Sirolimus-Eluting Electrospun-Produced Matrices as Coatings for Vascular Stents: Dependence of Drug Release on Matrix Structure and Composition of the External Environment
Abstract
1. Introduction
2. Materials and Methods
2.1. Production of 3H-Labeled Sirolimus
2.2. Fabrication of the ES Matrices
2.3. Characterization of the Matrices
2.3.1. Mechanical Characterization
2.3.2. Matrix Surface Characterization
2.3.3. Additional Characterization of the Matrices
2.4. Sirolimus Release Kinetics
2.5. Statistical Analysis
3. Results and Discussion
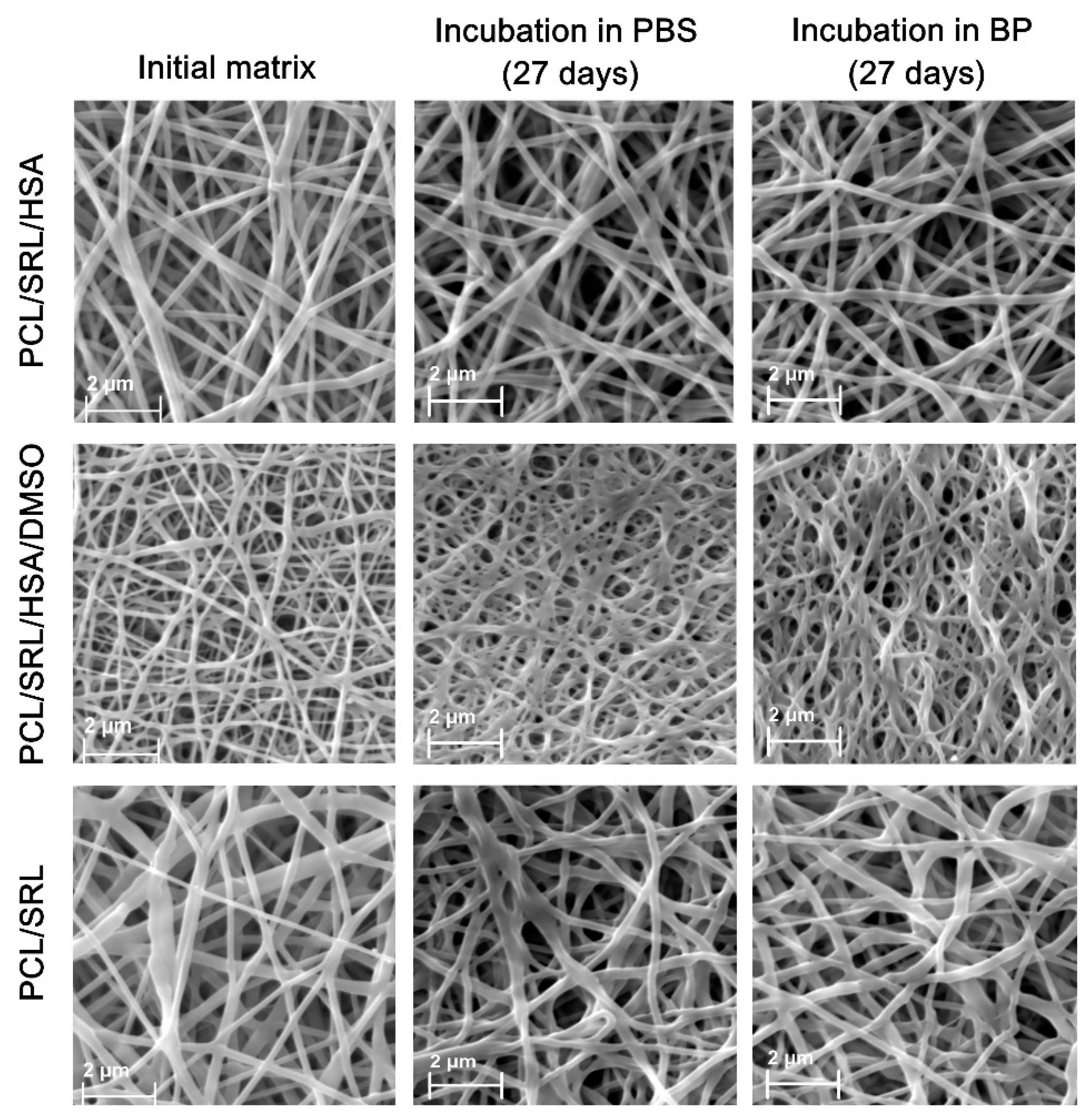
3.1. Preparation and Characterization of the SRL Matrices
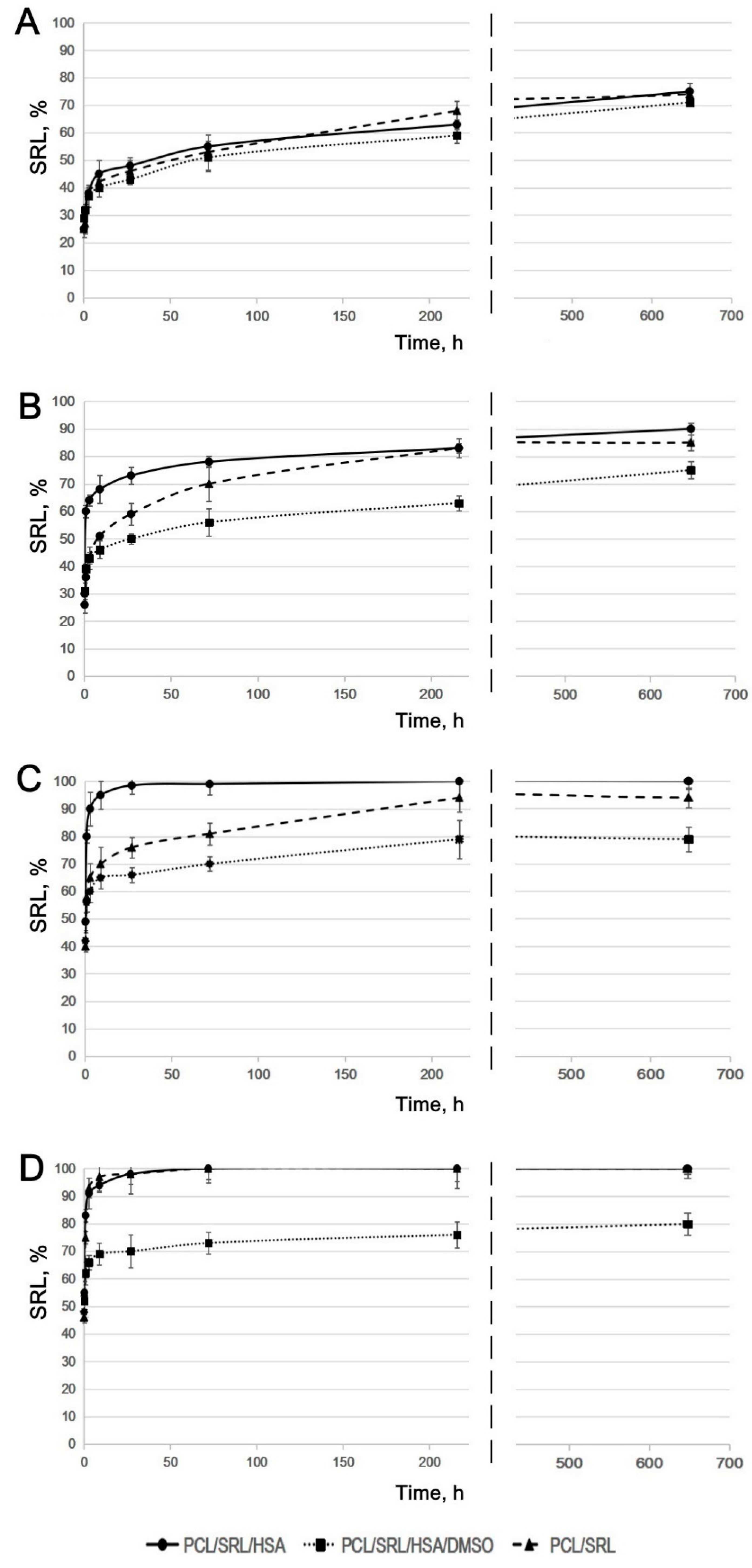
3.2. Study of SRL Release
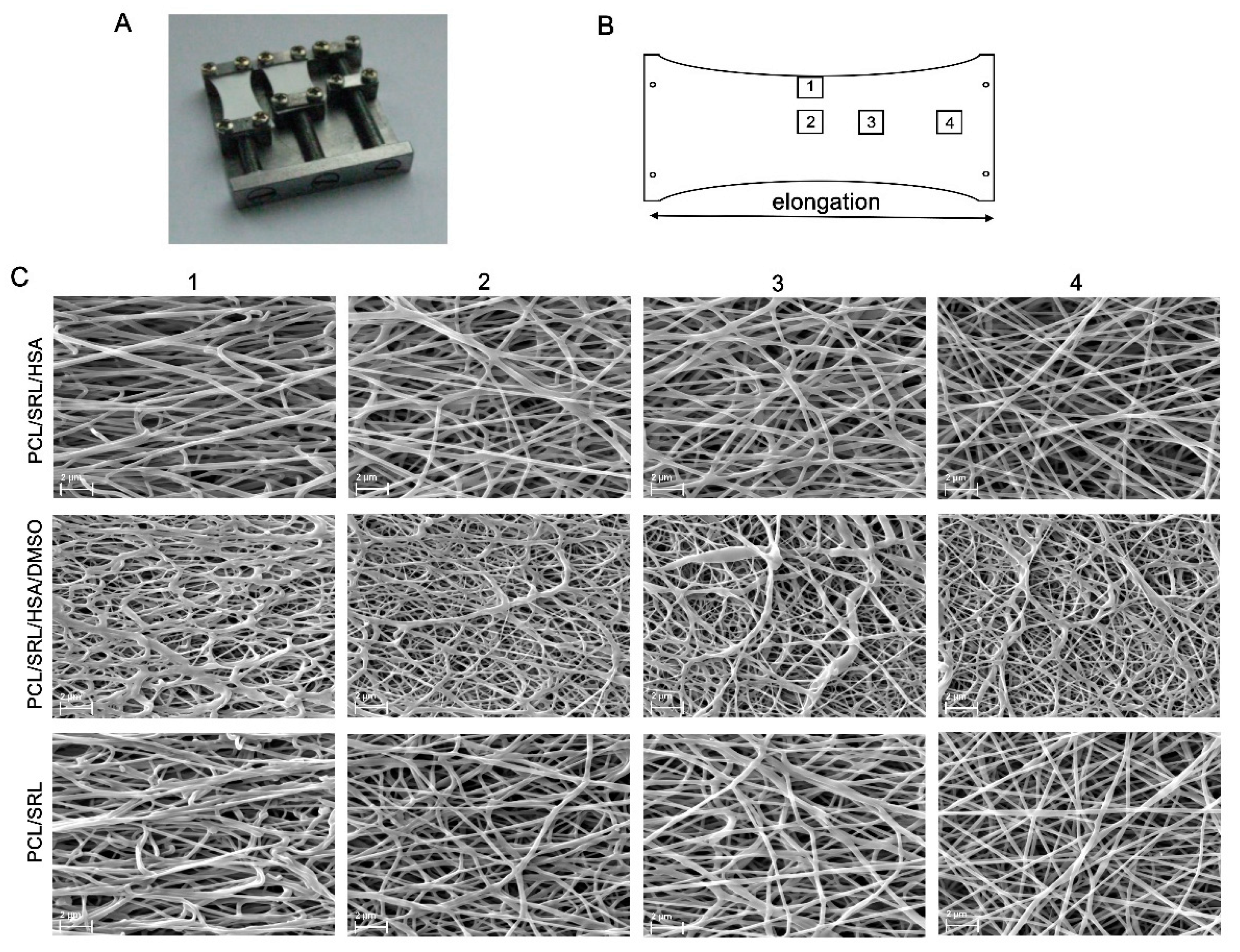
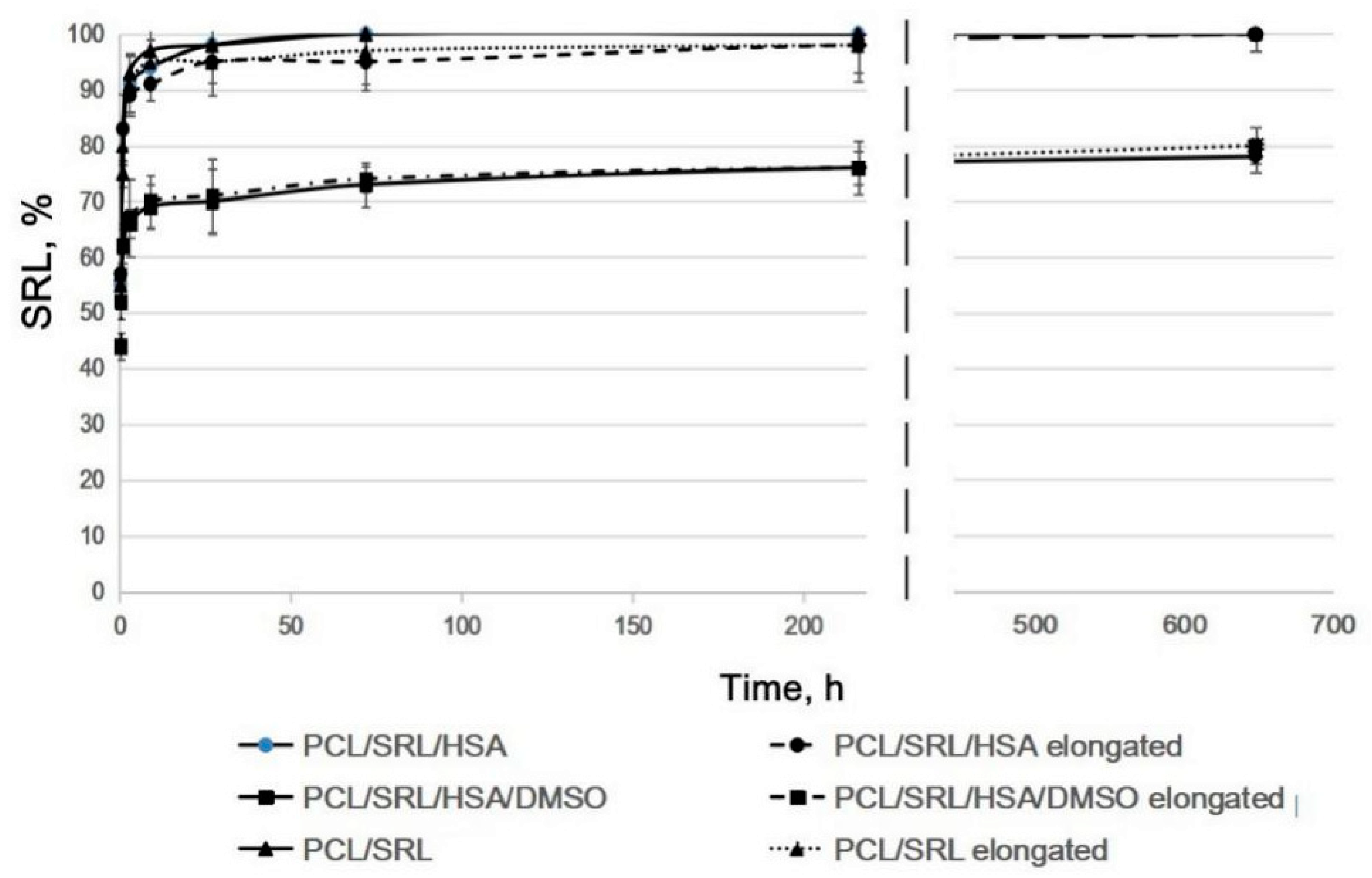
3.3. Influence of Matrix Elongation on SRL Release
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Costa, M.A.; Simon, D.I. Molecular basis of restenosis and drug-eluting stents. Circulation 2005, 111, 2257–2273. [Google Scholar] [CrossRef] [PubMed]
- Puranik, A.S.; Dawson, E.R.; Peppas, N.A. Recent advances in drug eluting stents. Int. J. Pharm. 2013, 441, 665–679. [Google Scholar] [CrossRef] [PubMed]
- Scott, N.A. Restenosis following implantation of bare metal coronary stents: Pathophysiology and pathways involved in the vascular response to injury. Adv. Drug Deliv. Rev. 2006, 58, 358–376. [Google Scholar] [CrossRef] [PubMed]
- Abizaid, A. Sirolimus-eluting coronary stents: A review. Vasc. Health. Risk Manag. 2007, 3, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Cheng-Lai, A.; Frishman, W.H. Sirolimus-eluting coronary stents: Novel devices for the management of coronary artery disease. Am. J. Ther. 2004, 11, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Piscione, F.; Piccolo, R.; Cassese, S.; Galasso, G.; Chiariello, M. Clinical impact of sirolimus-eluting stent in ST-segment elevation myocardial infarction: A meta-analysis of randomized clinical trials. Catheter Cardiovasc. Interv. 2009, 74, 323–332. [Google Scholar] [CrossRef]
- Sehgal, S.N. Sirolimus: Its discovery, biological properties, and mechanism of action. Transpl. Proc. 2003, 35, 7S–14S. [Google Scholar] [CrossRef]
- Haeri, A.; Osouli, M.; Bayat, F.; Alavi, S.; Dadashzadeh, S. Nanomedicine approaches for sirolimus delivery: A review of pharmaceutical properties and preclinical studies. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1–14. [Google Scholar] [CrossRef]
- Sehgal, S.N.; Camardo, J.S.; Scarola, J.A.; Maida, B.T. Rapamycin (sirolimus, rapamune). Curr. Opin. Nephrol. Hypertens. 1995, 4, 482–487. [Google Scholar] [CrossRef]
- Hafizi, S.; Mordi, V.N.; Andersson, K.M.; Chester, A.H.; Yacoub, M.H. Differential effects of rapamycin, cyclosporine A, and FK506 on human coronary artery smooth muscle cell proliferation and signalling. Vascul. Pharmacol. 2005, 41, 167–176. [Google Scholar] [CrossRef]
- Moes, D.J.; Guchelaar, H.J.; de Fijter, J.W. Sirolimus and everolimus in kidney transplantation. Drug Discov. Today 2015, 20, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, A.; Scarola, J.; Burke, J.T.; Zimmerman, J.J. Clinical pharmacokinetics and therapeutic drug monitoring of sirolimus. Clin. Ther. 2000, 22, B101–B121. [Google Scholar] [CrossRef]
- Mahalati, K.; Kahan, B.D. Clinical pharmacokinetics of sirolimus. Clin. Pharmacokinet. 2001, 40, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Tada, T.; Byrne, R.A.; Simunovic, I.; King, L.A.; Cassese, S.; Joner, M.; Fusaro, M.; Schneider, S.; Schulz, S.; Ibrahim, T.; et al. Risk of stent thrombosis among bare-metal stents, first-generation drug-eluting stents, and second-generation drug-eluting stents: Results from a registry of 18,334 patients. JACC Cardiovasc. Interv. 2013, 6, 1267–1274. [Google Scholar] [CrossRef] [PubMed]
- Vetrovec, G.W.; Rizik, D.; Williard, C.; Snead, D.; Piotrovski, V.; Kopia, G. Sirolimus PK trial: A pharmacokinetic study of the sirolimus-eluting Bx velocity stent in patients with de novo coronary lesions. Catheter Cardiovasc. Interv. 2006, 67, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Van Der Giessen, W.J.; Sorop, O.; Serruys, P.W.; Peters- Krabbendam, I.; Van Beusekom, H.M.M. Lowering the dose of sirolimus, released from a nonpolymeric hydroxyapatite coated coronary stent, reduces signs of delayed healing. J. Am. Coll. Cardiol. Intv. 2009, 2, 284–290. [Google Scholar] [CrossRef][Green Version]
- Nakamura, M.; Abizaid, A.; Hirohata, A.; Honda, Y.; Sousa, J.E.; Fitzgerald, P.J. Efficacy of reduced-dose sirolimus-eluting stents in the human coronaryartery: Serial IVUS analysis of neointimal hyperplasia and luminal dimension. Catheter Cardiovasc. Interv. 2007, 70, 946–951. [Google Scholar] [CrossRef]
- Onuma, Y.; Kogame, N.; Serruys, P.W. Unconfirmed very long-term clinical benefit of Xience over Cypher: Should we reset our vision? JACC Cardiovasc Interv. 2019, 12, 648–650. [Google Scholar] [CrossRef]
- Daemen, J.; Wenaweser, P.; Tsuchida, K.; Abrecht, L.; Vaina, S.; Morger, C.; Kukreja, N.; Jüni, P.; Sianos, G.; Hellige, G.; et al. Early and late coronary stent thrombosis of sirolimuseluting and paclitaxel-eluting stents in routine clinical practice: Data from a large two-institutional cohort study. Lancet 2007, 369, 667–678. [Google Scholar] [CrossRef]
- Bangalore, S.; Toklu, B.; Patel, N.; Feit, F.; Stone, G.W. Newer-generation ultrathin strut drug-eluting stents versus older second-generation thicker strut drug-eluting stents for coronary artery disease. Circulation 2018, 138, 2216–2226. [Google Scholar] [CrossRef]
- Tijssen, R.Y.G.; Kraak, R.P.; Lu, H.; Mifek, J.G.; Carlyle, W.C.; Donohoe, D.J.; De Winter, R.J.; Koch, K.T.; Wykrzykowska, J.J. Evaluation of the MiStent sustained sirolimus eluting biodegradable polymer coated stent for the treatment of coronary artery disease: Does uniform sustained abluminal drug release result in earlier strut coverage and better safety profile? Expert Rev. Med. Devices 2017, 14, 325–334. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ajmera, P.; Pothineni, R.; Chawla, K.K.; Mantravadi, S.S.; Jariwala, P.V.; Vijan, V.; Vijan, V. Real-world use of ultrathin-strut biodegradable polymer-coated sirolimus-eluting stents in patients with coronary artery disease: 6-month clinical outcomes. Vasc. Health Risk Manag. 2019, 18, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Mauri, L.; Doros, G.; Rao, S.V.; Cohen, D.J.; Yakubov, S.; Lasala, J.; Wong, S.C.; Zidar, J.; Kereiakes, D.J. The OPTIMIZE randomized trial to assess safety and efficacy of the Svelte IDS and RX Sirolimus-eluting coronary stent Systems for the Treatment of atherosclerotic lesions: Trial design and rationale. Am. Heart J. 2019, 216, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Bae, I.H.; Lim, K.S.; Park, D.S.; Shim, J.W.; Lee, S.Y.; Jang, E.J.; Park, J.K.; Kim, J.H.; Jeong, M.H. Sirolimus coating on heparinized stents prevents restenosis and thrombosis. J. Biomater. Appl. 2017, 31, 1337–1345. [Google Scholar] [CrossRef] [PubMed]
- Jelonek, K.; Jaworska, J.; Pastusiak, M.; Sobota, M.; Włodarczyk, J.; Karpeta-Jarzabek, P.; Kaczmarczyk, B.; Kasperczyk, J.; Dobrzyński, P. Effect of vascular scaffold composition on release of sirolimus. Eur. J. Pharm. Biopharm. 2018, 132, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Raval, A.; Parikh, J.; Engineer, C. Mechanism and in vitro release kinetic study of sirolimus from a biodegradable polymeric matrix coated cardiovascular stent. Ind. Eng. Chem. Res. 2011, 50, 9539–9549. [Google Scholar] [CrossRef]
- Livingston, M.; Tan, A. Coating techniques and release kinetics of drug-eluting stents. J Med. Device. 2019, 10, 1. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Xiao, J.; Fang, T.; Hu, N.; Li, M.; Deng, L.; Cheng, Y.; Zhu, Y.; Cui, W. An electrospun fiber-covered stent with programmable dual drug release for endothelialization acceleration and lumen stenosis prevention. Acta Biomater. 2019, 94, 295–305. [Google Scholar] [CrossRef]
- Kuznetsov, K.A.; Stepanova, A.O.; Kvon, R.I.; Douglas, T.E.L.; Kuznetsov, N.A.; Chernonosova, V.S.; Zaporozhchenko, I.A.; Kharkova, M.V.; Romanova, I.V.; Karpenko, A.A.; et al. Electrospun produced 3D matrices for covering of vascular stents: Paclitaxel release depending on fiber structure and composition of the external environment. Materials 2018, 11, 2176. [Google Scholar] [CrossRef]
- Rychter, M.; Baranowska-Korczyc, A.; Luleka, J. Progress and perspectives in bioactive agent delivery via electrospun vascular grafts. RSC Adv. 2017, 7, 32164. [Google Scholar] [CrossRef]
- Mirbagheri, M.S.; Mohebbi-Kalhori, D.; Jirofti, N. Evaluation of mechanical properties and medical applications of polycaprolactone small diameter artificial blood vessels. Int. J. Basic Sci. Med. 2017, 2, 58–70. [Google Scholar] [CrossRef]
- Rychter, M.; Milanowski, B.; Grzeskowiak, B.F.; Jarek, M.; Kempinski, M.; Coy, E.L.; Borysiak, S.; Baranowska-Korczyc, A.; Lulek, J. Cilostazol-loaded electrospun three-dimensional systems for potential cardiovascular application: Effect of fibers hydrophilization on drug release, and cytocompatibility. J. Colloid Interface Sci. 2019, 15, 310–327. [Google Scholar] [CrossRef] [PubMed]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Michel, S.A.; Knetsch, M.L.; Koole, L.H. Adsorption of albumin on flax fibers increases endothelial cell adhesion and blood compatibility in vitro. J. Biomater Sci. Polym. Ed. 2014, 25, 698–712. [Google Scholar] [CrossRef] [PubMed]
- Chernonosova, V.S.; Kvon, R.I.; Stepanova, A.O.; Larichev, Y.V.; Karpenko, A.A.; Chelobanov, B.P.; Kiseleva, E.V.; Laktionov, P.P. Human serum albumin in electrospun PCL fibers: Structure, release, and exposure on fibersurface. Polym. Adv. Technol. 2017, 28, 819–827. [Google Scholar] [CrossRef]
- Shen, M.; Martinson, L.; Wagner, M.S.; Castner, D.G.; Ratner, B.D.; Horbett, T.A. PEO-like plasma polymerized tetraglyme surface interactions with leukocytes and proteins: In vitro and in vivo studies. J. Biomater. Sci. Polym. Ed. 2002, 13, 367–390. [Google Scholar] [CrossRef] [PubMed]
- Denizli, F.K.; Guven, O. Competitive adsorption of blood proteins on gamma-irradiated-polycarbonate fifilms. J. Biomater. Sci. Polym. Ed. 2002, 13, 127–139. [Google Scholar] [CrossRef]
- Sidorov, V.N.; Polak, Y.V.; Laktionov, P.P.; Roshcke, V.V.; Kist, A.G. Method of production of tritium labeled organic compounds and the device for its implementation. SU Patent 1823961 A3, 18 January 1991. [Google Scholar]
- Cardiovascular Implants-Tubular Vacuum Prostheses. International Patent Application No. ISO/FDIS 7198:1998, July 1998.
- Tiwari, A.P.; Joshi, M.K.; Park, C.H.; Kim, C.S. Nano-nets covered composite nanofibers with enhanced biocompatibility and mechanical properties for bone tissue engineering. J. Nanosci. Nanotechnol. 2018, 18, 529–537. [Google Scholar] [CrossRef]
- Carter, D.C.; He, X.M.; Munson, S.H.; Twigg, P.D.; Gernert, K.M.; Broom, M.B. Three-dimensional structure of human serum albumin. Science 1989, 244, 1195–1198. [Google Scholar] [CrossRef]
- Jr, T.P. Ligand binding by albumin. In All about Albumin; Academic Press: San Diego, CA, USA, 1995. [Google Scholar]
- Chernonosova, V.S.; Gostev, A.A.; Gao, Y.; Chesalov, Y.A.; Shutov, A.V.; Pokushalov, E.A.; Karpenko, A.A.; Laktionov, P.P. Mechanical properties and biological behavior of 3D matrices produced by electrospinning from protein-enriched polyurethane. BioMed Res. Int. 2018, 2018, 1380606. [Google Scholar] [CrossRef]
- Yasukawa, T.; Ogura, Y.; Sakurai, E.; Tabata, Y.; Kimura, H. Intraocular sustained drug delivery using implantable polymeric devices. Adv. Drug Deliv. Rev. 2005, 57, 2033–2046. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-S.; Kim, J.-S.; Park, H.J.; Cho, W.K.; Cha, K.-H.; Hwan, S.-J. Enhanced bioavailability of sirolimus via preparation of solid dispersion nanoparticles using a supercritical antisolvent process. Int. J. Nanomed. 2011, 6, 2997–3009. [Google Scholar]
- Rahman, M.; Prianka, F.; Shohel, M.; Mazid, M.A. Interaction of palmitic acid with metoprolol succinate at the binding sites of bovine serum albumin. Adv. Pharm. Bull. 2014, 4, 379–383. [Google Scholar] [PubMed]
- Khodaei, A.; Bolandnazar, S.; Valizadeh, H.; Hasani, L.; Zakeri-Milani, P. Interactions between sirolimus and anti-inflammatory drugs: Competitive binding for human serum albumin. Adv. Pharm. Bull. 2016, 6, 227–233. [Google Scholar] [CrossRef] [PubMed]
| Matrix Type | Thickness (µm) | Strength (MPa) | Mass Per Unit Area (mg/cm2) | Fiber Diameter (µm) | Pore Diameter (µm) | Porosity (%) | Contact Angle (°) |
|---|---|---|---|---|---|---|---|
| 5% PCL/SRL/10% HSA | 111 | 10.45 ± 1.48 | 2.51 | 0.27 ± 0.8 | 1.94 ± 0.33 | 63.3 | 117.63 ± 2.72 |
| 5% PCL/SRL/10% HSA/3%DMSO | 125 | 12.41 ± 3.11 | 2.47 | 0.14 ± 0.05 | 1.12 ± 0.18 | 64.7 | 109.52 ± 2.22 |
| 5% PCL/SRL | 114 | 15.04 ± 2.50 | 2.44 | 0.36 ± 0.09 | 2.11 ± 0.34 | 60.9 | 127.37 ± 3.41 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazarkina, Z.K.; Chelobanov, B.P.; Chernonosova, V.S.; Romanova, I.V.; Karpenko, A.A.; Laktionov, P.P. Sirolimus-Eluting Electrospun-Produced Matrices as Coatings for Vascular Stents: Dependence of Drug Release on Matrix Structure and Composition of the External Environment. Materials 2020, 13, 2692. https://doi.org/10.3390/ma13122692
Nazarkina ZK, Chelobanov BP, Chernonosova VS, Romanova IV, Karpenko AA, Laktionov PP. Sirolimus-Eluting Electrospun-Produced Matrices as Coatings for Vascular Stents: Dependence of Drug Release on Matrix Structure and Composition of the External Environment. Materials. 2020; 13(12):2692. https://doi.org/10.3390/ma13122692
Chicago/Turabian StyleNazarkina, Zhanna K., Boris P. Chelobanov, Vera S. Chernonosova, Irina V. Romanova, Andrey A. Karpenko, and Pavel P. Laktionov. 2020. "Sirolimus-Eluting Electrospun-Produced Matrices as Coatings for Vascular Stents: Dependence of Drug Release on Matrix Structure and Composition of the External Environment" Materials 13, no. 12: 2692. https://doi.org/10.3390/ma13122692
APA StyleNazarkina, Z. K., Chelobanov, B. P., Chernonosova, V. S., Romanova, I. V., Karpenko, A. A., & Laktionov, P. P. (2020). Sirolimus-Eluting Electrospun-Produced Matrices as Coatings for Vascular Stents: Dependence of Drug Release on Matrix Structure and Composition of the External Environment. Materials, 13(12), 2692. https://doi.org/10.3390/ma13122692





