Deposition of Thin Alumina Films Containing 3D Ordered Network of Nanopores on Porous Substrates
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation Parameters
2.2. Characterization Techniques
3. Results
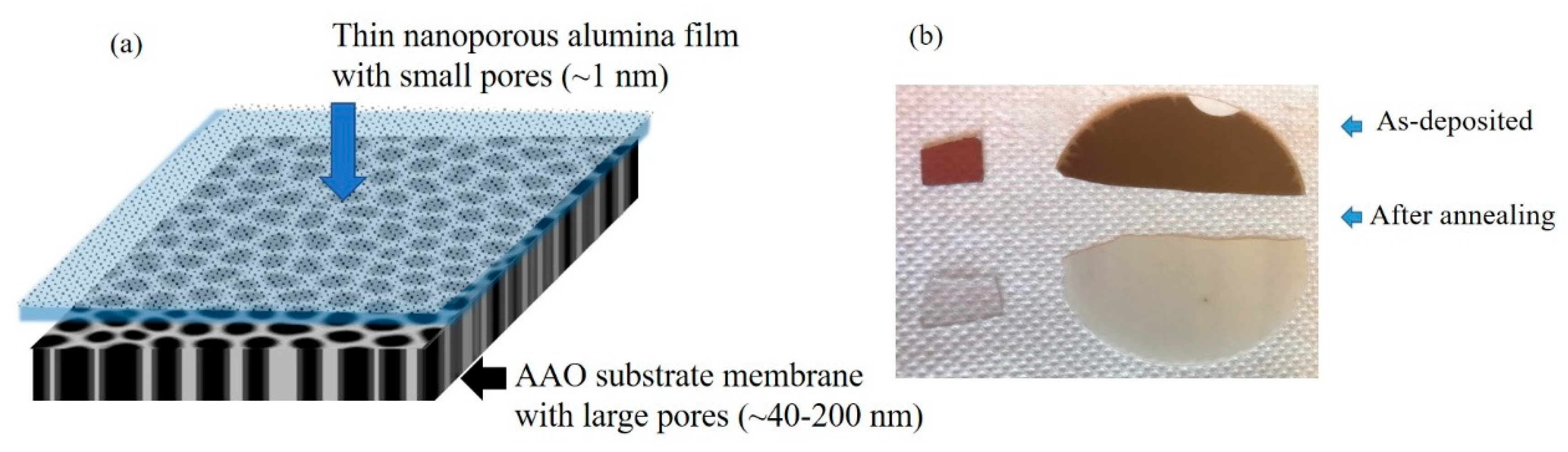
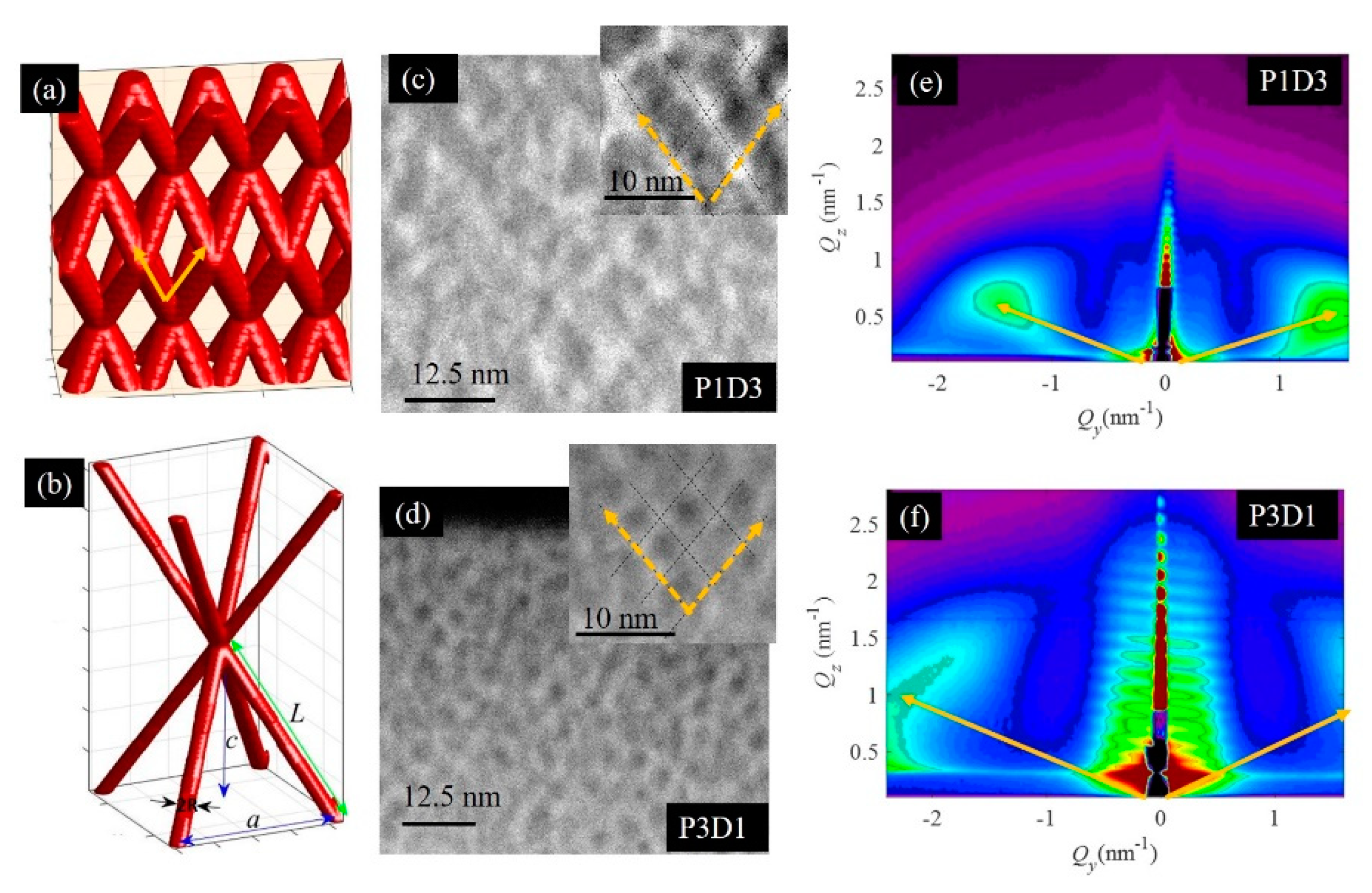
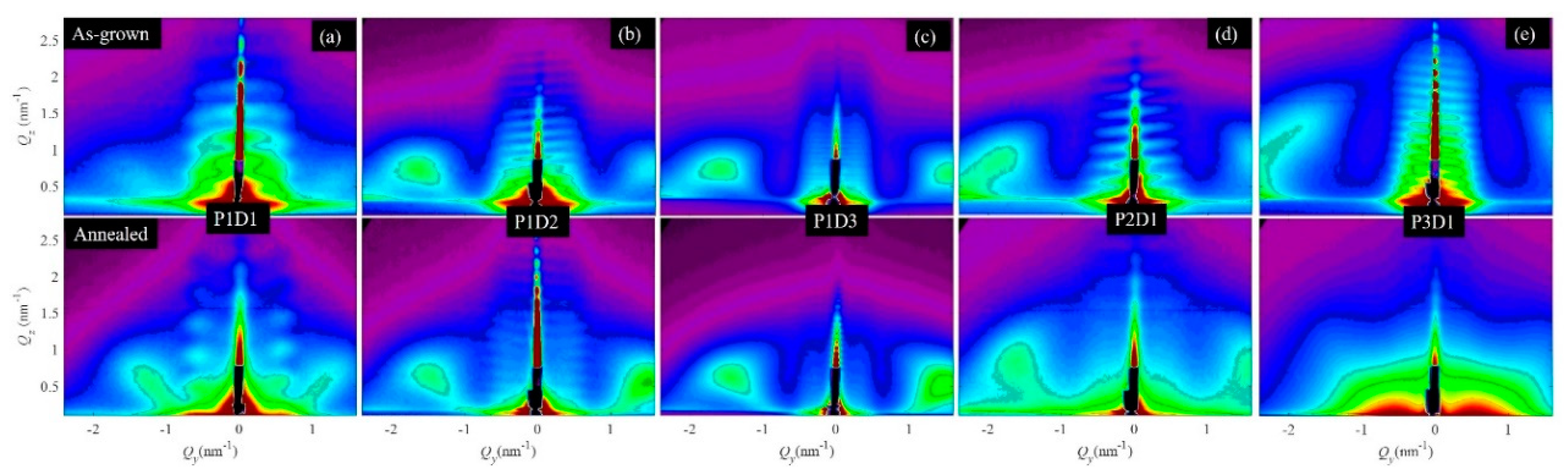
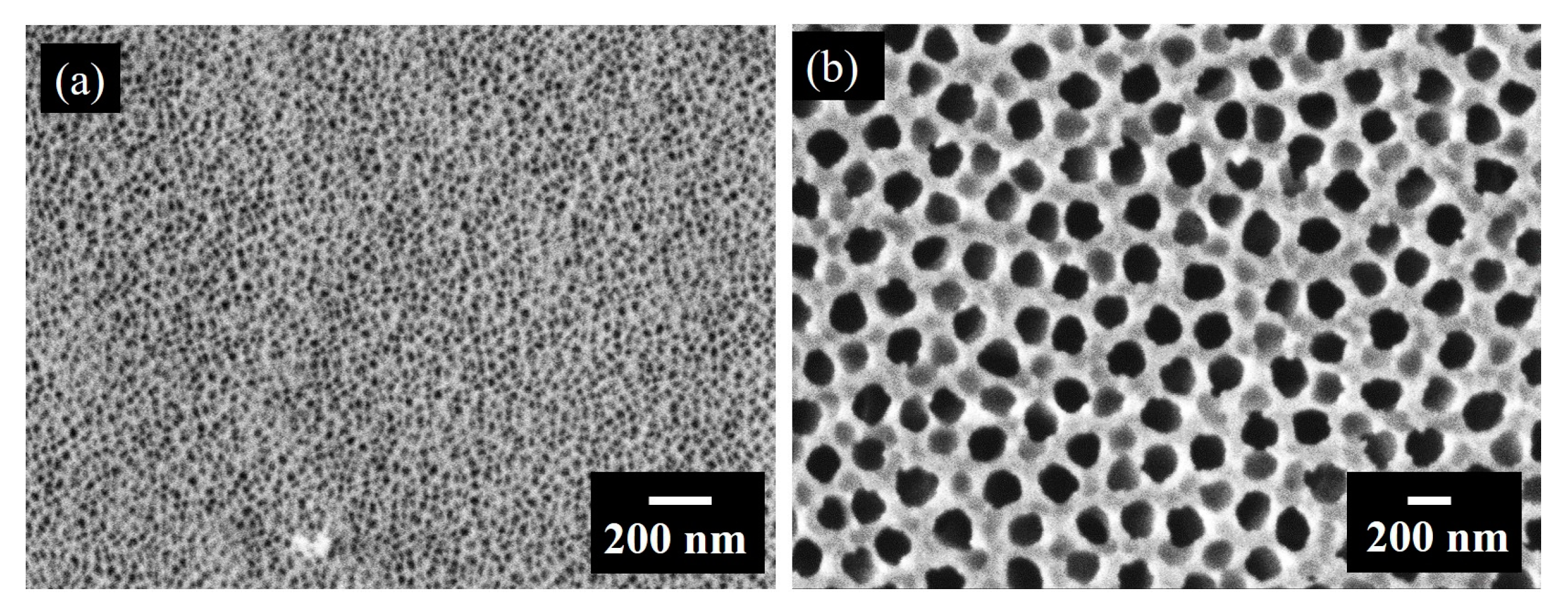
3.1. Structural Properties of Thin Nanoporous Films
3.2. Growth of Nanoporous Thin Films on Alumina Substrate with Larger Pores
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, Z.; Zhao, X.; Tang, Y. Thin Films for Energy Harvesting, Conversion, and Storage; Multidisciplinary Digital Publishing Institute: Basel, Switzerland, 2019; Volume 9, ISBN 9783039217243. [Google Scholar]
- Yang, Y.; Fan, X.; Casillas, G.; Peng, Z.; Ruan, G.; Wang, G. Three-Dimensional Nanoporous Thin Films for Lithium-Ion Batteries. ACS Nano 2015, 8, 3939–3946. [Google Scholar] [CrossRef]
- Перспективный, В.; Для, М.; Энергетики, В. Nanocrystalline porous vn x hydrogen storage as the promising material for hydrogen energy. Mach. Technol. Mater. 2016, 2, 75–77. [Google Scholar]
- Veronica Sofianos, M.; Sheppard, D.A.; Ianni, E.; Humphries, T.D.; Rowles, M.R.; Liu, S.; Buckley, C.E. Novel synthesis of porous aluminium and its application in hydrogen storage. J. Alloy. Compd. 2017, 702, 309–317. [Google Scholar] [CrossRef]
- Li, B.; Japip, S.; Chung, T.S. Molecularly tunable thin-film nanocomposite membranes with enhanced molecular sieving for organic solvent forward osmosis. Nat. Commun. 2020, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sano, T.; Iguchi, N.; Iida, K.; Sakamoto, T.; Baba, M.; Kawaura, H. Size-exclusion chromatography using self-organized nanopores in anodic porous alumina. Appl. Phys. Lett. 2003, 83, 4438–4440. [Google Scholar] [CrossRef]
- Qiu, X.Y.; Yu, H.Z.; Peinemann, K.V. Selective separation of similarly sized proteins with tunable nanoporous block copolymer membranes. Procedia Eng. 2012, 44, 461–463. [Google Scholar] [CrossRef]
- Osmanbeyoglu, H.U.; Hur, T.B.; Kim, H.K. Thin alumina nanoporous membranes for similar size biomolecule separation. J. Memb. Sci. 2009, 343, 1–6. [Google Scholar] [CrossRef]
- Nehache, S.; Semsarilar, M.; Deratani, A.; Quemener, D. Negatively charged porous thin film from ABA triblock copolymer assembly. Polymers 2018, 10, 733. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wu, A.; Ciacchi, L.C.; Wei, G. Recent advances in Nanoporous Membranes for Water Purification. Nanomaterials 2018, 8, 65. [Google Scholar] [CrossRef]
- Dong, J.; Lin, Y.S.; Kanezashi, M.; Tang, Z. Microporous inorganic membranes for high temperature hydrogen purification. J. Appl. Phys. 2008, 104, 13. [Google Scholar] [CrossRef]
- Korelskiy, D.; Grahn, M.; Ye, P.; Zhou, M.; Hedlund, J. A study of CO2/CO separation by sub-micron b-oriented MFI membranes. RSC Adv. 2016, 6, 65475–65482. [Google Scholar] [CrossRef]
- Heiranian, M.; Farimani, A.B.; Aluru, N.R. Water desalination with a single-layer MoS 2 nanopore. Nat. Commun. 2015, 6, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, Z.; Gupta, K.M.; Shi, Q.; Lu, R. Molecular dynamics study on water desalination through functionalized nanoporous graphene. Carbon N. Y. 2017, 116, 120–127. [Google Scholar] [CrossRef]
- Macha, M.; Marion, S.; Nandigana, V.V.R.; Radenovic, A. 2D materials as an emerging platform for nanopore-based power generation. Nat. Rev. Mater. 2019, 4, 588–605. [Google Scholar] [CrossRef]
- Yan, F.; Yao, L.; Chen, K.; Yang, Q.; Su, B. An ultrathin and highly porous silica nanochannel membrane: Toward highly efficient salinity energy conversion. J. Mater. Chem. A 2019, 7, 2385–2391. [Google Scholar] [CrossRef]
- Smilek, J.; Kynclová, H.; Sedláček, P.; Prášek, J.; Klučáková, M. Specific permeability of nanoporous alumina membranes studied by diffusion cell technique. In Proceedings of the Nanocon 2015-7th Int. Conf. Nanomater.-Res. Appl. Conf. Proc., Brno, Czech Republic, 14–16 October 2015; pp. 348–353. [Google Scholar]
- Brüggemann, D. Nanoporous aluminium oxide membranes as cell interfaces. J. Nanomater. 2013, 2013, 18. [Google Scholar] [CrossRef]
- Bragazzi, N.L.; Gasparini, R.; Amicizia, D.; Panatto, D.; Larosa, C. Porous Alumina as a Promising Biomaterial for Public Health, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2015; Volume 101. [Google Scholar]
- Yamaguchi, A.; Hotta, K.; Teramae, N. Optical waveguide sensor based on a porous anodic alumina/aluminum multilayer film. Anal. Chem. 2009, 81, 105–111. [Google Scholar] [CrossRef]
- Sciuto, A.; Mazzillo, M.C.; Di Franco, S.; Mannino, G.; Badala, P.; Renna, L.; Caruso, C.; Korotcenkov, G.; Cho, B.K.; Zargar, Z.H.; et al. Alcohol sensor based on gold-coated nanoporous anodic alumina membrane. Anal. Chim. Acta 2019, 81, 105–111. [Google Scholar]
- Juhász, L.; Mizsei, J. A simple humidity sensor with thin film porous alumina and integrated heating. Procedia Eng. 2010, 5, 701–704. [Google Scholar] [CrossRef]
- Jin, Z.; Meng, F.; Liu, J.; Li, M.; Kong, L.; Liu, J. A novel porous anodic alumina based capacitive sensor towards trace detection of PCBs. Sens. Actuators B Chem. 2011, 157, 641–646. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, W.; Lin, Y.; Cai, Y.; Qiu, M.; Fan, Y. Preparation of high-flux γ-alumina nanofiltration membranes by using a modified sol-gel method. Microporous Mesoporous Mater. 2015, 214, 195–203. [Google Scholar] [CrossRef]
- Alami-Younssi, S.; Larbot, A.; Persin, M.; Sarrazin, J.; Cot, L. Gamma alumina nanofiltration membrane. Application to the rejection of metallic cations. J. Memb. Sci. 1994, 91, 87–95. [Google Scholar] [CrossRef]
- Younssi, S.A.; Breida, M.; Achiou, B. Alumina Membranes for Desalination and Water Treatment. In Desalination and Water Treatment; IntechOpen: London, UK, 2018. [Google Scholar]
- Silva, K.K.O.S.; Paskocimas, C.A.; Oliveira, F.R.; Nascimento, J.H.O.; Zille, A. Development of porous alumina membranes for treatment of textile effluent. Desalin. Water Treat. 2016, 57, 2640–2648. [Google Scholar] [CrossRef]
- Jye, L.W.; Ismail, A.F. Nanofiltration Membranes: Synthesis, Characterization, and Applications; CRC Press: Boca Raton, FL, USA, 2016; ISBN 9781498751377. [Google Scholar]
- Breida, M.; Alami Younssi, S.; Bouazizi, A.; Achiou, B.; Ouammou, M.; El Rhazi, M. Nitrate removal from aqueous solutions by γ-Al2O3 ultrafiltration membranes. Heliyon 2018, 4, e00498. [Google Scholar] [CrossRef] [PubMed]
- Yeom, H.J.; Kim, S.C.; Kim, Y.W.; Song, I.H. Processing of alumina-coated clay-diatomite composite membranes for oily wastewater treatment. Ceram. Int. 2016, 42, 5024–5035. [Google Scholar] [CrossRef]
- Šereš, Z.; Maravić, N.; Takači, A.; Nikolić, I.; Šoronja-Simović, D.; Jokić, A.; Hodur, C. Treatment of vegetable oil refinery wastewater using alumina ceramic membrane: Optimization using response surface methodology. J. Clean. Prod. 2016, 112, 3132–3137. [Google Scholar] [CrossRef]
- Poinern, G.E.J.; Ali, N.; Fawcett, D. Progress in Nano-Engineered Anodic Aluminum Oxide Membrane Development. Materials 2010, 4, 487–526. [Google Scholar] [CrossRef]
- Said, S.; Mikhail, S.; Riad, M. Recent progress in preparations and applications of meso-porous alumina. Mater. Sci. Energy Technol. 2019, 2, 288–297. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, W.; Guan, K.; Peng, C.; Wu, J. Preparation of high permeable alumina ceramic membrane with good separation performance: Via UV curing technique. RSC Adv. 2018, 8, 13567–13577. [Google Scholar] [CrossRef]
- Wang, S.K.; Kita, K.; Lee, C.H.; Tabata, T.; Nishimura, T.; Nagashio, K.; Toriumi, A. Desorption kinetics of GeO from GeO2 /Ge structure. J. Appl. Phys. 2010, 108, 054104. [Google Scholar]
- Kita, K.; Suzuki, S.; Nomura, H.; Takahashi, T.; Nishimura, T.; Toriumi, A. Direct evidence of GeO volatilization from GeO2/Ge and impact of its suppression on GeO2/Ge metal-insulator-semiconductor characteristics. Jpn. J. Appl. Phys. 2008, 47, 2349–2353. [Google Scholar] [CrossRef]
- Copetti, G.; Soares, G.V.; Radtke, C. Stabilization of the GeO2/Ge Interface by Nitrogen Incorporation in a One-Step NO Thermal Oxynitridation. ACS Appl. Mater. Interfaces 2016, 8, 27339–27345. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Nishimura, T.; Yajima, T.; Toriumi, A. Thermal oxidation kinetics of germanium. Appl. Phys. Lett. 2017, 111, 052101. [Google Scholar] [CrossRef]
- Pinto, S.R.C.; Rolo, A.G.; Gomes, M.J.M.; Ivanda, M.; Bogdanović-Radović, I.; Grenzer, J.; Mücklich, A.; Barber, D.J.; Bernstorff, S.; Buljan, M. Formation of void lattice after annealing of Ge quantum dot lattice in alumina matrix. Appl. Phys. Lett. 2010, 97, 1–3. [Google Scholar] [CrossRef]
- Pinto, S.R.C.; Buljan, M.; Marques, L.; Martín-Sánchez, J.; Conde, O.; Chahboun, A.; Ramos, A.R.; Barradas, N.P.; Alves, E.; Bernstorff, S.; et al. Influence of annealing conditions on the formation of regular lattices of voids and Ge quantum dots in an amorphous alumina matrix. Nanotechnology 2012, 23, 405605. [Google Scholar] [CrossRef][Green Version]
- Nekić, N.; Šarić, I.; Salamon, K.; Basioli, L.; Sancho-Parramon, J.; Grenzer, J.; Hübner, R.; Bernstorff, S.; Petravić, M.; Mičetić, M. Preparation of non-oxidized Ge quantum dot lattices in amorphous Al2O3, Si3N4 and SiC matrices. Nanotechnology 2019, 30, 335601. [Google Scholar] [CrossRef]
- Chen, F.; Amekura, H.; Jia, Y. Photonic Structures Based on Thin Films Produced by Ion Beams. In Ion Irradiation of Dielectrics for Photonic Applications; Springer: Singapore, 2020; pp. 45–60. ISBN 978-981-15-4607-5. [Google Scholar]
- Buljan, M.; Radić, N.; Ivanda, M.; Bogdanović-Radović, I.; Karlušić, M.; Grenzer, J.; Prucnal, S.; Dražić, G.; Pletikapić, G.; Svetličić, V.; et al. Ge quantum dot lattices in Al2O3 multilayers. J. Nanoparticle Res. 2013, 15, 1485. [Google Scholar] [CrossRef]
- Buljan, M.; Radić, N.; Bernstorff, S.; Draić, G.; Bogdanović-Radović, I.; Hol, V. Grazing-incidence small-angle X-ray scattering: Application to the study of quantum dot lattices. Acta Crystallogr. Sect. A Found. Crystallogr. 2012, 68, 124–138. [Google Scholar] [CrossRef]
- Basioli, L.; Salamon, K.; Tkalčević, M.; Mekterović, I.; Bernstorff, S.; Mičetić, M. Application of GISAXS in the investigation of three-dimensional lattices of nanostructures. Crystals 2019, 9, 479. [Google Scholar] [CrossRef]
- Basioli, L.; Tkalčević, M.; Bogdanović-Radović, I.; Goran, D.; Salamon, K.; Mičetić, M. 3D Networks of Ge Quantum Wires in Amorphous Alumina Matrix. Nanomaterials 2020. (accepted). [Google Scholar]
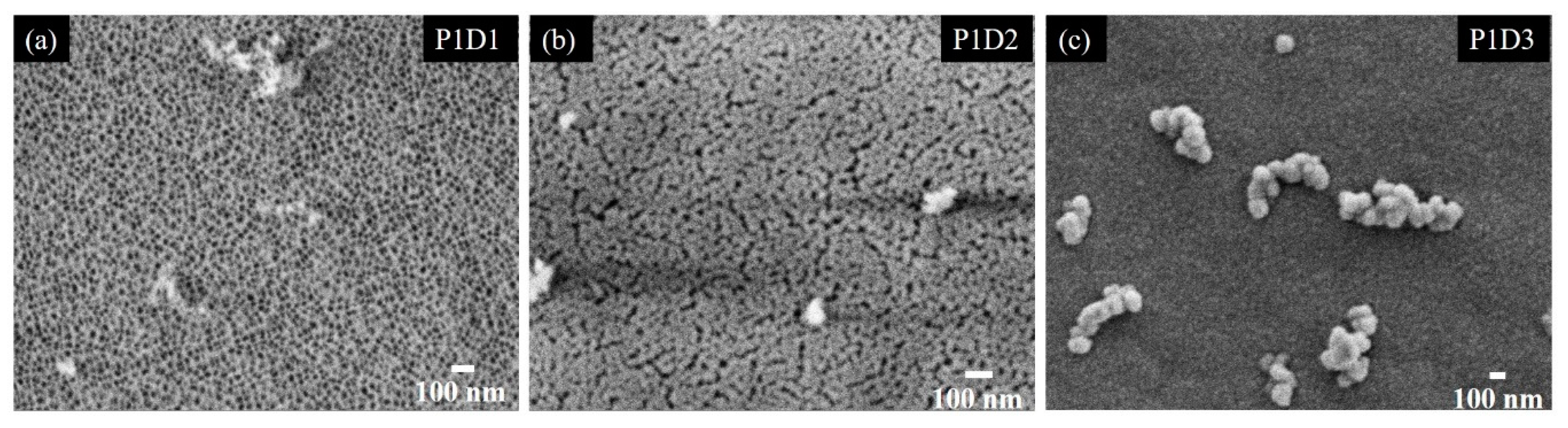
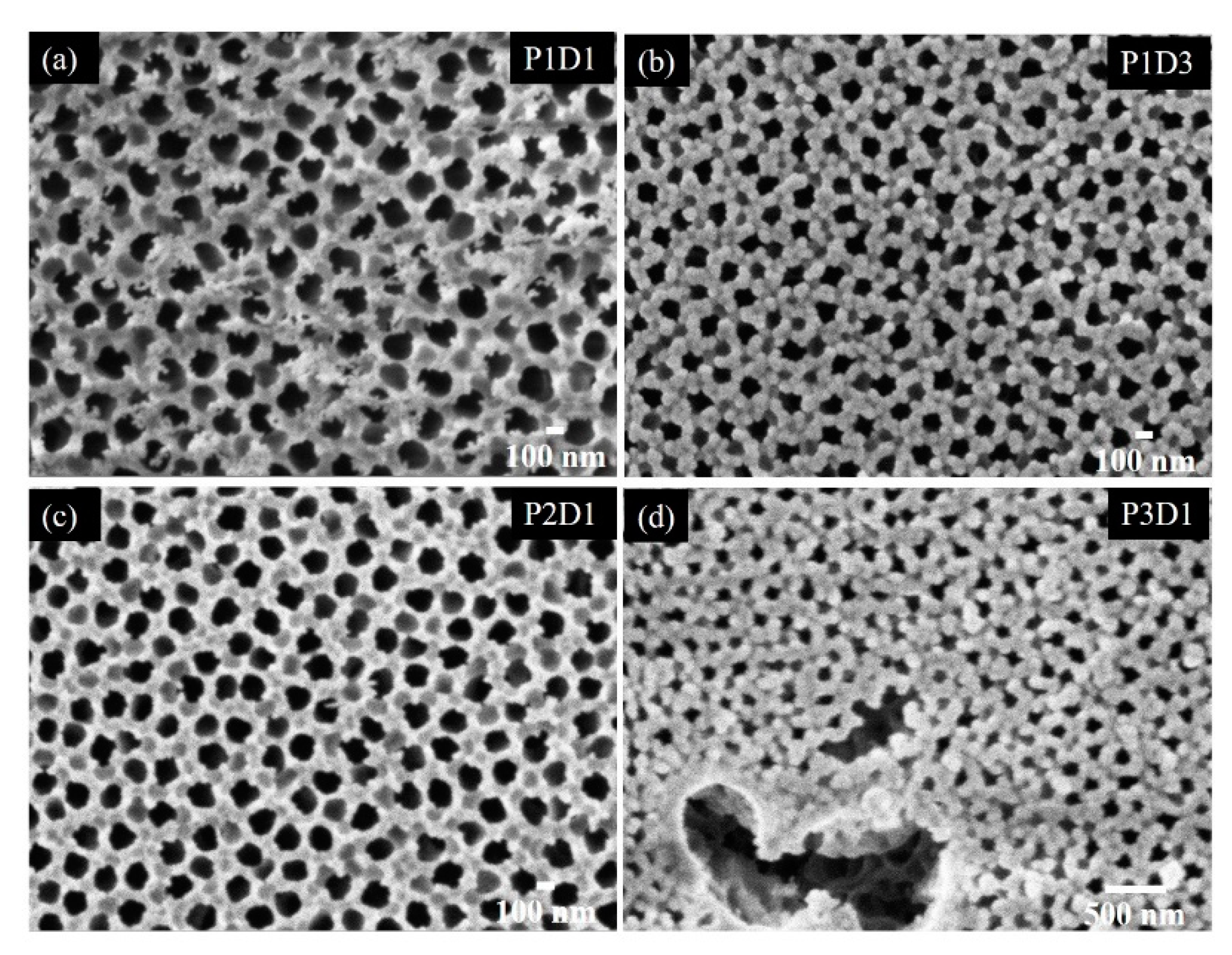
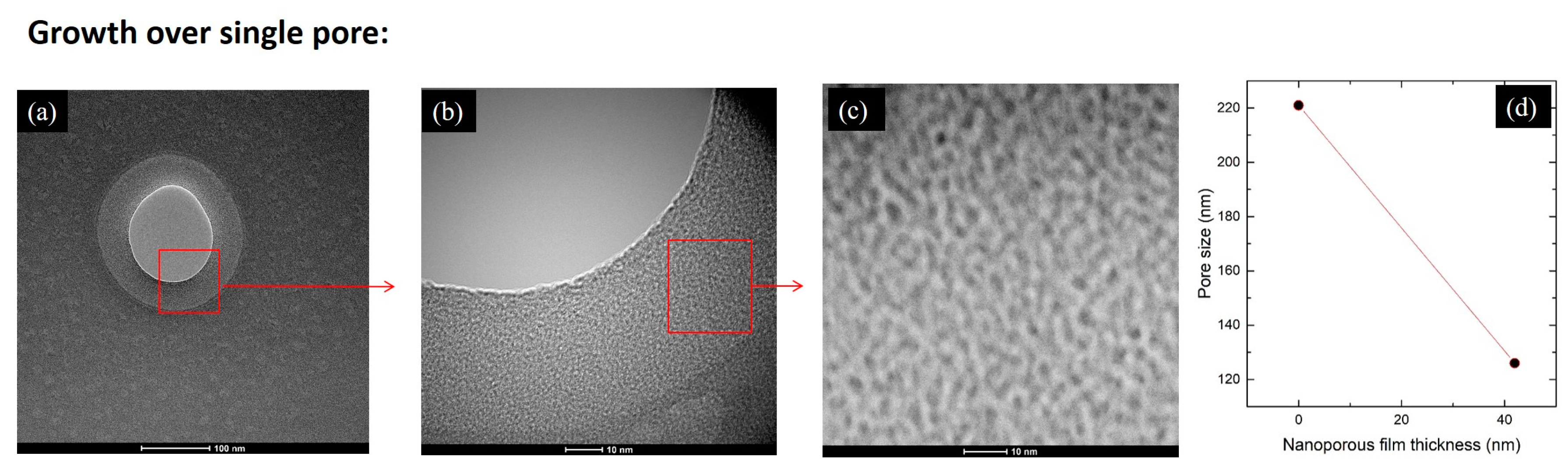
| Name/Par | P(Ge)/W | |
|---|---|---|
| P1D1 | 30 | 3 |
| P1D2 | 60 | 3 |
| P1D3 | 120 | 3 |
| P2D1 | 30 | 6 |
| P3D1 | 30 | 13 |
| Name/Par | a | c | R | L | D |
|---|---|---|---|---|---|
| P1D1 | 2.9 | 4.2 | 0.6 | 4.7 | 20 |
| P1D2 | 3.1 | 4.0 | 0.6 | 4.7 | 40 |
| P1D3 | 3.4 | 4.1 | 0.6 | 4.7 | 80 |
| P2D1 | 3.0 | 3.3 | 0.6 | 3.9 | 26 |
| P3D1 | 2.8 | 2.4 | 0.6 | 3.1 | 42 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tkalčević, M.; Gotić, M.; Basioli, L.; Lihter, M.; Dražić, G.; Bernstorff, S.; Vuletić, T.; Mičetić, M. Deposition of Thin Alumina Films Containing 3D Ordered Network of Nanopores on Porous Substrates. Materials 2020, 13, 2883. https://doi.org/10.3390/ma13132883
Tkalčević M, Gotić M, Basioli L, Lihter M, Dražić G, Bernstorff S, Vuletić T, Mičetić M. Deposition of Thin Alumina Films Containing 3D Ordered Network of Nanopores on Porous Substrates. Materials. 2020; 13(13):2883. https://doi.org/10.3390/ma13132883
Chicago/Turabian StyleTkalčević, Marija, Marijan Gotić, Lovro Basioli, Martina Lihter, Goran Dražić, Sigrid Bernstorff, Tomislav Vuletić, and Maja Mičetić. 2020. "Deposition of Thin Alumina Films Containing 3D Ordered Network of Nanopores on Porous Substrates" Materials 13, no. 13: 2883. https://doi.org/10.3390/ma13132883
APA StyleTkalčević, M., Gotić, M., Basioli, L., Lihter, M., Dražić, G., Bernstorff, S., Vuletić, T., & Mičetić, M. (2020). Deposition of Thin Alumina Films Containing 3D Ordered Network of Nanopores on Porous Substrates. Materials, 13(13), 2883. https://doi.org/10.3390/ma13132883









