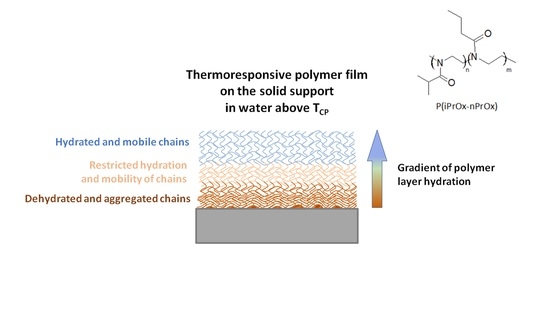
Poly(2-oxazoline) Matrices with Temperature-Dependent Solubility—Interactions with Water and Use for Cell Culture
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Behavior of TDS-Matrices in Water
3.1.1. Wettability of P(iPrOx-nPrOx) Films
3.1.2. Thickness of P(iPrOx-nPrOx) Films
3.1.3. Water Uptake by P(iPrOx-nPrOx) Films
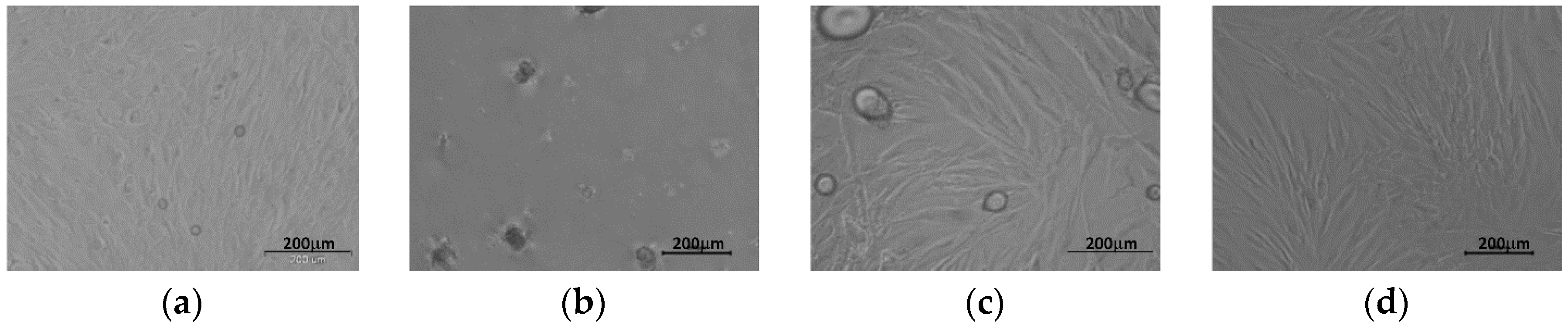
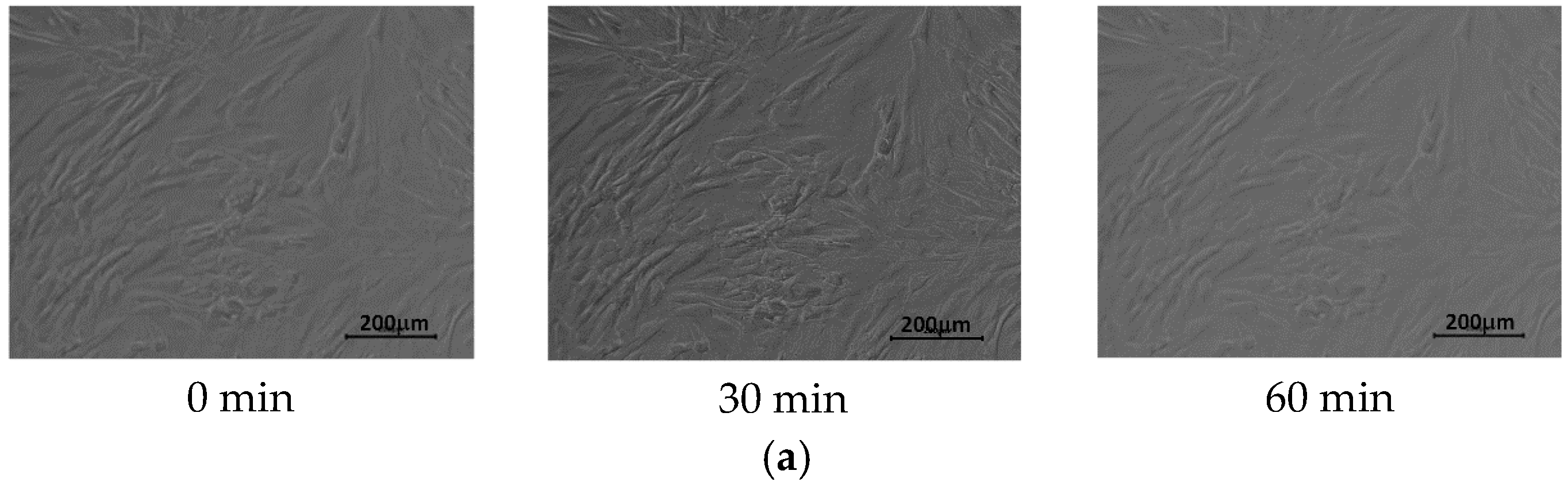
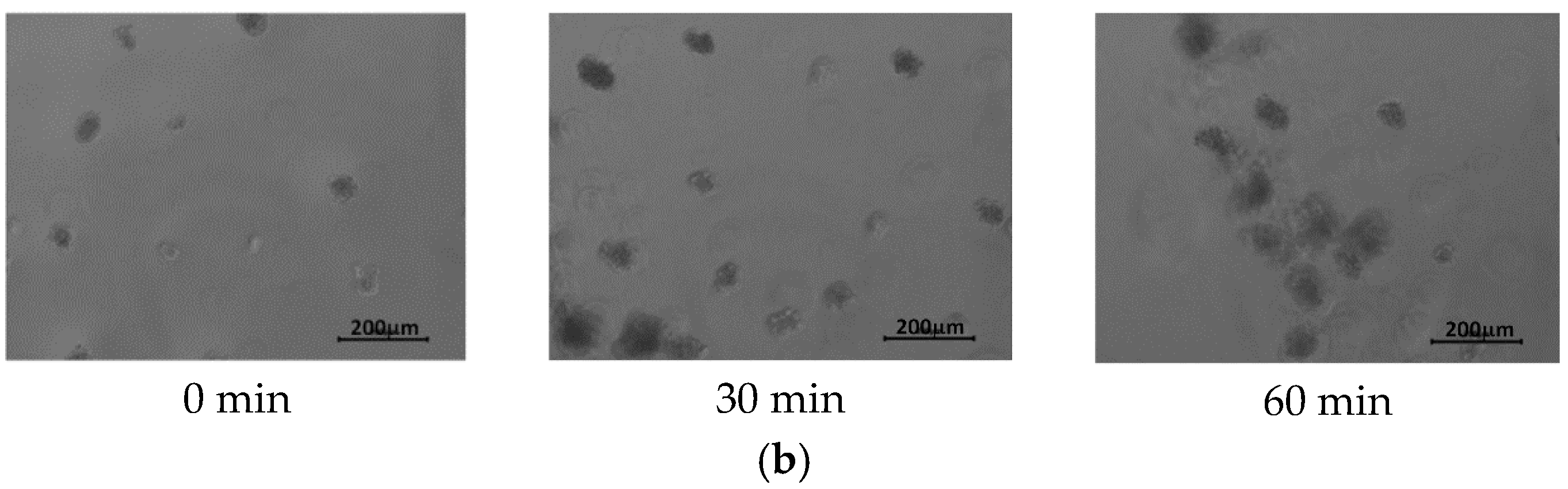
3.2. Cell Culture on P(iPrOx-nPrOx) Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Stratton, S.; Shelke, N.B.; Hoshino, K.; Rudraiah, S.; Kumbar, S.G. Bioactive polymeric scaffolds for tissue engineering. Bioact. Mater. 2016, 1, 93–108. [Google Scholar] [CrossRef]
- Kim, M.S.; Kim, J.H.; Min, B.H.; Chun, H.J.; Han, D.K.; Lee, H.B. Polymeric scaffolds for regenerative medicine. Polym. Rev. 2011, 51, 23–52. [Google Scholar] [CrossRef]
- Valappil, S.P.; Misra, S.K.; Boccaccini, A.; Roy, I. Biomedical applications of polyhydroxyalkanoates, an overview of animal testing and in vivo responses. Expert Rev. Med. Devices 2006, 3, 853–868. [Google Scholar] [CrossRef]
- Borselli, C.; Cezar, C.A.; Shvartsman, D.; Vandenburgh, H.H.; Mooney, D.J. The role of multifunctional delivery scaffold in the ability of cultured myoblasts to promote muscle regeneration. Biomaterials 2011, 32, 8905–8914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rychter, P.; Pamula, E.; Orchel, A.; Posadowska, U.; Krok-Borkowicz, M.; Kaps, A.; Smigiel-Gac, N.; Smola, A.; Kasperczyk, J.; Prochwicz, W.; et al. Scaffolds with shape memory behavior for the treatment of large bone defects. J. Biomed. Mater. Res. Part A 2015, 103, 3503–3515. [Google Scholar] [CrossRef] [PubMed]
- Jelonek, K.; Kaczmarczyk, B.; Jaworska, J.; Pastusiak, M.; Sobota, M.; Dobrzyński, P.; Kasperczyk, J. The influence of drug-polymer interactions on release of antirestenotic agent from bioresorbable scaffolds. Mater. Lett. 2018, 223, 82–85. [Google Scholar] [CrossRef]
- Kosik-Kozioł, A.; Costantini, M.; Bolek, T.; Szöke, K.; Barbetta, A.; Brinchmann, J.; Święszkowski, W. PLA short sub-micron fiber reinforcement of 3D bioprinted alginate constructs for cartilage regeneration. Biofabrication 2017, 9, 044105. [Google Scholar] [CrossRef]
- Kim, K.; Yu, M.; Zong, X.; Chiu, J.; Fang, D.; Seo, Y.S.; Hsiao, B.S.; Chu, B.; Hadjiargyrou, M. Control of degradation rate and hydrophilicity in electrospun non-woven poly(D,L-lactide) nanofiber scaffolds for biomedical applications. Biomaterials 2003, 24, 4977–4985. [Google Scholar] [CrossRef]
- Saito, N.; Okada, T.; Horiuchi, H.; Murakami, N.; Takahashi, J.; Nawata, M.; Ota, H.; Nozaki, K.; Takaoka, K. A biodegradable polymer as a cytokine delivery system for inducing bone formation. Nat. Biotechnol. 2001, 19, 332–335. [Google Scholar] [CrossRef]
- Nazemi, K.; Moztarzadeh, F.; Jalali, N.; Asgari, S.; Mozafari, M. Synthesis and characterization of poly(lactic-co-glycolic) acid nanoparticles-loaded chitosan/bioactive glass scaffolds as a localized delivery system in the bone defects. BioMed Res. Int. 2014, 2014, 898930. [Google Scholar] [CrossRef]
- Sultana, N.; Khan, T.H. Water absorption and diffusion characteristics of nanohydroxyapatite (nHA) and poly(hydroxybutyrate-co-hydroxyvalerate-) based composite tissue engineering scaffolds and nonporous thin films. J. Nanomater. 2013, 2013, 479109. [Google Scholar] [CrossRef] [Green Version]
- Sultana, N.; Wang, M. PHBV/PLLA-based composite scaffolds fabricated using an emulsion freezing/freeze-drying technique for bone tissue engineering: Surface modification and in vitro biological evaluation. Biofabrication 2012, 4, 015003. [Google Scholar] [CrossRef] [PubMed]
- Kawalec, M.; Sitkowska, A.; Sobota, M.; Sieroń, A.L.; Komar, P.; Kurcok, P. Human procollagen type I surface-modified PHB-based non-woven textile scaffolds for cell growth: Preparation and short-term biological tests. Biomed. Mater. 2014, 9, 065005. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Cheng, L.; Hu, C.; Li, H.; Zhang, Y.; Chang, J. Electrospun Poly(L-Lactide) Fiber with Ginsenoside Rg3 for Inhibiting Scar Hyperplasia of Skin. PLoS ONE 2013, 8, e68771. [Google Scholar] [CrossRef] [Green Version]
- Ishaug, S.L.; Crane, G.M.; Miller, M.J.; Yasko, A.W.; Yaszemski, M.J.; Mikos, A.G. Bone formation by three-dimensional stromal osteoblast culture in biodegradable polymer scaffolds. J. Biomed. Mater. Res. 1997, 36, 17–28. [Google Scholar] [CrossRef]
- Chang, K.Y.; Hung, L.H.; Chu, I.M.; Ko, C.S.; Lee, Y. Der The application of type II collagen and chondroitin sulfate grafted PCL porous scaffold in cartilage tissue engineering. J. Biomed. Mater. Res. Part A 2010, 92, 712–723. [Google Scholar] [CrossRef]
- Mandal, B.B.; Kundu, S.C. Cell proliferation and migration in silk fibroin 3D scaffolds. Biomaterials 2009, 30, 2956–2965. [Google Scholar] [CrossRef]
- Oliveira, S.M.; Ringshia, R.A.; Legeros, R.Z.; Clark, E.; Yost, M.J.; Terracio, L.; Teixeira, C.C. An improved collagen scaffold for skeletal regeneration. J. Biomed. Mater. Res. Part A 2010, 94, 371–379. [Google Scholar] [CrossRef] [Green Version]
- Gaetani, R.; Feyen, D.A.M.; Verhage, V.; Slaats, R.; Messina, E.; Christman, K.L.; Giacomello, A.; Doevendans, P.A.F.M.; Sluijter, J.P.G. Epicardial application of cardiac progenitor cells in a 3D-printed gelatin/hyaluronic acid patch preserves cardiac function after myocardial infarction. Biomaterials 2015, 61, 339–348. [Google Scholar] [CrossRef]
- Neves, S.C.; Moreira Teixeira, L.S.; Moroni, L.; Reis, R.L.; Van Blitterswijk, C.A.; Alves, N.M.; Karperien, M.; Mano, J.F. Chitosan/Poly(e{open}-caprolactone) blend scaffolds for cartilage repair. Biomaterials 2011, 32, 1068–1079. [Google Scholar] [CrossRef] [Green Version]
- Kubota, K.; Fujishige, S.; Ando, I. Solution properties of poly(N-isopropylacrylamide) in water. Polym. J. 1990, 22, 15–20. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Wang, X. Globule-to-coil transition of a single homopolymer chain in solution. Phys. Rev. Lett. 1998, 80, 4092–4094. [Google Scholar] [CrossRef] [Green Version]
- Schild, H.G. Poly(N-isopropylacrylamide): Experiment, theory and application. Prog. Polym. Sci. 1992, 17, 163–249. [Google Scholar] [CrossRef]
- Lutz, J.F. Polymerization of oligo(ethylene glycol) (meth)acrylates: Toward new generations of smart biocompatible materials. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 3459–3470. [Google Scholar] [CrossRef]
- Christova, D.; Ivanova, S.; Ivanova, G. Water-soluble temperature-responsive poly(vinyl alcohol-co-vinyl acetal)s. Polym. Bull. 2003, 50, 367–372. [Google Scholar] [CrossRef]
- Jamróz-Piegza, M.; Utrata-Wesołek, A.; Trzebicka, B.; Dworak, A. Hydrophobic modification of high molar mass polyglycidol to thermosensitive polymers. Eur. Polym. J. 2006, 42, 2497–2506. [Google Scholar] [CrossRef]
- Mortensen, K.; Schwahn, D.; Janssen, S. Pressure-induced melting of micellar crystal. Phys. Rev. Lett. 1993, 71, 1728–1731. [Google Scholar] [CrossRef]
- Lorson, T.; Lübtow, M.M.; Wegener, E.; Haider, M.S.; Borova, S.; Nahm, D.; Jordan, R.; Sokolski-Papkov, M.; Kabanov, A.V.; Luxenhofer, R. Poly(2-oxazoline)s based biomaterials: A comprehensive and critical update. Biomaterials 2018, 178, 204–280. [Google Scholar] [CrossRef]
- Adams, N.; Schubert, U.S. Poly(2-oxazolines) in biological and biomedical application contexts. Adv. Drug Deliv. Rev. 2007, 59, 1504–1520. [Google Scholar] [CrossRef]
- Schlaad, H.; Diehl, C.; Gress, A.; Meyer, M.; Levent Demirel, A.; Nur, Y.; Bertin, A. Poly(2-oxazoline)s as smart bioinspired polymers. Macromol. Rapid Commun. 2010, 31, 511–525. [Google Scholar] [CrossRef]
- De La Rosa, V.R. Poly(2-oxazoline)s as materials for biomedical applications. J. Mater. Sci. Mater. Med. 2014, 25, 1211–1225. [Google Scholar] [CrossRef] [PubMed]
- Hoogenboom, R.; Schlaad, H. Bioinspired Poly(2-oxazoline)s. Polymers (Basel) 2011, 3, 467–488. [Google Scholar] [CrossRef] [Green Version]
- Hoogenboom, R. Poly(2-oxazoline)s: A polymer class with numerous potential applications. Angew. Chem.-Int. Ed. 2009, 48, 7978–7994. [Google Scholar] [CrossRef] [PubMed]
- Luxenhofer, R.; Han, Y.; Schulz, A.; Tong, J.; He, Z.; Kabanov, A.V.; Jordan, R. Poly(2-oxazoline)s as polymer therapeutics. Macromol. Rapid Commun. 2012, 33, 1613–1631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jerca, F.A.; Anghelache, A.M.; Ghibu, E.; Cecoltan, S.; Stancu, I.C.; Trusca, R.; Vasile, E.; Teodorescu, M.; Vuluga, D.M.; Hoogenboom, R.; et al. Poly(2-isopropenyl-2-oxazoline) Hydrogels for Biomedical Applications. Chem. Mater. 2018, 30, 7938–7949. [Google Scholar] [CrossRef] [Green Version]
- Lück, S.; Schubel, R.; Rüb, J.; Hahn, D.; Mathieu, E.; Zimmermann, H.; Scharnweber, D.; Werner, C.; Pautot, S.; Jordan, R. Tailored and biodegradable poly(2-oxazoline) microbeads as 3D matrices for stem cell culture in regenerative therapies. Biomaterials 2016, 79, 1–14. [Google Scholar] [CrossRef]
- Kronek, J.; Kroneková, Z.; Lustoň, J.; Paulovičová, E.; Paulovičová, L.; Mendrek, B. In vitro bio-immunological and cytotoxicity studies of poly(2-oxazolines). J. Mater. Sci. Mater. Med. 2011, 22, 1725–1734. [Google Scholar] [CrossRef]
- Kronek, J.; Lustoň, J.; Kroneková, Z.; Paulovičová, E.; Farkaš, P.; Petrenčíková, N.; Paulovičová, L.; Janigová, I. Synthesis and bioimmunological efficiency of poly(2-oxazolines) containing a free amino group. J. Mater. Sci. Mater. Med. 2010, 21, 879–886. [Google Scholar] [CrossRef]
- Gaertner, F.C.; Luxenhofer, R.; Blechert, B.; Jordan, R.; Essler, M. Synthesis, biodistribution and excretion of radiolabeled poly(2-alkyl-2-oxazoline)s. J. Control. Release 2007, 119, 291–300. [Google Scholar] [CrossRef]
- Diehl, C.; Schlaad, H. Thermo-responsive polyoxazolines with widely tuneable LCST. Macromol. Biosci. 2009, 9, 157–161. [Google Scholar] [CrossRef]
- Park, J.S.; Kataoka, K. Precise control of lower critical solution temperature of thermosensitive poly(2-isopropyl-2-oxazoline) via gradient copolymerization with 2-ethyl-2-oxazoline as a hydrophilic comonomer. Macromolecules 2006, 39, 6622–6630. [Google Scholar] [CrossRef]
- Huber, S.; Jordan, R. Modulation of the lower critical solution temperature of 2-Alkyl-2-oxazoline copolymers. Colloid Polym. Sci. 2008, 286, 395–402. [Google Scholar] [CrossRef]
- Jerca, F.A.; Jerca, V.V.; Anghelache, A.M.; Vuluga, D.M.; Hoogenboom, R. Poly(2-isopropenyl-2-oxazoline) as a versatile platform towards thermoresponsive copolymers. Polym. Chem. 2018, 9, 3473–3478. [Google Scholar] [CrossRef]
- Oleszko-Torbus, N.; Utrata-Wesołek, A.; Bochenek, M.; Lipowska-Kur, D.; Dworak, A.; Wałach, W. Thermal and crystalline properties of poly(2-oxazoline)s. Polym. Chem. 2020, 11, 15–33. [Google Scholar] [CrossRef]
- Dworak, A.; Utrata-Wesołek, A.; Oleszko, N.; Wałach, W.; Trzebicka, B.; Anioł, J.; Sieroń, A.L.; Klama-Baryła, A.; Kawecki, M. Poly(2-substituted-2-oxazoline) surfaces for dermal fibroblasts adhesion and detachment. J. Mater. Sci. Mater. Med. 2014, 25, 1149–1163. [Google Scholar] [CrossRef]
- Oleszko, N.; Wałach, W.; Utrata-Wesołek, A.; Kowalczuk, A.; Trzebicka, B.; Klama-Baryła, A.; Hoff-Lenczewska, D.; Kawecki, M.; Lesiak, M.; Sieroń, A.L.; et al. Controlling the crystallinity of thermoresponsive poly(2-oxazoline)-based nanolayers to cell adhesion and detachment. Biomacromolecules 2015, 16, 2805–2813. [Google Scholar] [CrossRef]
- Wałach, W.; Oleszko-Torbus, N.; Utrata-Wesołek, A.; Bochenek, M.; Kijeńska-Gawrońska, E.; Górecka, Ż.; Święszkowski, W.; Dworak, A. Processing of (Co)Poly(2-oxazoline)s by Electrospinning and Extrusion from Melt and the Postprocessing Properties of the (Co)Polymers. Polymers (Basel) 2020, 12, 295. [Google Scholar] [CrossRef] [Green Version]
- Witte, H.; Seeliger, W. Cyclische Imidsäureester aus Nitrilen und Aminoalkoholen. Justus Liebigs Ann. Chem. 1974, 1974, 996–1009. [Google Scholar] [CrossRef]
- Oleszko-Torbus, N.; Wałach, W.; Utrata-Wesołek, A.; Dworak, A. Control of the Crystalline Properties of 2-Isopropyl-2-oxazoline Copolymers in Condensed State and in Solution Depending on the Composition. Macromolecules 2017, 50, 7636–7645. [Google Scholar] [CrossRef]
- Utrata-Wesołek, A.; Oleszko, N.; Trzebicka, B.; Anioł, J.; Zagdańska, M.; Lesiak, M.; Sieroń, A.; Dworak, A. Modified polyglycidol based nanolayers of switchable philicity and their interactions with skin cells. Eur. Polym. J. 2013, 49, 106–117. [Google Scholar] [CrossRef]
- Fukumori, K.; Akiyama, Y.; Kumashiro, Y.; Kobayashi, J.; Yamato, M.; Sakai, K.; Okano, T. Characterization of Ultra-Thin Temperature-Responsive Polymer Layer and Its Polymer Thickness Dependency on Cell Attachment/Detachment Properties. Macromol. Biosci. 2010, 10, 1117–1129. [Google Scholar] [CrossRef] [PubMed]
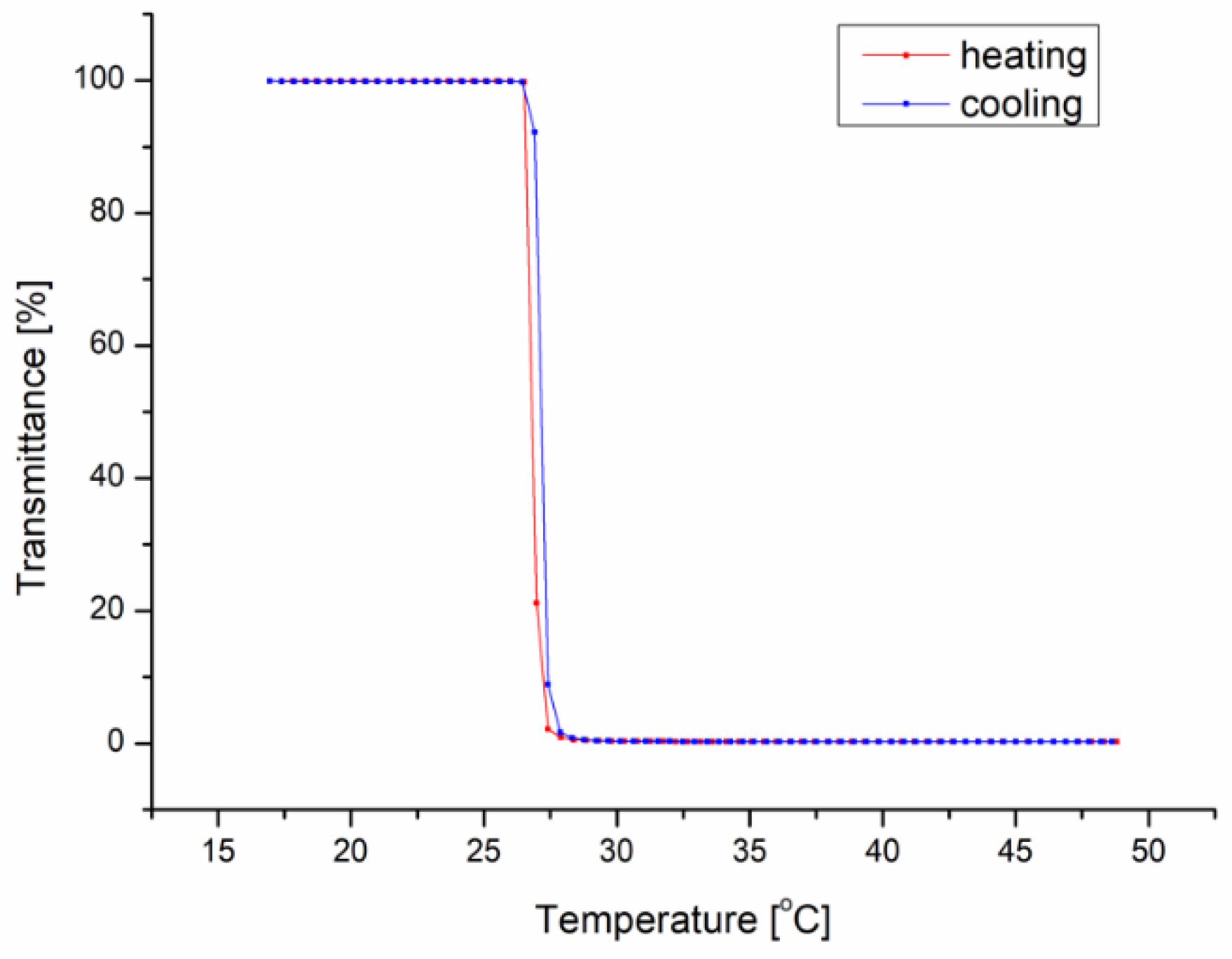
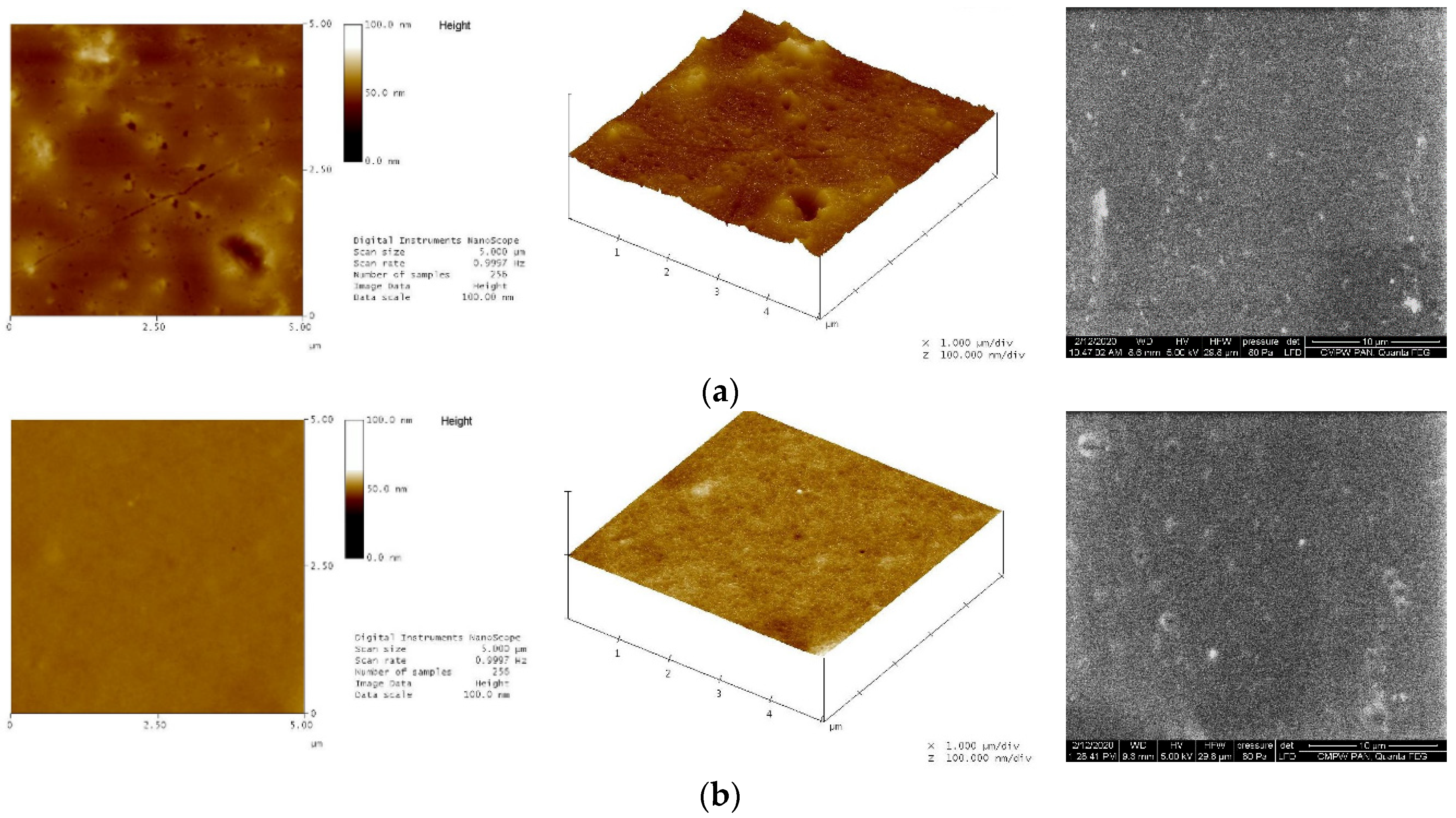
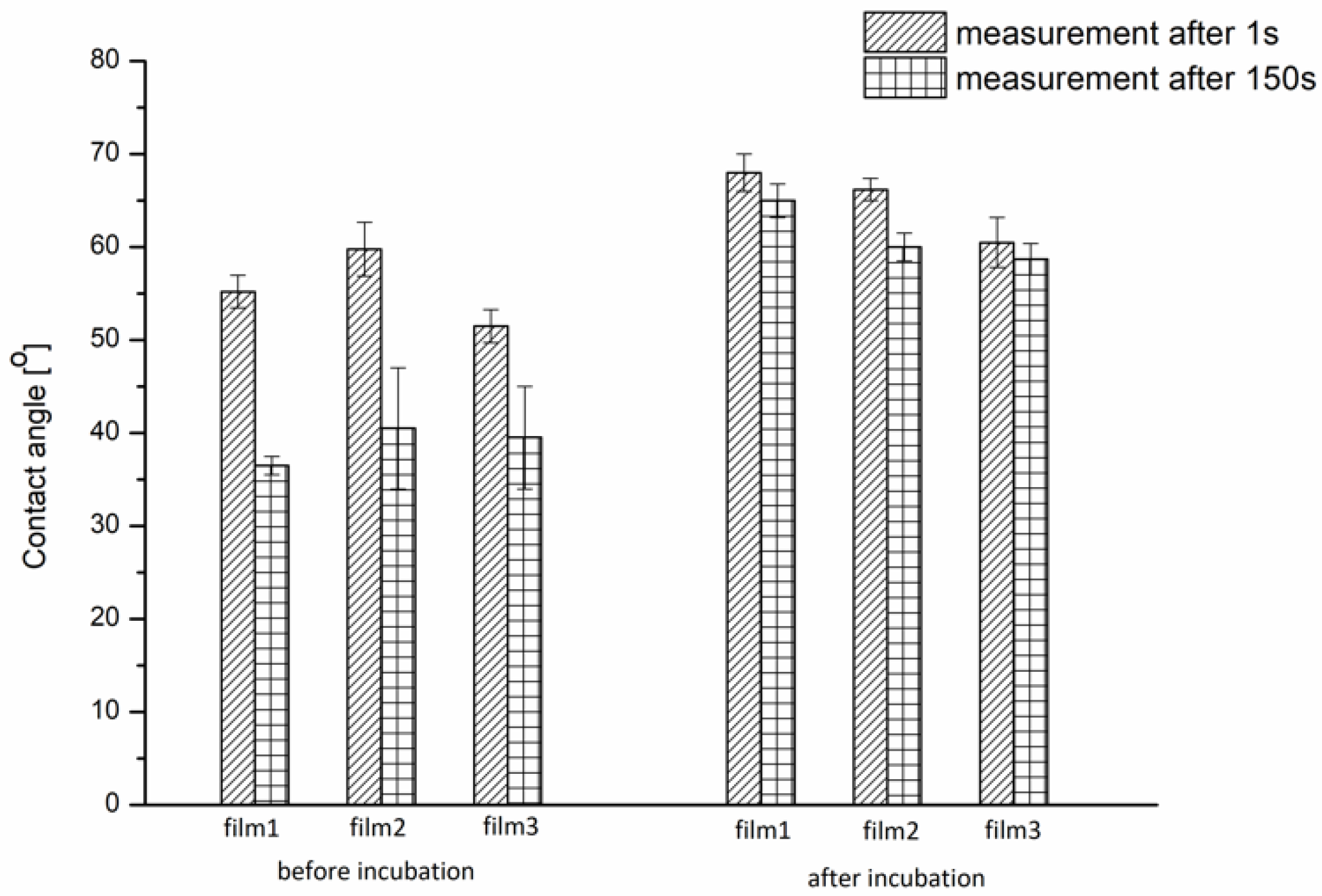
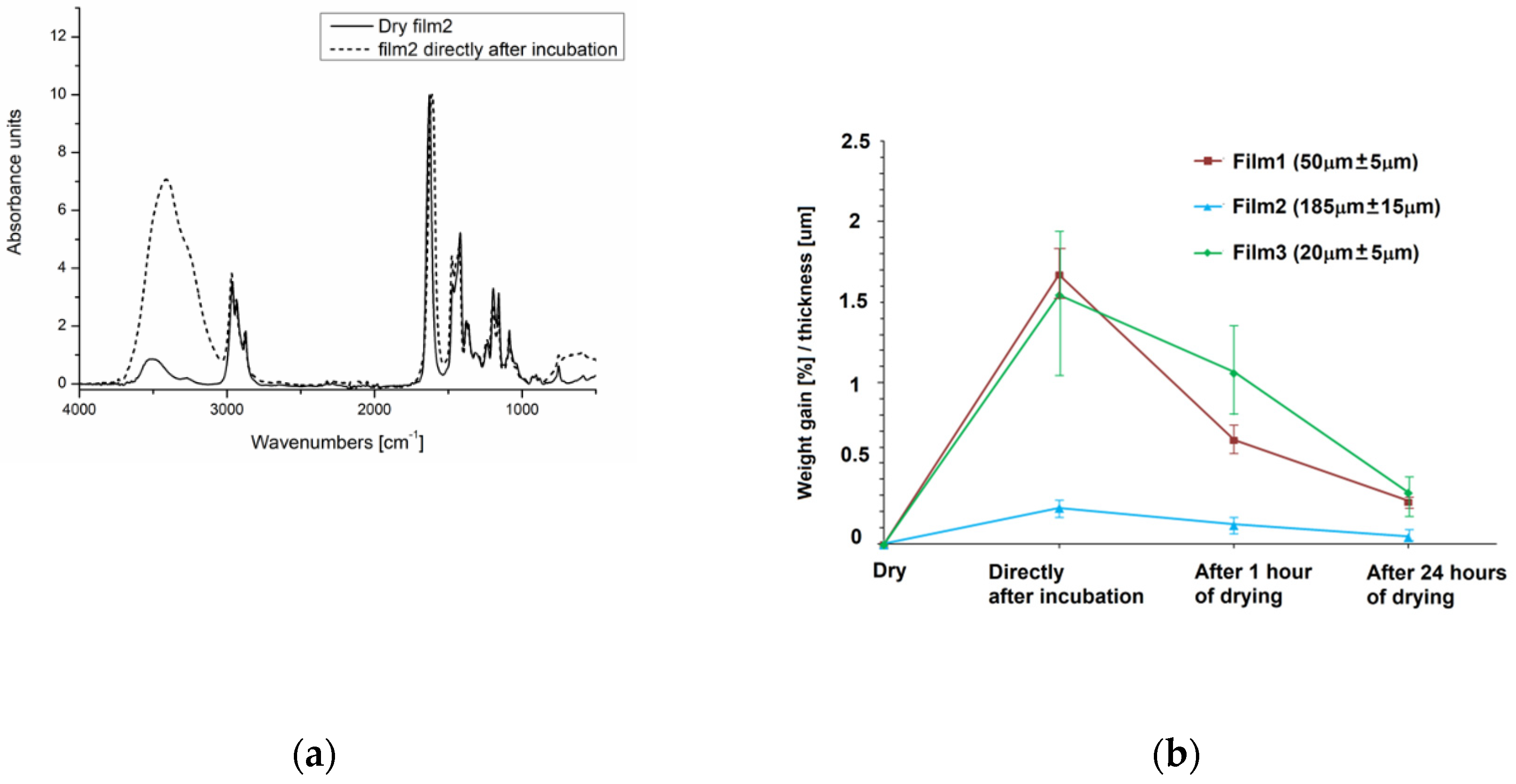
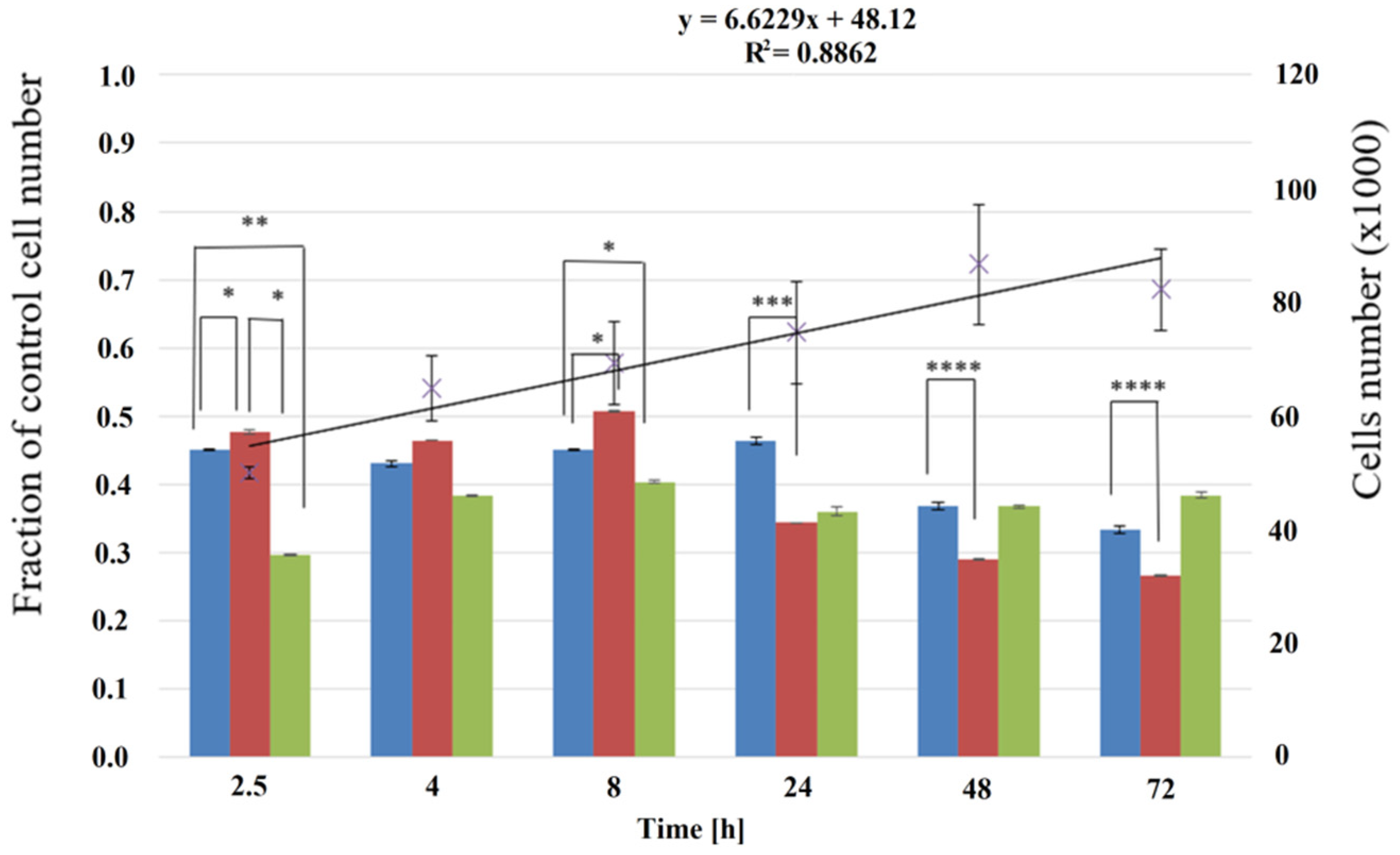
| iPrOx:nPrOx | Mtheoret | Mn (g/mol) | Ð | DP | TCP (°C) | Tg (°C) |
|---|---|---|---|---|---|---|
| 47:53 | 52,000 | 51,000 | 1.32 | 450 | 27 | 50 |
| Sample Description | Film 1 | Film 2 | Film 3 |
|---|---|---|---|
| In the dry state | 50 µm ± 5 µm | 185 µm ± 15 µm | 20 µm ± 5 µm |
| After incubation in water at 40 °C | 70 µm ± 15 µm | 220 µm ± 20 µm | 30 µm ± 5 µm |
| Increase in thickness/initial thickness | 0.4 | 0.19 | 0.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oleszko-Torbus, N.; Bochenek, M.; Utrata-Wesołek, A.; Kowalczuk, A.; Marcinkowski, A.; Dworak, A.; Fus-Kujawa, A.; Sieroń, A.L.; Wałach, W. Poly(2-oxazoline) Matrices with Temperature-Dependent Solubility—Interactions with Water and Use for Cell Culture. Materials 2020, 13, 2702. https://doi.org/10.3390/ma13122702
Oleszko-Torbus N, Bochenek M, Utrata-Wesołek A, Kowalczuk A, Marcinkowski A, Dworak A, Fus-Kujawa A, Sieroń AL, Wałach W. Poly(2-oxazoline) Matrices with Temperature-Dependent Solubility—Interactions with Water and Use for Cell Culture. Materials. 2020; 13(12):2702. https://doi.org/10.3390/ma13122702
Chicago/Turabian StyleOleszko-Torbus, Natalia, Marcelina Bochenek, Alicja Utrata-Wesołek, Agnieszka Kowalczuk, Andrzej Marcinkowski, Andrzej Dworak, Agnieszka Fus-Kujawa, Aleksander L. Sieroń, and Wojciech Wałach. 2020. "Poly(2-oxazoline) Matrices with Temperature-Dependent Solubility—Interactions with Water and Use for Cell Culture" Materials 13, no. 12: 2702. https://doi.org/10.3390/ma13122702
APA StyleOleszko-Torbus, N., Bochenek, M., Utrata-Wesołek, A., Kowalczuk, A., Marcinkowski, A., Dworak, A., Fus-Kujawa, A., Sieroń, A. L., & Wałach, W. (2020). Poly(2-oxazoline) Matrices with Temperature-Dependent Solubility—Interactions with Water and Use for Cell Culture. Materials, 13(12), 2702. https://doi.org/10.3390/ma13122702