Crystallization of TiO2-MoS2 Hybrid Material under Hydrothermal Treatment and Its Electrochemical Performance
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
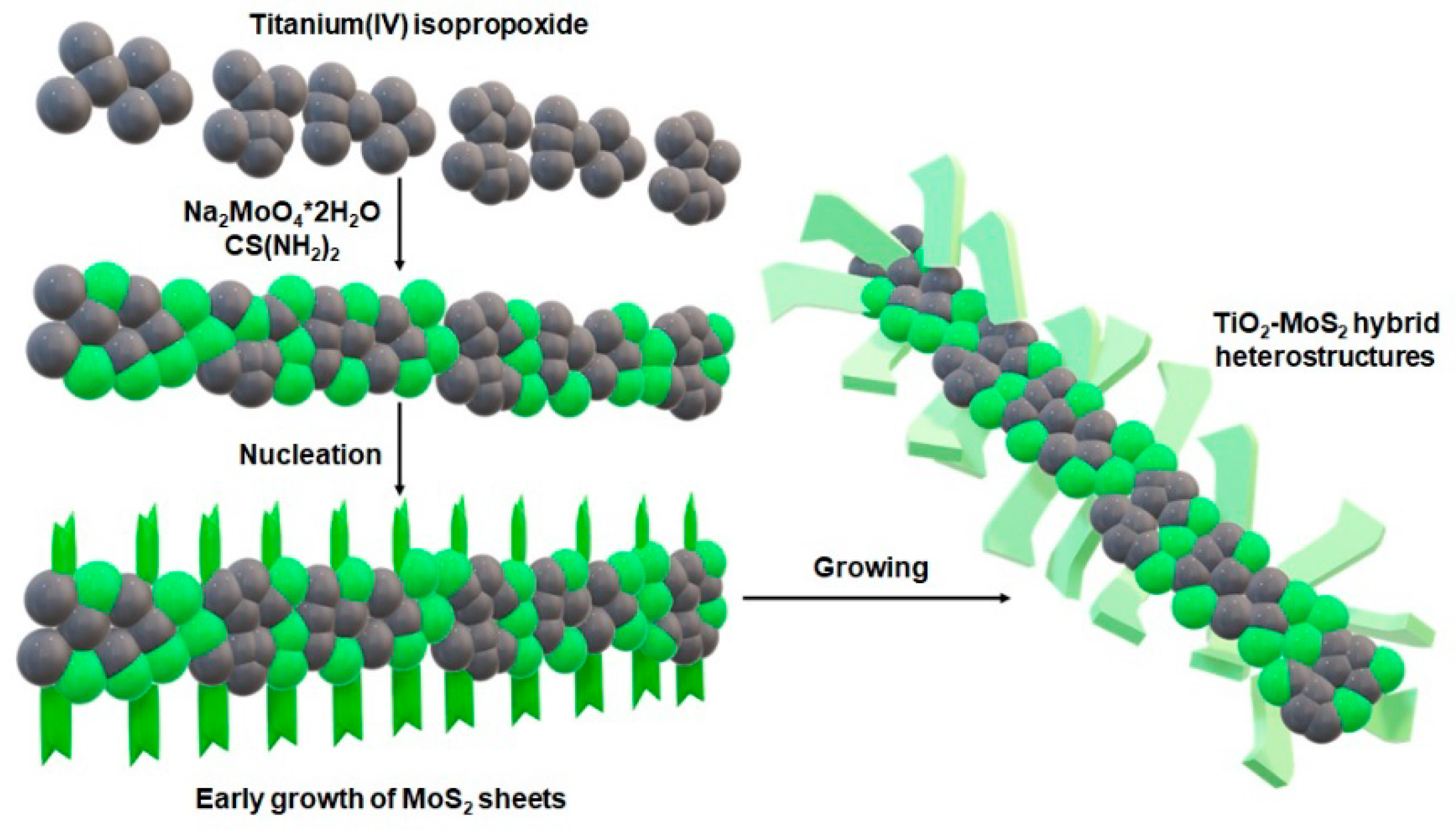
2.2. Fabrication of TiO2-MoS2 Hybrid Systems
2.3. Characterization of Synthesized Hybrid Materials
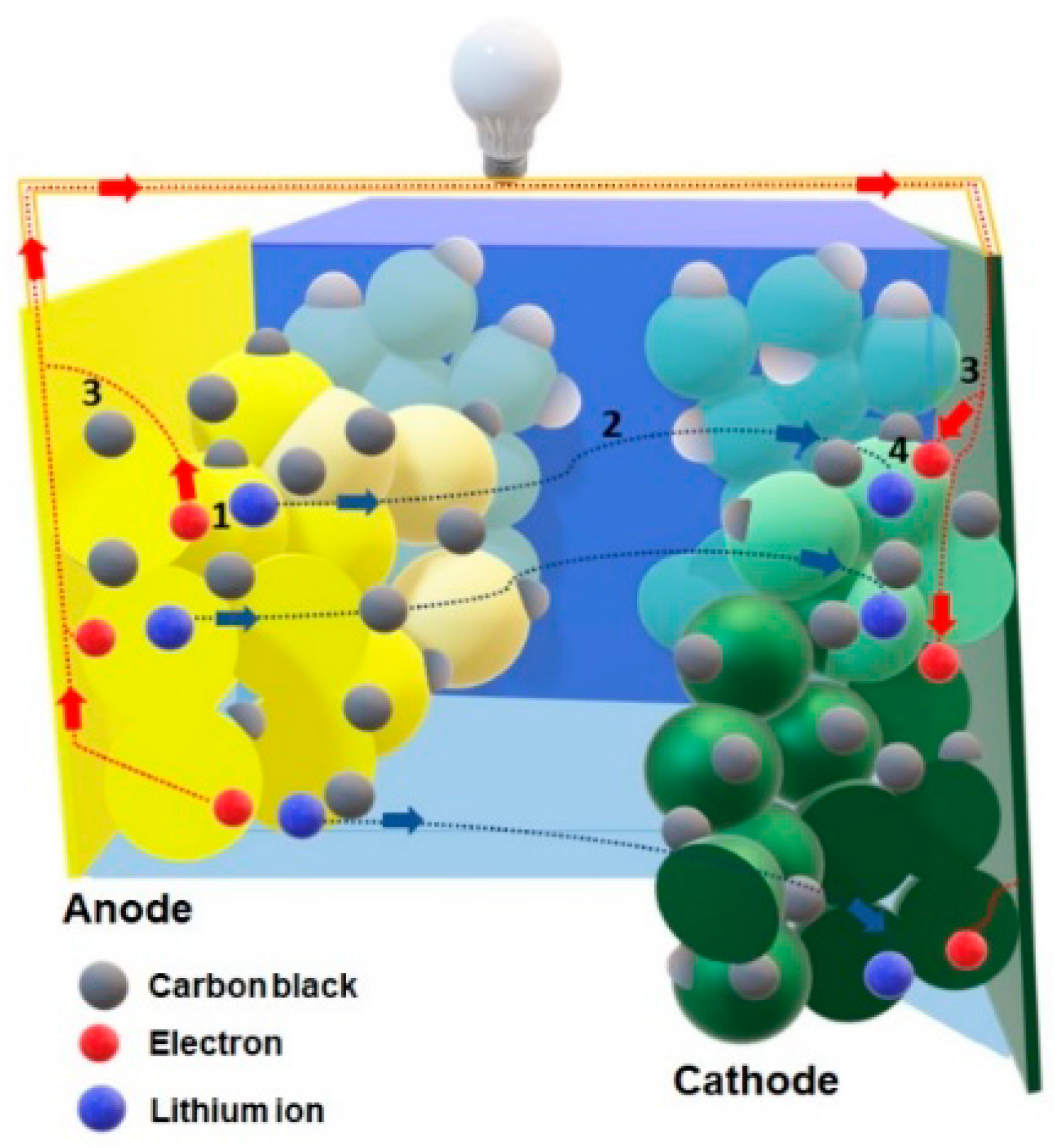
2.4. Electrochemical Performance
3. Results and Discussion
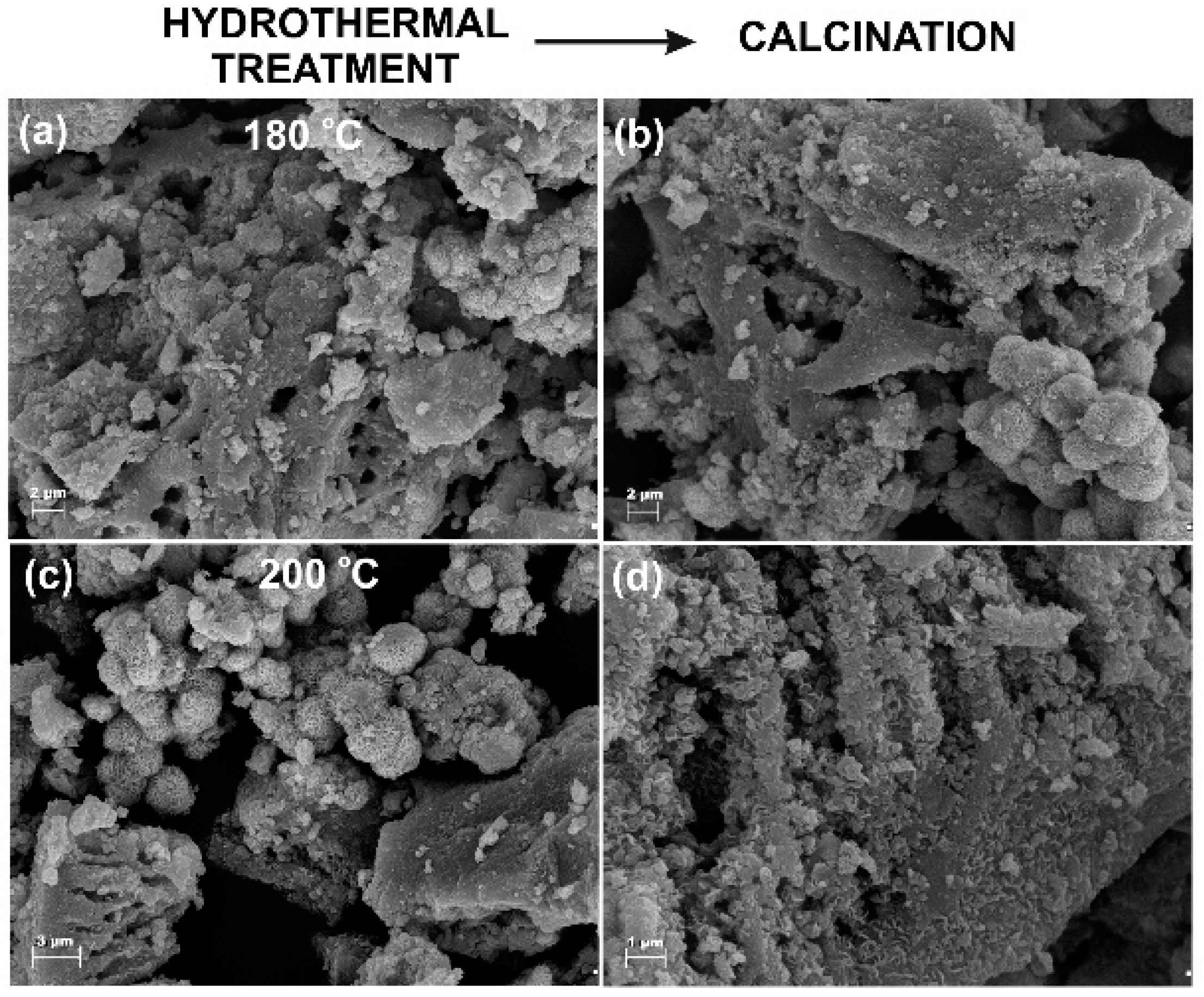
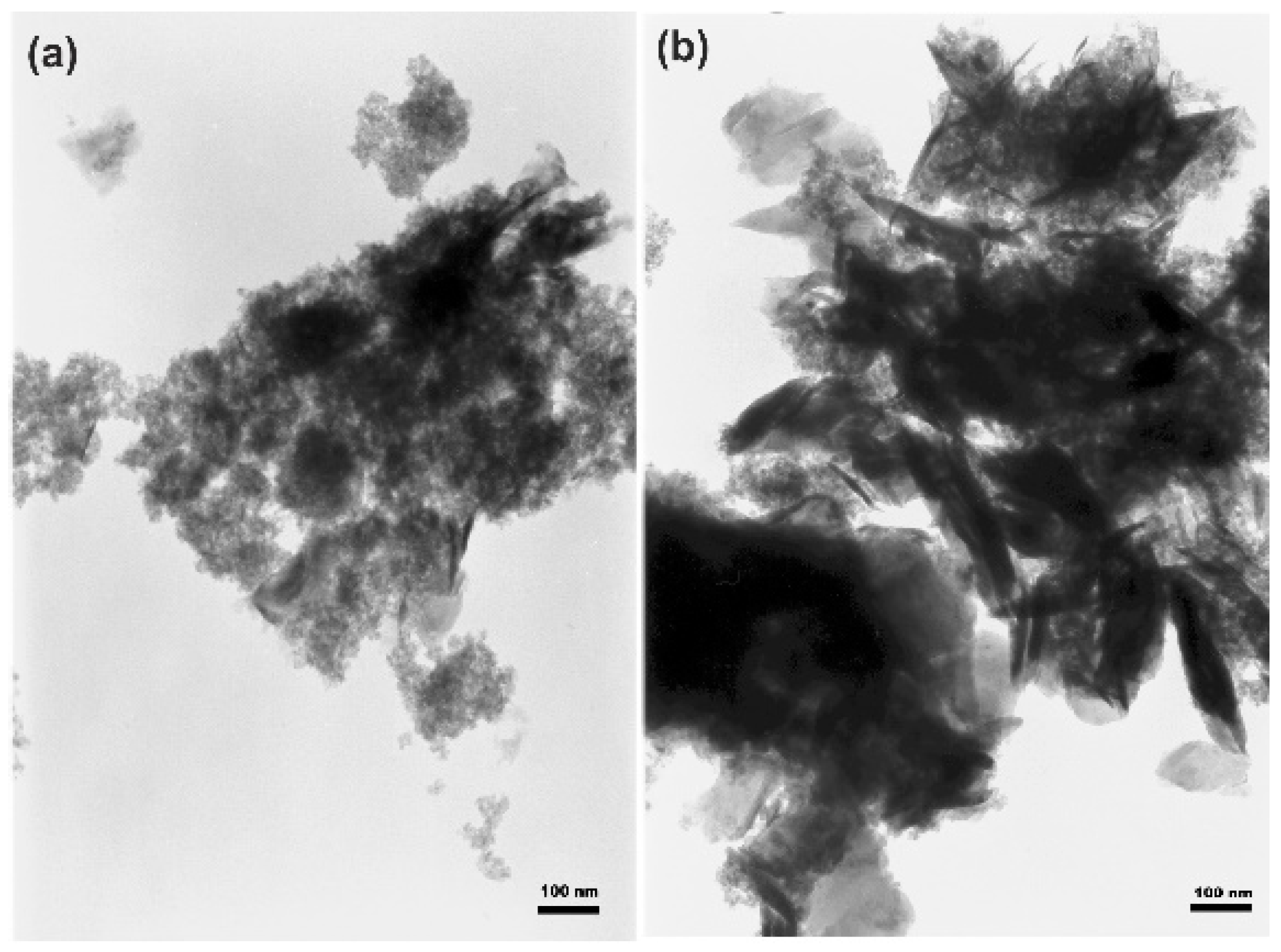
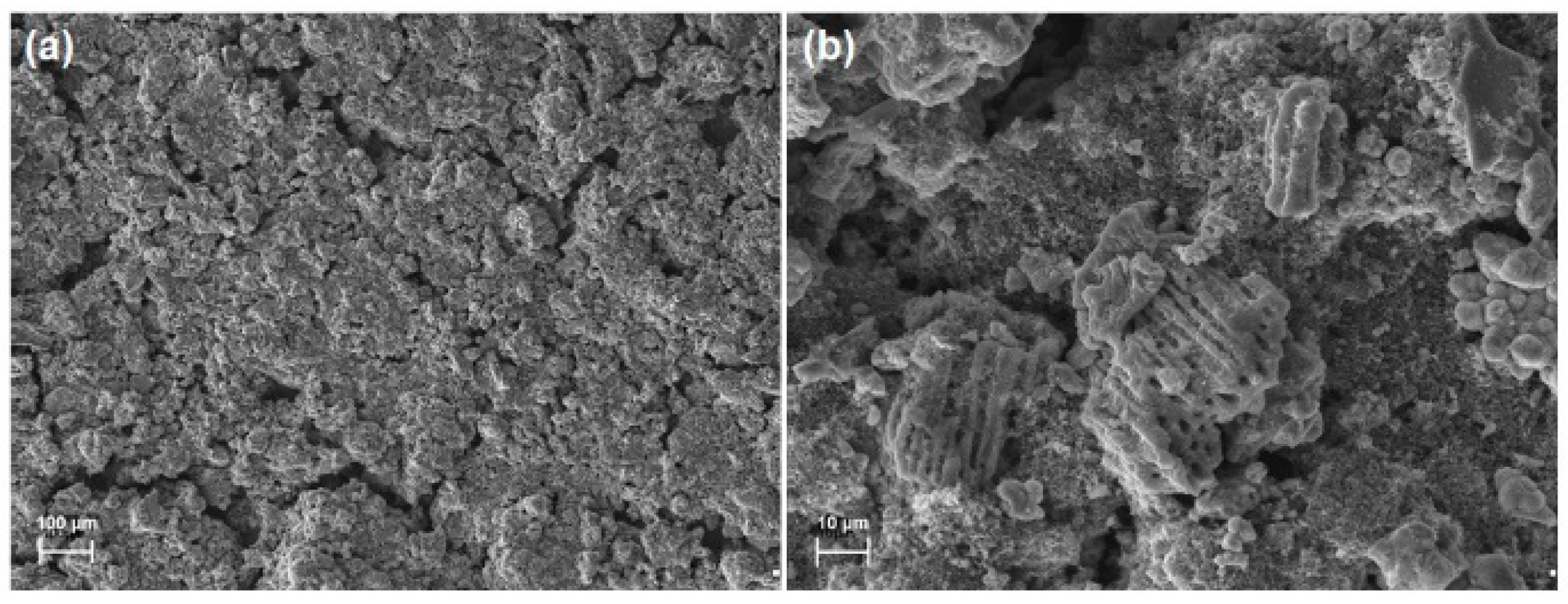
3.1. Microstructure and Morphology
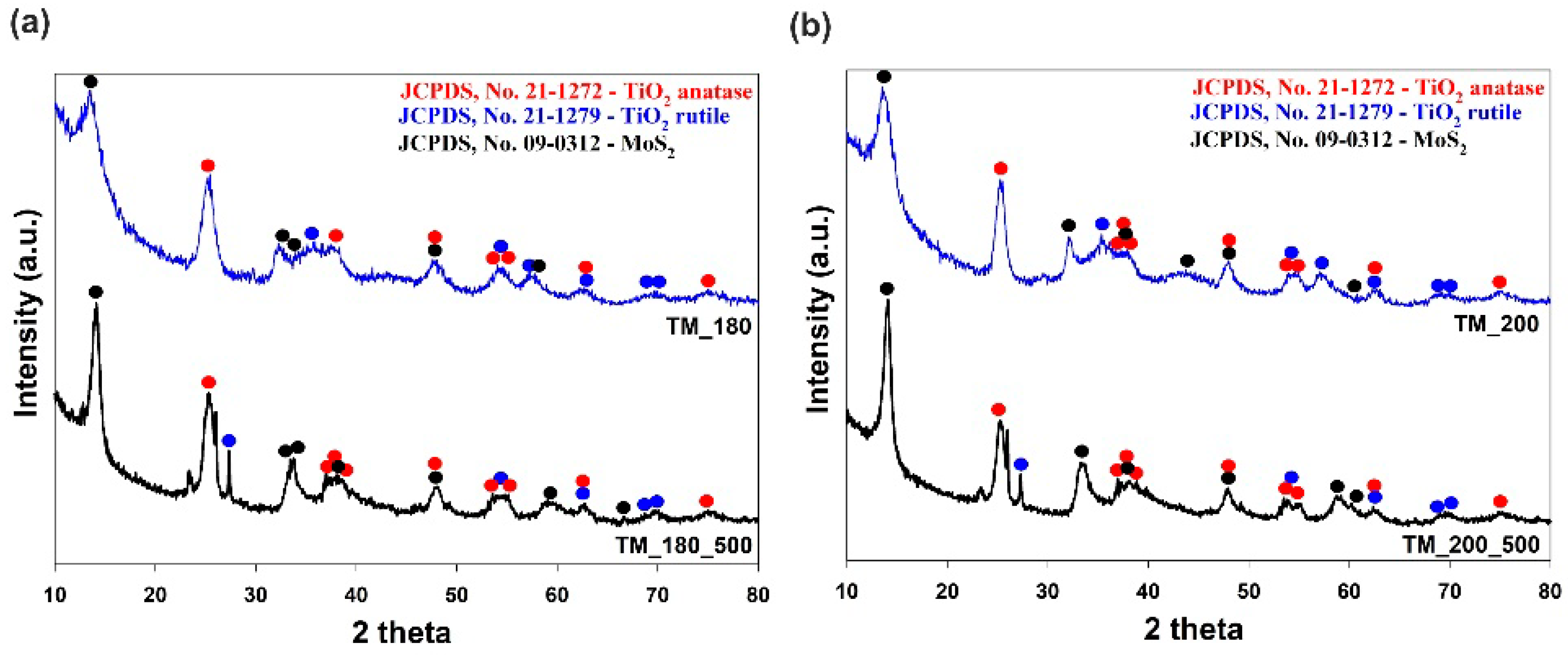
3.2. Crystalline Structure
3.3. Surface Chemical Composition
3.4. Textural Properties
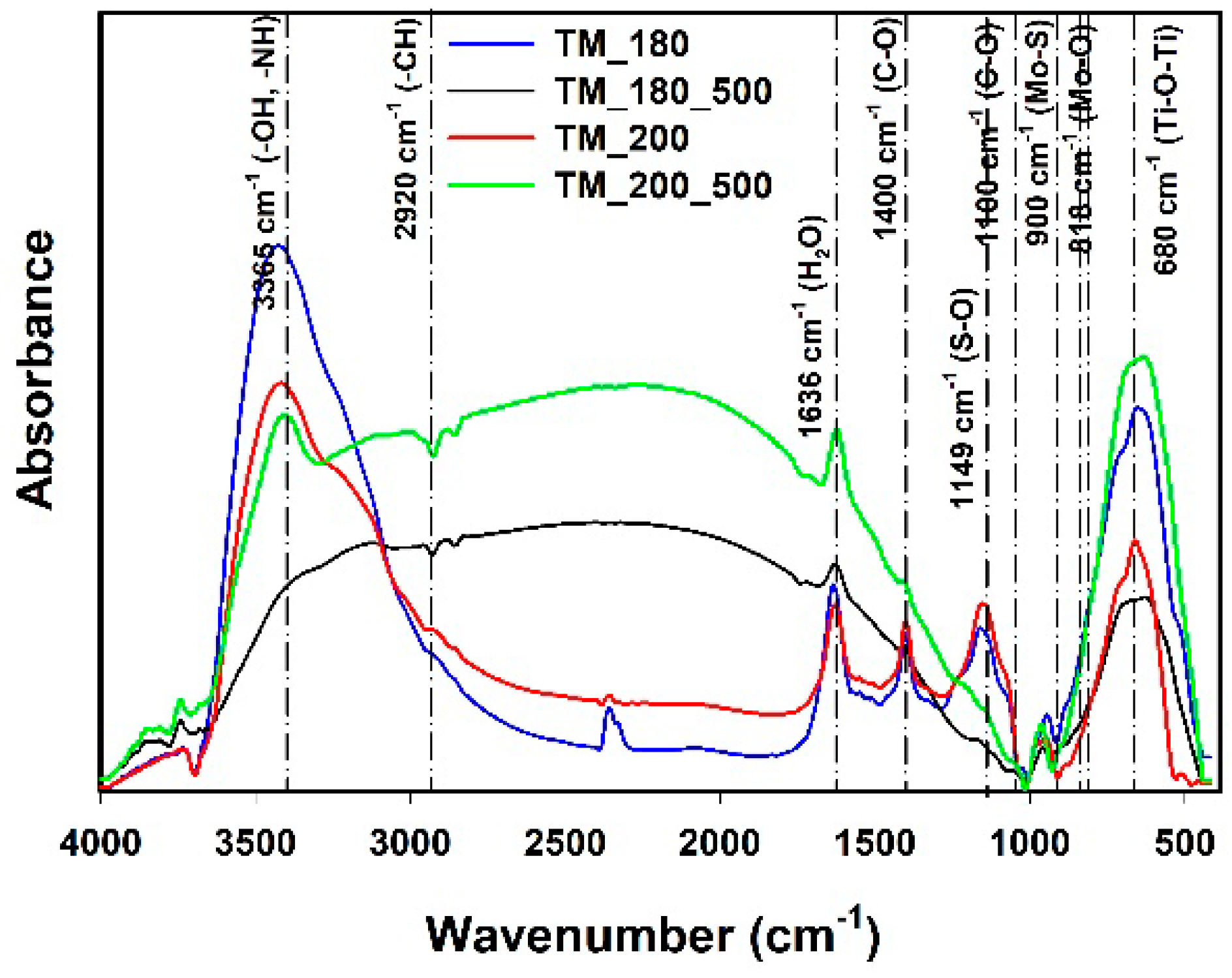
3.5. FTIR Analysis
3.6. XPS Analysis
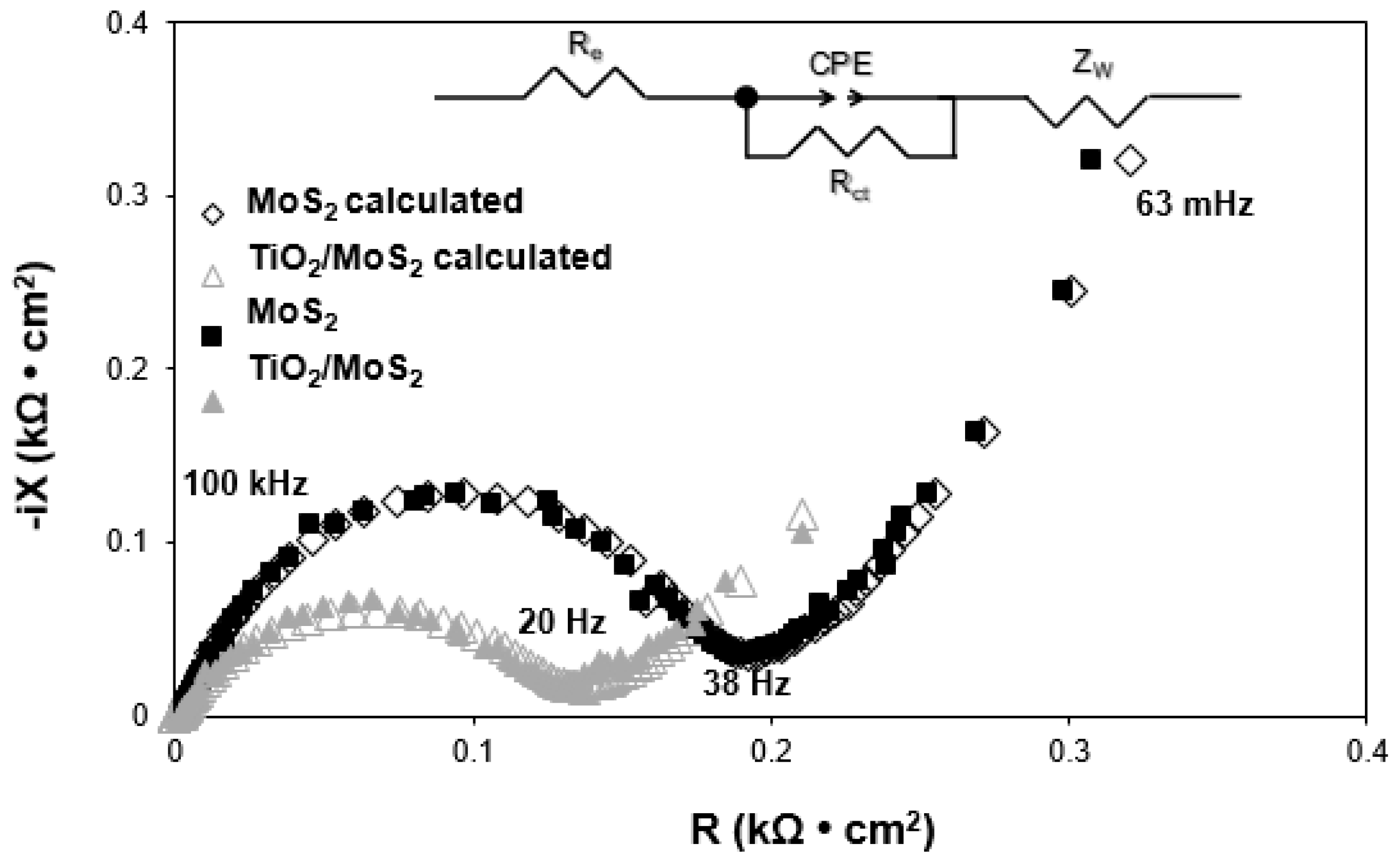
3.7. Electrochemical Performance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the development of advanced Li-ion batteries: A review. Energy Environ. Sci. 2011, 4, 3243–3262. [Google Scholar] [CrossRef]
- Scrosati, B.; Hassoun, J.; Sun, Y. Lithium-ion batteries. A look into the future. Energy Environ. Sci. 2011, 4, 3287–3295. [Google Scholar] [CrossRef]
- Goriparti, S.; Miele, E.; De Angelis, F.; Di Fabrizio, E.; Zaccaria, R.P.; Capiglia, C. Review on recent progress of nanostructured anode materials for Li-ion batteries. J. Power Sources 2014, 257, 421–443. [Google Scholar] [CrossRef]
- Roy, P.; Srivastava, S.K. Nanostructured anode materials for lithium ion batteries. J. Mater. Chem. A 2015, 3, 2454–2484. [Google Scholar] [CrossRef]
- Yuan, C.; Wu, H.B.; Xie, Y.; Lou, X.W. Mixed transition-metal oxides: Design, synthesis, and energy-related applications. Angew. Chem. Int. Ed. 2014, 53, 1488–1504. [Google Scholar] [CrossRef]
- Reddy, M.V.; Subba Rao, G.V.; Chowdari, B.V.R. Metal oxides and oxysalts as anode materials for li ion batteries. Chem. Rev. 2013, 113, 5364–5457. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Marom, R.; Amalraj, F.; Leifer, N.; Jacob, D.; Aurbach, D. A review of advanced and practical lithium battery materials. J. Mater. Chem. 2011, 21, 9938–9954. [Google Scholar] [CrossRef]
- Xu, W.; Wang, T.; Yu, Y.; Wang, S. Synthesis of core-shell TiO2@MoS2 composites for lithium-ion battery anodes. J. Alloys Compd. 2016, 689, 460–467. [Google Scholar] [CrossRef]
- Qiu, J.; Zhang, P.; Ling, M.; Li, S.; Liu, P.; Zhao, H.; Zhang, S. Photocatalytic synthesis of TiO2 and reduced graphene oxide nanocomposite for lithium ion battery. ACS Appl. Mater. Interfaces 2012, 4, 3636–3642. [Google Scholar] [CrossRef]
- Qiu, J.; Lai, C.; Wang, Y.; Li, S.; Zhang, S. Resilient mesoporous TiO2/graphene nanocomposite for high rate performance lithium-ion batteries. Chem. Eng. J. 2014, 256, 247–254. [Google Scholar] [CrossRef]
- Wei, T.; Lau, W.M.; An, X.; Yu, X. Interfacial charge transfer in MoS2/TiO2 heterostructured photocatalysts: The impact of crystal facets and defects. Molecules 2019, 24, 1769. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, L.; Huang, F. Black titanium dioxide (TiO2) nanomaterials. Chem. Soc. Rev. 2015, 44, 1861–1885. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Lian, Z.; Wang, W.; Zhang, D.; Li, H. Nanotube-confinement induced size-controllable g-C3N4 quantum dots modified single-crystalline TiO2 nanotube arrays for stable synergetic photoelectrocatalysis. Nano Energy 2016, 19, 446–454. [Google Scholar] [CrossRef]
- Wang, S.L.; Luo, X.; Zhou, X.; Zhu, Y.; Chi, X.; Chen, W.; Wu, K.; Liu, Z.; Quek, S.Y.; Xu, G.Q. Fabrication and properties of a free-standing two-dimensional titania. J. Am. Chem. Soc. 2017, 139, 15414–15419. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wang, L.; Kim, J.K.; Ma, M.; Veerappan, G.; Lee, C.-L.; Kong, K.-J.; Lee, H.; Park, J.H. An order/disorder/water junction system for highly efficient co-catalyst-free photocatalytic hydrogen generation. Energy Environ. Sci. 2016, 9, 499–503. [Google Scholar] [CrossRef]
- Li, H.; Dong, W.; Xi, J.; Du, G.; Ji, Z. 3D flowerlike TiO2/GO and TiO2/MoS2 heterostructures with enhanced photoelectrochemical water splitting. J. Mater. Sci. 2018, 53, 7609–7620. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Jaroniec, M. Synergetic effect of MoS2 and graphene as cocatalysts for enhanced photocatalytic H2 production activity of TiO2 nanoparticles. J. Am. Chem. Soc. 2012, 134, 6575–6578. [Google Scholar] [CrossRef] [PubMed]
- Di Lupo, F.; Tuel, A.; Mendez, V.; Francia, C.; Meligrana, G.; Bodoardo, S.; Gerbaldi, C. Mesoporous TiO2 nanocrystals produced by a fast hydrolytic process as high-rate long-lasting Li-ion battery anodes. Acta Mater. 2014, 69, 60–67. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Senthil, R.A.; Senthilkumar, B.; Polu, A.R.; Madhavan, J.; Ashokkumar, M. Recent advances in MoS2 nanostructured materials for energy and environmental applications–A review. J. Solid State Chem. 2017, 252, 43–71. [Google Scholar] [CrossRef]
- Song, I.; Park, C.; Choi, H.C. Synthesis and properties of molybdenum disulphide: From bulk to atomic layers. RSC Adv. 2015, 5, 7495–7514. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Wang, X.; Hu, J.; Fang, X.; Chu, X.; Wei, Z.; Shan, J.; Ding, X. Preparation, applications of two-dimensional graphene-like molybdenum disulfide. Integr. Ferroelectr. 2014, 158, 26–42. [Google Scholar] [CrossRef]
- Choi, W.; Choudhary, N.; Han, G.H.; Park, J.; Akinwande, D.; Lee, Y.H. Recent development of two-dimensional transition metal dichalcogenides and their applications. Mater. Today 2017, 20, 116–130. [Google Scholar] [CrossRef]
- He, Z.; Que, W. Molybdenum disulfide nanomaterials: Structures, properties, synthesis and recent progress on hydrogen evolution reaction. Appl. Mater. Today 2016, 3, 23–56. [Google Scholar] [CrossRef]
- Li, X.; Zhu, H. Two-dimensional MoS2: Properties, preparation, and applications. J. Mater. 2015, 1, 33–44. [Google Scholar] [CrossRef]
- Parzinger, E.; Miller, B.; Blaschke, B.; Garrido, J.A.; Ager, J.W.; Holleitner, A.; Wurstbauer, U. Photocatalytic stability of single- and few-layer MoS2. ACS Nano 2015, 9, 11302–11309. [Google Scholar] [CrossRef]
- Wang, T.; Chen, S.; Pang, H.; Xue, H.; Yu, Y. MoS2-based nanocomposites for electrochemical energy storage. Adv. Sci. 2017, 4, 1600289. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, S.; Zeng, X.; Xiao, J.; Dong, P.; Zhao, J.; Sun, S.; Huang, L.; Li, X. TiO2-MoS2 hybrid nano composites with 3D network architecture as binder-free flexible electrodes for lithium ion batteries. J. Mater. Sci. Mater. Electron. 2017, 28, 9519–9527. [Google Scholar] [CrossRef]
- Chen, B.; Meng, Y.; Sha, J.; Zhong, C.; Hu, W.; Zhao, N. Preparation of MoS2/TiO2 based nanocomposites for photocatalysis and rechargeable batteries: Progress, challenges, and perspective. Nanoscale 2018, 10, 34–64. [Google Scholar] [CrossRef]
- Guo, B.; Yu, K.; Fu, H.; Hua, Q.; Qi, R.; Li, H.; Song, H.; Guo, S.; Zhu, Z. Firework-shaped TiO2 microspheres embedded with few-layer MoS2 as an anode material for excellent performance lithium-ion batteries. J. Mater. Chem. A 2015, 3, 6392–6401. [Google Scholar] [CrossRef]
- Zhu, X.; Yang, C.; Xiao, F.; Wang, J.; Su, X. Synthesis of nano-TiO2-decorated MoS2 nanosheets for lithium ion batteries. New J. Chem. 2015, 39, 683–688. [Google Scholar] [CrossRef]
- Li, X.; Li, W.; Li, M.; Cui, P.; Chen, D.; Gengenbach, T.; Chu, L.; Liu, H.; Song, G. Glucose-assisted synthesis of the hierarchical TiO2 nanowires@MoS2 nanosheets nanocomposite and its synergistic lithium storage performance. J. Mater. Chem. A 2015, 3, 2762–2769. [Google Scholar] [CrossRef]
- Zhu, X.; Liang, X.; Fan, X.; Su, X. Fabrication of flower-like MoS2/TiO2 hybrid as an anode material for lithium ion batteries. RSC Adv. 2017, 7, 38119–38124. [Google Scholar] [CrossRef]
- Pang, Q.; Zhao, Y.; Bian, X.; Ju, Y.; Wang, X.; Wei, Y.; Liu, B.; Du, F.; Wang, C.; Chen, G. Hybrid graphene@MoS2@TiO2 microspheres for use as a high performance negative electrode material for lithium ion batteries. J. Mater. Chem. A 2017, 5, 3667–3674. [Google Scholar] [CrossRef]
- Siwińska-Ciesielczyk, K.; Świgoń, D.; Rychtowski, R.; Moszyński, D.; Zgoła-Grześkowiak, A.; Jesionowski, T. The performance of multicomponent oxide systems based on TiO2, ZrO2 and SiO2 in the photocatalytic degradation of Rhodamine B: Mechanism and kinetic studies. Colloids Surf. A Physicochem. Eng. Asp. 2020, 586, 124272. [Google Scholar] [CrossRef]
- Siwińska-Stefańska, K.; Kubiak, A.; Piasecki, A.; Dobrowolska, A.; Czaczyk, K.; Motylenko, M.; Rafaja, D.; Ehrlich, H.; Jesionowski, T. Hydrothermal synthesis of multifunctional TiO2-ZnO oxide systems with desired antibacterial and photocatalytic properties. Appl. Surf. Sci. 2019, 463, 791–801. [Google Scholar] [CrossRef]
- Zhu, W.; Chi, M.; Gao, M.; Wang, C.; Lu, X. Controlled synthesis of titanium dioxide/molybdenum disulfide core-shell hybrid nanofibers with enhanced peroxidase-like activity for colorimetric detection of glutathione. J. Colloid Interf. Sci. 2018, 528, 410–418. [Google Scholar] [CrossRef]
- Prabhakar Vattikuti, S.V.; Nagajyothi, P.C.; Kumar Reddy, P.A.; Kumar, M.K.; Shim, J.; Byon, C. Tiny MoO3 nanocrystals self-assembled on folded molybdenum disulfide nanosheets via a hydrothermal method for supercapacitor. Mater. Res. Lett. 2018, 6, 432–441. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Phys. B: Condens. Matter 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierott, R.A.; Rouquérol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Naderi, M. Surface area: Brunauer-Emmett-Teller (BET). In Progress in Filtration and Separation, 1st ed.; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Suzuki, M.; Tanaka, K.; Kato, Y.; Hanabusa, K. Metal oxide/TiO2 hybrid nanotubes fabricated through the organogel route. Gels 2017, 3, 24. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xiao, X.; Zheng, L.; Wan, C. Fabrication of TiO2/MoS2 composite photocatalyst and its photocatalytic mechanism for degradation of methyl orange under visible light. Can. J. Chem. Eng. 2015, 93, 1594–1602. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Ke, J.; Wang, Z.; Xiao, W. Synergically improving light harvesting and charge transportation of TiO2 nanobelts by deposition of MoS2 for enhanced photocatalytic removal of Cr(VI). Catalysts 2017, 7, 30. [Google Scholar] [CrossRef]
- Weber, T.; Muijsers, J.C.; van Wolput, J.H.M.C.; Verhagen, C.P.J.; Niemantsverdriet, J.W. Basic reaction steps in the sulfidation of crystalline MoO3 to MoS2, as studied by X-ray photoelectron and infrared emission spectroscopy. J. Phys. Chem. 1996, 100, 14144–14150. [Google Scholar] [CrossRef]
- Maugé, F.; Lamotte, J.; Nesterenko, N.S.; Manoilova, O.; Tsyganenko, A.A. FT-IR study of surface properties of unsupported MoS2. Catal. Today 2001, 70, 271–284. [Google Scholar] [CrossRef]
- Dolat, D.; Moszyński, D.; Guskos, N.; Ohtani, B.; Morawski, A.W. Preparation of photoactive nitrogen-doped rutile. Appl. Surf. Sci. 2013, 266, 410–419. [Google Scholar] [CrossRef]
- Reddy, M.V.; Sharma, N.; Adams, S.; Prasada Rao, R.; Peterson, V.K.; Chowdari, B.V.R. Evaluation of undoped and M-doped TiO2, where M=Sn, Fe, Ni/Nb, Zr, V, and Mn, for lithium-ion battery applications prepared by the molten-salt method. RSC Adv. 2015, 5, 29535–29544. [Google Scholar] [CrossRef]
- Santoni, A.; Rondino, F.; Malerba, C.; Valentini, M.; Mittiga, A. Electronic structure of Ar+ ion-sputtered thin-film MoS2: A XPS and IPES study. Appl. Surf. Sci. 2017, 392, 795–800. [Google Scholar] [CrossRef]
- Kondekar, N.P.; Boebinger, M.G.; Woods, E.V.; McDowell, M.T. In situ XPS investigation of transformations at crystallographically oriented MoS2 interfaces. ACS Appl. Mater. Interfaces 2017, 9, 32394–32404. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.E.; Sobol, P.E.; Bomben, K.E. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer: Eden Prairie, MN, USA, 1992. [Google Scholar]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Jiang, Z.; Tang, Y.; Tay, Q.; Zhang, Y.; Malyi, O.I.; Wang, D.; Deng, J.; Lai, Y.; Zhou, H.; Chen, X.; et al. Understanding the role of nanostructures for efficient hydrogen generation on immobilized photocatalysts. Adv. Energy Mater. 2013, 3, 1368–1380. [Google Scholar] [CrossRef]
- Zhu, G.-N.; Wang, Y.-G.; Xia, Y.-Y. Ti-based compounds as anode materials for Li-ion batteries. Energy Environ. Sci. 2012, 5, 6652–6667. [Google Scholar] [CrossRef]
- Yang, Z.; Choi, D.; Kerisit, S.; Rosso, K.M.; Wang, D.; Zhang, J.; Graff, G.; Liu, J. Nanostructures and lithium electrochemical reactivity of lithium titanites and titanium oxides: A review. J. Power Sources 2009, 192, 588–598. [Google Scholar] [CrossRef]
- Song, T.; Paik, U. TiO2 as an active or supplemental material for lithium batteries. J. Mater. Chem. A 2016, 4, 14–31. [Google Scholar] [CrossRef]
- Mei, Y.; Huang, Y.; Hu, X. Nanostructured Ti-based anode materials for Na-ion batteries. J. Mater. Chem. A 2016, 4, 12001–12013. [Google Scholar] [CrossRef]
- Han, B.; Hu, Y.H. MoS2 as a co-catalyst for photocatalytic hydrogen production from water. Energy Sci. Eng. 2016, 4, 285–304. [Google Scholar] [CrossRef]
- Yuan, Y.-J.; Lu, H.-W.; Yu, Z.-T.; Zou, Z.-G. Noble-metal-free molybdenum disulfide cocatalyst for photocatalytic hydrogen production. ChemSusChem 2015, 8, 4113–4127. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Y.-L.; Sa, B.; Ahuja, R. Review of two-dimensional materials for photocatalytic water splitting from a theoretical perspective. Catal. Sci. Technol. 2017, 7, 545–559. [Google Scholar] [CrossRef]
- Laursen, A.B.; Kegnaes, S.; Dahl, S.; Chorkendorff, I. Molybdenum sulfides—Efficient and viable materials for electro—And photoelectrocatalytic hydrogen evolution. Energy Environ. Sci. 2012, 5, 5577–5591. [Google Scholar] [CrossRef]
- Chen, S.; Duan, J.; Tang, Y.; Jin, B.; Qiao, S.Z. Molybdenum sulfide clusters-nitrogen-doped graphene hybrid hydrogel film as an efficient three-dimensional hydrogen evolution electrocatalyst. Nano Energy 2015, 11, 11–18. [Google Scholar] [CrossRef]
- Ran, J.; Gao, G.; Li, F.T.; Ma, T.Y.; Du, A.; Qiao, S.Z. Ti3C2 MXene co-catalyst on metal sulfide photo-absorbers for enhanced visible-light photocatalytic hydrogen production. Nat. Commun. 2017, 8, 13907. [Google Scholar] [CrossRef]
- Stephenson, T.; Li, Z.; Olsen, B.; Mitlin, D. Lithium ion battery applications of molybdenum disulfide (MoS2) nanocomposites. Energy Environ. Sci. 2014, 7, 209–231. [Google Scholar] [CrossRef]
- Zhang, X.; Lai, Z.; Tan, C.; Zhang, H. Solution-processed two-dimensional MoS2 nanosheets: Preparation, hybridization, and applications. Angew. Chem. Int. Ed. Engl. 2016, 55, 8816–8838. [Google Scholar] [CrossRef]
- Xiao, Y.; Lee, S.H.; Sun, Y.-K. The application of metal sulfides in sodium ion batteries. Adv. Energy Mater 2017, 7, 1601329. [Google Scholar] [CrossRef]
- Li, H.; Shi, Y.; Chiu, M.-H.; Li, L.-J. Emerging energy applications of two-dimensional layered transition metal dichalcogenides. Nano Energy 2015, 18, 293–305. [Google Scholar] [CrossRef]
- Xu, X.; Liu, W.; Kim, Y.; Cho, J. Nanostructured transition metal sulfides for lithium ion batteries: Progress and challenges. Nano Today 2014, 9, 604–630. [Google Scholar] [CrossRef]
- Tian, J.; Zhao, Z.; Kumar, A.; Boughton, R.I.; Liu, H. Recent progress in design, synthesis, and applications of one-dimensional TiO2 nanostructured surface heterostructures: A review. Chem. Soc. Rev. 2014, 43, 6920–6937. [Google Scholar] [CrossRef] [PubMed]
- Peining, Z.; Yongzhi, W.; Reddy, M.V.; Sreekumaran Nair, A.; Shengjie, P.; Sharma, N.; Peterson, V.K.; Chowdari, B.V.R.; Ramakrishna, S. TiO2 nanoparticles synthesized by the molten salt method as a dual functional material for dye-sensitized solar cells. RSC Adv. 2012, 2, 5123–5126. [Google Scholar] [CrossRef]
- Shi, W.; Song, S.; Zhang, H. Hydrothermal synthetic strategies of inorganic semiconducting nanostructures. Chem. Soc. Rev. 2013, 42, 5714–5743. [Google Scholar] [CrossRef]
- Reddy, M.V.; Valerie Teoh, X.W.; Nguyen, T.B.; Michelle Lim, Y.Y.; Chowdari, B.V.R. Effect of 0.5 M NaNO3: 0.5 M KNO3 and 0.88 M LiNO3:0.12 M LiCl molten salts, and heat treatment on electrochemical properties of TiO2. J. Electrochem. Soc. 2012, 159, A762–A769. [Google Scholar] [CrossRef]
- Petnikota, S.; Teo, K.W.; Chen, L.; Sim, A.; Kumar Marka, S.; Reddy, M.V.; Srikanth, V.V.S.S.; Adams, S.; Chowdari, B.V.R. Exfoliated graphene oxide/MoO2 composites as anode materials in lithium-ion batteries: An insight into intercalation of Li and conversion mechanism of MoO2. ACS Appl. Mater. Interfaces 2016, 8, 10884–10896. [Google Scholar] [CrossRef]
- Cherian, C.T.; Zheng, M.; Reddy, M.V.; Chowdari, B.V.R.; Sow, C.H. Zn2SnO4 nanowires versus nanoplates: Electrochemical performance and morphological evolution during Li-cycling. ACS Appl Mater Interfaces 2013, 10, 6054–6060. [Google Scholar] [CrossRef]
- Zhou, W.; Yin, Z.; Du, Y.; Huang, X.; Zeng, Z.; Fan, Z.; Liu, H.; Wang, J.; Zhang, H. Synthesis of few-layer MoS2 nanosheet-coated TiO2 nanobelt heterostructures for enhanced photocatalytic activities. Small 2013, 9, 140–147. [Google Scholar] [CrossRef]
- Mao, M.; Mei, L.; Guo, D.; Wu, L.; Zhang, D.; Li, Q.; Wang, T. High electrochemical performance based on the TiO2 nanobelt@few-layered MoS2 structure for lithium-ion batteries. Nanoscale 2014, 6, 12350–12353. [Google Scholar] [CrossRef]
- Liu, C.; Wang, L.; Tang, Y.; Luo, S.; Liu, Y.; Zhang, S.; Zeng, Y.; Xu, Y. Vertical single or few-layer MoS2 nanosheets rooting into TiO2 nanofibers for highly efficient photocatalytic hydrogen evolution. Appl. Catal. B 2015, 164, 1–9. [Google Scholar] [CrossRef]
- Liu, H.; Lv, T.; Zhu, C.; Su, X.; Zhu, Z. Efficient synthesis of MoS2 nanoparticles modified TiO2 nanobelts with enhanced visible-light-driven photocatalytic activity. J. Mol. Catal. A Chem. 2015, 396, 136–142. [Google Scholar] [CrossRef]
- Zhang, X.; Shao, C.; Li, X.; Miao, F.; Wang, K.; Lu, N.; Liu, Y. 3D MoS2 nanosheet/TiO2 nanofiber heterostructures with enhanced photocatalytic activity under UV irradiation. J. Alloy. Compd. 2016, 686, 137–144. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Chen, G.; Sang, Y.; Jiang, H.; He, J.; Li, X.; Liu, H. Few-layered MoS2 nanosheets wrapped ultrafine TiO2 nanobelts with enhanced photocatalytic property. Nanoscale 2016, 8, 6101–6109. [Google Scholar] [CrossRef] [PubMed]
- Ivanishcheva, I.A.; Ivanishchev, A.V.; Dixit, A. Positive effect of surface modification with titanium carbosilicide on performance of lithium-transition metal phosphate cathode materials. Monatshefte für Chem.-Chem. Mon. 2019, 150, 489–498. [Google Scholar] [CrossRef]
- Ren, M.M.; Zhou, Z.; Gao, X.P.; Peng, W.X.; Wei, J.P. Core-shell Li3V2(PO4)3@C composites as cathode materials for lithium-ion batteries. J. Phys. Chem. C. 2008, 112, 5689–5693. [Google Scholar] [CrossRef]
- Ataherian, F.; Lee, K.-T.; Wu, N.-L. Long-term electrochemical behaviors of manganese oxide aqueous electrochemical capacitor under reducing potentials. Electrochim. Acta 2010, 55, 7429–7435. [Google Scholar] [CrossRef]
- Yamin, H.; Peled, E. Electrochemistry of a nonaqueous lithium/sulfur cell. J. Power Sources 1983, 9, 281–287. [Google Scholar] [CrossRef]
- Cheon, S.E.; Ko, K.-S.; Cho, J.-H.; Kim, S.-W.; Chin, E.-Y.; Kim, H.-T. Rechargeable lithium sulfur battery I. Structural change of sulfur cathode during discharge and charge. J. Electrochem. Soc. 2003, 150, A796–A799. [Google Scholar] [CrossRef]
- Cheon, S.E.; Ko, K.-S.; Cho, J.-H.; Kim, S.-W.; Chin, E.-Y.; Kim, H.-T. Rechargeable lithium sulfur battery II. Rate capability and cycle characteristics. J. Electrochem. Soc. 2003, 150, A800–A805. [Google Scholar] [CrossRef]
- Cheon, S.E.; Cho, J.-H.; Ko, K.-S.; Kwon, C.-W.; Chang, D.-R.; Kim, H.-T.; Kim, S.-W. Structural factors of sulfur cathodes with poly(ethylene oxide) binder for performance of rechargeable lithium sulfur batteries. J. Electrochem. Soc. 2002, 149, A1437–A1441. [Google Scholar] [CrossRef]
- Elazari, R.; Salitra, G.; Talyosef, Y.; Grinblat, J.; Scordilis-Kelley, C.; Xiao, A.; Affinito, J.; Aurbach, D. Morphological and structural studies of composite sulfur electrodes upon cycling by HRTEM, AFM and raman spectroscopy. J. Electrochem. Soc. 2010, 157, A1131–A1138. [Google Scholar] [CrossRef]
- Ivanishchev, A.V.; Churikov, A.V.; Ivanishcheva, I.A. Modelling of electrochemically stimulated ionic transport in lithium intercalation compounds. Monatshefte für Chem.-Chem. Mon. 2017, 148, 481–487. [Google Scholar] [CrossRef]
- Ivanishchev, A.V.; Ushakov, A.V.; Ivanishcheva, I.A.; Churikov, A.V.; Mironov, A.V.; Fedotov, S.S.; Khasanova, N.R.; Antipov, E.V. Structural and electrochemical study of fast Li diffusion in Li3V2(PO4)3-based electrode material. Electrochim. Acta 2017, 230, 479–491. [Google Scholar] [CrossRef]
- Ivanishchev, A.V.; Ivanishcheva, I.A.; Dixit, A. LiFePO4-based composite electrode material: Synthetic approaches, peculiarities of the structure, and regularities of ionic transport processes. Russ. J. Electrochem. 2019, 55, 719–737. [Google Scholar] [CrossRef]
- Churikov, A.V.; Pridatko, K.I.; Ivanishchev, A.V.; Ivanishcheva, I.A.; Gamayunova, I.M.; Zapsis, K.V.; Sycheva, V.O. Impedance spectroscopy of lithium–tin film electrodes. Russ. J. Electrochem. 2008, 44, 550–557. [Google Scholar] [CrossRef]
- Ivanishchev, A.V.; Bobrikov, I.A.; Ivanishcheva, I.A.; Ivanshina, O.Y. Study of structural and electrochemical characteristics of LiNi0.33Mn0.33Co0.33O2 electrode at lithium content variation. J. Electroanal. Chem. 2018, 821, 140–151. [Google Scholar] [CrossRef]
- Ivanishchev, A.V.; Churikov, A.V.; Ivanishcheva, I.A.; Ushakov, A.V. Lithium diffusion inLi3V2(PO4)3-based electrodes: A joint analysis of electrochemical impedance, cyclic volt-ammetry, pulse chronoamperometry, and chronopotentiometry data. Ionics 2016, 22, 483–501. [Google Scholar] [CrossRef]
- Reddy, M.V.; Wei Wen, B.L.; Ping Loh, K.; Chowdari, B.V.R. Energy storage studies on InVO4 as high performance anode material for Li-ion batteries. ACS Appl. Mater. Interfaces 2013, 5, 7777–7785. [Google Scholar] [CrossRef]
- Reddy, M.V.G.; Subba Rao, V.; Chowdari, B.V.R. Nano-(V1/2Sb1/2Sn)O4: A high capacity, high rate anode material for Li-ion batteries. J. Mater. Chem. 2011, 21, 10003–10011. [Google Scholar] [CrossRef]




| Sample | TM_180 | TM_180_500 | TM_200 | TM_200_500 | ||
|---|---|---|---|---|---|---|
| Lattice parameter | anatase | a (Å) | 3.8390(1) | 3.7977(9) | 3.7946(1) | 3.7674(9) |
| c (Å) | 9.5863(5) | 9.4957(2) | 95355(2) | 9.4556(2) | ||
| rutile | a (Å) | - | 4.6248(1) | - | 4.6336(1) | |
| c (Å) | - | 3.1342(2) | - | 3.1146(2) | ||
| MoS2 | a (Å) | 3.1332(1) | 3.1104(1) | 3.0994(2) | 3.0704(1) | |
| c (Å) | 13.378(4) | 13.265(4) | 13.384(1) | 13.242(4) | ||
| Composition (% wt.) | anatase | 61(1) | 54(1) | 77(1) | 52(1) | |
| rutile | - | 22(1) | - | 20(2) | ||
| MoS2 | 39(1) | 24(1) | 23(2) | 28(2) | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siwińska-Ciesielczyk, K.; Kurc, B.; Rymarowicz, D.; Kubiak, A.; Piasecki, A.; Moszyński, D.; Jesionowski, T. Crystallization of TiO2-MoS2 Hybrid Material under Hydrothermal Treatment and Its Electrochemical Performance. Materials 2020, 13, 2706. https://doi.org/10.3390/ma13122706
Siwińska-Ciesielczyk K, Kurc B, Rymarowicz D, Kubiak A, Piasecki A, Moszyński D, Jesionowski T. Crystallization of TiO2-MoS2 Hybrid Material under Hydrothermal Treatment and Its Electrochemical Performance. Materials. 2020; 13(12):2706. https://doi.org/10.3390/ma13122706
Chicago/Turabian StyleSiwińska-Ciesielczyk, Katarzyna, Beata Kurc, Dominika Rymarowicz, Adam Kubiak, Adam Piasecki, Dariusz Moszyński, and Teofil Jesionowski. 2020. "Crystallization of TiO2-MoS2 Hybrid Material under Hydrothermal Treatment and Its Electrochemical Performance" Materials 13, no. 12: 2706. https://doi.org/10.3390/ma13122706
APA StyleSiwińska-Ciesielczyk, K., Kurc, B., Rymarowicz, D., Kubiak, A., Piasecki, A., Moszyński, D., & Jesionowski, T. (2020). Crystallization of TiO2-MoS2 Hybrid Material under Hydrothermal Treatment and Its Electrochemical Performance. Materials, 13(12), 2706. https://doi.org/10.3390/ma13122706









