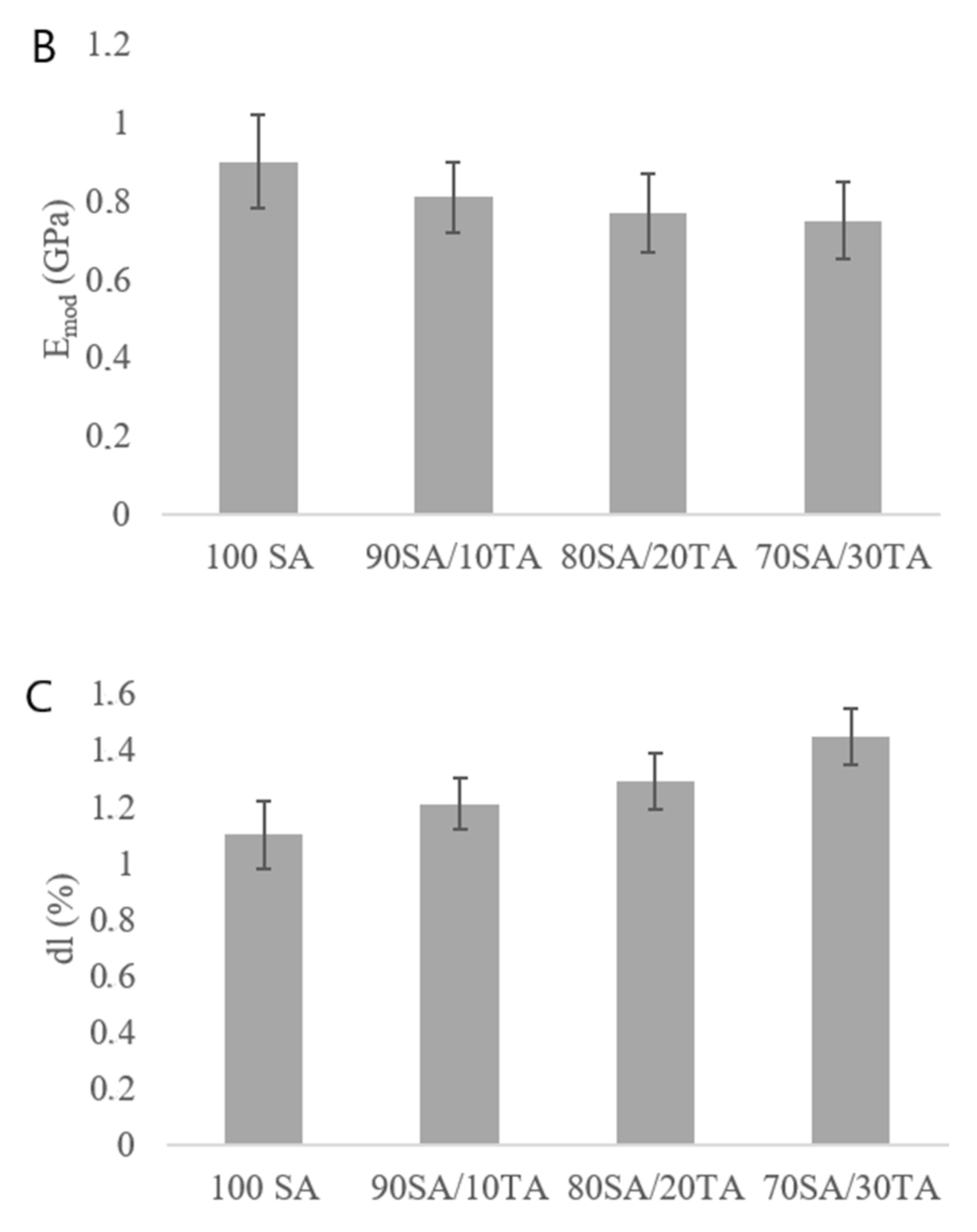
3.2. Mechanical Parameters
3.3. Wettability
3.4. DPPH Radical Scavenging Assay
3.5. Color Change
3.6. Water Vapor Permeation Rate (WVPR)
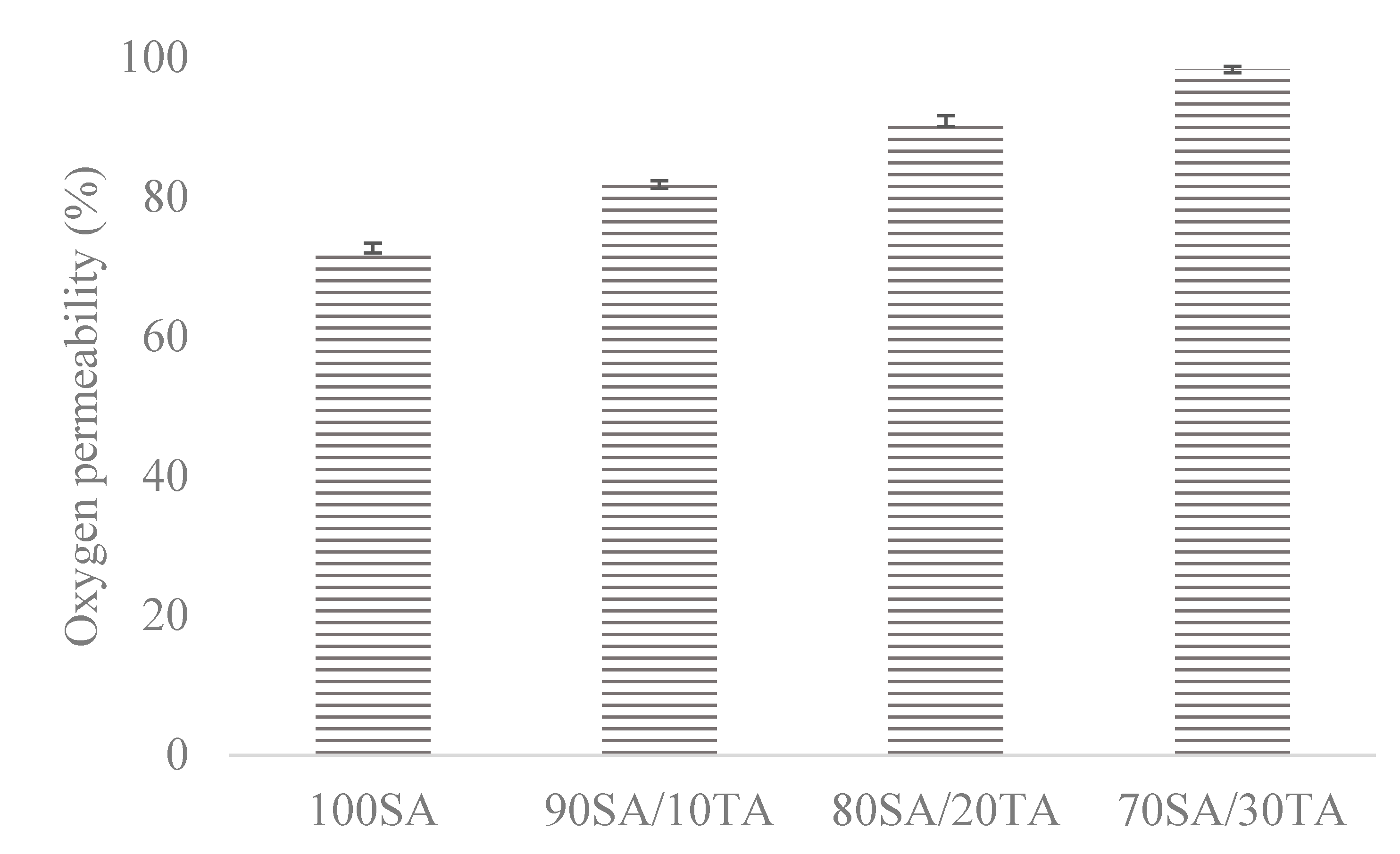
3.7. Oxygen Permeability
4. Discussion
4.1. Infrared Spectroscopy (FTIR-ATR)
4.2. Mechanical Parameters
4.3. Wettability
4.4. DPPH Radical Scavenging Assay
4.5. Color Change
4.6. Water Vapor Permeation Rate (WVPR)
4.7. Oxygen Permeability
5. Conclusion
Funding
Conflicts of Interest
References
- Marsz, K.; Bugusu, B. Food packaging—Roles, materials, and environmental issues. J. Food Sci. 2007, 72, R39–R55. [Google Scholar] [CrossRef] [PubMed]
- Muthuraj, R.; Misra, M.; Mohanty, A.K. Biodegradable compatibilized polymer blends for packaging applications: A literature review. J. Appl. Polym. Sci. 2018, 135, 45726. [Google Scholar] [CrossRef]
- Moustafa, H.; Youssef, A.M.; Darwish, N.A.; Abou-Kandil, A.I. Eco-friendly polymer composites for green packaging: Future vision and challenges. Compos. Part B Eng. 2019, 172, 16–25. [Google Scholar] [CrossRef]
- Dang, X.; Yuan, H.; Shan, Z. An eco-friendly material based on graft copolymer of gelatin extracted from leather solid waste for potential application in chemical sand-fixation. J. Clean. Prod. 2018, 188, 416–424. [Google Scholar] [CrossRef]
- Lie, J.; Yang, S.; Li, D.; Chalivendra, V. Numerical and experimental studies of additively manufactured polymers for enhanced fracture properties. Eng. Fract. Mech. 2018, 204, 557–569. [Google Scholar] [CrossRef]
- Danso, D.; Chow, J.; Streit, W.R. Plastics: Environmental and biotechnological perspectives on microbial degradation. Appl. Environ. Microbiol. 2019, 85, e01095-19. [Google Scholar] [CrossRef]
- Piergiovanni, L.; Limbo, S. Plastic packaging materials. Food Packag. Mater. 2015, 5, 33–49. [Google Scholar]
- Garcia Ibarra, V.; Quiros, A.; Losada, P.P.; Sendon, R. Non-target analysis of intentionally and non intentionally added substances from plastic packaging materials and their migration into food simulants. Food Packag. Shelf Life 2019, 21, 100325. [Google Scholar] [CrossRef]
- Siracusa, V.; Rocculi, P.; Romani, S.; Rosa, M. Biodegradable polymers for food packaging: A review. Trends Food Sci. Tech. 2008, 19, 634–643. [Google Scholar] [CrossRef]
- Tanan, W.; Panichpakdee, J.; Saengsuwan, S. Novel biodegradable hydrogel based on natural polymers: Synthesis, characterization, swelling/reswelling and biodegradability. Eur. Polym. J. 2019, 112, 678–687. [Google Scholar] [CrossRef]
- Sam, S.T.; Nuradibah, M.A.; Chin, K.M.; Hani, N. Current application and challenges on packaging industry based on natural polymer blending. Nat. Polym. 2015, 163–184. [Google Scholar] [CrossRef]
- Cui, X.; Lee, J.; Chen, W.N. Eco-friendly and biodegradable cellulose hydrogels produced from low cost okara: Towards non-toxic flexible electronics. Sci. Rep. 2019, 9, 18166. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, J.; Sobral, P.J.A. Antioxidant and physicochemical properties of blended films based on gelatin-sodium caseinate activated with natural extracts. J. Appl. Polym. Sci. 2016, 134. [Google Scholar] [CrossRef]
- Noorbakhsh-Soltani, S.M.; Zerafat, M.M.; Sabbaghi, S. A comparative study of gelatin and starch-based nano-composite films modified by nano-cellulose and chitosan for food packaging applications. Carbohyd. Polym. 2018, 189, 48–55. [Google Scholar] [CrossRef]
- Lopusiewicz, L.; Jedra, F.; Bartkowiak, A. New active packaging films made from gelatin modified with fungal melanin. World Sci. News 2018, 101, 1–30. [Google Scholar]
- Taheddin, B.; Ahmadi, B.; Sohrab, F.; Chenarbon, H.A. Polymers for modified atmosphere packaging applications. Food Packag. Preserv. 2018, 457–499. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Kenawy, I.M.; Hafez, M.A.H.; Ismail, M.A.; Hashem, M.A. Adsorption of Cu(II), Cd(II), Hg(II), Pb(II) and Zn(II) from aqueous single metal solutions by guanyl-modified cellulose. Int. J. Biol. Macromol. 2018, 107, 1538–1549. [Google Scholar] [CrossRef]
- Yang, J.; Xia, Y.; Xu, P.; Chem, B. Super-elastic and highly hydrophobic/superoleophilic sodium alginate/cellulose aerogel for oil/water separation. Cellulose 2018, 25, 3533–3544. [Google Scholar] [CrossRef]
- Zhang, L.; Fang, H.; Zhang, K.; Yin, J. Homologous sodium alginate/chitosan-based scaffolds, but contrasting effect on stem cell shape and osteogenesis. ACS Appl. Mater. Interfaces 2018, 10, 6930–6941. [Google Scholar] [CrossRef]
- Chaudhari, S.; Arshad, S.; Amjad, M.S.; Akhtar, M.S. Natural compounds extracted from medicinal plants and their applications. Nat. Bio-active Compd. 2019, 193–207. [Google Scholar] [CrossRef]
- Wan-Fei, L.; Chik, W.I.; Dong-Ying, W.; Lu-Tai, P. Plant phenolic compounds as potential lead compounds in functional foods for antiviral drug discovery. Curr. Org. Chem. 2017, 21, 1847–1860. [Google Scholar]
- Dewi, I.C.; Falaise, C.; Hellio, C.; Bourgougnon, N.; Mouget, J.L. Anticancer, antiviral, antibacterial, and antifungal properties in microalgae. Microalgae Health Dis. Prev. 2018, 235–261. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Waldron, K.W. Crosslinking in polysaccharide and protein films and coatings for food contact—A review. Trends Food Sci. Technol. 2016, 52, 109–122. [Google Scholar] [CrossRef]
- Roberts, J.J.; Naudiyal, P.; Lim, K.S.; Poole-Warren, L.A.; Martens, P.J. A comparative study of enzyme initiators for crosslinking phenol-functionalized hydrogels for cell encapsulation. Biomater. Res. 2016, 20, 30. [Google Scholar] [CrossRef]
- Kaczmarek, B.; Nadolna, K.; Owczarek, A.; Michalska-Sionkowska, M.; Sionkowska, A. The characterization of thin films based on chitosan and tannic acid mixture for potential applications as wound dressings. Polym. Test. 2019, 78, 106007. [Google Scholar] [CrossRef]
- Yang, S.; Wu, W.; Jiao, Y.; Cai, Z.; Fan, H. Preparation of NBR/Tannic acid composites by assembling a weak IPN structure. Compos. Sci. Technol. 2017, 153, 40–47. [Google Scholar] [CrossRef]
- Kaczmarek, B.; Sionkowska, A.; Osyczka, A.M. Scaffolds based on chitosan and collagen with glycosaminoglycans cross-linked by tannic acid. Polym. Test. 2018, 65, 163–168. [Google Scholar] [CrossRef]
- Kaczmarek, B.; Sionkowska, A.; Otrocka-Domagala, I.; Polkowska, I. In vivo studies of novel scaffolds with tannic acid addition. Polym. Deg. Stab. 2018, 158, 26–30. [Google Scholar] [CrossRef]
- Yang, J.; Li, M.; Wang, Y.; Wu, H.; Zhen, T.; Xiong, L.; Sun, Q. Double cross-linked chitosan composite films developed with oxidized tannic acid and ferric ions exhibit high strength and excellent water resistance. Biomacromolecules 2019, 20, 801–812. [Google Scholar] [CrossRef]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Riaz, T.; Zeeshan, R.; Zarif, F.; Ilyas, K.; Muhammad, N.; Safi, S.Z.; Rafin, A.; Rizvi, S.A.A.; Rehman, I. FTIR analysis of natural and synthetic collagen. Appl. Spectro. Rev. 2018, 53, 703–746. [Google Scholar] [CrossRef]
- Liu, C.; Liu, H.; Xiong, T.; Xu, A.; Pan, B.; Tang, K. Graphene oxide reinforced alginate/PVA double network hydrogels for efficient dye removal. Polymers 2018, 10, 835. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Pei, Y.; Li, J.; Xiong, W.; He, Y.; Liu, S.; Li, Y.; Li, B. pH-Degradable antioxidant nanoparticles based on hydrogen-bonded tannic acid assembly. RSC Adv. 2016, 6, 31374–31385. [Google Scholar] [CrossRef]
- Michalska-Sionkowska, M.; Kaczmarek, B.; Walczak, M.; Sionkowska, A. Antimicrobial activity of new materials based on the blends of collagen/chitosan/hyaluronic acid with gentamicin sulfate addition. Mater. Sci. Eng. C 2018, 86, 103–108. [Google Scholar] [CrossRef]
- Bastarrachea, L.J.; Dhawan, S.; Sablani, S.S. Engineering properties of polymeric-based antimicrobial films for food packaging: A review. Food Eng. Rev. 2011, 3, 79–93. [Google Scholar] [CrossRef]
- Jakubowska, E.; Gierszewska, M.; Nowaczyk, J.; Olewnik-Kruszkowska, E. Physicochemical and storage properties of chitosan-based films plasticized with deep eutectic solvent. Food Hydrocoll. 2020, 108, 106007. [Google Scholar] [CrossRef]
- Nie, L.; Liu, C.; Wang, J.; Shuai, Y.; Ciu, X.; Liu, L. Effects of surface functionalized graphene oxide on the behavior of sodium alginate. Carbohyd. Polym. 2015, 117, 616–623. [Google Scholar] [CrossRef]
- Gotoh, K.; Yasukawa, A.; Kobayashi, Y. Wettability characteristics of poly(ethylene terephthalate) films treated by atmospheric pressure plasma and ultraviolet excimer light. Polym. J. 2011, 53, 545–551. [Google Scholar] [CrossRef][Green Version]
- Junkar, I.; Cvelbar, U.; Lehocky, M. Plasma treatment of biomedical materials. Mater. Tehnol. 2011, 45, 221–226. [Google Scholar]
- Farghal, H.H.; Karabagias, I.; Sayed, M.; Kontominas, M.G. Determination of antioxidant activity of surface-treated PET films coated with rosemary and clove extracts. Packag. Tech. Sci. 2017, 30, 799–808. [Google Scholar] [CrossRef]
- Dou, L.; Li, B.; Zhang, K.; Chu, X.; Hou, H. Physical properties and antioxidant activity of gelatin’sodium alginate edible films with tea polyphenol. Int. J. Biol. Macromol. 2018, 118, 1377–1383. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Jiang, H.; Wu, H.; Tong, C.; Pang, J. Multifunctional bionanocomposite films based on konjac glucomannan/chitosan with nano-ZnO and mulberry anthocyanin extract for active food packaging. Food Hydrocoll. 2020, 107, 105942. [Google Scholar] [CrossRef]
- Liu, H.; Huang, M.; Cui, G.; Luo, M.R.; Melgosa, M. Color-difference evaluation for digital images using a categorical judgment method. J. Opt. Soc. Am. A 2013, 30, 616–626. [Google Scholar] [CrossRef] [PubMed]
- Sothornvit, R.; Pitak, N. Oxygen permeability and mechanical properties of banana films. Food Res. Int. 2007, 40, 365–370. [Google Scholar] [CrossRef]

| Specimen | 0 s | 5 s | 10 s | 15 s |
|---|---|---|---|---|
| 100SA | 73.67 ± 1.16 | 67.33 ± 0.47 | 60.73 ± 1.66 | 51.17 ± 1.25 |
| 90SA/10TA | 90.10 ± 1.55 | 78.85 ± 2.05 | 74.70 ± 0.42 | 66.90 ± 0.28 |
| 80SA/20TA | 85.00 ± 1.47 | 76.97 ± 1.55 | 73.97 ± 1.58 | 68.20 ± 0.94 |
| 70SA/30TA | 76.30 ± 1.40 | 70.15 ± 0.89 | 68.80 ± 1.77 | 65.90 ± 0.72 |
| Specimen | RSA% |
|---|---|
| 100SA | 5.87 ± 1.12 |
| 90SA/10TA | 28.42 ± 0.98 |
| 80SA/20TA | 43.74 ± 1.07 |
| 70SA/30TA | 50.05 ± 0.94 |
| Specimen | Color Parameter | Solution | ||
|---|---|---|---|---|
| HCl | NaOH | Water | ||
| 100SA | L | −7.73 ± 1.24 | −5.90 ± 1.03 | −7.29 ± 0.91 |
| a | 2.15 ± 0.78 | 2.46 ± 0.77 | 2.22 ± 0.73 | |
| b | 6.54 ± 1.09 | 1.69 ± 0.82 | 7.08 ± 1.02 | |
| ΔE | 10.35 ± 0.91 | 6.61 ± 0.67 | 10.40 ± 0.88 | |
| 90SA/10TA | L | −6.98 ± 0.68 | −13.05 ± 1.56 | −10.27 ± 0.62 |
| a | 2.48 ± 0.90 | 7.90 ± 1.71 | 3.17 ± 1.08 | |
| b | 5.00 ± 0.65 | 30.98 ± 0.76 | 2.00 ± 0.77 | |
| ΔE | 8.94 ± 0.61 | 34.53 ± 0.61 | 10.93 ± 1.04 | |
| 80SA/20TA | L | −8.25 ± 0.44 | −12.11 ± 1.11 | −7.10 ± 0.66 |
| a | 3.46 ± 1.31 | 12.50 ± 0.95 | 3.22 ± 1.01 | |
| b | 3.91 ± 0.98 | 31.89 ± 0.74 | 6.42 ± 0.99 | |
| ΔE | 8.76 ± 1.01 | 36.32 ± 1.03 | 10.09 ± 0.73 | |
| 70SA/30TA | L | −7.02 ± 0.94 | −18.39 ± 0.98 | −6.48 ± 0.96 |
| a | 1.70 ± 0.67 | 14.79 ± 1.03 | 2.58 ± 1.07 | |
| b | 3.23 ± 0.54 | 24.88 ± 0.77 | 4.86 ± 0.95 | |
| ΔE | 7.91 ± 1.09 | 34.29 ± 0.54 | 10.05 ± 1.43 | |
| Specimen | WVPR (g/m2/h) |
|---|---|
| 100SA | 16.13 |
| 90SA/10TA | 21.45 |
| 80SA/20TA | 22.54 |
| 70SA/30TA | 24.29 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaczmarek, B. Improving Sodium Alginate Films Properties by Phenolic Acid Addition. Materials 2020, 13, 2895. https://doi.org/10.3390/ma13132895
Kaczmarek B. Improving Sodium Alginate Films Properties by Phenolic Acid Addition. Materials. 2020; 13(13):2895. https://doi.org/10.3390/ma13132895
Chicago/Turabian StyleKaczmarek, Beata. 2020. "Improving Sodium Alginate Films Properties by Phenolic Acid Addition" Materials 13, no. 13: 2895. https://doi.org/10.3390/ma13132895
APA StyleKaczmarek, B. (2020). Improving Sodium Alginate Films Properties by Phenolic Acid Addition. Materials, 13(13), 2895. https://doi.org/10.3390/ma13132895




