Three-Dimensional Printing Constructs Based on the Chitosan for Tissue Regeneration: State of the Art, Developing Directions and Prospect Trends
Abstract
1. Introduction
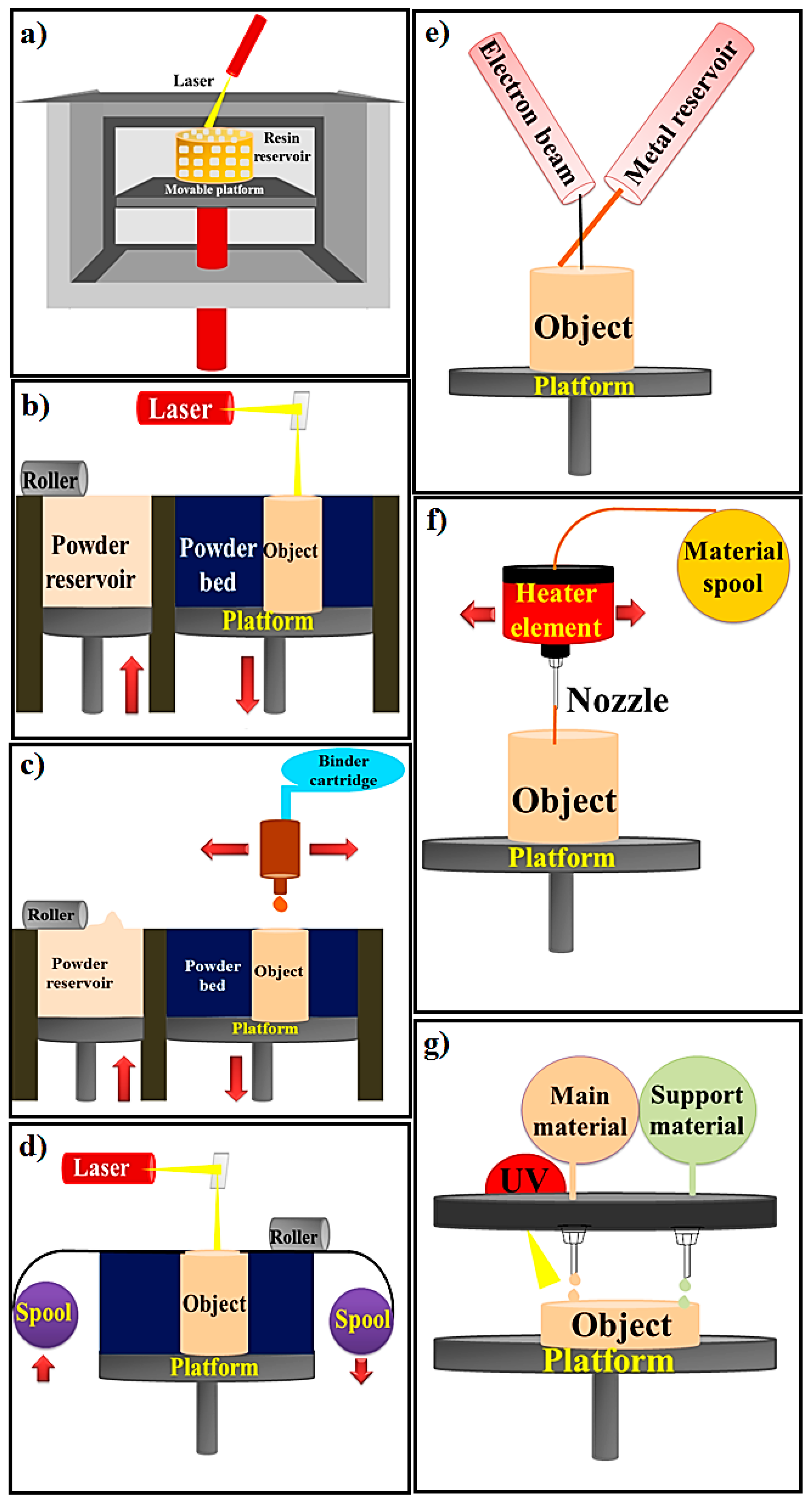
2. 3D Printing Methods
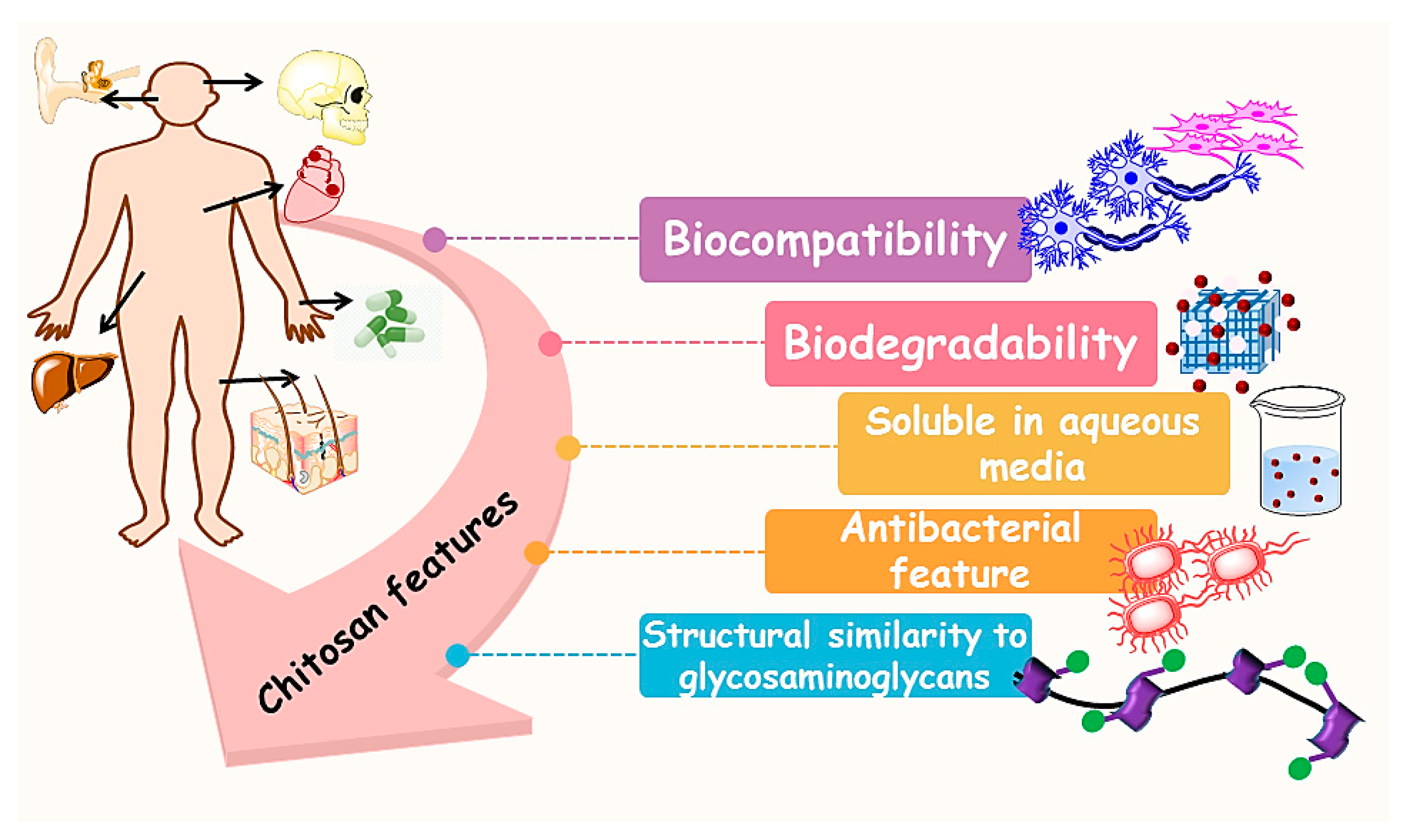
3. Chitosan (CS)
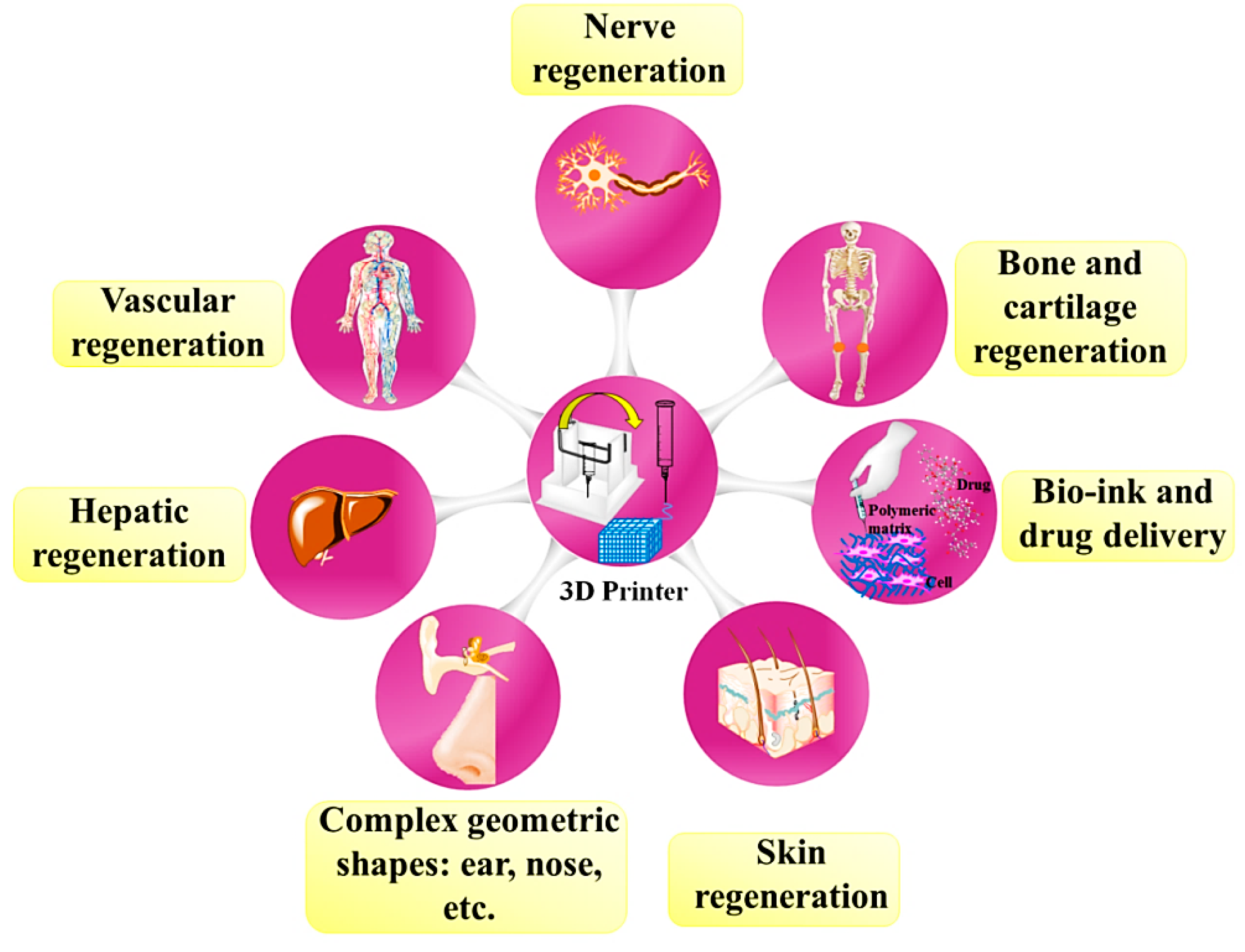
4. Chitosan-Based 3D Printed Construct for Hard Tissues Application
4.1. Bone Regeneration
4.2. Cartilage Regeneration
5. Chitosan-Based 3D Printed Construct for Soft Tissue Application
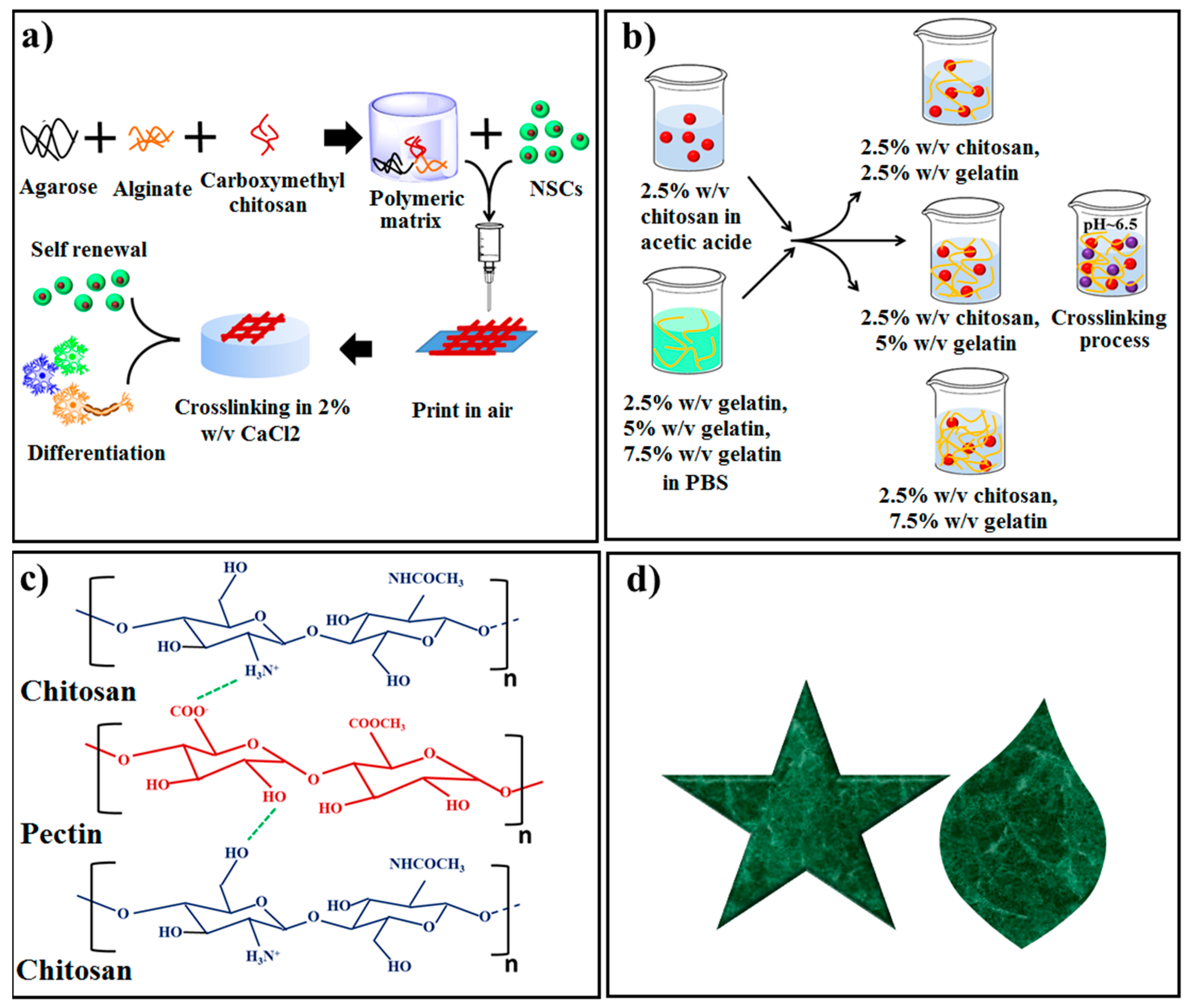
5.1. Nerve Regeneration
5.2. Skin Regeneration
5.3. Vascular Regeneration
5.4. Hepatic Regeneration
6. Drug Delivery
7. Bio-Inks
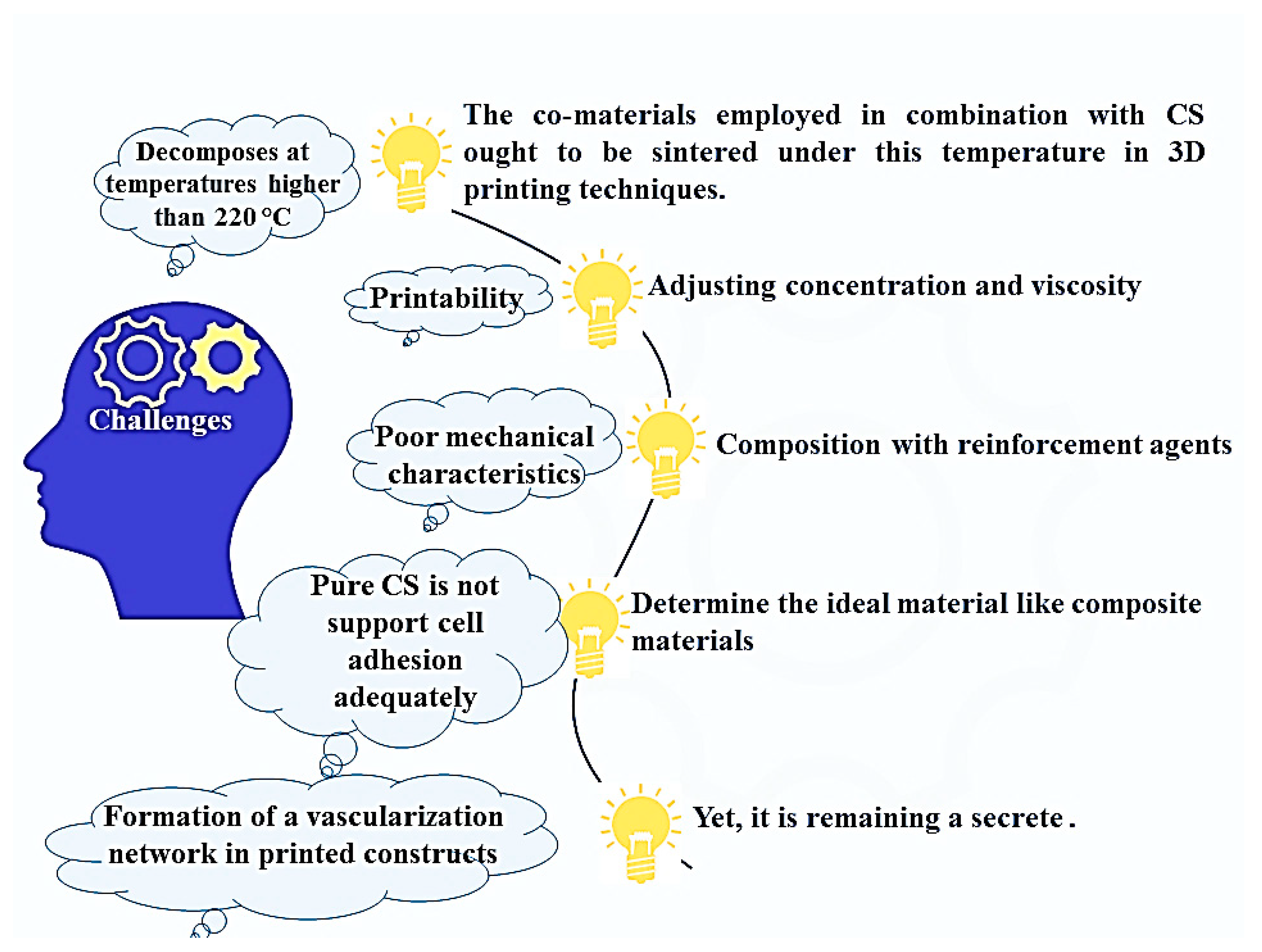
8. Benefits, Limitations, and Future Prospects
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| LOM | Laminated object manufacturing |
| MAC-Lp | Methacrylated chitosan-laponite |
| MAG-Lp | Methacrylated gelatin-laponite |
| MJM | Material jetting multijet |
| nBA | Nano bioactive glass |
| NVE | Nerve tissue engineering |
| NSCs | Neural stem cells |
| OCN | Osteocalcin |
| PBS | Phosphate buffer saline |
| PBF | Powder Bed Fusion |
| PCL-DA | Poly (ϵ-caprolactone) diacrylate |
| PDA | Polydopamine |
| PEC | Pectin |
| PEG | Poly ethylene glycol |
| PEGDA | Polyethylene glycol diacrylate |
| PLA | Poly (lactide acid) |
| PLLA | Poly (L-lactide) |
| PSL | Projection based stereolithography |
| PVA | Poly (vinyl alcohol) |
| Qu | Quercetin |
| RDMAM | Reflective dynamic mask additive manufacturing |
| RP | Rapid prototyping |
| SAL | Stereolithography |
| SCI | Spinal cord injury |
| SCs | Schwann cells |
| SLA | Stereolithography |
| SLM | Selective laser melting |
| SLS | Selective laser sintering |
| SFF | Solid free-form fabrication |
| SPSL | Scanning-projection based stereolithography |
| SSL | Scanning-based stereolithography |
| 3D | Three dimensional |
| 2D | Two dimensional |
| UAM | Ultrasonic additive manufacturing |
| UV | Ultraviolet |
| WD | Wound dressing |
| AL | Alginate |
| ALP | Alkaline phosphatase |
| AM | Additive manufacturing |
| ARS | Alizarin Red Staining |
| BGC | Bioactive glass ceramic |
| BJ | Binder jetting |
| BMP-2 | Bone morphogenetic protein-2 |
| BMSCs | Bone mesenchymal stem cells |
| BTE | Bone tissue engineering |
| CAD | Computer aided design |
| CMC | Carboxy methyl chitosan |
| CNT | Carbon nano tube |
| Col | Collagen |
| CPCs | Cartilage progenitor cells |
| CS | Chitosan |
| CSG | CS hydrogel |
| CT | Computed tomography |
| CTE | Cartilage tissue engineering |
| DA | Deacetylation |
| DBRP | Dispensing-based rapid prototyping |
| DED | Direct energy deposition |
| DMD | Digital micromirror device |
| DMLS | Direct metal laser Sintering |
| DRG | Dorsal root ganglion |
| EBM | Electron beam melting |
| EBW | Electron beam welding |
| FDM | Fuse deposition manufacturing |
| FFF | Fused filament fabrication |
| GAG | Glycosaminoglycans |
| GE | Genipin |
| Gel | Gelatin |
| GLY | Glycerol |
| HA | Hydroxyapatite |
| hMSCs | Human mesenchymal stem cells |
| hNSC | Human neural stem cells |
| IPFP-ASCs | Infrapatellar fat pad adipose stem cells |
References
- Eisenbarth, E. Biomaterials for Tissue Engineering. Adv. Eng. Mater. 2007, 9, 1051–1060. [Google Scholar] [CrossRef]
- Bose, S.; Ke, D.; Sahasrabudhe, H.; Bandyopadhyay, A. Additive manufacturing of biomaterials. Prog. Mater. Sci. 2018, 93, 45–111. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Bristow, J.F.; Smith, P.J.; Payne, G.F. Enzymatic gelation of the natural polymer chitosan. Polymer 2000, 41, 2157–2168. [Google Scholar] [CrossRef]
- Olatunji, O. Classification of Natural Polymers. In Natural Polymers; Springer Science and Business Media LLC: New York, NY, USA, 2016; pp. 1–17. [Google Scholar]
- Crini, G. Historical review on chitin and chitosan biopolymers. Environ. Chem. Lett. 2019, 17, 1623–1643. [Google Scholar] [CrossRef]
- Madihally, S.V.; Matthew, H. Porous chitosan scaffolds for tissue engineering. Biomaterials 1999, 20, 1133–1142. [Google Scholar] [CrossRef]
- Ivanova, E.P.; Bazaka, K.; Crawford, R. Natural polymer biomaterials: Advanced applications. In New Functional Biomaterials for Medicine and Healthcare; Woodhead Publishing: New Delhi, India, 2014; pp. 32–70. [Google Scholar]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Kean, T.J.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef]
- Singh, D.K.; Ray, A.R. Biomedical Applications of Chitin, Chitosan, and Their Derivatives. J. Macromol. Sci. Part C 2000, 40, 69–83. [Google Scholar] [CrossRef]
- Sahranavard, M.; Zamanian, A.; Ghorbani, F.; Shahrezaee, M.H. A critical review on three dimensional-printed chitosan hydrogels for development of tissue engineering. Bioprinting 2020, 17, e00063. [Google Scholar] [CrossRef]
- Zhu, C.; Fan, D.; Ma, X.; Xue, W.-J.; Yu, Y.; Luo, Y.; Liu, B.; Chen, L. Effects of Chitosan on Properties of Novel Human-like Collagen/Chitosan Hybrid Vascular Scaffold. J. Bioact. Compat. Polym. 2009, 24, 560–576. [Google Scholar] [CrossRef]
- Eltom, A.; Zhong, G.; Muhammad, A. Scaffold Techniques and Designs in Tissue Engineering Functions and Purposes: A Review. Adv. Mater. Sci. Eng. 2019, 2019, 1–13. [Google Scholar] [CrossRef]
- Yeong, W.Y.; Chua, C.K.; Leong, K.-F.; Chandrasekaran, M. Rapid prototyping in tissue engineering: Challenges and potential. Trends Biotechnol. 2004, 22, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Dong, H.-H.; Su, J.; Han, J.; Song, B.; Wei, Q.; Shi, Y. A Review of 3D Printing Technology for Medical Applications. Engineering 2018, 4, 729–742. [Google Scholar] [CrossRef]
- Cheng, Y.L.; Chen, F. Preparation and characterization of photocured poly (ε-caprolactone) diacrylate/poly (ethylene glycol) diacrylate/chitosan for photopoly merization-type 3D printing tissue engineering scaffold application. Mater. Sci. Eng. C 2017, 1, 66–73. [Google Scholar] [CrossRef]
- Feilden, E.; Blanca, E.G.-T.; Giuliani, F.; Saiz, E.; Vandeperre, L. Robocasting of structural ceramic parts with hydrogel inks. J. Eur. Ceram. Soc. 2016, 36, 2525–2533. [Google Scholar] [CrossRef]
- Manapat, J.Z.; Chen, Q.; Ye, P.; Advincula, R.C. 3D Printing of Polymer Nanocomposites via Stereolithography. Macromol. Mater. Eng. 2017, 302, 1600553. [Google Scholar] [CrossRef]
- Ngo, T.; Kashani, A.; Imbalzano, G.; Nguyen, Q.T.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B: Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Franchin, G. Additive Manufacturing of Ceramics. Printing Beyond the Binder. Ph.D. Thesis, University of Padova, Padova, Italy, 2013. [Google Scholar]
- Balla, V.K.; Kate, K.H.; Satyavolu, J.; Singh, P.; Tadimeti, J.G.D. Additive manufacturing of natural fiber reinforced polymer composites: Processing and prospects. Compos. Part B Eng. 2019, 174, 106956. [Google Scholar] [CrossRef]
- Duda, T.; Raghavan, L.V. 3D Metal Printing Technology. IFAC-PapersOnLine 2016, 49, 103–110. [Google Scholar] [CrossRef]
- Patra, S.; Young, V. A Review of 3D Printing Techniques and the Future in Biofabrication of Bioprinted Tissue. Cell Biophys. 2016, 74, 93–98. [Google Scholar] [CrossRef]
- Du, W.; Ren, X.; Ma, C.; Pei, Z. Binder Jetting Additive Manufacturing of Ceramics: A Literature Review. Energy 2017, 6. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, M.; Zhou, Z.; Gou, J.; Hui, D. 3D printing of polymer matrix composites: A review and prospective. Compos. Part B Eng. 2017, 110, 442–458. [Google Scholar] [CrossRef]
- Aimar, A.; Palermo, A.; Innocenti, B. The Role of 3D Printing in Medical Applications: A State of the Art. J. Healthc. Eng. 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Fergani, O.; Berto, F.; Welo, T.; Liang, S.Y. Analytical modelling of residual stress in additive manufacturing. Fatigue Fract. Eng. Mater. Struct. 2016, 40, 971–978. [Google Scholar] [CrossRef]
- Aramian, A.; Razavi, S.M.J.; Sadeghian, Z.; Berto, F. A review of additive manufacturing of cermets. Addit. Manuf. 2020, 33, 101130. [Google Scholar] [CrossRef]
- Ventola, C.L. Medical Applications for 3D Printing: Current and Projected Uses. Pharm. Ther. 2014, 39, 704–711. [Google Scholar]
- Su, A.; Al’Aref, S.J. History of 3D Printing. In 3D Printing Applications in Cardiovascular Medicine; Academic Press: Cambridge, MA, USA, 2018; pp. 1–10. [Google Scholar]
- Bourell, D.L.; Beaman, J.J.; Wohlers, T.; Frazier, W.; Kuhn, H.; Seifi, M. History of Additive Manufacturing. Addit. Manuf. Process. 2020, 24, 1–8. [Google Scholar] [CrossRef]
- Apparatus for Production of Three-Dimensional Objects by Stereolithography. UVP, INC., A CORP OF CALIFORNIA. U.S. Patent 4,575,330, 11 March 1986.
- Modeling Apparatus for Three-Dimensional Objects. STRATASYS, INC. A CORP. OF DELAWARE, MINNESOTA. U.S. Patent 5,340,433, 23 August 1994.
- Self-Assembling Cell Aggregates and Methods of Making Engineered. The Curators of the University of Missouri, Missou, Musc Foundation for Research Development, South CA, Medical University of South Carolina, South Caroli. U.S. Patent 8,241,905, 14 August 2012.
- Leukers, B.; Gülkan, H.; Irsen, S.H.; Milz, S.; Tille, C.; Schieker, M.; Seitz, H. Hydroxyapatite scaffolds for bone tissue engineering made by 3D printing. J. Mater. Sci. Mater. Electron. 2005, 16, 1121–1124. [Google Scholar] [CrossRef]
- Ahsan, S.M.; Thomas, M.; Reddy, K.K.; Sooraparaju, S.G.; Asthana, A.; Bhatnagar, I. Chitosan as biomaterial in drug delivery and tissue engineering. Int. J. Biol. Macromol. 2018, 110, 97–109. [Google Scholar] [CrossRef]
- Lavanya, K.; Chandran, S.V.; Balagangadharan, K.; Selvamurugan, N. Temperature-and pH-responsive chitosan-based injectable hydrogels for bone tissue engineering. Mater. Sci. Eng. C 2020, 111, 110862. [Google Scholar] [CrossRef]
- Ahmed, S.; Annu; Ali, A.; Sheikh, J. A review on chitosan centred scaffolds and their applications in tissue engineering. Int. J. Biol. Macromol. 2018, 116, 849–862. [Google Scholar] [CrossRef]
- Islam, M.; Shahruzzaman, M.; Biswas, S.; Sakib, N.; Rashid, T.U. Chitosan based bioactive materials in tissue engineering applications—A review. Bioact. Mater. 2020, 5, 164–183. [Google Scholar] [CrossRef] [PubMed]
- Tao, F.; Cheng, Y.; Shi, X.; Zheng, H.; Du, Y.; Xiang, W.; Deng, H. Applications of chitin and chitosan nanofibers in bone regenerative engineering. Carbohydr. Polym. 2019, 230, 115658. [Google Scholar] [CrossRef] [PubMed]
- Croisier, F.; Jérôme, C. Chitosan-Based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef]
- Ranganathan, S.; Balagangadharan, K.; Selvamurugan, N. Chitosan and gelatin-based electrospun fibers for bone tissue engineering. Int. J. Biol. Macromol. 2019, 133, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Vukajlovic, D.; Parker, J.; Bretcanu, O.; Novakovic, K. Chitosan based polymer/bioglass composites for tissue engineering applications. Mater. Sci. Eng. C 2019, 96, 955–967. [Google Scholar] [CrossRef]
- Balagangadharan, K.; Dhivya, S.; Selvamurugan, N. Chitosan based nanofibers in bone tissue engineering. Int. J. Biol. Macromol. 2017, 104, 1372–1382. [Google Scholar] [CrossRef]
- American Society for Testing and Materials. Standard Technology for Additive Manufacturing-General Principles/Terminology; ASTM ISO/ASTM 52900: 2015 (E); ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- Singh, S.; Ramakrishna, S.; Solberg, K. 3D Printing of polymer composites: A short review. Mater. Des. Process. Commun. 2019, 2, 97. [Google Scholar] [CrossRef]
- Sireesha, M.; Lee, J.; Kiran, A.S.K.; Babu, V.J.; Kee, B.B.T.; Ramakrishna, S. A review on additive manufacturing and its way into the oil and gas industry. RSC Adv. 2018, 8, 22460–22468. [Google Scholar] [CrossRef]
- He, Y.; Wildman, R.D.; Tuck, C.; Christie, S.D.R.; Edmondson, S. An Investigation of the Behavior of Solvent based Polycaprolactone ink for Material Jetting. Sci. Rep. 2016, 6, 20852. [Google Scholar] [CrossRef]
- Gibson, I.; Rosen, D.; Stucker, B. Material jetting. In Additive Manufacturing Technologies; Springer: New York, NY, USA, 2015; pp. 175–203. [Google Scholar]
- Levengood, S.K.L.; Zhang, M. Chitosan-based scaffolds for bone tissue engineering. J. Mater. Chem. B 2014, 2, 3161–3184. [Google Scholar] [CrossRef] [PubMed]
- Poshina, D.N.; Raik, S.; Poshin, A.N.; Skorik, Y. Accessibility of chitin and chitosan in enzymatic hydrolysis: A review. Polym. Degrad. Stab. 2018, 156, 269–278. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Chen, X.; Kong, M.; Liu, C.; Cha, D.S.; Kennedy, J.F. Effect of molecular weight and degree of chitosan deacetylation on the preparation and characteristics of chitosan thermosensitive hydrogel as a delivery system. Carbohydr. Polym. 2008, 73, 265–273. [Google Scholar] [CrossRef]
- Januariyasa, I.K.; Ana, I.D.; Yusuf, Y. Nanofibrous poly (vinyl alcohol)/chitosan contained carbonated hydroxyapatite nanoparticles scaffold for bone tissue engineering. Mater. Sci. Eng. C 2020, 1, 110347. [Google Scholar] [CrossRef] [PubMed]
- Garakani, S.S.; Khanmohammadi, M.; Atoufi, Z.; Kamrava, S.K.; Setayeshmehr, M.; Alizadeh, R.; Faghihi, F.; Bagher, Z.; Davachi, S.M.; Abbaspourrad, A. Fabrication of chitosan/agarose scaffolds containing extracellular matrix for tissue engineering applications. Int. J. Biol. Macromol. 2019, 143, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Khalili, R.; Zarrintaj, P.; Jafari, S.H.; Vahabi, H.; Saeb, M.R. Electroactive poly (p-phenylene sulfide)/r-Graphene Oxide/Chitosan as a novel potential candidate for tissue engineering. Int. J. Biol. Macromol. 2020, 154, 18–24. [Google Scholar] [CrossRef] [PubMed]
- De Witte, T.; Wagner, A.M.; Fratila-Apachitei, L.E.; Zadpoor, A.A.; Peppas, N.A. Immobilization of nanocarriers within a porous chitosan scaffold for the sustained delivery of growth factors in bone tissue engineering applications. J. Biomed. Mater. Res. Part A 2020, 108, 1122–1135. [Google Scholar] [CrossRef]
- Ghorbani, M.; Nezhad-Mokhtari, P.; Sohrabi, H.; Roshangar, L. Electrospun chitosan/nanocrystalline cellulose-graft-poly(N-vinylcaprolactam) nanofibers as the reinforced scaffold for tissue engineering. J. Mater. Sci. 2019, 55, 2176–2185. [Google Scholar] [CrossRef]
- Rahmani, H.; Najafi, S.H.M.; Ashori, A.; Fashapoyeh, M.A.; Mohseni, F.A.; Torkaman, S. Preparation of chitosan-Based composites with urethane cross linkage and evaluation of their properties for using as wound healing dressing. Carbohydr. Polym. 2019, 230, 115606. [Google Scholar] [CrossRef]
- Das, P.; Remigy, J.C.; Lahitte, J.F.; van der Meer, A.D.; Garmy-Susini, B.; Coetsier, C.; Desclaux, S.; Bacchin, P. Development of double porous poly (ε-caprolactone)/chitosan polymer as tissue engineering scaffold. Mater. Sci. Eng. C 2020, 107, 110257. [Google Scholar] [CrossRef]
- Abdel-Mohsen, A.M.; Abdel-Rahman, R.M.; Kubena, I.; Kobera, L.; Spotz, Z.; Zboncak, M.; Prikryl, R.; Brus, J.; Jancar, J. Chitosan-Glucan complex hollow fibers reinforced collagen wound dressing embedded with aloe vera. Part I: Preparation and characterization. Carbohydr. Polym. 2020, 15, 115708. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, B.; Li, M.; Li, J.; Wan, Y. Enhanced dual network hydrogels consisting of thiolated chitosan and silk fibroin for cartilage tissue engineering. Carbohydr. Polym. 2020, 227, 115335. [Google Scholar] [CrossRef] [PubMed]
- Zou, P.; Lee, W.-H.; Gao, Z.; Qin, D.; Wang, Y.; Liu, J.; Sun, T.; Gao, Y. Wound dressing from polyvinyl alcohol/chitosan electrospun fiber membrane loaded with OH-CATH30 nanoparticles. Carbohydr. Polym. 2020, 232, 115786. [Google Scholar] [CrossRef] [PubMed]
- Tabesh, E.; Salimijazi, H.; Kharaziha, M.; Mahmoudi, M.; Hejazi, M. Development of an in-Situ chitosan-Copper nanoparticle coating by electrophoretic deposition. Surf. Coat. Technol. 2019, 364, 239–247. [Google Scholar] [CrossRef]
- Mokhtari, H.; Ghasemi, Z.; Kharaziha, M.; Karimzadeh, F.; Alihosseini, F. Chitosan-58S bioactive glass nanocomposite coatings on TiO2 nanotube: Structural and biological properties. Appl. Surf. Sci. 2018, 441, 138–149. [Google Scholar] [CrossRef]
- Tabesh, E.; Salimijazi, H.; Kharaziha, M.; Hejazi, M. Antibacterial chitosan-copper nanocomposite coatings for biomedical applications. Mater. Today Proc. 2018, 5, 15806–15812. [Google Scholar] [CrossRef]
- Mohammadi, F.; Golafshan, N.; Kharaziha, M.; Ashrafi, A. Chitosan-heparin nanoparticle coating on anodized NiTi for improvement of blood compatibility and biocompatibility. Int. J. Biol. Macromol. 2019, 127, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Karimi, N.; Kharaziha, M.; Raeissi, K. Electrophoretic deposition of chitosan reinforced graphene oxide-hydroxyapatite on the anodized titanium to improve biological and electrochemical characteristics. Mater. Sci. Eng. C 2018, 98, 140–152. [Google Scholar] [CrossRef]
- Kumar, M.N. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Jayakumar, R.; Menon, D.; Koyakutty, M.; Nair, S.; Tamura, H. Biomedical applications of chitin and chitosan based nanomaterials—A short review. Carbohydr. Polym. 2010, 82, 227–232. [Google Scholar] [CrossRef]
- Sayyar, S.; Murray, E.; Thompson, B.C.; Chung, J.; Officer, D.L.; Gambhir, S.; Spinks, G.M.; Wallace, G.G. Processable conducting graphene/chitosan hydrogels for tissue engineering. J. Mater. Chem. B 2014, 3, 481–490. [Google Scholar] [CrossRef]
- Yang, X.; Tu, Y.; Li, L.; Shang, S.; Tao, X.-M. Well-Dispersed Chitosan/Graphene Oxide Nanocomposites. ACS Appl. Mater. Interfaces 2010, 2, 1707–1713. [Google Scholar] [CrossRef]
- Wang, S.-F.; Shen, L.; Zhang, W.-D.; Tong, Y.-J. Preparation and Mechanical Properties of Chitosan/Carbon Nanotubes Composites. Biomacromolecules 2005, 6, 3067–3072. [Google Scholar] [CrossRef]
- Wu, C.-S. Modulation, functionality, and cytocompatibility of three-dimensional printing materials made from chitosan-based polysaccharide composites. Mater. Sci. Eng. C 2016, 69, 27–36. [Google Scholar] [CrossRef]
- Carpinteri, A.; Berto, F.; Fortese, G.; Ronchei, C.; Scorza, D.; Vantadori, S. Modified two-parameter fracture model for bone. Eng. Fract. Mech. 2017, 174, 44–53. [Google Scholar] [CrossRef]
- Akbardoost, J.; Amirafshari, R.; Mohsenzade, O.; Berto, F. Scaling effect on the fracture toughness of bone materials using MMTS criterion. J. Mech. Behav. Biomed. Mater. 2018, 85, 72–79. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Hamzah, E.; Shuang, C.P.; Berto, F. Preparation of poly(ε-caprolactone)-hydroxyapatite composite coating for improvement of corrosion performance of biodegradable magnesium. Mater. Des. Process. Commun. 2020, 1–7. [Google Scholar] [CrossRef]
- Anitha, A.; Sowmya, S.; Jayakumar, R.; Deepthi, S.; Chennazhi, K.; Ehrlich, H.; Tsurkan, M.; Jayakumar, R. Chitin and chitosan in selected biomedical applications. Prog. Polym. Sci. 2014, 39, 1644–1667. [Google Scholar] [CrossRef]
- Pahlevanzadeh, F.; Bakhsheshi-Rad, H.R.; Ismail, A.F.; Aziz, M. Apatite-Forming ability, cytocompatibility, and mechanical properties enhancement of poly methyl methacrylate-based bone cements by incorporating of baghdadite nanoparticles. Int. J. Appl. Ceram. Technol. 2019, 16, 2006–2019. [Google Scholar] [CrossRef]
- Pahlevanzadeh, F.; Bakhsheshi-Rad, H.; Hamzah, E. In-vitro biocompatibility, bioactivity, and mechanical strength of PMMA-PCL polymer containing fluorapatite and graphene oxide bone cements. J. Mech. Behav. Biomed. Mater. 2018, 82, 257–267. [Google Scholar] [CrossRef]
- Pahlevanzadeh, F.; Bakhsheshi-Rad, H.R.; Ismail, A.; Aziz, M.; Chen, X. Development of PMMA-Mon-CNT bone cement with superior mechanical properties and favorable biological properties for use in bone-defect treatment. Mater. Lett. 2019, 240, 9–12. [Google Scholar] [CrossRef]
- Lee, C.-M.; Yang, S.-W.; Jung, S.-C.; Kim, M.-S.; Lee, J.-H.; Min, C.-; Seong-Won, Y.; Sang-Chul, J.; Byung-Hoon, K. Oxygen Plasma Treatment on 3D-Printed Chitosan/Gelatin/Hydroxyapatite Scaffolds for Bone Tissue Engineering. J. Nanosci. Nanotechnol. 2017, 17, 2747–2750. [Google Scholar] [CrossRef] [PubMed]
- Demirtaş, T.T.; Irmak, G.; Gumusderelioglu, M. A bioprintable form of chitosan hydrogel for bone tissue engineering. Biofabrication 2017, 9, 035003. [Google Scholar] [CrossRef] [PubMed]
- Chavanne, P.; Stevanovic, S.; Wuethrich, A.; Braissant, O.; Pieles, U.; Gruner, P.; Schumacher, R. 3D printed chitosan/hydroxyapatite scaffolds for potential use in regenerative medicine. Biomed. Tech. Eng. 2013. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Caballero, S.S.R.; Saiz, E.; Montembault, A.; Tadier, S.; Maire, E.; David, L.; Delair, T.; Gremillard, L. 3-D printing of chitosan-calcium phosphate inks: Rheology, interactions and characterization. J. Mater. Sci. Mater. Electron. 2018, 30, 6. [Google Scholar] [CrossRef]
- Dong, L.; Wang, S.; Zhao, X.-R.; Zhu, Y.; Yu, J.-K. 3D-Printed Poly(ε-caprolactone) Scaffold Integrated with Cell-laden Chitosan Hydrogels for Bone Tissue Engineering. Sci. Rep. 2017, 7, 13412. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, S.; Liu, K.; Wen, W.; Lu, L.; Ding, S.; Zhou, C.; Luo, B. 3D poly (L-lactide)/chitosan micro/nano fibrous scaffolds functionalized with quercetin-polydopamine for enhanced osteogenic and anti-inflammatory activities. Chem. Eng. J. 2019, 18, 123524. [Google Scholar] [CrossRef]
- Sedghi, R.; Shaabani, A.; Sayyari, N. Electrospun triazole-based chitosan nanofibers as a novel scaffolds for bone tissue repair and regeneration. Carbohydr. Polym. 2019, 230, 115707. [Google Scholar] [CrossRef]
- Ye, H.; Zhu, J.; Deng, D.; Jin, S.; Li, J.; Man, Y. Enhanced osteogenesis and angiogenesis by PCL/chitosan/Sr-doped calcium phosphate electrospun nanocomposite membrane for guided bone regeneration. J. Biomater. Sci. Polym. Ed. 2019, 30, 1505–1522. [Google Scholar] [CrossRef]
- Sharifi, F.; Atyabi, S.-M.; Irani, S.; Bakhshi, H. Bone morphogenic protein-2 immobilization by cold atmospheric plasma to enhance the osteoinductivity of carboxymethyl chitosan-based nanofibers. Carbohydr. Polym. 2020, 231, 115681. [Google Scholar] [CrossRef]
- Arabahmadi, S.; Pezeshki, M.M.; Irani, S.; Zandi, M. Electrospun biocompatible Gelatin-Chitosan/Polycaprolactone/Hydroxyapatite nanocomposite scaffold for bone tissue engineering. Int. J. Nano Dimens. 2019, 1, 169–179. [Google Scholar]
- Kaliva, M.; Georgopoulou, A.; Dragatogiannis, D.; Charitidis, C.A.; Chatzinikolaidou, M.; Vamvakaki, M. Biodegradable Chitosan-graft-Poly(l-lactide) Copolymers For Bone Tissue Engineering. Polymers 2020, 12, 316. [Google Scholar] [CrossRef]
- Ergul, N.M.; Unal, S.; Kalkandelen, C.; Ekren, N.; Kilic, O.; Chi-Chang, L.; Gunduz, O.; Kartal, I.; Kılıç, O. 3D printing of chitosan/ poly(vinyl alcohol) hydrogel containing synthesized hydroxyapatite scaffolds for hard-tissue engineering. Polym. Test. 2019, 79, 106006. [Google Scholar] [CrossRef]
- Cebe, T.; Ahuja, N.; Monte, F.A.; Awad, K.; Vyavhare, K.; Aswath, P.; Huang, J.; Brotto, M.; Varanasi, V. Novel 3D-printed methacrylated chitosan-laponite nanosilicate composite scaffolds enhance cell growth and biomineral formation in MC3T3 pre-osteoblasts. J. Mater. Res. 2018, 35, 58–75. [Google Scholar] [CrossRef]
- Rogina, A.; Pribolšan, L.; Hanžek, A.; Gόmez-Estrada, L.; Ferrer, G.G.; Marijanović, I.; Ivanković, M.; Ivanković, H. Macroporous poly(lactic acid) construct supporting the osteoinductive porous chitosan-based hydrogel for bone tissue engineering. Polymer 2016, 98, 172–181. [Google Scholar] [CrossRef]
- Abazari, M.F.; Nejati, F.; Nasiri, N.; Khazeni, Z.A.S.; Nazari, B.; Enderami, S.E.; Mohajerani, H. Platelet-rich plasma incorporated electrospun PVA-chitosan-HA nanofibers accelerates osteogenic differentiation and bone reconstruction. Gene 2019, 720, 144096. [Google Scholar] [CrossRef]
- Ang, T.; Sultana, F.; Hutmacher, D.W.; Wong, Y.; Fuh, J.; Mo, X.; Loh, H.; Burdet, E.; Teoh, S. Fabrication of 3D chitosan–hydroxyapatite scaffolds using a robotic dispensing system. Mater. Sci. Eng. C 2002, 20, 35–42. [Google Scholar] [CrossRef]
- Dorj, B.; Park, J.-H.; Kim, H.-W. Robocasting chitosan/nanobioactive glass dual-Pore structured scaffolds for bone engineering. Mater. Lett. 2012, 73, 119–122. [Google Scholar] [CrossRef]
- Ghorbani, M.; Roshangar, L.; Rad, J.S. Development of reinforced chitosan/pectin scaffold by using the cellulose nanocrystals as nanofillers: An injectable hydrogel for tissue engineering. Eur. Polym. J. 2020, 130, 109697. [Google Scholar] [CrossRef]
- Tan, H.; Chu, C.R.; Payne, K.; Marra, K.G. Injectable in situ forming biodegradable chitosan–hyaluronic acid based hydrogels for cartilage tissue engineering. Acta Biomater. 2009, 30, 2499–2506. [Google Scholar] [CrossRef]
- Da Silva, M.A.; Crawford, A.; Mundy, J.; Correlo, V.; Sol, P.; Bhattacharya, M.; Hatton, P.V.; Reis, R.L.; Neves, N.M. Chitosan/polyester-based scaffolds for cartilage tissue engineering: Assessment of extracellular matrix formation. Acta Biomater. 2010, 6, 1149–1157. [Google Scholar] [CrossRef]
- Mohammadalizadeh, Z.; Karbasi, S.; Arasteh, S. Physical, mechanical and biological evaluation of poly (3-hydroxybutyrate)-chitosan/MWNTs as a novel electrospun scaffold for cartilage tissue engineering applications. Polym. Technol. Mater. 2019, 59, 417–429. [Google Scholar] [CrossRef]
- Tan, H.; Wu, J.; Lao, L.; Gaoa, C. Gelatin/chitosan/hyaluronan scaffold integrated with PLGA microspheres for cartilage tissue engineering. Acta Biomater. 2009, 5, 328–337. [Google Scholar] [CrossRef]
- Kim, S.E.; Park, J.H.; Cho, Y.W.; Chung, H.; Jeong, S.Y.; Lee, E.B.; Kwon, I.C. Porous chitosan scaffold containing microspheres loaded with transforming growth factor-β1: Implications for cartilage tissue engineering. J. Control. Release 2003, 91, 365–374. [Google Scholar] [CrossRef]
- Suh, J.-K.F.; Matthew, H. Application of chitosan-Based polysaccharide biomaterials in cartilage tissue engineering: A review. Biomaterials 2000, 21, 2589–2598. [Google Scholar] [CrossRef]
- Mirahmadi, F.; Tafazzoli-Shadpour, M.; Shokrgozar, M.A.; Bonakdar, S. Enhanced mechanical properties of thermosensitive chitosan hydrogel by silk fibers for cartilage tissue engineering. Mater. Sci. Eng. C 2013, 33, 4786–4794. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, M. Chitosan-Alginate as scaffolding material for cartilage tissue engineering. J. Biomed. Mater. Res. Part A 2005, 75, 485–493. [Google Scholar] [CrossRef]
- Ye, K.; Felimban, R.; Traianedes, K.; Moulton, S.E.; Wallace, G.G.; Chung, J.; Quigley, A.; Choong, P.F.M.; Myers, D.E. Chondrogenesis of Infrapatellar Fat Pad Derived Adipose Stem Cells in 3D Printed Chitosan Scaffold. PLoS ONE 2014, 9, e99410. [Google Scholar] [CrossRef]
- Morris, V.B.; Nimbalkar, S.; Younesi, M.; McClellan, P.; Akkus, O. Mechanical Properties, Cytocompatibility and Manufacturability of Chitosan:PEGDA Hybrid-Gel Scaffolds by Stereolithography. Ann. Biomed. Eng. 2016, 45, 286–296. [Google Scholar] [CrossRef]
- Reed, S.; Lau, G.; Delattre, B.; Lopez, D.D.; Tomsia, A.P.; Wu, B.M. Macro-and micro-designed chitosan-alginate scaffold architecture by three-dimensional printing and directional freezing. Biofabrication 2016, 8, 015003. [Google Scholar] [CrossRef]
- Saderi, N.; Rajabi, M.; Akbari, B.; Firouzi, M.; Hassannejad, Z. Fabrication and characterization of gold nanoparticle-doped electrospun PCL/chitosan nanofibrous scaffolds for nerve tissue engineering. J. Mater. Sci. Mater. Electron. 2018, 29, 134. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Ao, Q.; He, Q.; Gong, X.; Gong, K.; Gong, Y.; Zhao, N.; Zhang, X. Neural Stem Cell Affinity of Chitosan and Feasibility of Chitosan-Based Porous Conduits as Scaffolds for Nerve Tissue Engineering. Tsinghua Sci. Technol. 2006, 11, 415–420. [Google Scholar] [CrossRef]
- Wang, A.; Ao, Q.; Cao, W.; Yu, M.; He, Q.; Kong, L.; Zhang, L.; Gong, Y.; Zhang, X. Porous chitosan tubular scaffolds with knitted outer wall and controllable inner structure for nerve tissue engineering. J. Biomed. Mater. Res. Part A 2006, 79, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sun, C.; Guan, S.; Li, W.; Xu, J.; Ge, D.; Zhuang, M.; Liu, T.; Ma, X. Chitosan/gelatin porous scaffolds assembled with conductive poly(3,4-ethylenedioxythiophene) nanoparticles for neural tissue engineering. J. Mater. Chem. B 2017, 5, 4774–4788. [Google Scholar] [CrossRef]
- Wang, A.; Ao, Q.; Wei, Y.; Gong, K.; Liu, X.; Zhao, N.; Gong, Y.; Zhang, X. Physical properties and biocompatibility of a porous chitosan-based fiber-reinforced conduit for nerve regeneration. Biotechnol. Lett. 2007, 29, 1697–1702. [Google Scholar] [CrossRef]
- Soltani, S.; Ebrahimian-Hosseinabadi, M.; Kharazi, A.Z. Chitosan/graphene and poly (D, L-lactic-co-glycolic acid)/graphene nano-composites for nerve tissue engineering. Tissue Eng. Regen. Med. 2016, 1, 684–690. [Google Scholar] [CrossRef]
- Saravani, S.; Ebrahimian-Hosseinabadi, M.; Mohebbi-Kalhori, D. Polyglycerol sebacate/chitosan/gelatin nano-Composite scaffolds for engineering neural construct. Mater. Chem. Phys. 2019, 15, 147–151. [Google Scholar] [CrossRef]
- Baniasadi, H.; Mashayekhan, S.; Ramazani, S.A.A. Fabrication and characterization of conductive chitosan/gelatin-based scaffolds for nerve tissue engineering. Int. J. Biol. Macromol. 2015, 74, 360–366. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, C.; Zhu, X.; Wang, J.; Liu, X.; Yang, X.; An, X.; Liang, J.; Dong, H.; Jiang, W.; et al. 3D printing collagen/chitosan scaffold ameliorated axon regeneration and neurological recovery after spinal cord injury. J. Biomed. Mater. Res. Part A 2019, 107, 1898–1908. [Google Scholar] [CrossRef]
- Bardakova, K.; Demina, T.S.; Grebenik, E.A.; Minaev, N.; Akopova, T.; Bagratashvili, V.N.; Timashev, P.S. 3D printing biodegradable scaffolds with chitosan materials for tissue engineering. IOP Conf. Ser. Mater. Sci. Eng. 2018, 347, 012009. [Google Scholar] [CrossRef]
- Zhu, N.; Li, M.G.; Guan, Y.J.; Schreyer, D.J.; Chen, X.B. Effects of laminin blended with chitosan on axon guidance on patterned substrates. Biofabrication 2010, 2, 045002. [Google Scholar] [CrossRef]
- Gu, Q.; Tomaskovic-Crook, E.; Lozano, R.; Chen, Y.; Kapsa, R.M.; Zhou, Q.; Wallace, G.G.; Crook, J.M. Functional 3D Neural Mini-Tissues from Printed Gel-Based Bioink and Human Neural Stem Cells. Adv. Healthc. Mater. 2016, 5, 1429–1438. [Google Scholar] [CrossRef]
- Hafezi, F.; Scoutaris, N.; Douroumis, D.; Boateng, J.S. 3D printed chitosan dressing crosslinked with genipin for potential healing of chronic wounds. Int. J. Pharm. 2019, 560, 406–415. [Google Scholar] [CrossRef]
- Long, J.; Etxeberria, A.E.; Nand, A.; Bunt, C.R.; Ray, S.; Seyfoddin, A. A 3D printed chitosan-pectin hydrogel wound dressing for lidocaine hydrochloride delivery. Mater. Sci. Eng. C 2019, 104, 109873. [Google Scholar] [CrossRef]
- Han, F.; Dong, Y.; Su, Z.; Yin, R.; Song, A.-H.; Li, S. Preparation, characteristics and assessment of a novel gelatin–chitosan sponge scaffold as skin tissue engineering material. Int. J. Pharm. 2014, 476, 124–133. [Google Scholar] [CrossRef]
- Dhandayuthapani, B.; Krishnan, U.M.; Sethuraman, S. Fabrication and characterization of chitosan-gelatin blend nanofibers for skin tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 94, 264–272. [Google Scholar] [CrossRef]
- Liu, H.; Mao, J.; Yao, K.; Yang, G.; Cui, L.; Cao, Y. A study on a chitosan-Gelatin-Hyaluronic acid scaffold as artificial skin in vitro and its tissue engineering applications. J. Biomater. Sci. Polym. Ed. 2004, 15, 25–40. [Google Scholar] [CrossRef]
- Choi, S.M.; Singh, D.; Kumar, A.; Oh, T.H.; Cho, Y.W.; Han, S.S. Porous Three-Dimensional PVA/Gelatin Sponge for Skin Tissue Engineering. Int. J. Polym. Mater. 2013, 62, 384–389. [Google Scholar] [CrossRef]
- Ehterami, A.; Salehi, M.; Farzamfar, S.; Samadian, H.; Vaez, A.; Ghorbani, S.; Ai, J.; Sahrapeyma, H.; Vaez, A. Chitosan/alginate hydrogels containing Alpha-tocopherol for wound healing in rat model. J. Drug Deliv. Sci. Technol. 2019, 51, 204–213. [Google Scholar] [CrossRef]
- Sharma, S.; Batra, S. Recent advances of chitosan composites in artificial skin: The next era for potential biomedical application. Mater. Biomed. Eng. 2019, 1, 97–119. [Google Scholar] [CrossRef]
- Boucard, N.; Viton, C.; Agay, D.; Mari, E.; Roger, T.; Chancerelle, Y.; Domard, A. The use of physical hydrogels of chitosan for skin regeneration following third-degree burns. Biomaterials 2007, 28, 3478–3488. [Google Scholar] [CrossRef]
- Madni, A.; Khan, R.; Ikram, M.; Naz, S.S.; Khan, T.; Wahid, F. Fabrication and Characterization of Chitosan–Vitamin C–Lactic Acid Composite Membrane for Potential Skin Tissue Engineering. Int. J. Polym. Sci. 2019, 2019, 1–8. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Hadisi, Z.; Ismail, A.; Aziz, M.; Akbari, M.; Berto, F.; Chen, X. In vitro and in vivo evaluation of chitosan-alginate/gentamicin wound dressing nanofibrous with high antibacterial performance. Polym. Test. 2020, 82, 106298. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, D.; Chen, X.; Xu, Q.; Lu, F.M.; Nie, J. Electrospun Water-Soluble Carboxyethyl Chitosan/Poly(vinyl alcohol) Nanofibrous Membrane as Potential Wound Dressing for Skin Regeneration. Biomacromolecules 2008, 9, 349–354. [Google Scholar] [CrossRef]
- Tchemtchoua, V.T.; Atanasova, G.; Aqil, A.; Filée, P.; Garbacki, N.; Vanhooteghem, O.; Deroanne, C.; Noel, A.; Jérome, C.; Nusgens, B.; et al. Development of a Chitosan Nanofibrillar Scaffold for Skin Repair and Regeneration. Biomacromolecules 2011, 12, 3194–3204. [Google Scholar] [CrossRef]
- Behera, S.S.; Das, U.; Kumar, A.; Bissoyi, A.; Singh, A.K. Chitosan/TiO2 composite membrane improves proliferation and survival of L929 fibroblast cells: Application in wound dressing and skin regeneration. Int. J. Biol. Macromol. 2017, 98, 329–340. [Google Scholar] [CrossRef]
- Yao, C.-H.; Chen, K.-Y.; Cheng, M.-H.; Chen, Y.-S.; Huang, C.-H. Effect of genipin crosslinked chitosan scaffolds containing SDF-1 on wound healing in a rat model. Mater. Sci. Eng. C 2019, 109, 110368. [Google Scholar] [CrossRef]
- Ng, W.L.; Yeong, W.Y.; Naing, M.W. Polyelectrolyte gelatin-Chitosan hydrogel optimized for 3D bioprinting in skin tissue engineering. Int. J. BioPrint. 2016, 2. [Google Scholar] [CrossRef]
- Ng, W.L.; Yeong, W.Y.; Naing, M.W. Development of Polyelectrolyte Chitosan-gelatin Hydrogels for Skin Bioprinting. Procedia CIRP 2016, 49, 105–112. [Google Scholar] [CrossRef]
- Intini, C.; Elviri, L.; Cabral, J.; Mros, S.; Bergonzi, C.; Bianchera, A.; Flammini, L.; Govoni, P.; Barocelli, E.; Bettini, R.; et al. 3D-Printed chitosan-Based scaffolds: An in vitro study of human skin cell growth and an in-vivo wound healing evaluation in experimental diabetes in rats. Carbohydr. Polym. 2018, 199, 593–602. [Google Scholar] [CrossRef]
- Muzzarelli, R. Genipin-Crosslinked chitosan hydrogels as biomedical and pharmaceutical aids. Carbohydr. Polym. 2009, 77, 1–9. [Google Scholar] [CrossRef]
- Harris, R.; Lecumberri, E.; Heras, A. Chitosan-Genipin Microspheres for the Controlled Release of Drugs: Clarithromycin, Tramadol and Heparin. Mar. Drugs 2010, 8, 1750–1762. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, W.; Kim, H.-I. pH-responsive release behavior of genipin-crosslinked chitosan/poly(ethylene glycol) hydrogels. J. Appl. Polym. Sci. 2012, 125, 290–298. [Google Scholar] [CrossRef]
- Ulag, S.; Kalkandelen, C.; Oktar, F.N.; Uzun, M.; Sahin, Y.M.; Karademir, B.; Arslan, S.; Ozbolat, I.T.; Mahirogullari, M.; Gunduz, O. 3D Printing Artificial Blood Vessel Constructs Using PCL/Chitosan/Hydrogel Biocomposites. Chem. Select. 2019, 4, 2387–2391. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, Y.; Ozbolat, I.T. Direct Bioprinting of Vessel-Like Tubular Microfluidic Channels. J. Nanotechnol. Eng. Med. 2013, 4, 0210011–0210017. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, J.; Cui, Y.; Xu, R.; Wang, Z.; Zhang, J.; Wang, K.; Li, Y.; Zhao, Q.; Kong, D. Effect of sustained heparin release from PCL/chitosan hybrid small-diameter vascular grafts on anti-thrombogenic property and endothelialization. Acta Biomater. 2014, 10, 2739–2749. [Google Scholar] [CrossRef]
- Yin, A.; McClure, M.J.; Wu, J.; Bowlin, G.L.; El-Newehy, M.; Zhang, K.; Huang, C.; Fang, J.; Mo, X.; Al-Deyab, S.S. Electrospinning collagen/chitosan/poly(L-lactic acid-co-ϵ-caprolactone) to form a vascular graft: Mechanical and biological characterization. J. Biomed. Mater. Res. Part A 2012, 101, 1292–1301. [Google Scholar] [CrossRef]
- Deng, C.; Li, F.; Griffith, M.; Ruel, M.; Suuronen, E.J. Application of Chitosan-Based Biomaterials for Blood Vessel Regeneration. Macromol. Symp. 2010, 297, 138–146. [Google Scholar] [CrossRef]
- Wang, X.; Yan, Y.; Xiong, Z.; Lin, F.; Wu, R.; Zhang, R.; Lu, Q. Preparation and evaluation of ammonia-treated collagen/chitosan matrices for liver tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2005, 75, 91–98. [Google Scholar] [CrossRef]
- Wang, X.; Yan, Y.; Lin, F.; Xiong, Z.; Wu, R.; Zhang, R.; Lu, Q. Preparation and characterization of a collagen/chitosan/heparin matrix for an implantable bioartificial liver. J. Biomater. Sci. Polym. Ed. 2005, 16, 1063–1080. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Q.S.; Yan, K.; Qi, Y.; Wang, G.F.; Cui, Y.L. Preparation, characterization, and evaluation of genipin crosslinked chitosan/gelatin three-dimensional scaffolds for liver tissue engineering applications. J. Biomed. Mater. Res. Part A 2016, 104, 1863–1870. [Google Scholar] [CrossRef] [PubMed]
- Semnani, D.; Naghashzargar, E.; Hadjianfar, M.; Manshadi, F.D.; Mohammadi, S.; Karbasi, S.; Effaty, F. Evaluation of PCL/Chitosan Electrospun Nanofibers for Liver Tissue Engineering. Int. J. Polym. Mater. 2016, 66, 149–157. [Google Scholar] [CrossRef]
- Jiankang, H.; Dichen, L.; Yaxiong, L.; Bo, Y.; Hanxiang, Z.; Qin, L.; Bingheng, L.; Yi, L. Preparation of chitosan–gelatin hybrid scaffolds with well-organized microstructures for hepatic tissue engineering. Acta Biomater. 2009, 5, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Dodane, V.; Vilivalam, V.D. Pharmaceutical applications of chitosan. Pharm. Sci. Technol. Today 1998, 1, 246–253. [Google Scholar] [CrossRef]
- Heidenreich, A.C.; Pérez-Recalde, M.; Wusener, A.G.; Hermida, É.B. Collagen and chitosan blends for 3D bioprinting: A rheological and printability approach. Polym. Test. 2020, 82, 106297. [Google Scholar] [CrossRef]
- Wu, Q.; Therriault, D.; Heuzey, M.-C. Processing and Properties of Chitosan Inks for 3D Printing of Hydrogel Microstructures. ACS Biomater. Sci. Eng. 2018, 4, 2643–2652. [Google Scholar] [CrossRef]
- Huang, J.; Fu, H.; Wang, Z.; Meng, Q.; Liu, S.; Wang, H.; Zheng, X.; Dai, J.; Zhang, Z. BMSCs-laden gelatin/sodium alginate/carboxymethyl chitosan hydrogel for 3D bioprinting. RSC Adv. 2016, 6, 108423–108430. [Google Scholar] [CrossRef]
- Ferreira, M.; Lima, I.; Ribeiro, A.; Lobo, A.D.O.; Rizzo, M.; Osajima, J.A.; Estevinho, L.; Filho, E.C.S. Biocompatible Gels of Chitosan–Buriti Oil for Potential Wound Healing Applications. Materials 2020, 13, 1977. [Google Scholar] [CrossRef]
- Sedyakina, N.; Kuskov, A.; Velonia, K.; Feldman, N.; Lutsenko, S.; Avramenko, G. Modulation of Entrapment Efficiency and In Vitro Release Properties of BSA-Loaded Chitosan Microparticles Cross-Linked with Citric Acid as a Potential Protein–Drug Delivery System. Materials 2020, 13, 1989. [Google Scholar] [CrossRef]
- Cirillo, G.; Vittorio, O.; Kunhardt, D.; Valli, E.; Voli, F.; Farfalla, A.; Curcio, M.; Spizzirri, U.G.; Hampel, S. Combining Carbon Nanotubes and Chitosan for the Vectorization of Methotrexate to Lung Cancer Cells. Materials 2019, 12, 2889. [Google Scholar] [CrossRef]
- Charitidis, C.A.; Dragatogiannis, D.A.; Milioni, E.; Kaliva, M.; Vamvakaki, M.; Chatzinikolaidou, M. Synthesis, Nanomechanical Characterization and Biocompatibility of a Chitosan-Graft-Poly(ε-caprolactone) Copolymer for Soft Tissue Regeneration. Materials 2019, 12, 150. [Google Scholar] [CrossRef] [PubMed]
- Casimiro, M.H.; Gomes, S.R.; Rodrigues, G.; Leal, J.P.; Ferreira, L. Chitosan/Poly(vinylpyrrolidone) Matrices Obtained by Gamma-Irradiation for Skin Scaffolds: Characterization and Preliminary Cell Response Studies. Materials 2018, 11, 2535. [Google Scholar] [CrossRef] [PubMed]
- Tamer, T.M.; Collins, M.N.; Katarína, V.; Hassan, M.A.; Omer, A.M.; Mohyeldin, M.S.; Švík, K.; Jurčík, R.; Ondruska, L.; Biró, C.; et al. MitoQ Loaded Chitosan-Hyaluronan Composite Membranes for Wound Healing. Materials 2018, 11, 569. [Google Scholar] [CrossRef] [PubMed]
- Georgopoulou, A.; Kaliva, M.; Vamvakaki, M.; Chatzinikolaidou, M. Osteogenic Potential of Pre-Osteoblastic Cells on a Chitosan-graft-Polycaprolactone Copolymer. Materials 2018, 11, 490. [Google Scholar] [CrossRef]
- Forero, J.C.; Roa, E.; Reyes, J.G.; Acevedo, C.A.; Osses, N. Development of Useful Biomaterial for Bone Tissue Engineering by Incorporating Nano-Copper-Zinc Alloy (nCuZn) in Chitosan/Gelatin/Nano-Hydroxyapatite (Ch/G/nHAp) Scaffold. Materials 2017, 10, 1177. [Google Scholar] [CrossRef]
- Su, C.-J.; Tu, M.-G.; Wei, L.-J.; Hsu, T.-T.; Kao, C.-T.; Chen, T.-H.; Huang, T.-H. Calcium Silicate/Chitosan-Coated Electrospun Poly (Lactic Acid) Fibers for Bone Tissue Engineering. Materials 2017, 10, 501. [Google Scholar] [CrossRef]
- González-Henríquez, C.M.; Sarabia-Vallejos, M.A.; Rodriguez-Hernandez, J. Advances in the Fabrication of Antimicrobial Hydrogels for Biomedical Applications. Materials 2017, 10, 232. [Google Scholar] [CrossRef]
- Liu, J.; Sun, L.; Xu, W.; Wang, Q.; Yu, S.; Sun, J. Current advances and future perspectives of 3D printing natural-derived biopolymers. Carbohydr. Polym. 2019, 207, 297–316. [Google Scholar] [CrossRef]
- Berger, J.; Reist, M.; Mayer, J.; Felt, O.; Peppas, N.; Gurny, R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 19–34. [Google Scholar] [CrossRef]
- Ozbolat, I.T.; Hospodiuk, M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials 2016, 76, 321–343. [Google Scholar] [CrossRef] [PubMed]
- Mironov, V.; Visconti, R.P.; Kasyanov, V.; Forgacs, G.; Drake, C.J.; Markwald, R.R. Organ printing: Tissue spheroids as building blocks. Biomaterials 2009, 30, 2164–2174. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Maire, M.; Lerouge, S.; Therriault, D.; Heuzey, M.-C. 3D Printing of Microstructured and Stretchable Chitosan Hydrogel for Guided Cell Growth. Adv. Biosyst. 2017, 1, 1700058. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Chen, X.; Ismail, A.F.; Aziz, M.; Abdolahi, E.; Mahmoodiyan, F. Improved antibacterial properties of an Mg-Zn-Ca alloy coated with chitosan nanofibers incorporating silver sulfadiazine multiwall carbon nanotubes for bone implants. Polym. Adv. Technol. 2019, 30, 1333–1339. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Ismail, A.F.; Aziz, M.; Akbari, M.; Hadisi, Z.; Khoshnava, S.M.; Pagan, E.; Chen, X. Co-incorporation of graphene oxide/silver nanoparticle into poly-L-lactic acid fibrous: A route toward the development of cytocompatible and antibacterial coating layer on magnesium implants. Mater. Sci. Eng. C 2020, 111, 110812. [Google Scholar] [CrossRef]
- Hadisi, Z.; Farokhi, M.; Bakhsheshi-Rad, H.R.; Jahanshahi, M.; Hasanpour, S.; Pagan, E.; Dolatshahi-Pirouz, A.; Zhang, Y.S.; Kundu, S.C.; Akbari, M. Hyaluronic Acid (HA)-Based Silk Fibroin/Zinc Oxide Core–Shell Electrospun Dressing for Burn Wound Management. Macromol. Biosci. 2020, 20. [Google Scholar] [CrossRef] [PubMed]
- Bakhsheshi-Rad, H.R.; Akbari, M.; Ismail, A.F.; Aziz, M.; Hadisi, Z.; Pagan, E.; Daroonparvar, M.; Chen, X. Coating biodegradable magnesium alloys with electrospun poly-L-lactic acid-åkermanite-doxycycline nanofibers for enhanced biocompatibility, antibacterial activity, and corrosion resistance. Surf. Coat. Technol. 2019, 377, 124898. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Ismail, A.F.; Aziz, M.; Hadisi, Z.; Omidi, M.; Chen, X.; Omidi, M. Antibacterial activity and corrosion resistance of Ta2O5 thin film and electrospun PCL/MgO-Ag nanofiber coatings on biodegradable Mg alloy implants. Ceram. Int. 2019, 45, 11883–11892. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Ismail, A.F.; Aziz, M.; Akbari, M.; Hadisi, Z.; Omidi, M.; Chen, X. Development of the PVA/CS nanofibers containing silk protein sericin as a wound dressing: In vitro and in vivo assessment. Int. J. Biol. Macromol. 2020, 149, 513–521. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Hadisi, Z.; Hamzah, E.; Ismail, A.F.; Aziz, M.; Kashefian, M. Drug delivery and cytocompatibility of ciprofloxacin loaded gelatin nanofibers-coated Mg alloy. Mater. Lett. 2017, 207, 179–182. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Ismail, A.F.; Aziz, M.; Akbari, M.; Hadisi, Z.; Daroonparvar, M.; Chen, X. Antibacterial activity and in vivo wound healing evaluation of polycaprolactone-gelatin methacryloyl-cephalexin electrospun nanofibrous. Mater. Lett. 2019, 256, 126618. [Google Scholar] [CrossRef]
- Parham, S.; Kharazi, A.Z.; Bakhsheshi-Rad, H.R.; Ghayour, H.; Ismail, A.F.; Nur, H.; Berto, F. Electrospun Nano-Fibers for Biomedical and Tissue Engineering Applications: A Comprehensive Review. Materials 2020, 13, 2153. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Wang, R.; Wang, M.; Zhong, S.; Ding, L.; Chen, S. Effect of Reconstruction Algorithm on the Identification of 3D Printing Polymers Based on Hyperspectral CT Technology Combined with Artificial Neural Network. Materials 2020, 13, 1963. [Google Scholar] [CrossRef]
- Ziminska, M.; Wilson, J.J.; McErlean, E. Synthesis and Evaluation of a Thermoresponsive DegradableChitosan-Grafted PNIPAAm Hydrogel as a “Smart” Gene Delivery System. Materials 2020, 13, 2530. [Google Scholar] [CrossRef]
- Fafenrot, S.; Grimmelsmann, N.; Wortmann, M.; Ehrmann, A. Three-Dimensional (3D) Printing of Polymer-Metal Hybrid Materials by Fused Deposition Modeling. Materials 2017, 10, 1199. [Google Scholar] [CrossRef]
- Bae, B.; Lee, J.; Cha, J.; Kim, I.-W.; Jung, H.-D.; Yoon, C.-B. Preliminary Characterization of Glass/Alumina Composite Using Laser Powder Bed Fusion (L-PBF) Additive Manufacturing. Materials 2020, 13, 2156. [Google Scholar] [CrossRef] [PubMed]
- Reverte, J.; Caminero, M.Á.; Chacón, J.; García-Plaza, E.; Núñez, P.; Becar, J. Mechanical and Geometric Performance of PLA-Based Polymer Composites Processed by the Fused Filament Fabrication Additive Manufacturing Technique. Materials 2020, 13, 1924. [Google Scholar] [CrossRef] [PubMed]
- Söhling, N.; Neijhoft, J.; Nienhaus, V.; Acker, V.; Harbig, J.; Menz, F.; Ochs, J.; Verboket, R.D.; Ritz, U.; Blaeser, A.; et al. 3D-Printing of Hierarchically Designed and Osteoconductive Bone Tissue Engineering Scaffolds. Materials 2020, 13, 1836. [Google Scholar] [CrossRef]
- Polley, C.; Distler, T.; Detsch, R.; Lund, H.; Springer, A.; Boccaccini, A.R.; Seitz, H. 3D Printing of Piezoelectric Barium Titanate-Hydroxyapatite Scaffolds with Interconnected Porosity for Bone Tissue Engineering. Materials 2020, 13, 1773. [Google Scholar] [CrossRef]
- Baino, F.; Fiume, E. 3D Printing of Hierarchical Scaffolds Based on Mesoporous Bioactive Glasses (MBGs)—Fundamentals and Applications. Materials 2020, 13, 1688. [Google Scholar] [CrossRef]
- Pitjamit, S.; Thunsiri, K.; Nakkiew, W.; Wongwichai, T.; Pothacharoen, P.; Wattanutchariya, W. The Possibility of Interlocking Nail Fabrication from FFF 3D Printing PLA/PCL/HA Composites Coated by Local Silk Fibroin for Canine Bone Fracture Treatment. Materials 2020, 13, 1564. [Google Scholar] [CrossRef]
- Schönherr, J.; Baumgartner, S.; Hartmann, M.; Stampfl, J. Stereolithographic Additive Manufacturing of High Precision Glass Ceramic Parts. Materials 2020, 13, 1492. [Google Scholar] [CrossRef] [PubMed]
- Dorigato, A.; Rigotti, D.; Pegoretti, A. Novel Poly(Caprolactone)/Epoxy Blends by Additive Manufacturing. Materials 2020, 13, 819. [Google Scholar] [CrossRef] [PubMed]
- De Toro, E.V.; Sobrino, J.C.; Martínez, A.M.; Eguía, V.M.; Perez, J.A. Investigation of a Short Carbon Fibre-Reinforced Polyamide and Comparison of Two Manufacturing Processes: Fused Deposition Modelling (FDM) and Polymer Injection Moulding (PIM). Materials 2020, 13, 672. [Google Scholar] [CrossRef]
- Wibowo, A.; Vyas, C.; Cooper, G.; Qulub, F.; Suratman, R.; Mahyuddin, A.I.; Dirgantara, T.; Fernandes, P.R. 3D Printing of Polycaprolactone–Polyaniline Electroactive Scaffolds for Bone Tissue Engineering. Materials 2020, 13, 512. [Google Scholar] [CrossRef]
- Wang, Y.; Müller, W.-D.; Rumjahn, A.; Schwitalla, A. Parameters Influencing the Outcome of Additive Manufacturing of Tiny Medical Devices Based on PEEK. Materials 2020, 13, 466. [Google Scholar] [CrossRef]
- Wang, Z.; Florczyk, S.J. Freeze-FRESH: A 3D Printing Technique to Produce Biomaterial Scaffolds with Hierarchical Porosity. Materials 2020, 13, 354. [Google Scholar] [CrossRef]
- Barrios-Muriel, J.; Romero-Sánchez, F.; Alonso-Sánchez, F.J.; Salgado, D.R. Advances in Orthotic and Prosthetic Manufacturing: A Technology Review. Materials 2020, 13, 295. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Hosny, A.; Keating, S.; Dilley, J.D.; Ripley, B.; Kelil, T.; Pieper, S.; Kolb, D.; Bader, C.; Pobloth, A.-M.; Griffin, M.; et al. From Improved Diagnostics to Presurgical Planning: High-Resolution Functionally Graded Multimaterial 3D Printing of Biomedical Tomographic Data Sets. 3D Print. Addit. Manuf. 2018, 5, 103–113. [Google Scholar] [CrossRef]
- Amirpour, M.; Bickerton, S.; Calius, E.; Das, R.; Mace, B. Numerical and experimental study on deformation of 3D-printed polymeric functionally graded plates: 3D-Digital Image Correlation approach. Compos. Struct. 2019, 211, 481–489. [Google Scholar] [CrossRef]


| 3D Printing Method | Materials | Device Components | Manufacturing Process | Advantage | Disadvantage | Ref. |
|---|---|---|---|---|---|---|
| Stereolithography (SLA); Bottom-up SLA; Top-down SLA | A resin with photo-active monomers | Laser, Vat of resin, UV light, Platform | The SLA technique is classified to top-down and bottom-up (based on build platform movement and laser motion). The laser is utilized for initiating photopolymerization and converting liquid resin to solid shape via photocuring process. | Various applications, Printing living tissues, Having the highest resolution among other printing methods, SLA has the ability of making structures with a resolution of 20 μm or less, which is the highest resolution among other printing methods (with resolution of 50-200 μm) | Lack of monolithic mechanical structure due to layer by layer fabrication process. Time consuming process caused by low photopoly-merization rates | [18,25] |
| PBF (SLS, SLM, 3DP) | Metals and alloys, Limited polymers, Ceramic | Laser, Powder roller, Powder bed, Powder stock, Platform | The working method is to spray powder materials on the previous layers and laser is utilized for fusing powders together. | Good resolution, High quality | Slow printing rate, Expensive process, High porosity | [20,22] |
| Binder Jetting or indirect 3D printing | Metals, Polymers, Ceramics | Powder roller, Powder stock, Build Platform, Powder bed, Binder cartridge, and inkjet print head. | The binder jetting techniques is used for powders and powder layers binds together with adhesive. The powder is sprayed on the platform via roller. The head of print sprinkled the adhesive on top of the powder according to the structure designed by the computer. The platform comes down by the thickness of object’s layer. Next layer is made by spraying powder on the previous layer. The object is fabricated via powder and the liquid bounding. | Ceramics has more challenges to use by additive manufacturing than polymers and metals technologies due to high melting temperature; Hence, binder jetting can be a promising method to fabricate ceramic based materials. Cost effective, No shrinkage | Low mechanical properties | [24,47] |
| Sheet Lamination | Metals (aluminum, copper, stainless steel and titanium), Ceramic, and Composite | Laser, Platform, Mirror, Material spool, Cross hatched material, Support material, | UAM and LOM are two strategies of sheet lamination. The material is placed on platform and bonded to the previous layer by the adhesive materials. The designed structure is cut from the layer via laser. Then next layer is made. | Low shrinkage and residual stresses, Quick process | Difficulty of precision in the Z-dimension control, Lack of mechanical homogeneity in products because of utilizing adhesive in fabrication process | [21] |
| Directed Energy Deposition | A resin with photo-active monomers, Hybrid polymer-ceramics, Metals and alloys in the form of powder or wire, Ceramics and polymers | Electron beam, Metal Wire supply, Metal wire, Platform | The powder or wire is placed in the pool of melt which is glued to a lower part or layers via source of energy (laser or electron beam). | Cost effective and quick process, Favorable mechanical properties, Control on microstructure | Low accuracy and surface quality, Restrictions on printing complex geometric shapes with precise details | [19] |
| Material extrusion FDM and FFF | Plastics, Polymers | Material spool, Heater element, Nozzle, Heater element | Thermoplastic materials are melted and extruded and create layers by moving the nozzle according to the computer design. | Ease of use, Suitable mechanical properties | Filament required, Restriction of raw materials, Inability to print live cells | [26] |
| Material jetting | Plastics, Polymers | UV light, Elevator, Platform, | The MJM mechanism of action is similar to ink jet printer. Material jetting on platform is done (drop or continuous) | High accuracy, Low waste of material | Restriction of raw materials: polymers and waxes, Required support material | [48,49] |
| Biobased-Material | 3D Printing Method | Solvent | Printed Structure | Porosity, Pore Size | Mechanical Properties | Cellular Assay | Cell Type | Target Tissue | Ref |
|---|---|---|---|---|---|---|---|---|---|
| CS, PCL-DA and PEG-DA | RDMAM system | Benzene, acetone and acetic acid. | Multi-layer scaffolds | Pore size = 300 μm | PCL-DA/PEG-DA/CS 5% tensile strength = 0.75 ± 0.05, PCL-DA/PEG-DA/CS 10% tensile strength = 0.53 ± 0.04, PCL-DA/PEG-DA/CS 15% tensile strength= 0.29 ± 0.09 Elastic Modulus= 14.97 ± 3.99 kPa | Well cell viability and proliferation | L929 cells | TE | [16] |
| CS (6% w/v) and CS modified with raffinose | FDM | 2% acetic acid | 3D scaffolds | Feret diameter: scaffold without raffinose 10 ± 20 μm; scaffold with raffinose 3.5 ± 3 μm | - | Well cell adhesion and proliferation | Fibroblasts | Soft tissue engineering | [51] |
| PLA, CS and Maleic anhydride-grafted PLA (PLA-g-MA) | An extruder (by heating and melting) | - | (3D) printing strips | - | Tensile strength of PLA-g-MA/CS (20 wt%) ≈ 52 | Well cell viability | Human foreskin fibroblasts | Biomedical material | [73] |
| CS, Gel and HA | FDM | 2% acetic acid | 3D scaffolds | Pore size ≈ 200–500 µm | - | Well cell viability and proliferation | MC3T3-E1 cells | BTE | [81] |
| AL, AL-HA, CS, CS-HA | The Fab@Home™ (The Seraph Robotics) open source RP platform Model | PBS, 0.1 M acetic acid | Scaffolds with disc shape (6 mm diameter × 1 mm thickness) | Average pore size of pure CS ≈ 200 μm and CS-HA ≈ 100 μm | - | Well cell viability, proliferation and osteogenic differentiation | MC3T3-E1 pre-osteoblast | BTE | [82] |
| CS, HA | Z-Corp, Z-510 Solvent/dispensing | Lactic acid, citric acid, acetic acid | 3D scaffolds | Porosity = 37.1% | Compressive strength = 16.32 ± 2.8 MPa Elastic Modulus = 4.4 ± 2.1 GPa | - | - | BTE | [83] |
| CS, calcium phosphate | Robocasting | Acetic acid | 3D scaffolds | Porosity = 22% | - | - | - | Filler for large bone defects | [84] |
| PCL, CS | FDM | 0.1 M acetic acid | 3D scaffolds | PCL/CS porosity = 62.4 ± 0.23% The pore size of PCL scaffolds = 325.2 ± 26.3 μm | Compressive strength ≈ 6.7 MPa | Well Cell viability, Proliferation and expressions of Osteogenic gene | Rabbit BMMSCs | BTE | [85] |
| PLLA, CS and bioactive Qu, PDA | 3D printer (MakerBot Replicator Z18) via a FDM) | 0.1% (v/v) acetic acid aqueous solution | Cylindrical scaffolds | - | Compressive strength of PLLA/CS-D/Qu ≈ 15 MPa and elastic modulus ≈ 0.140 GPa (dry condition) | Well cell attachment, osteogenic activity and good anti-inflammatory feature | MC3T3-E1 cell | BTE | [86] |
| CS, PVA and various ratio of HA (2.5, 5, 10, and 15 wt %) And BMP-2 | Pushing of Hydrogel from the syringe (by computer controlling) and spraying the crosslinking agent | Acetic acid, distilled water | 3D scaffolds | Pore size = 800 to 1300 μm | Elastic modulus of CS/PVA containing 15 wt% HA ≈ 91.14 MPa | Well cell viability and adhesion | hMSCs | BTE | [92] |
| MAG-Lp, MAC-Lp | Robocast-assisted deposition system | Acetic acid | 3D scaffolds | Average pore size = 389 ± 58 µm based on horizontal, 385 ± 38 µm based on vertical for MAC-Lp. | Compressive strength ≈ 14–15 MPa for MAC-Lp | Enhanced osteoblast growth and biomineral formation | MC3T3-E1 | Osteoblast growth | [93] |
| PLA, CS and HA | FDM | 0.36% of acetic acid | 3D scaffolds | Very large pore diameter ≈ 960 ± 50 mm, Porosity ≈ 60% | PLA/CS-HA modulus = 16.4 ± 2.5 MPa | Well cell viability and osteogenic differentiation | hMSCs | BTE | [94] |
| CS, HA | Robotic dispensing System Solvent/dispensing | Acetic acid/NaOH ethanol | 3D scaffolds | Macropore = 400–1000 µm for CS scaffolds, macropore size = 200–400 µm for the CS–HA scaffolds | - | Well cell adhesion and distribution | Osteoblasts | BTE | [96] |
| CS, nBA | Robocasting | Acetic acid | 3D scaffolds | Macro structure (hundreds of micrometers) and highly micro-pore = a few to 10 μm | - | Well cell adhesion and spread | MC3T3-E1 preosteoblastic cells | BTE | [97] |
| CS scaffolds + IPFP-ASCs + TGFb3 and BMP6 | Extrusion printed onto a glass slide, immersion in bath of isopropyl alcohol. | Acetic acid | Scaffolds | - | - | A shiny cartilage-like tissue ‘cap’, positive staining of collagen I, II and cartilage proteoglycans | IPFP-ASCs | Osteochondral graft | [107] |
| Resin, CS and PEGDA | Stereolithography | 1% acetic acid | 3D printed ear scaffold | Pore size ≈ 50 µm | Elastic modulus ≈ 400 kPa | Long term cell viability and spreading | hMSCs | Complex tissue geometries, such as human ear | [108] |
| CS, AL | Uprint, Z402 | Acetic acid | 3D scaffolds | Pore size ≈ 100 μm pores | - | Improvement of cell suspension uptake | Mouse bone marrow stromal cells | CTE | [109] |
| Col, CS | A 3D bioprinter | 1% acetic acid | 3D scaffolds | Porosity = 83.5% pore size 60–200 μm | Compressive | Implementing | NSCs were obtained from embryonic brains at day 14 | SCI | [118] |
| strength of 3D-Col/CS = 345.20, 29.60 KPa and Compressive modulus = 3.82 ± 0.25 MPa | 3D-C/C scaffold enhanced the number of biotin dextran amine fibers and led to smaller cavity and a more linear-ordered structure | ||||||||
| CS-g-oligo (L,L-lactide) copolymer and PEGDA as a cross linker | Two-photon-induced micro stereolithography | 3 vol.% acetic acid | A truncated cylinder scaffolds | - | - | A high survival rate of cortical neurons and the formation of neural networks | Dissociated rat cortical neurons | NTE | [119] |
| CS, laminin | DBRP | Acetic acid | 3D nerve conduit scaffolds | - | - | Laminin improves the viability of neurons grown and the length of neurite growth | Adult DRG neurons | NTE | [120] |
| Al, CMC and agarose | Direct write printing (Extrusion-based-3DBioplotter System) | PBS | 3D scaffolds | - | - | Well hNSC expansion and differentiation | hNSC | NTE | [121] |
| CS, GE as a cross linker, GLY and PEG as plasticizer | A 3D printer with jet dispenser | 0.5% v/v acetic acid | Film | - | - | Well cell viability | Human skin fibroblast cell | Chronic wound healing | [122] |
| CS, PEC | Extrusion-based 3D printing | 0.1M HCl | A mesh scaffold model | - | Self-adhesion to skin with bioadhesion strength in the range of 86.5–126.9 g | - | - | Wound healing, local LDC release | [123] |
| Polyelectrolyte Gel, CS | A 3D bioprinter, (extrusion-based print-head) | Acetic acid, PBS solution | A 3-layered grid-like patterns | - | - | Well cell viability and proliferation, spindle-like morphology | Fibroblast skin cells (HFF-1) | STE | [137] |
| Polyelectrolyte CS, Gel | A 3D bioprinter, Biofactory | CS in acetic acid, gelatin in PBS | Multi-layered hydrogel construct | - | - | Well cell viability and proliferation, spindle-like morphology, | Naonatal human foreskin fibroblasts (HFF-1) | STE | [138] |
| CS | FDM | Acetic acid 2% (v/v) containing D-(+) raffinose pentahydrate | 3D scaffolds with grid of orthogonal filament | Pore size ranges = from 4 to 9 μm | - | An early skin-like layer consisting of fibroblast and keratinocyte | Human fibroblast (Nhdf) and keratinocyte (HaCaT) | STE | [139] |
| PCL, CS | Materials extrusion, (by melting materials) | - | Vessel-like scaffolds | - | Elastic modulus for PCL/7 wt%CS/5 wt%H = 174 MPa | Well cell viability and growth | HUVEC cell | Cardiovascular diseases | [143] |
| Al, CS | A single arm robotic printing | Deionized water, 1.0 M acetic acid | Channels in form of hollow tubes | - | Maximum tensile stress = 5.65 ± 1.78 kPa and Young’s modulus = 5.91 ± 1.12 kPa | Well cell viability | CPCs | Vascular networks | [144] |
| CS and Gel hybrid, glutaraldehyde as a cross linker | Combining rapid prototyping, microreplication and freeze–drying | 1 wt% acetic acid | 3D scaffolds | Porosity = 90–95%, pore size = 100 µm | Compressive strength ≈ 264 ± 10.1 KPa | Well hepatocyte attachment and viability ≈ a bove 90% Well albumin secretion and urea synthesis | Hepatocytes | HTE | [152] |
| Col, CS | A bioprinter with two syringes | 0.10 M acetic acid | Meshes design | Square holes of 4 mm on each side | - | No cell morphology change, Non-cellular toxicity | NIH/3T3 fibroblasts monolayers | TE | [154] |
| CS | Extrusion-based 3D printing | Acidic mixture (40 vol% acetic acid, 20 vol% lactic acid, 40 vol% distilledwater | 30-layer scaffolds, starfish, leaf, and spider shapes | Pore size ≈ 220 µm | Maximum tensile strength ≈ 97 MPa (dry condition) and high strain at break ~360% in the wet condition | - | - | Inks for 3D Printing, tissue engineering, drug delivery | [155] |
| BMSCs-laden Gel, sodium alginate and CMC | Micro extrusion-based 3D printer equipped with z-axis-controlled ink reservoirs | water | 3D scaffolds | - | Young modulus ≈ 120 MPa | Well cell viability | BMSCs | TE | [156] |
| CS | Direct printing of chitosan ink in air (Extrusion-based method) and partial hardening via solvent evaporation | Acidic mixture: 40 vol% acetic acid, 10 vol% lactic acid, and 3 wt% citric acid). | 3D scaffolds | Microfiber networks, pore size ≈ 220 μm | Tensile strength ≈ 7.5 MPa | Well cell Survival and proliferation | L929 fibroblasts | Biomedical materialS | [161] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pahlevanzadeh, F.; Emadi, R.; Valiani, A.; Kharaziha, M.; Poursamar, S.A.; Bakhsheshi-Rad, H.R.; Ismail, A.F.; RamaKrishna, S.; Berto, F. Three-Dimensional Printing Constructs Based on the Chitosan for Tissue Regeneration: State of the Art, Developing Directions and Prospect Trends. Materials 2020, 13, 2663. https://doi.org/10.3390/ma13112663
Pahlevanzadeh F, Emadi R, Valiani A, Kharaziha M, Poursamar SA, Bakhsheshi-Rad HR, Ismail AF, RamaKrishna S, Berto F. Three-Dimensional Printing Constructs Based on the Chitosan for Tissue Regeneration: State of the Art, Developing Directions and Prospect Trends. Materials. 2020; 13(11):2663. https://doi.org/10.3390/ma13112663
Chicago/Turabian StylePahlevanzadeh, Farnoosh, Rahmatollah Emadi, Ali Valiani, Mahshid Kharaziha, S. Ali Poursamar, Hamid Reza Bakhsheshi-Rad, Ahmad Fauzi Ismail, Seeram RamaKrishna, and Filippo Berto. 2020. "Three-Dimensional Printing Constructs Based on the Chitosan for Tissue Regeneration: State of the Art, Developing Directions and Prospect Trends" Materials 13, no. 11: 2663. https://doi.org/10.3390/ma13112663
APA StylePahlevanzadeh, F., Emadi, R., Valiani, A., Kharaziha, M., Poursamar, S. A., Bakhsheshi-Rad, H. R., Ismail, A. F., RamaKrishna, S., & Berto, F. (2020). Three-Dimensional Printing Constructs Based on the Chitosan for Tissue Regeneration: State of the Art, Developing Directions and Prospect Trends. Materials, 13(11), 2663. https://doi.org/10.3390/ma13112663








