Introduction of a Methodology to Enhance the Stabilization Process of PAN Fibers by Modeling and Advanced Characterization
Abstract
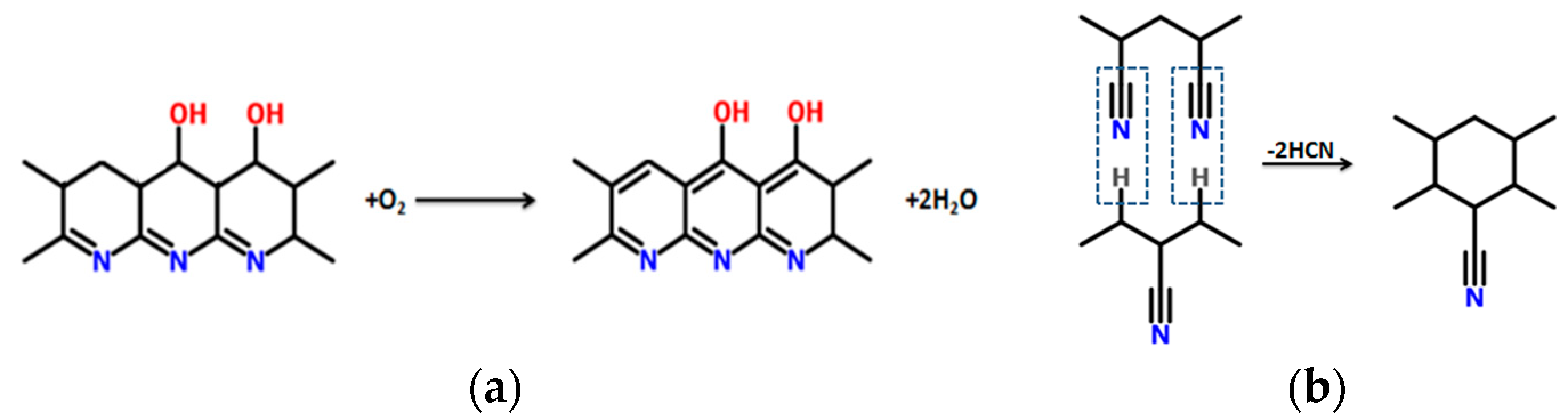
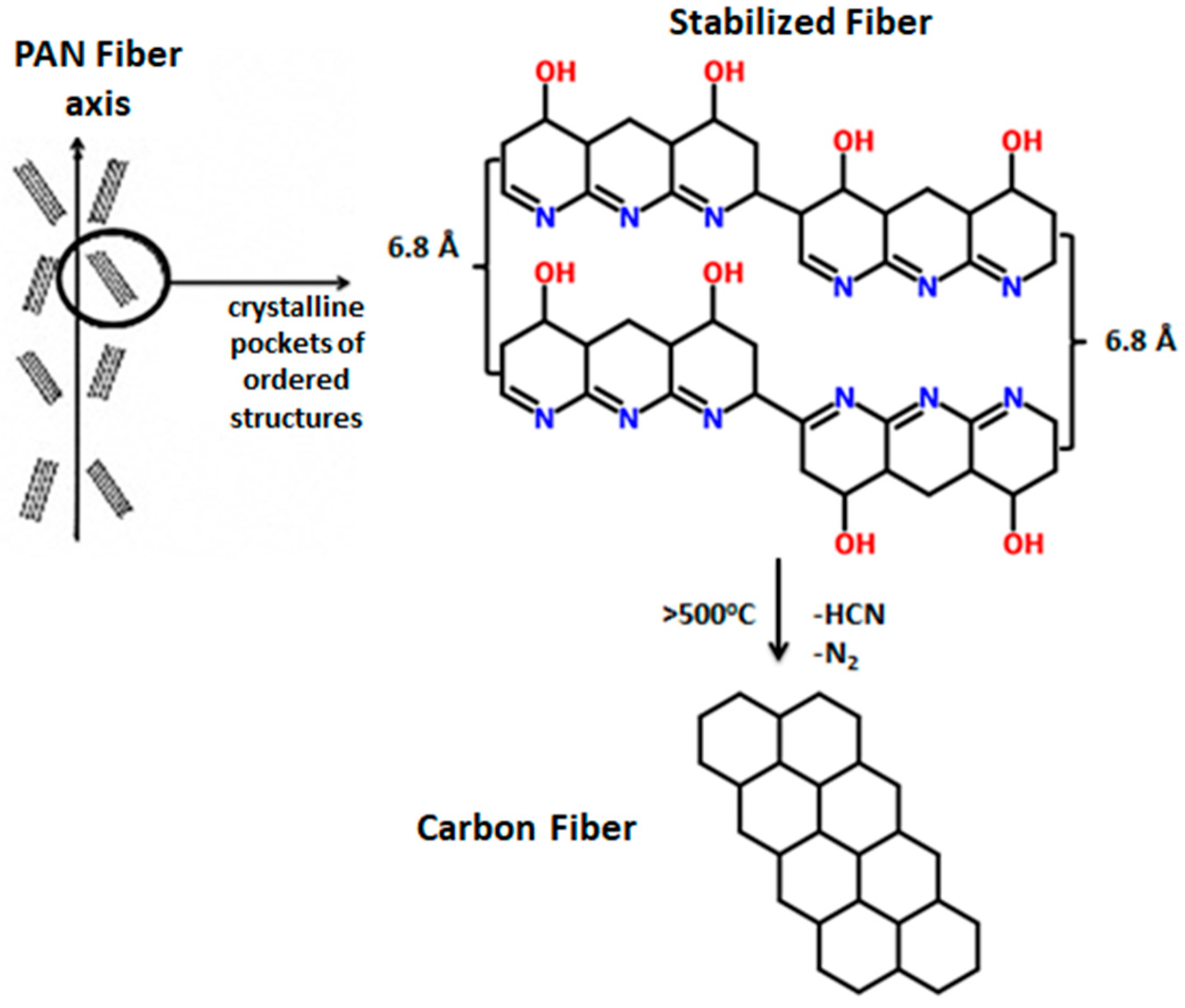
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Isothermal Stabilization Process
2.3. Characterization of Stabilized YTD PAN Fibers
3. Results and Discussion
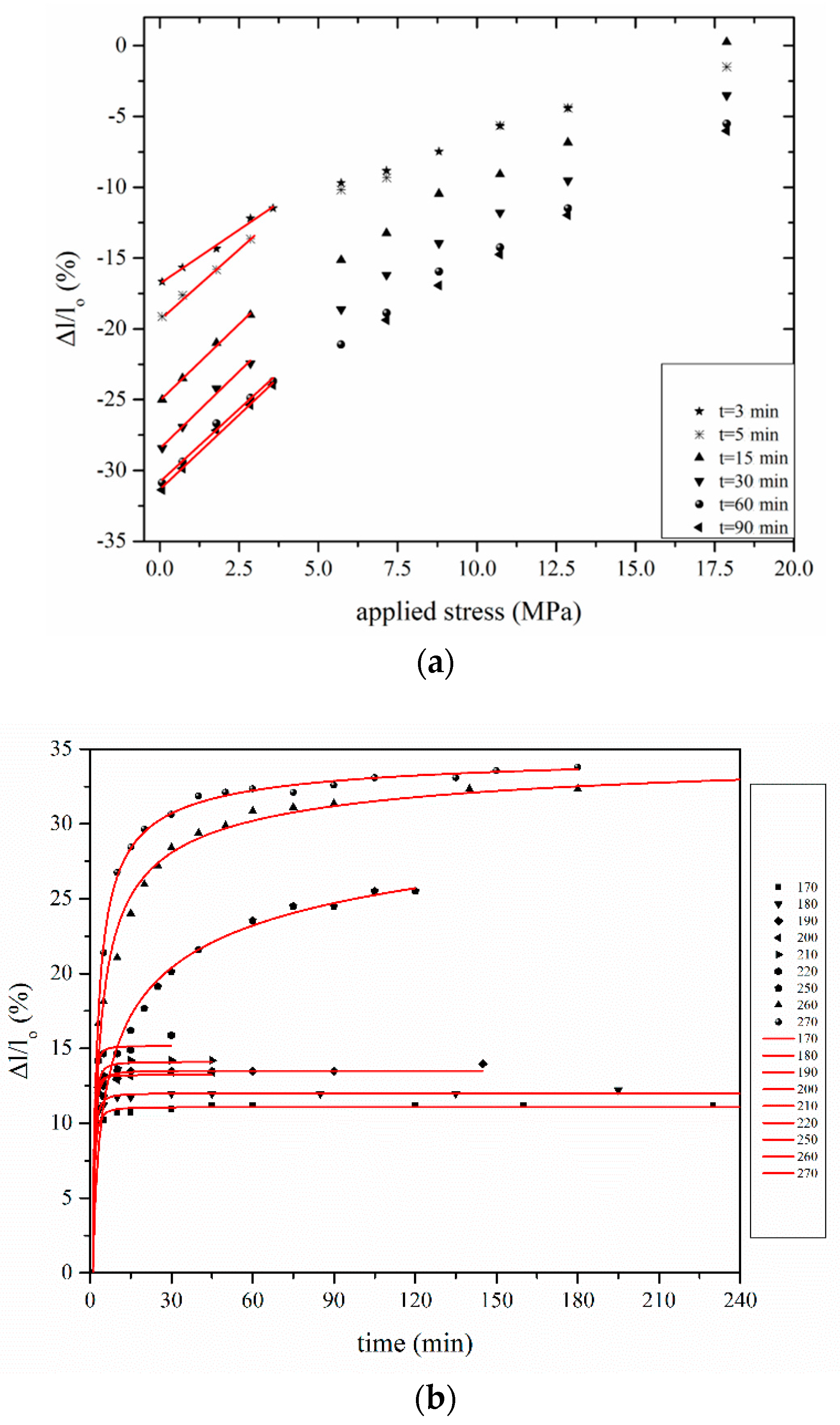
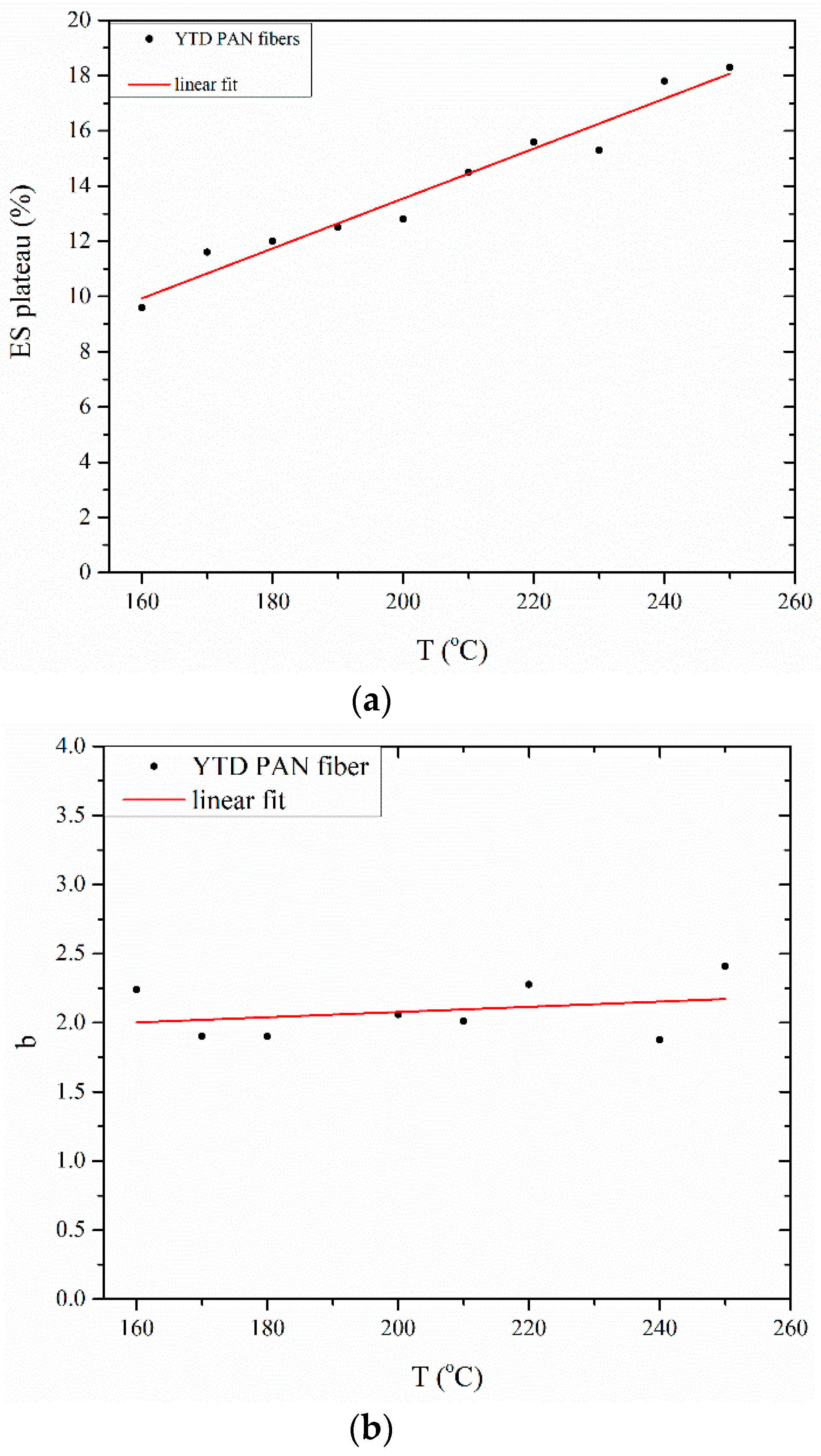
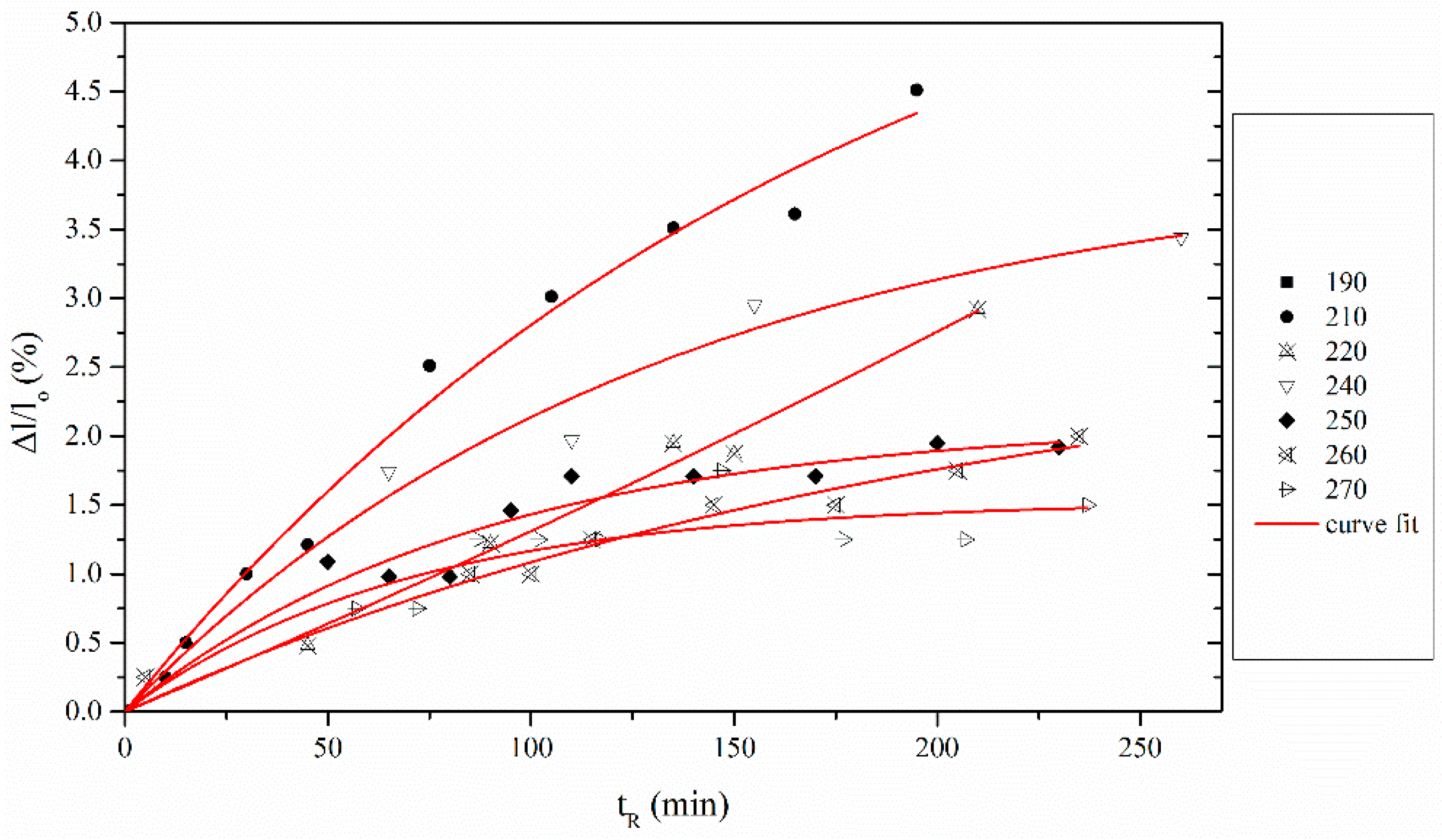
3.1. Determination of Entropic Shrinkage (ES)
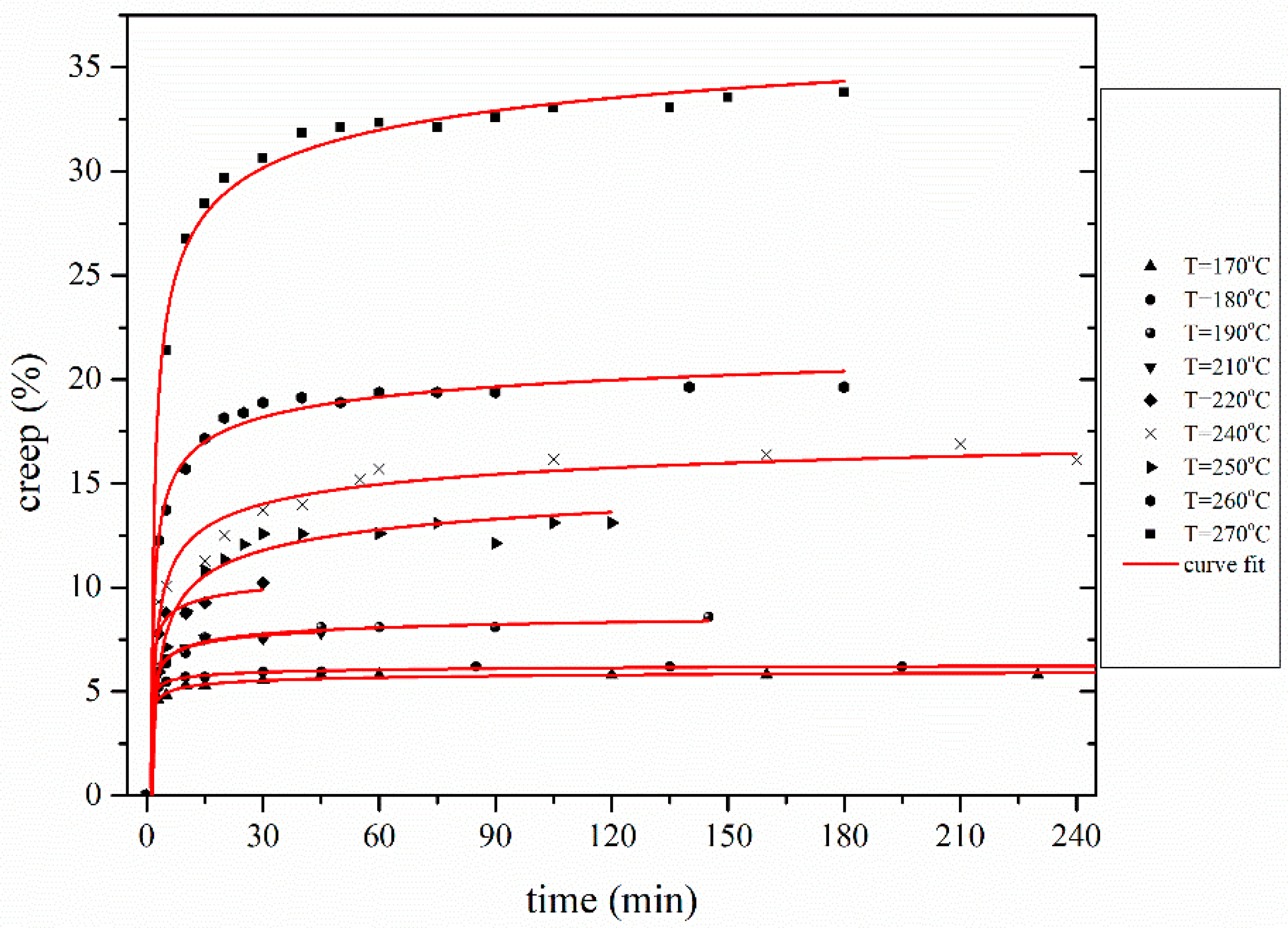
3.2. Deconvolution of Creep Effect
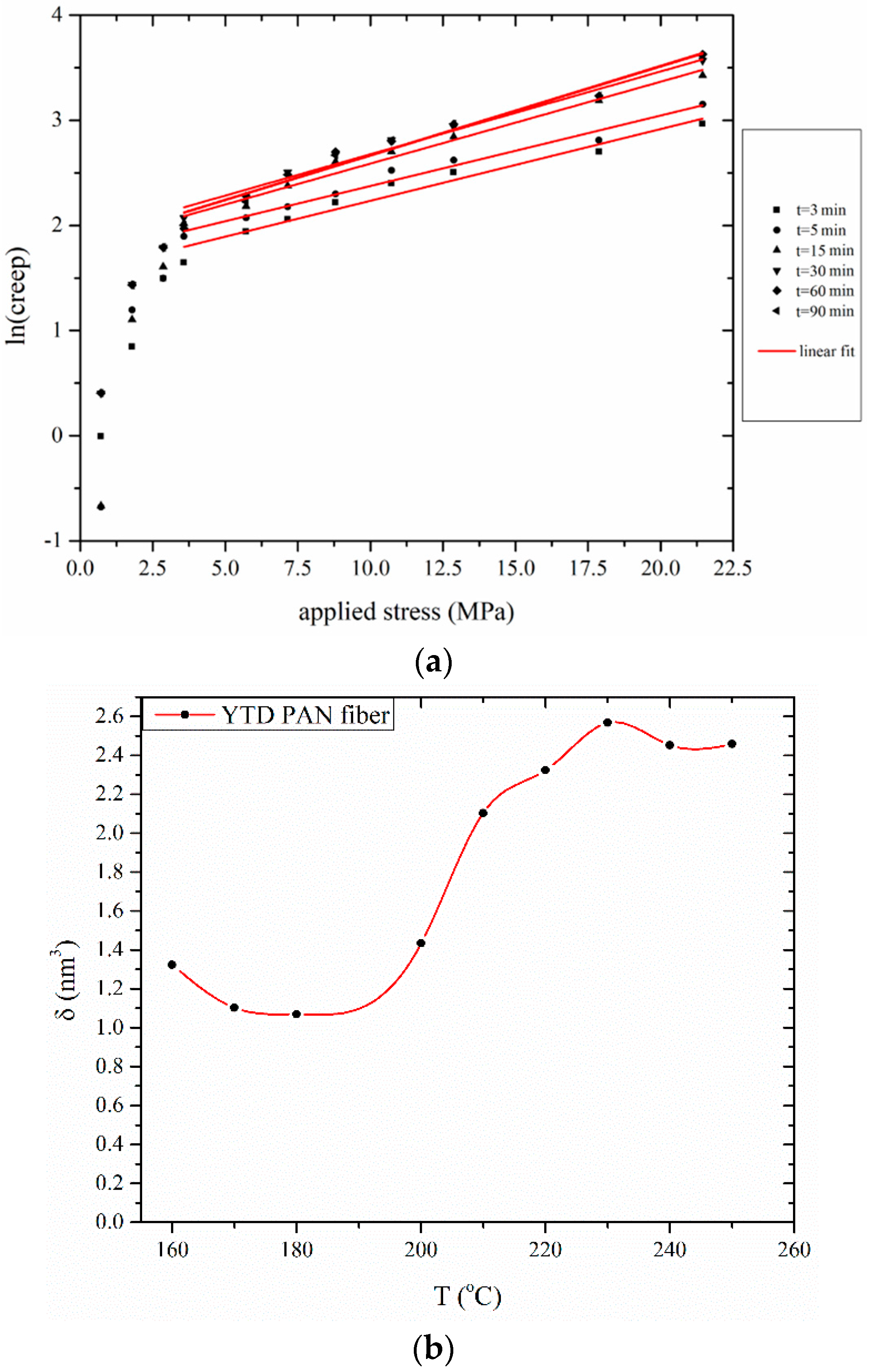
3.2.1. Determination of Creep Activation Volume δ
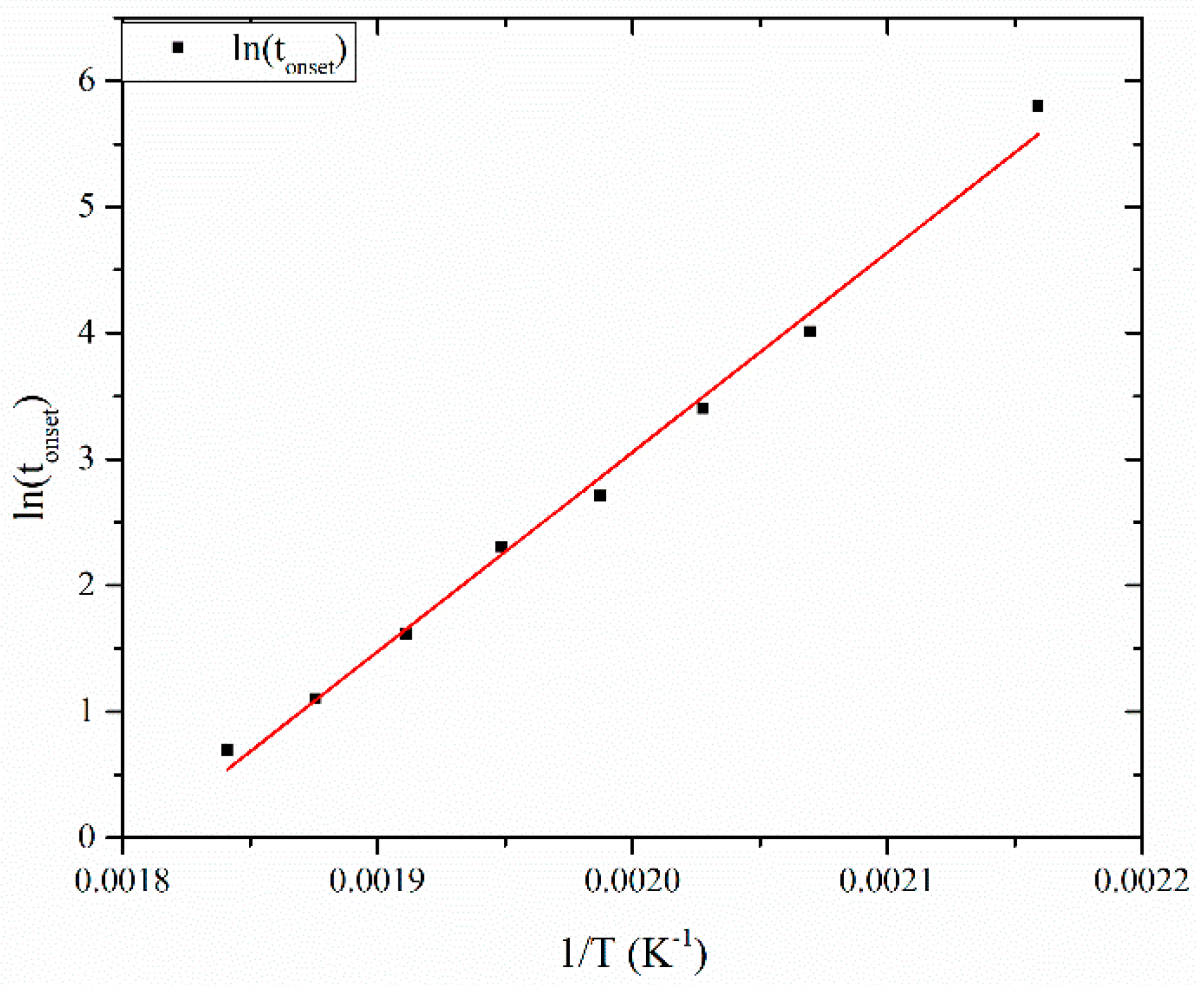
3.2.2. Evaluation of Creep Activation Energy
3.3. Modeling of Chemical Shrinkage (CS)
3.4. Fourier Transform Infrared Spectroscopy
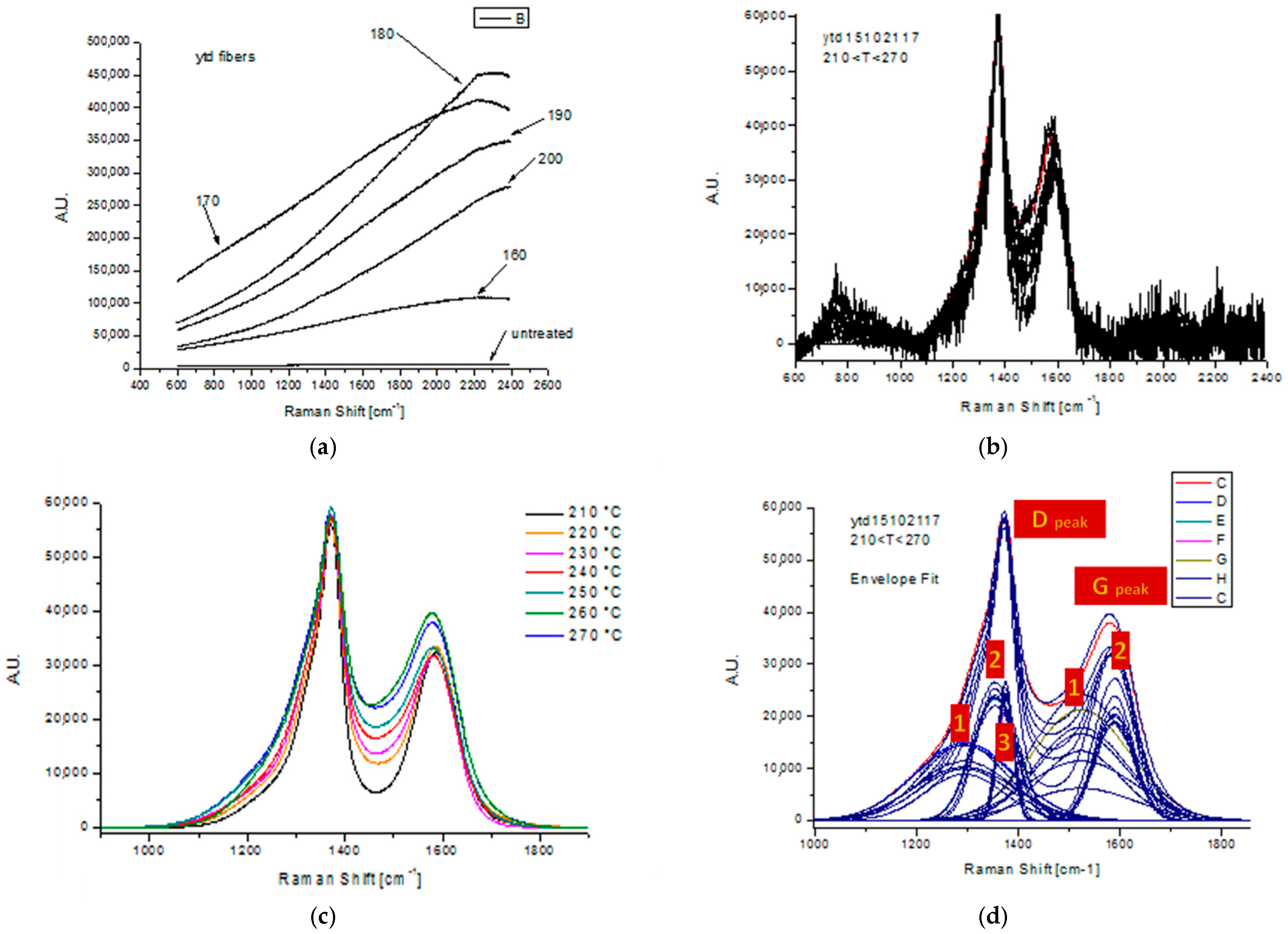
3.5. Raman Spectroscopy
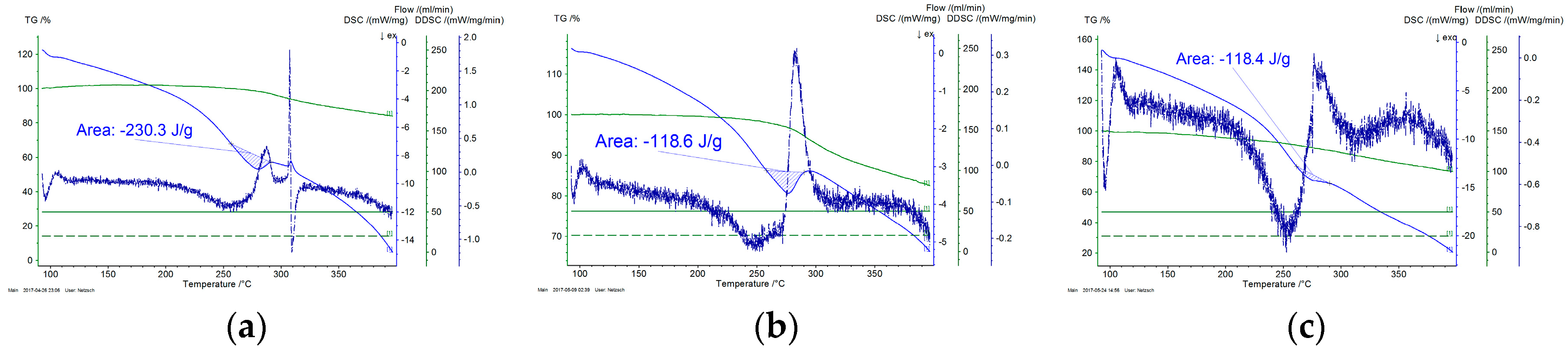
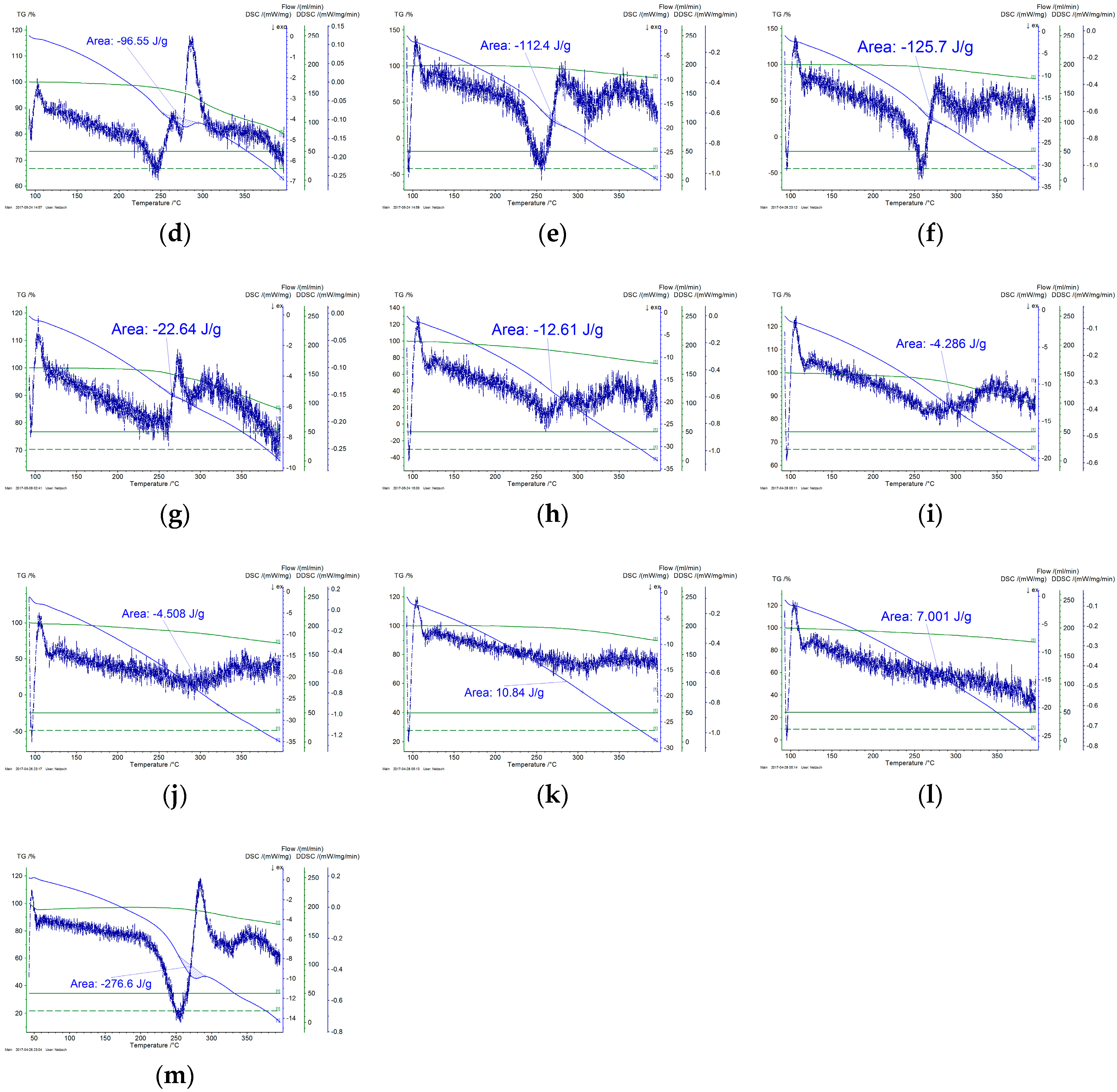
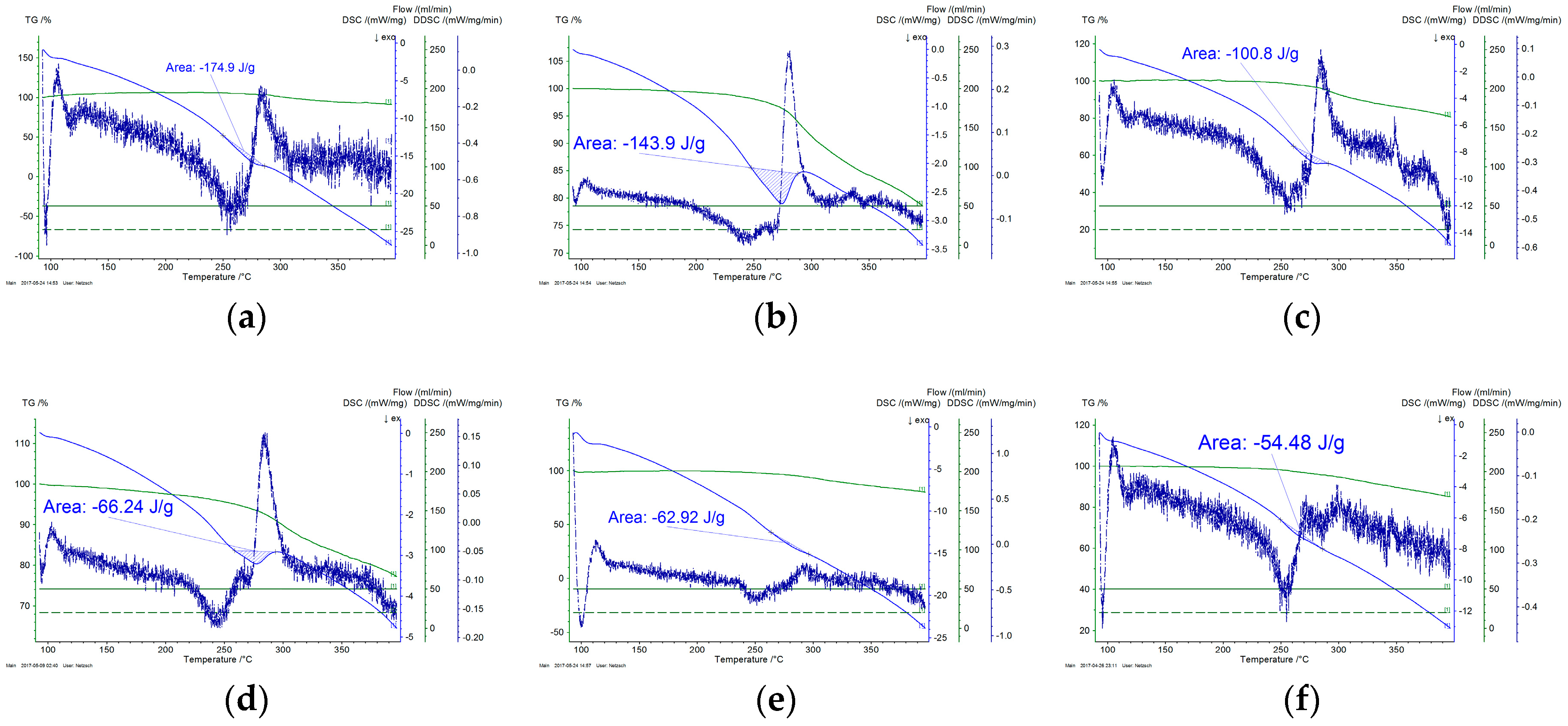
3.6. Thermal Analysis
3.7. Comparison of the Cyclization Yield Derived from DSC, FTIR, and Chemical Shrinkage Model
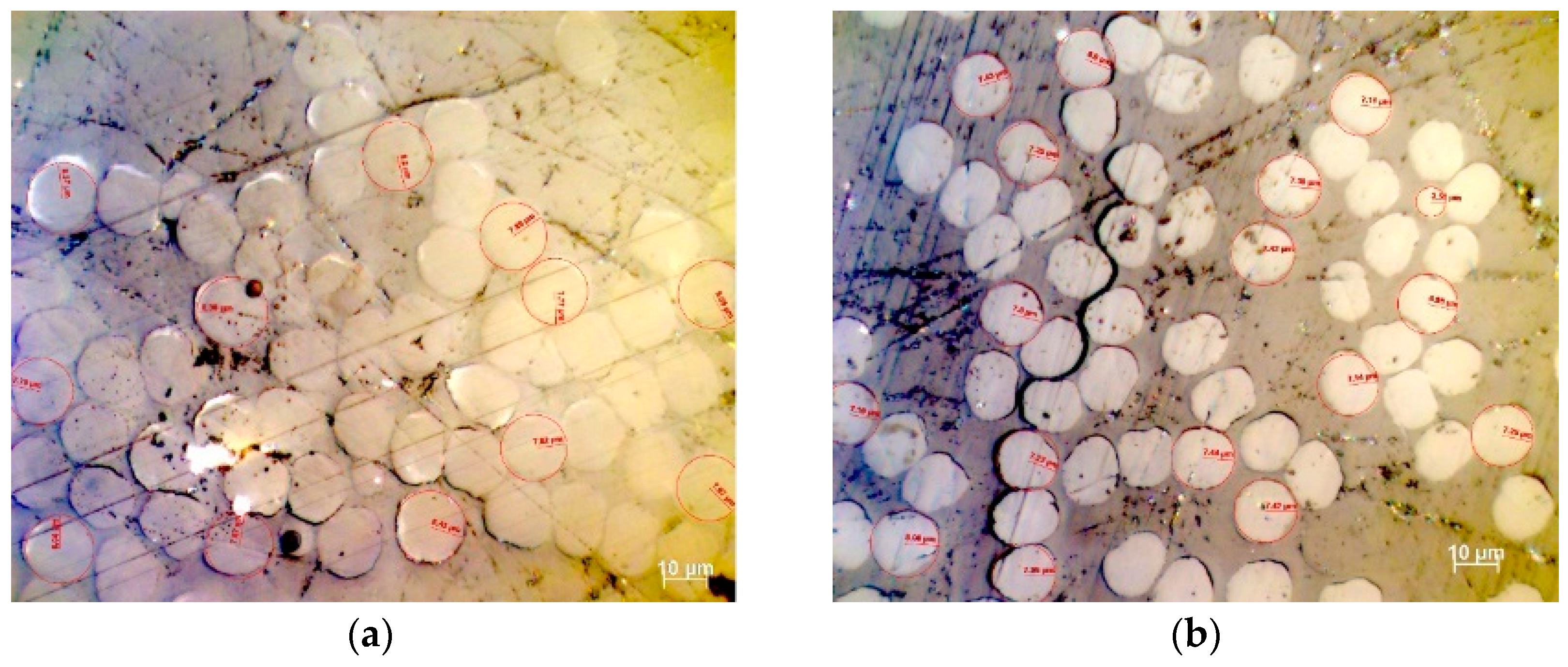
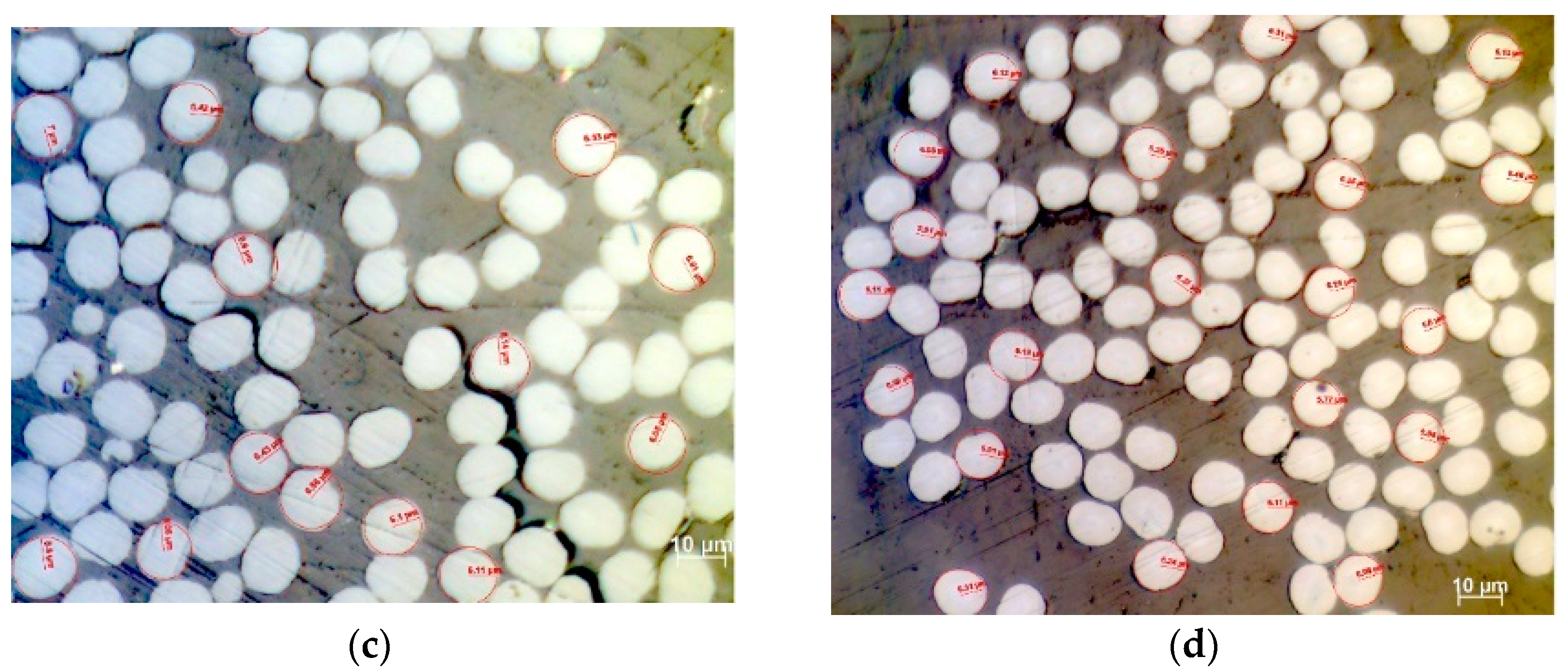
3.8. Investigation of Cross-Section Circularity after Thermal Treatment
4. Conclusions
- Cyclization yield can be accurately calculated at high temperature by the model implementation, wherein macroscopic change in length was involved. The results were further confirmed by FTIR and DSC-derived reaction yields.
- Control of cyclization yield via adjustment of stabilization variables could optimize mechanical properties after carbonization via the optimization of ladder polymer structure.
- A further gain is the applicability of this model for oxidative stabilization at both batch and continuous processing plants, as the employed values of temperature, tension, and residence time are taken into consideration to measure cyclization index.
- Using FTIR and Raman spectroscopy, it is feasible to observe the evolution of chemical structure and ladder polymer formation, using aromatic index, and G-peak activity.
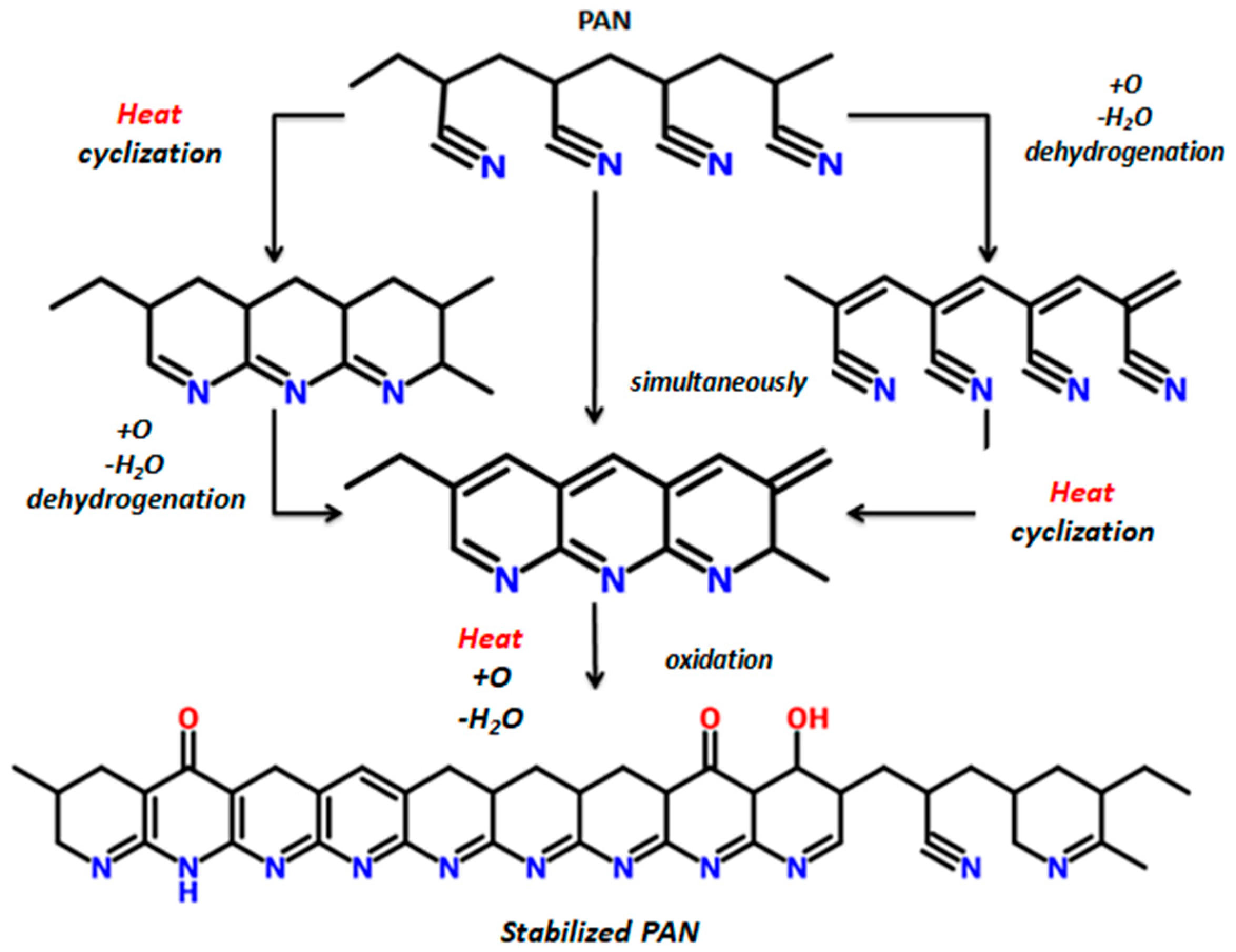
- Thermal analysis provided additional insight regarding the progress of cyclization and the overall progress of stabilization reaction. It was also confirmed by FTIR analysis that dehydrogenation is the basic reaction that occurs below 200 °C. Rate of both cyclization and oxidation is increased at higher temperatures.
- Raman confirmed the structural transformation of linear PAN molecule to ladder polymer, where the formation of graphite-like sp2 planes is evidenced as a result of stabilization at temperatures above 200 °C.
- Optical Microscopy demonstrated that thermal treatment had minor effect in fibers microstructure, as proved by the observation of the area and the circularity of thermal treated PAN fibers.
Author Contributions
Funding
Conflicts of Interest
Appendix A
| ytd15102117_210 | ||||
| Area | Center | Width | Shape | ID/IG |
| 1.714 × 106 | 1293.353 | 76.866 | Gaussian | 1.14 |
| 2.322 × 106 | 1354.051 | 36.573 | Gaussian | |
| 9.812 × 105 | 1374.569 | 14.640 | Gaussian | |
| 1.643 × 106 | 1523.361 | 106.078 | Gaussian | |
| 2.750 × 106 | 1589.261 | 40.364 | Gaussian | |
| ytd15102117_220 | ||||
| Area | Center | Width | Shape | ID/IG |
| 2.184 × 106 | 1293.353 | 87.784 | Gaussian | 1.04 |
| 2.374 × 106 | 1354.051 | 39.445 | Gaussian | |
| 9.444 × 105 | 1374.569 | 14.738 | Gaussian | |
| 3.052 × 106 | 1523.361 | 106.417 | Gaussian | |
| 2.227 × 106 | 1589.261 | 37.194 | Gaussian | |
| ytd15102117_230 | ||||
| Area | Center | Width | Shape | ID/IG |
| 2.561 × 106 | 1293.353 | 99.535 | Gaussian | 1.35 |
| 2.896 × 106 | 1354.051 | 43.540 | Gaussian | |
| 9.155 × 105 | 1374.569 | 14.965 | Gaussian | |
| 2.656 × 106 | 1523.361 | 79.408 | Gaussian | |
| 2.062 × 106 | 1589.261 | 37.930 | Gaussian | |
| ytd15102117_240 | ||||
| Area | Center | Width | Shape | ID/IG |
| 2.648 × 106 | 1293.353 | 92.829 | Gaussian | 1.15 |
| 2.662 × 106 | 1354.051 | 44.313 | Gaussian | |
| 9.778 × 105 | 1374.569 | 16.475 | Gaussian | |
| 3.672 × 106 | 1523.361 | 88.085 | Gaussian | |
| 1.772 × 106 | 1589.261 | 37.850 | Gaussian | |
| ytd15102117_250 | ||||
| Area | Center | Width | Shape | ID/IG |
| 3.537 × 106 | 1293.353 | 98.379 | Gaussian | 1.21 |
| 2.593 × 106 | 1354.051 | 44.092 | Gaussian | |
| 9.361 × 105 | 1374.569 | 15.943 | Gaussian | |
| 3.965 × 106 | 1523.361 | 88.573 | Gaussian | |
| 1.866 × 106 | 1589.261 | 39.126 | Gaussian | |
| ytd15102117_260 | ||||
| Area | Center | Width | Shape | ID/IG |
| 3.034 × 106 | 1293.353 | 83.041 | Gaussian | 0.86 |
| 2.503 × 106 | 1354.051 | 45.529 | Gaussian | |
| 1.018 × 106 | 1374.569 | 18.630 | Gaussian | |
| 5.655 × 106 | 1523.361 | 93.225 | Gaussian | |
| 1.961 × 106 | 1589.261 | 39.163 | Gaussian | |
| ytd15102117_270 | ||||
| Area | Center | Width | Shape | ID/IG |
| 3.650 × 106 | 1293.354 | 97.955 | Gaussian | 1.00 |
| 2.638 × 106 | 1354.051 | 45.256 | Gaussian | |
| 8.770 × 105 | 1374.569 | 16.601 | Gaussian | |
| 5.119 × 106 | 1523.361 | 96.119 | Gaussian | |
| 2.049 × 106 | 1589.261 | 40.142 | Gaussian |

References
- Taylor, M.P. Temperature and Strain Controlled Optimization of Stabilization of Polyacrylonitrile Precursor Fibers; University of Kentucky: Lexington, KY, USA, 2012. [Google Scholar]
- Konstantopoulos, G.; Koumoulos, E.P.; Charitidis, C.A. Classification of mechanism of reinforcement in the fiber-matrix interface: Application of machine learning on nanoindentation data. Mater. Des. 2020, 192, 108705. [Google Scholar] [CrossRef]
- Koumoulos, E.; Konstantopoulos, G.; Charitidis, C. Applying machine learning to nanoindentation data of (nano-) enhanced composites. Fibers 2020, 8, 3. [Google Scholar] [CrossRef]
- Park, S.; Yoo, S.H.; Kang, H.R.; Jo, S.M.; Joh, H.I.; Lee, S. Comprehensive stabilization mechanism of electron-beam irradiated polyacrylonitrile fibers to shorten the conventional thermal treatment. Sci. Rep. 2016, 6, 27330. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Lee, S.; Park, S.; Jo, S.M.; Lee, H.-S.; Joh, H.-I. Continuous and rapid stabilization of polyacrylonitrile fiber bundles assisted by atmospheric pressure plasma for fabricating large-tow carbon fibers. Carbon 2015, 94, 412–416. [Google Scholar] [CrossRef]
- Cipriani, E.; Zanetti, M.; Bracco, P.; Brunella, V.; Luda, M.P.; Costa, L. Crosslinking and carbonization processes in PAN films and nanofibers. Polym. Degrad. Stab. 2016, 123, 178–188. [Google Scholar] [CrossRef]
- Xue, Y.; Liu, J.; Liang, J. Correlative study of critical reactions in polyacrylonitrile based carbon fiber precursors during thermal-oxidative stabilization. Polym. Degrad. Stab. 2013, 98, 219–229. [Google Scholar] [CrossRef]
- Shin, H.K.; Park, M.; Kim, H.-Y.; Park, S.-J. An overview of new oxidation methods for polyacrylonitrile-based carbon fibers. Carbon Lett. 2015, 16, 11–18. [Google Scholar] [CrossRef]
- Morris, E.A.; Weisenberger, M.C.; Abdallah, M.G.; Vautard, F.; Grappe, H.; Ozcan, S.; Paulauskas, F.L.; Eberle, C.; Jackson, D.; Mecham, S.J.; et al. High performance carbon fibers from very high molecular weight polyacrylonitrile precursors. Carbon 2016, 101, 245–252. [Google Scholar] [CrossRef]
- Liu, W.; Wang, M.; Xing, Z.; Qi, Y.; Wu, G. Radiation-induced crosslinking of polyacrylonitrile fibers and the subsequent regulative effect on the preoxidation process. Radiat. Phys. Chem. 2012, 81, 622–627. [Google Scholar] [CrossRef]
- Rahaman, M.S.A.; Ismail, A.F.; Mustafa, A. A review of heat treatment on polyacrylonitrile fiber. Polym. Degrad. Stab. 2007, 92, 1421–1432. [Google Scholar] [CrossRef]
- Zhao, W.; Lu, Y.; Wang, J.; Chen, Q.; Zhou, L.; Jiang, J.; Chen, L. Improving crosslinking of stabilized polyacrylonitrile fibers and mechanical properties of carbon fibers by irradiating with γ-ray. Polym. Degrad. Stab. 2016, 133, 16–26. [Google Scholar] [CrossRef]
- Nunna, S.; Naebe, M.; Hameed, N.; Creighton, C.; Naghashian, S.; Jennings, M.J.; Atkiss, S.; Setty, M.; Fox, B.L. Investigation of progress of reactions and evolution of radial heterogeneity in the initial stage of thermal stabilization of PAN precursor fibres. Polym. Degrad. Stab. 2016, 125, 105–114. [Google Scholar] [CrossRef]
- Goulis, P.; Konstantopoulos, G.; Kartsonakis, I.A.; Mpalias, K.; Anagnou, S.; Dragatogiannis, D.; Charitidis, C.A. Thermal treatment of melt-spun fibers based on high density polyethylene and lignin. C—J. Carbon Res. 2017, 3, 35. [Google Scholar] [CrossRef]
- Goulis, P.; Kartsonakis, I.A.; Konstantopoulos, G.; Charitidis, C.A. Synthesis and processing of melt spun materials from esterified lignin with lactic acid. Appl. Sci. 2019, 9, 5361. [Google Scholar] [CrossRef]
- Santhana Krishnan, G.; Thomas, P.; Naveen, S.; Murali, N. Molecular and thermal studies of carbon fiber precursor polymers with low thermal-oxidative stabilization characteristics. J. Appl. Polym. Sci. 2018, 135, 46381. [Google Scholar] [CrossRef]
- Liu, Y.; Kumar, S. Recent progress in fabrication, structure, and properties of carbon fibers. Polym. Rev. 2012, 52, 234–258. [Google Scholar] [CrossRef]
- Hiremath, N.; Mays, J.; Bhat, G. Recent developments in carbon fibers and carbon nanotube-based fibers: A review. Polym. Rev. 2016, 57, 339–368. [Google Scholar] [CrossRef]
- Huang, X. Fabrication and properties of carbon fibers. Materials 2009, 2, 2369–2403. [Google Scholar] [CrossRef]
- Soulis, S.; Dragatogiannis, D.A.; Charitidis, C.A. A novel methodology for designing thermal processes in order to optimize stabilization of polyacrylonitrile (PAN) fibers. Polym. Adv. Technol. 2020, 31, 1403–1413. [Google Scholar] [CrossRef]
- Gilbert, M. Brydson’s Plastics Materials, 8th ed.; Payne, E., Matthew, D., Eds.; Oxford: Oxford, UK, 2017. [Google Scholar]
- Soulis, S.; Simitzis, J. Thermomechanical behaviour of poly[acrylonitrile-co-(methyl acrylate)] fibres oxidatively treated at temperatures up to 180 °C. Polym. Int. 2005, 54, 1474–1483. [Google Scholar] [CrossRef]
- Sabet, E.N.; Nourpanah, P.; Arbab, S. Quantitative analysis of entropic stress effect on the structural rearrangement during pre-stabilization of PAN precursor fibers. Polymer 2016, 90, 138–146. [Google Scholar] [CrossRef]
- Donnet, J.-B. Carbon Fibers, 3rd ed.; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]
- Mathur, R.B.; Bahl, O.P.; Mittal, J. A new approach to thermal stabilisation of PAN fibres. Carbon 1992, 30, 657–663. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kim, J.-H.; Ku, B.-C.; Kim, J.-K.; Chung, Y.-S. Effect of process condition on tensile properties of carbon fiber. Carbon Lett. 2011, 12, 26–30. [Google Scholar] [CrossRef]
- Qin, X.; Lu, Y.; Xiao, H.; Hao, Y.; Pan, D. Improving preferred orientation and mechanical properties of PAN-based carbon fibers by pretreating precursor fibers in nitrogen. Carbon 2011, 49, 4598–4600. [Google Scholar] [CrossRef]
- Wu, G.; Lu, C.; Ling, L.; Hao, A.; He, F. Influence of tension on the oxidative stabilization process of polyacrylonitrile fibers. J. Appl. Polym. Sci. 2005, 96, 1029–1034. [Google Scholar] [CrossRef]
- Arbab, S.; Zeinolebadi, A. A procedure for precise determination of thermal stabilization reactions in carbon fiber precursors. Polym. Degrad. Stab. 2013, 98, 2537–2545. [Google Scholar] [CrossRef]
- Fitzer, E.; Müller, D.J. Zur bildung von gewinkelten leiterpolymeren in polyacrylnitril-fasern. Die Makromol. Chem. 1971, 144, 117–133. [Google Scholar] [CrossRef]
- Simitzis, J.; Soulis, S. Correlation of chemical shrinkage of polyacrylonitrile fibres with kinetics of cyclization. Polym. Int. 2008, 57, 99–105. [Google Scholar] [CrossRef]
- Jing, M.; Wang, C.-G.; Zhu, B.; Wang, Y.-X.; Gao, X.-P.; Chen, W.-N. Effects of preoxidation and carbonization technologies on tensile strength of PAN-based carbon fiber. J. Appl. Polym. Sci. 2008, 108, 1259–1264. [Google Scholar] [CrossRef]
- Xiao, S.; Lv, H.; Tong, Y.; Xu, L.; Chen, B. Thermal behavior and kinetics during the stabilization of polyacrylonitrile precursor in inert gas. J. Appl. Polym. Sci. 2011, 122, 480–488. [Google Scholar] [CrossRef]
- Nunna, S.; Creighton, C.; Hameed, N.; Naebe, M.; Henderson, L.C.; Setty, M.; Fox, B.L. Radial structure and property relationship in the thermal stabilization of PAN precursor fibres. Polym. Test. 2017, 59, 203–211. [Google Scholar] [CrossRef]
- Liu, H.C.; Chien, A.-T.; Newcomb, B.A.; Bakhtiary Davijani, A.A.; Kumar, S. Stabilization kinetics of gel spun polyacrylonitrile/lignin blend fiber. Carbon 2016, 101, 382–389. [Google Scholar] [CrossRef]
- Golkarnarenji, G.; Naebe, M.; Church, J.S.; Badii, K.; Bab-Hadiashar, A.; Atkiss, S.; Khayyam, H. Development of a predictive model for study of skin-core phenomenon in stabilization process of PAN precursor. J. Ind. Eng. Chem. 2017, 49, 46–60. [Google Scholar] [CrossRef]
- Nunna, S.; Naebe, M.; Hameed, N.; Fox, B.L.; Creighton, C. Evolution of radial heterogeneity in polyacrylonitrile fibres during thermal stabilization: An overview. Polym. Degrad. Stab. 2017, 136, 20–30. [Google Scholar] [CrossRef]
- Chai, X.; Mi, H.; Zhu, C.; He, C.; Xu, J.; Zhou, X.; Liu, J. Low-temperature thermal stabilization of polyacrylontrile-based precursor fibers towards efficient preparation of carbon fibers with improved mechanical properties. Polymer 2015, 76, 131–139. [Google Scholar] [CrossRef]
- Xue, Y.; Liu, J.; Lian, F.; Liang, J. Effect of the oxygen-induced modification of polyacrylonitrile fibers during thermal-oxidative stabilization on the radial microcrystalline structure of the resulting carbon fibers. Polym. Degrad. Stab. 2013, 98, 2259–2267. [Google Scholar] [CrossRef]
- Bajaj, P.; Roopanwal, A.K. Thermal stabilization of acrylic precursors for the production of carbon fibers: An overview. J. Macromol. Sci. Part C: Polym. Rev. 1997, 37, 97–147. [Google Scholar] [CrossRef]
- Banaie, K.A.; Mirjalili, M.; Eslami-Farsani, R. The study of the correlation between ascending and descending rates of the imposed specific stress and transposition of chemical reactions during PAN precursor fibers stabilization. Polym. Degrad. Stab. 2018, 156, 234–244. [Google Scholar] [CrossRef]
- Sabet, E.N.; Nourpanah, P.; Arbab, S. A novel method for investigation of entropic stress in prestabilization of PAN-based precursor fibers. Adv. Polym. Technol. 2017, 36, 424–432. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, J.; Zhou, T.; Liu, X.; Yuan, Q.; Zhang, A. New understanding on the reaction pathways of the polyacrylonitrile copolymer fiber pre-oxidation: Online tracking by two-dimensional correlation FTIR spectroscopy. RSC Adv. 2016, 6, 4397–4409. [Google Scholar] [CrossRef]
- Suresh, K.I.; Thomas, K.S.; Rao, B.S.; Nair, C.P.R. Viscoelastic properties of polyacrylonitrile terpolymers during thermo-oxidative stabilization (cyclization). Polym. Adv. Technol. 2008, 19, 831–837. [Google Scholar] [CrossRef]
- Jain, M.K.; Abhiraman, A.S. Conversion of acrylonitrile-based precursor fibres to carbon fibres. J. Mater. Sci. 1987, 22, 278–300. [Google Scholar] [CrossRef]
- Bashir, Z. The hexagonal mesophase in atactic polyacrylonitrile: A new interpretation of the phase transitions in the polymer. J. Macromol. Sci. Part B 2007, 40, 41–67. [Google Scholar] [CrossRef]
- Yao, Z.; Wu, D.; Chen, C.; Zhang, M. Creep behavior of polyurethane nanocomposites with carbon nanotubes. Compos. Part A: Appl. Sci. Manuf. 2013, 50, 65–72. [Google Scholar] [CrossRef]
- McCrum, N.; Buckley, C.; Bucknall, C. Principles of Polymer Engineering, 2nd ed.; Cambridge University Press: Cambridge, UK, 2002. [Google Scholar]
- Arbab, S.; Zeinolebadi, A. Quantitative analysis of the effects of comonomers and heating conditions on the stabilization reactions of polyacrylonitrile fibers as carbon fiber precursors. Polym. Degrad. Stab. 2017, 139, 107–116. [Google Scholar] [CrossRef]
- Bashir, Z.; Rastogi, S. The Explanation of the increase in slope at the Tg in the plot of d-spacing versus temperature in polyacrylonitrile. J. Macromol. Sci. Part B 2005, 44, 55–78. [Google Scholar] [CrossRef]
- Arbab, S.; Teimoury, A.; Mirbaha, H.; Adolphe, D.C.; Noroozi, B.; Nourpanah, P. Optimum stabilization processing parameters for polyacrylonitrile-based carbon nanofibers and their difference with carbon (micro) fibers. Polym. Degrad. Stab. 2017, 142, 198–208. [Google Scholar] [CrossRef]
- Soulis, S.; Konstantopoulos, G.; Koumoulos, E.; Charitidis, C. Impact of alternative stabilization strategies for the production of PAN-based carbon fibers with high performance. Fibers 2020, 8, 33. [Google Scholar] [CrossRef]
- Fitzer, E.; Frohs, W.; Heine, M. Optimization of stabilization and carbonization treatment of PAN fibres and structural characterization of the resulting carbon fibres. Carbon 1986, 24, 387–395. [Google Scholar] [CrossRef]
- Kalashnik, A.; Panichkina, O.N.; Rudinskaya, G.Y. Shrinkage mechanisms in thermooxidative stabilization of acrylic fibres. Fibre Chem. 2001, 33, 132–139. [Google Scholar] [CrossRef]
- Gupta, A.; Harrison, I.R. New aspects in the oxidative stabilization of PAN-based carbon fibers. Carbon 1996, 34, 1427–1445. [Google Scholar] [CrossRef]
- Hou, Y.; Sun, T.; Wang, H.; Wu, D. A new method for the kinetic study of cyclization reaction during stabilization of polyacrylonitrile fibers. J. Mater. Sci. 2008, 43, 4910–4914. [Google Scholar] [CrossRef]
- Farsani, E.R.; Raissi, S.; Shokuhfar, A.; Sedghi, A. FTIR Study of stabilized PAN fibers for fabrication of carbon fibers. World Acad. Sci. Eng. Technol. 2009, 50, 430. [Google Scholar]
- Hameed, N.; Sharp, J.; Nunna, S.; Creighton, C.; Magniez, K.; Jyotishkumar, P.; Salim, N.V.; Fox, B. Structural transformation of polyacrylonitrile fibers during stabilization and low temperature carbonization. Polym. Degrad. Stab. 2016, 128, 39–45. [Google Scholar] [CrossRef]
- Cuesta, A.; Dhamelincourt, P.; Laureyns, J.; Martínez-Alonso, A.; Tascón, J.M.D. Raman microprobe studies on carbon materials. Carbon 1994, 32, 1523–1532. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, C.; Guo, J.; Liu, Q.; Dong, H.; Gu, Y.; Liu, R.; Zhao, N.; Zhang, Z.; Xu, J. Nanoscale dynamic mechanical imaging of the skin–core difference: From PAN precursors to carbon fibers. Mater. Lett. 2014, 128, 417–420. [Google Scholar] [CrossRef]
- Newcomb, B.A. Processing, structure, and properties of carbon fibers. Compos. Part A: Appl. Sci. Manuf. 2016, 91, 262–282. [Google Scholar] [CrossRef]
- Zhao, R.-X.; Sun, P.-f.; Liu, R.-j.; Ding, Z.-h.; Li, X.-s.; Liu, X.-y.; Zhao, X.-d.; Gao, Z.-m. Influence of heating procedures on the surface structure of stabilized polyacrylonitrile fibers. Appl. Surf. Sci. 2018, 433, 321–328. [Google Scholar] [CrossRef]
- Jin, S.; Guo, C.; Lu, Y.; Zhang, R.; Wang, Z.; Jin, M. Comparison of microwave and conventional heating methods in carbonization of polyacrylonitrile-based stabilized fibers at different temperature measured by an in-situ process temperature control ring. Polym. Degrad. Stab. 2017, 140, 32–41. [Google Scholar] [CrossRef]
- Musiol, P.; Szatkowski, P.; Gubernat, M.; Weselucha-Birczynska, A.; Blazewicz, S. Comparative study of the structure and microstructure of PAN-based nano- and micro-carbon fibers. Ceram. Int. 2016, 42, 11603–11610. [Google Scholar] [CrossRef]
- Park, O.-K.; Lee, S.; Joh, H.-I.; Kim, J.K.; Kang, P.-H.; Lee, J.H.; Ku, B.-C. Effect of functional groups of carbon nanotubes on the cyclization mechanism of polyacrylonitrile (PAN). Polymer 2012, 53, 2168–2174. [Google Scholar] [CrossRef]
- Meinl, J.; Schönfeld, K.; Kirsten, M.; Kittler, K.; Michaelis, A.; Cherif, C. Optimization of the temperature program to scale up the stabilization of polyacrylonitrile fibers. Compos. Part A: Appl. Sci. Manuf. 2017, 96, 37–45. [Google Scholar] [CrossRef]

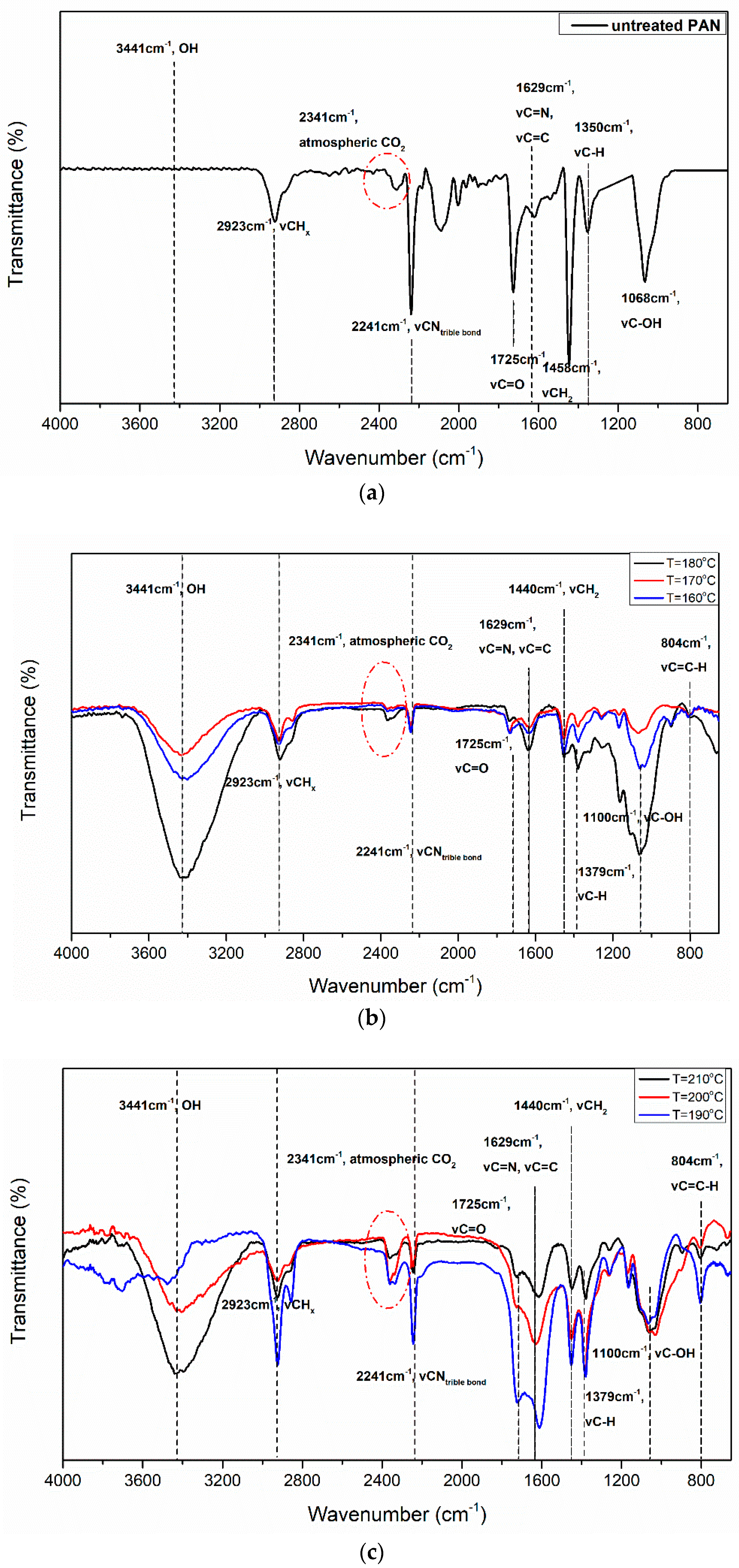


| Wavenumber (cm−1) | 2924 | 2239 | 2107 | 1725 | 1658 | 1583 | 1446 | 1357 & 1262 | 1163 | 1097 & 1064 | 804 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bonds | CHx | C≡N | C=NH | C=O | C=C asymmetral vibration | C=C,C=N | Tensile vibration CH2 | C-H vibration | C-O | C-OH | =C-H |
| Major FTIR peaks before stabilization | √ | √ | √ | √ | × | × | √ | × | √ | × | × |
| Major FTIR peaks after stabilization | √ | ↓ | √ | ↑ | √ | √ | ↓ | √ | √ | √ | √ |
| Stabilization Temperature (°C) | CI | rnm | Cyclization | Dehydrogenation |
|---|---|---|---|---|
| 160 | 0.43 | 2.50 | low | High |
| 170 | 0.44 | 1.91 | low | Medium |
| 190 | 0.65 | 2.24 | mid | High |
| 200 | 0.64 | 2.97 | mid | High |
| 210 | 0.64 | 1.84 | mid | High |
| 230 | 0.83 | 0.99 | high | High |
| 240 | 0.91 | 0.74 | high | High |
| 250 | 0.94 | 0.44 | high | High |
| 260 | 0.97 | 0.41 | high | High |
| 270 | 0.98 | 0.11 | high | High |
| Stabilization Temperature (°C) | ΔHtotal (J/g) | Stabilization Index (SI) |
|---|---|---|
| --- | 276.6 | 0.0% |
| 160 | 230.3 | 16.7% |
| 170 | 118.6 | 57.1% |
| 180 | 118.4 | 57.2% |
| 190 | 96.6 | 65.1% |
| 200 | 112.4 | 59.4% |
| 210 | 125.7 | 54.6% |
| 220 | 22.6 | 91.8% |
| 230 | 12.6 | 95.4% |
| 240 | 4.3 | 98.4% |
| 250 | 4.5 | 98.4% |
| 260 | 10.8 | 96.1% |
| 270 | 19.1 | 93.1% |
| Stabilization Temperature (°C). | ΔHcyclization (J/g) | Cyclization Index (CI) |
|---|---|---|
| --- | 184 | --- |
| 160 | 174.9 | 4.9% |
| 170 | 143.9 | 21.8% |
| 180 | 100.8 | 45.2% |
| 190 | 66.2 | 64.0% |
| 200 | 62.9 | 65.8% |
| 210 | 54.5 | 70.4% |
| 220 | 21.1 | 88.5% |
| 230 | 20.7 | 88.8% |
| 240 | 6.9 | 96.3% |
| 250 | 0.4 | 99.8% |
| 260 | 5.5 | 97.0% |
| 270 | 7 | 96.2% |
| Stabilization Temperature (°C) | 160 | 210 | 230 | 260 | ||||
|---|---|---|---|---|---|---|---|---|
| Software | OM | ImageJ | OM | ImageJ | OM | ImageJ | OM | ImageJ |
| Μean Radius (μm) | 7.76 | 7.35 | 7.22 | 6.90 | 6.43 | 6.18 | 6.01 | 5.69 |
| Standard Deviation | 0.48 | 0.34 | 0.30 | 0.33 | 0.31 | 0.30 | 0.29 | 0.16 |
| Mean monofilament area (μm2) | 188.9 | 169.8 | 163.6 | 149.8 | 129.8 | 120.3 | 113.5 | 101.6 |
| Standard Deviation of Monofilament Area | 0.71 | 15.94 | 0.29 | 14.28 | 0.31 | 11.72 | 0.26 | 5.71 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konstantopoulos, G.; Soulis, S.; Dragatogiannis, D.; Charitidis, C. Introduction of a Methodology to Enhance the Stabilization Process of PAN Fibers by Modeling and Advanced Characterization. Materials 2020, 13, 2749. https://doi.org/10.3390/ma13122749
Konstantopoulos G, Soulis S, Dragatogiannis D, Charitidis C. Introduction of a Methodology to Enhance the Stabilization Process of PAN Fibers by Modeling and Advanced Characterization. Materials. 2020; 13(12):2749. https://doi.org/10.3390/ma13122749
Chicago/Turabian StyleKonstantopoulos, George, Spyros Soulis, Dimitrios Dragatogiannis, and Costas Charitidis. 2020. "Introduction of a Methodology to Enhance the Stabilization Process of PAN Fibers by Modeling and Advanced Characterization" Materials 13, no. 12: 2749. https://doi.org/10.3390/ma13122749
APA StyleKonstantopoulos, G., Soulis, S., Dragatogiannis, D., & Charitidis, C. (2020). Introduction of a Methodology to Enhance the Stabilization Process of PAN Fibers by Modeling and Advanced Characterization. Materials, 13(12), 2749. https://doi.org/10.3390/ma13122749






