Adsorption and Release of Growth Factors from Four Different Porcine-Derived Collagen Matrices
Abstract
1. Introduction
2. Materials and Methods
2.1. Porcine-Derived Collagen Matrices and Their Exposure to EMD and Recombinant Growth Factors
2.2. ELISA Quantification of Protein Adsorption to Collagen Matrices
2.3. Analysis of Protein Release Kinetics from Collagen Matrices over Time
2.4. Statistical Analysis
3. Results
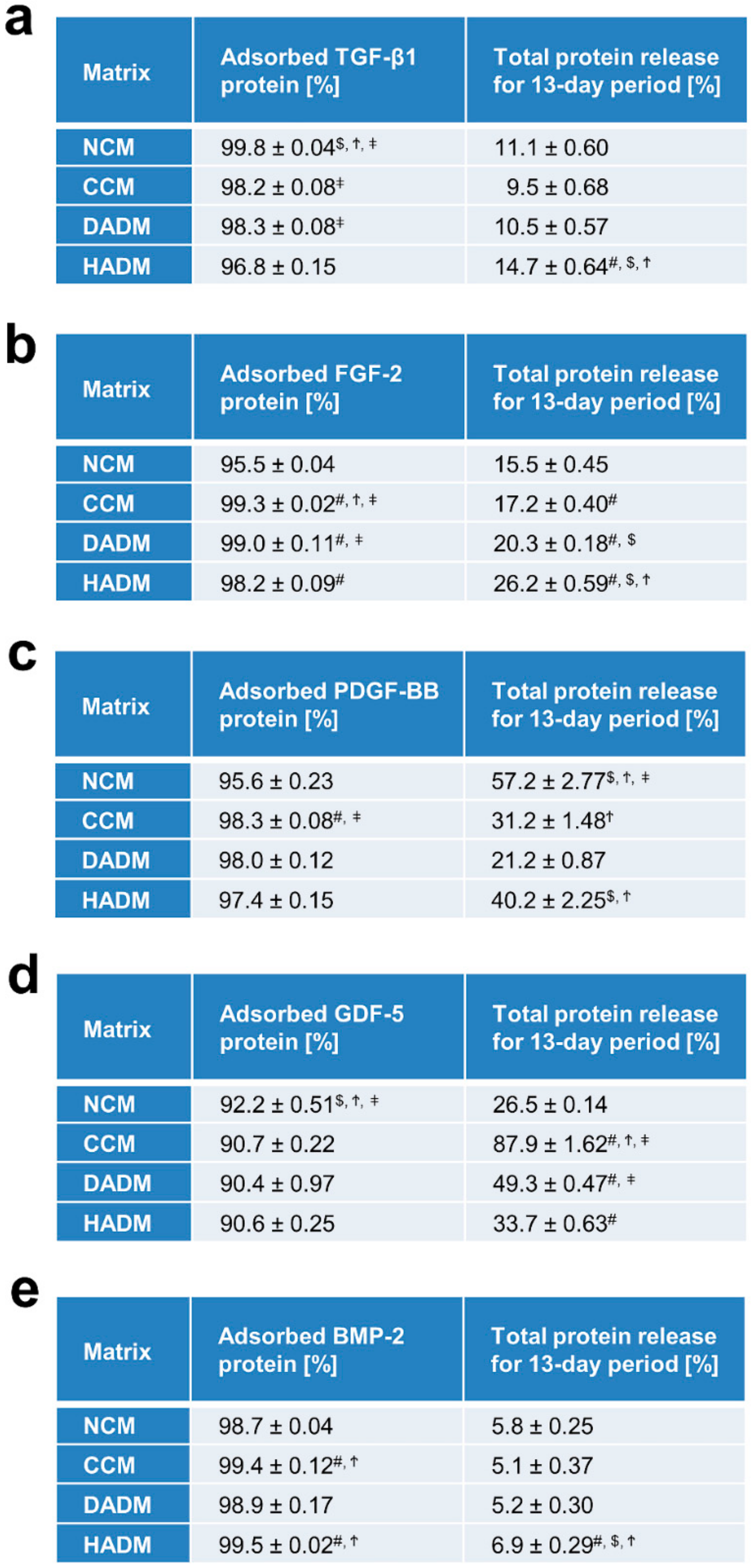
3.1. Adsorption Rate and Total Protein Release from Collagen Matrices Over 13-Day Period
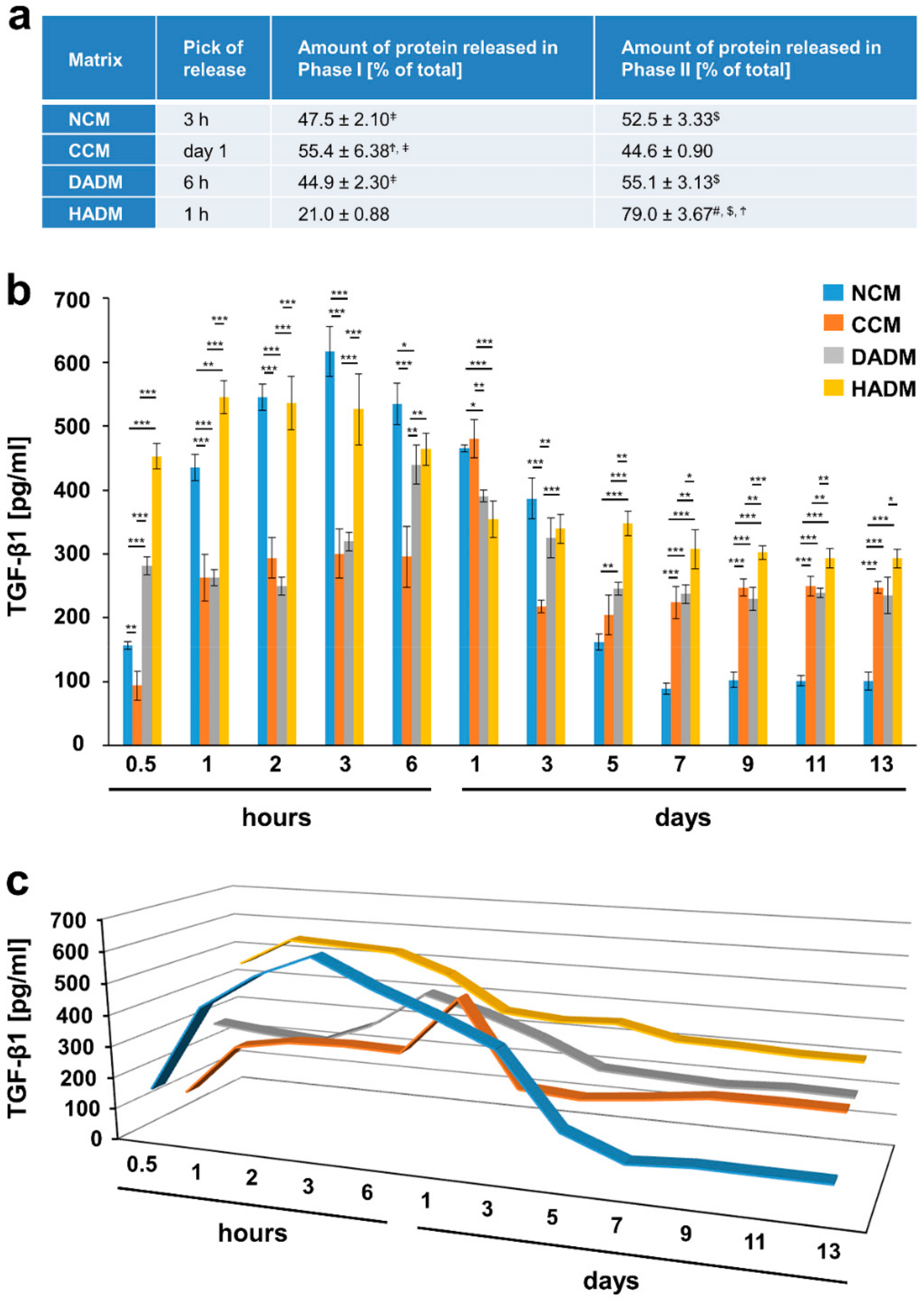
3.2. Detailed Analysis of the Release of TGF-β1 from Recombinant TGF-β1- or EMD-Coated Collagen Matrices over Time
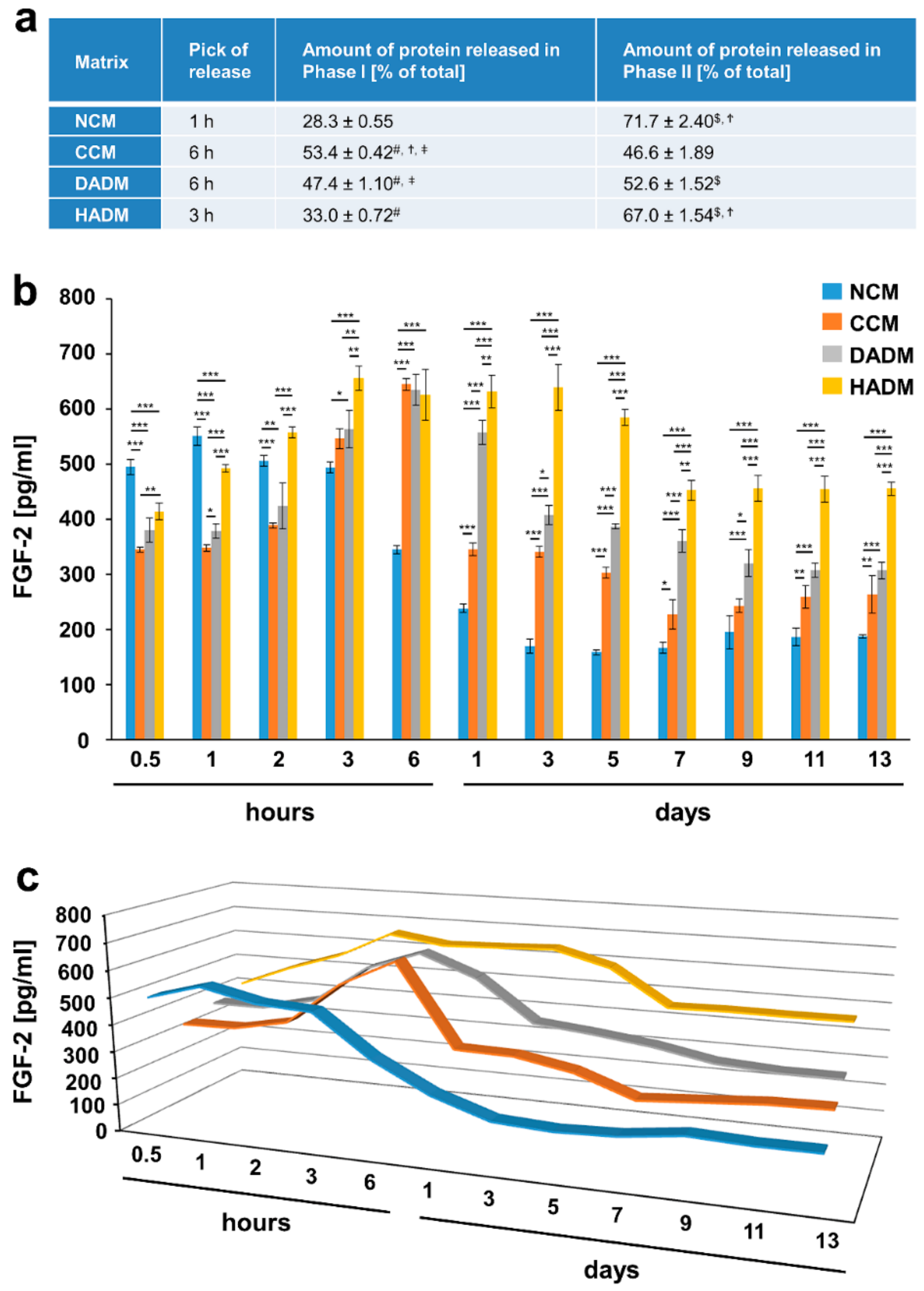
3.3. Detailed Analysis of the Release of FGF-2 from Recombinant FGF-2-Coated Collagen Matrices over Time
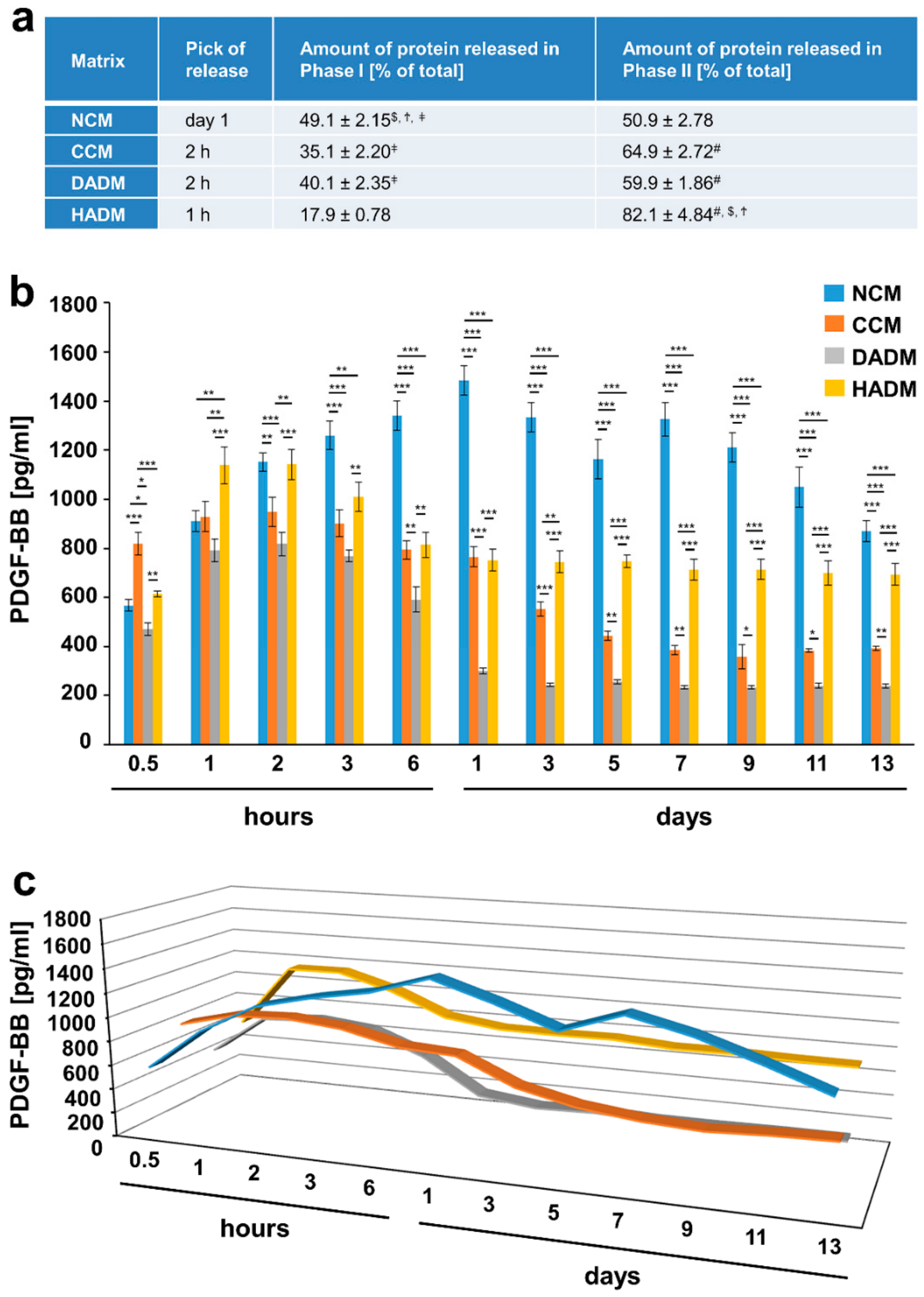
3.4. Detailed Analysis of the Release of PDGF-BB from Recombinant PDGF-BB-Coated Collagen Matrices over Time
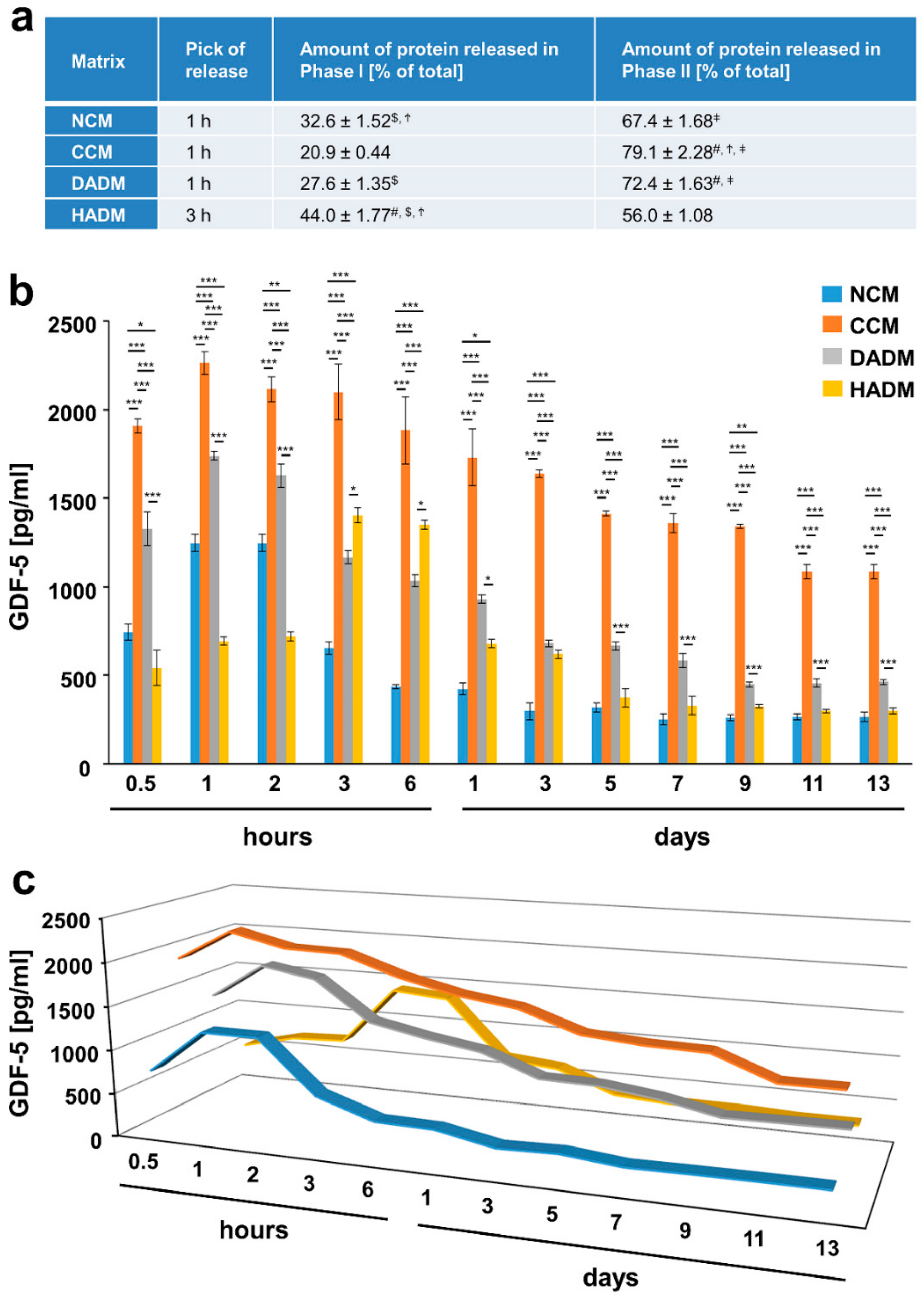
3.5. Detailed Analysis of the Release of GDF-5 from Recombinant GDF-5-Coated Collagen Matrices over Time
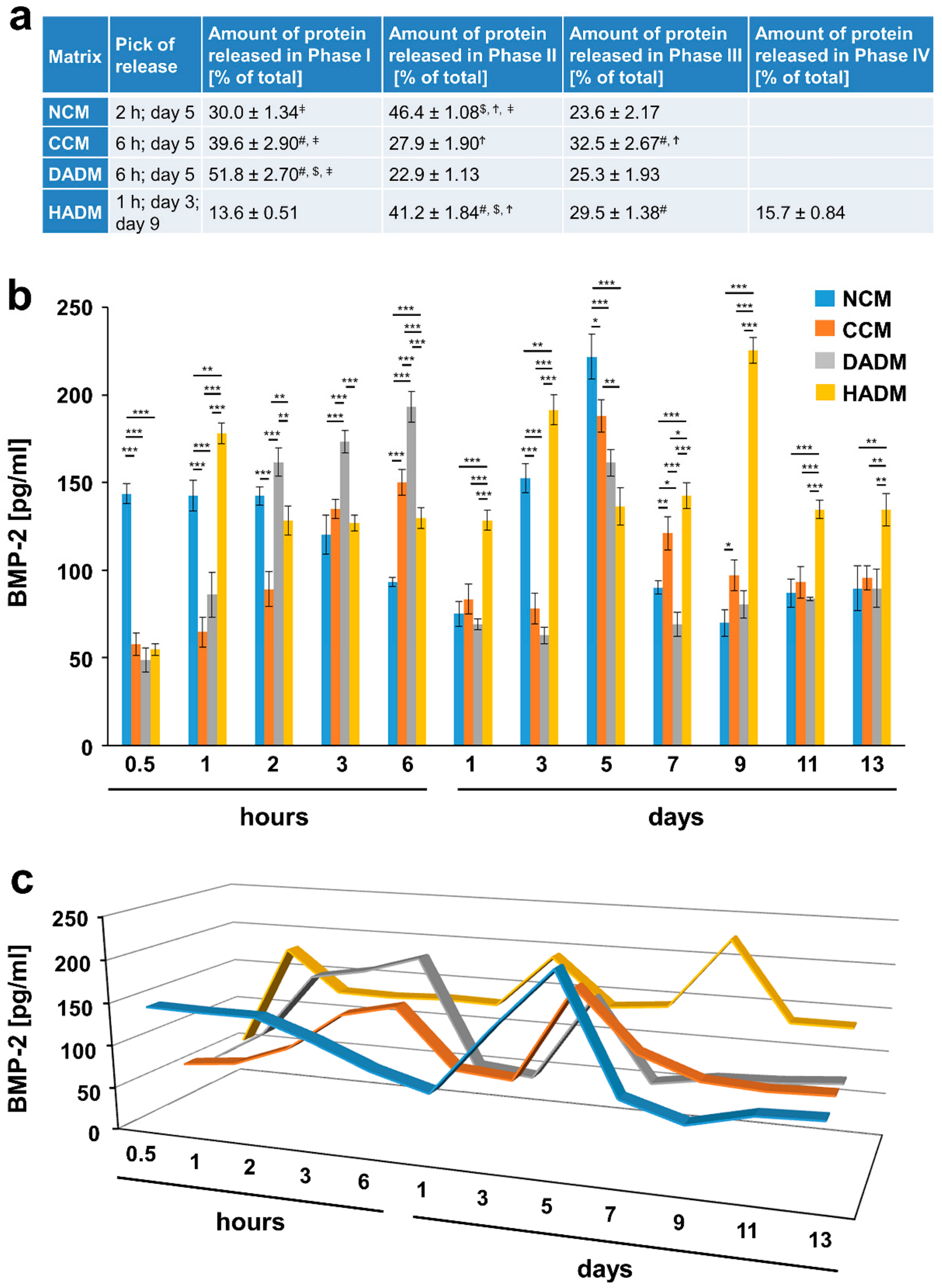
3.6. Detailed Analysis of the Release of BMP-2 from Recombinant BMP-2-coated Collagen Matrices over Time
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thoma, D.; Benić, G.; Zwahlen, M.; Hämmerle, C.; Jung, R. A systematic review assessing soft tissue augmentation techniques. Clin. Oral Implant. Res. 2009, 20, 146–165. [Google Scholar] [CrossRef]
- Tavelli, L.; McGuire, M.K.; Zucchelli, G.; Rasperini, G.; Feinberg, S.E.; Wang, H.-L.; Giannobile, W.V. Extracellular matrix-based scaffolding technologies for periodontal and peri-implant soft tissue regeneration. J. Periodontol. 2020, 91, 17–25. [Google Scholar] [CrossRef]
- Liang, Y.; Luan, X.; Liu, X. Recent advances in periodontal regeneration: A biomaterial perspective. Bioact. Mater. 2020, 5, 297–308. [Google Scholar] [CrossRef]
- Moharamzadeh, K.; Brook, I.M.; Van Noort, R.; Scutt, A.M.; Thornhill, M.H. Tissue-engineered Oral Mucosa: A Review of the Scientific Literature. J. Dent. Res. 2007, 86, 115–124. [Google Scholar] [CrossRef]
- Minuth, W.W.; Sittinger, M.; Kloth, S. Tissue engineering: Generation of differentiated artificial tissues for biomedical applications. Cell Tissue Res. 1997, 291, 1–11. [Google Scholar] [CrossRef]
- Wallace, D.G.; Rosenblatt, J. Collagen gel systems for sustained delivery and tissue engineering. Adv. Drug Deliv. Rev. 2003, 55, 1631–1649. [Google Scholar] [CrossRef]
- Coomes, A.M.; Mealey, B.L.; Huynh, B.G.; Barboza, A.C.; Moore, W.S.; Cochran, D.L. Buccal bone formation after flapless extraction: A randomized, controlled clinical trial comparing recombinant human bone morphogenetic protein 2/absorbable collagen carrier and collagen sponge alone. J. Periodontol. 2014, 85, 525–535. [Google Scholar] [CrossRef]
- Sculean, A.; Nikolidakis, D.; Nikou, G.; Ivanovic, A.; Chapple, I.L.C.; Stavropoulos, A. Biomaterials for promoting periodontal regeneration in human intrabony defects: A systematic review. Periodontol. 2000 2015, 68, 182–216. [Google Scholar] [CrossRef]
- Ma, L.; Gao, C.; Mao, Z.; Zhou, J.; Shen, J.; Hu, X.; Han, C. Collagen/chitosan porous scaffolds with improved biostability for skin tissue engineering. Biomaterials 2003, 24, 4833–4841. [Google Scholar] [CrossRef]
- Ghanaati, S.; Schlee, M.; Webber, M.; Willershausen, I.; Barbeck, M.; Balic, E.; Görlach, C.; Stupp, S.; Sader, R.; Kirkpatrick, C. Evaluation of the tissue reaction to a new bilayered collagen matrix in vivo and its translation to the clinic. Biomed. Mater. 2011, 6, 015010. [Google Scholar] [CrossRef]
- Pabst, A.M.; Happe, A.; Callaway, A.; Ziebart, T.; Stratul, S.I.; Ackermann, M.; Konerding, M.A.; Willershausen, B.; Kasaj, A. In vitro and in vivo characterization of porcine acellular dermal matrix for gingival augmentation procedures. J. Periodontal. Res. 2014, 49, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Kasaj, A.; Levin, L.; Stratul, S.-I.; Götz, H.; Schlee, M.; Rütters, C.B.; Konerding, M.A.; Ackermann, M.; Willershausen, B.; Pabst, A.M. The influence of various rehydration protocols on biomechanical properties of different acellular tissue matrices. Clin. Oral Investig. 2016, 20, 1303–1315. [Google Scholar] [CrossRef]
- Chia-Lai, P.-j.; Orlowska, A.; Al-Maawi, S.; Dias, A.; Zhang, Y.; Wang, X.; Zender, N.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. Sugar-based collagen membrane cross-linking increases barrier capacity of membranes. Clin. Oral Investig. 2018, 22, 1851–1863. [Google Scholar] [CrossRef] [PubMed]
- Sculean, A.; Mihatovic, I.; Shirakata, Y.; Bosshardt, D.D.; Schwarz, F.; Iglhaut, G. Healing of localized gingival recessions treated with coronally advanced flap alone or combined with either a resorbable collagen matrix or subepithelial connective tissue graft. A preclinical study. Clin. Oral Investig. 2015, 19, 903–909. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.R.O.; Santamaria, M.P.; Silvério, K.G.; Casati, M.Z.; Nociti Junior, F.H.; Sculean, A.; Sallum, E.A. Coronally advanced flap with or without porcine collagen matrix for root coverage: A randomized clinical trial. Clin. Oral Investig. 2016, 20, 2539–2549. [Google Scholar] [CrossRef]
- Rotundo, R.; Genzano, L.; Patel, D.; D’Aiuto, F.; Nieri, M. Adjunctive benefit of a xenogenic collagen matrix associated with coronally advanced flap for the treatment of multiple gingival recessions: A superiority, assessor-blind, randomized clinical trial. J. Clin. Periodontol. 2019, 46, 1013–1023. [Google Scholar] [CrossRef]
- Fischer, K.R.; Fickl, S.; Mardas, N.; Bozec, L.; Donos, N. Stage-two surgery using collagen soft tissue grafts: Clinical cases and ultrastructural analysis. Quintessence Int. 2014, 45, 853–860. [Google Scholar]
- Schmitt, C.M.; Moest, T.; Lutz, R.; Wehrhan, F.; Neukam, F.W.; Schlegel, K.A. Long-term outcomes after vestibuloplasty with a porcine collagen matrix (Mucograft®) versus the free gingival graft: A comparative prospective clinical trial. Clin. Oral Implant. Res. 2016, 27, e125–e133. [Google Scholar] [CrossRef]
- De Resende, D.R.B.; Greghi, S.L.A.; Siqueira, A.F.; Benfatti, C.A.M.; Damante, C.A.; Ragghianti Zangrando, M.S. Acellular dermal matrix allograft versus free gingival graft: A histological evaluation and split-mouth randomized clinical trial. Clin. Oral Investig. 2019, 23, 539–550. [Google Scholar] [CrossRef]
- Gruber, R.; Karreth, F.; Kandler, B.; Fuerst, G.; Rot, A.; Fischer, M.B.; Watzek, G. Platelet-released supernatants increase migration and proliferation, and decrease osteogenic differentiation of bone marrow-derived mesenchymal progenitor cells under in vitro conditions. Platelets 2004, 15, 29–35. [Google Scholar] [CrossRef]
- Mozgan, E.-M.; Edelmayer, M.; Janjić, K.; Pensch, M.; Fischer, M.B.; Moritz, A.; Agis, H. Release kinetics and mitogenic capacity of collagen barrier membranes supplemented with secretome of activated platelets—The in vitro response of fibroblasts of the periodontal ligament and the gingiva. BMC Oral Health 2017, 17, 66. [Google Scholar] [CrossRef]
- Lieberman, J.R.; Daluiski, A.; Einhorn, T.A. The Role of Growth Factors in the Repair of Bone: Biology and Clinical Applications. J. Bone Jt. Surg. Am. 2002, 84, 1032–1044. [Google Scholar] [CrossRef]
- Paralkar, V.M.; Vukicevic, S.; Reddi, A.H. Transforming growth factor β type 1 binds to collagen IV of basement membrane matrix: Implications for development. Dev. Biol. 1991, 143, 303–308. [Google Scholar] [CrossRef]
- Wang, A.Y.; Leong, S.; Liang, Y.-C.; Huang, R.C.C.; Chen, C.S.; Yu, S.M. Immobilization of growth factors on collagen scaffolds mediated by polyanionic collagen mimetic peptides and its effect on endothelial cell morphogenesis. Biomacromolecules 2008, 9, 2929–2936. [Google Scholar] [CrossRef] [PubMed]
- Spagnoli, D.; Choi, C. Extraction socket grafting and buccal wall regeneration with recombinant human bone morphogenetic protein-2 and acellular collagen sponge. Atlas Oral Maxillofac. Surg. Clin. N. A. 2013, 21, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Stähli, A.; Miron, R.J.; Bosshardt, D.D.; Sculean, A.; Gruber, R. Collagen membranes adsorb the transforming growth factor-β receptor I kinase-dependent activity of enamel matrix derivative. J. Periodontol. 2016, 87, 1–14. [Google Scholar] [CrossRef]
- Friess, W.; Uludag, H.; Foskett, S.; Biron, R.; Sargeant, C. Characterization of absorbable collagen sponges as recombinant human bone morphogenetic protein-2 carriers. Int. J. Pharm. 1999, 185, 51–60. [Google Scholar] [CrossRef]
- Geiger, M.; Li, R.H.; Friess, W. Collagen sponges for bone regeneration with rhBMP-2. Adv. Drug Deliv. Rev. 2003, 55, 1613–1629. [Google Scholar] [CrossRef]
- Sarban, S.; Senkoylu, A.; Isikan, U.E.; Korkusuz, P.; Korkusuz, F. Can rhBMP-2 containing collagen sponges enhance bone repair in ovariectomized rats?: A preliminary study. Clin. Orthop. Relat. Res. 2009, 467, 3113–3120. [Google Scholar] [CrossRef]
- Howell, T.; Fiorellini, J.; Jones, A.; Alder, M.; Nummikoski, P.; Lazaro, M.; Lilly, L.; Cochran, D. A feasibility study evaluating rhBMP-2/absorbable collagen sponge device for local alveolar ridge preservation or augmentation. Int. J. Periodontics Restor. Dent. 1997, 17, 124–139. [Google Scholar]
- Hempel, U.; Hintze, V.; Möller, S.; Schnabelrauch, M.; Scharnweber, D.; Dieter, P. Artificial extracellular matrices composed of collagen I and sulfated hyaluronan with adsorbed transforming growth factor β1 promote collagen synthesis of human mesenchymal stromal cells. Acta Biomater. 2012, 8, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.-R.; Won, J.E.; Jeon, E.; Lee, S.; Kang, W.; Jo, H.; Jang, J.-H.; Shin, U.S.; Kim, H.-W. Fibroblast growth factors: Biology, function, and application for tissue regeneration. J. Tissue Eng. 2010, 2010, 218142. [Google Scholar] [CrossRef] [PubMed]
- Akita, S.; Akino, K.; Hirano, A. Basic Fibroblast Growth Factor in Scarless Wound Healing. Adv. Wound Care (New Rochelle) 2013, 2, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Hart, C.; Bailey, M.; Curtis, D.; Osborn, S.; Raines, E.; Ross, R.; Forstrom, J. Purification of PDGF-AB and PDGF-BB from human platelet extracts and identification of all three PDGF dimers in human platelets. Biochemistry 1990, 29, 166–172. [Google Scholar] [CrossRef]
- Ozaki, Y.; Nishimura, M.; Sekiya, K.; Suehiro, F.; Kanawa, M.; Nikawa, H.; Hamada, T.; Kato, Y. Comprehensive analysis of chemotactic factors for bone marrow mesenchymal stem cells. Stem. Cells Dev. 2007, 16, 119–130. [Google Scholar] [CrossRef]
- Kelly, J.D.; Raines, E.W.; Ross, R.; Murray, M.J. The B chain of PDGF alone is sufficient for mitogenesis. EMBO J. 1985, 4, 3399–3405. [Google Scholar] [CrossRef]
- Gruber, R.; Karreth, F.; Frommlet, F.; Fischer, M.B.; Watzek, G. Platelets are mitogenic for periosteum-derived cells. J. Orthop. Res. 2003, 21, 941–948. [Google Scholar] [CrossRef]
- Hollinger, J.O.; Hart, C.E.; Hirsch, S.N.; Lynch, S.; Friedlaender, G.E. Recombinant human platelet-derived growth factor: Biology and clinical applications. J. Bone Jt. Surg. Am. 2008, 90, 48–54. [Google Scholar] [CrossRef]
- Young, C.S.; Bradica, G.; Hart, C.E.; Karunanidhi, A.; Street, R.M.; Schutte, L.; Hollinger, J.O. Preclinical Toxicology Studies of Recombinant Human Platelet-Derived Growth Factor-BB Either Alone or in Combination with Beta-Tricalcium Phosphate and Type I Collagen. J. Tissue Eng. 2011, 2010, 246215. [Google Scholar] [CrossRef]
- Solchaga, L.A.; Hee, C.K.; Roach, S.; Snel, L.B. Safety of recombinant human platelet-derived growth factor-BB in Augment(®) Bone Graft. J. Tissue Eng. 2012, 3, 2041731412442668. [Google Scholar] [CrossRef] [PubMed]
- Graham, S.; Leonidou, A.; Lester, M.; Heliotis, M.; Mantalaris, A.; Tsiridis, E. Investigating the role of PDGF as a potential drug therapy in bone formation and fracture healing. Expert Opin. Investig. Drugs 2009, 18, 1633–1654. [Google Scholar] [CrossRef] [PubMed]
- Javed, F.; Al-Askar, M.; Al-Rasheed, A.; Al-Hezaimi, K. Significance of the platelet-derived growth factor in periodontal tissue regeneration. Arch. Oral Biol. 2011, 56, 1476–1484. [Google Scholar] [CrossRef] [PubMed]
- Gruber, R.; Varga, F.; Fischer, M.B.; Watzek, G. Platelets stimulate proliferation of bone cells: Involvement of platelet-derived growth factor, microparticles and membranes. Clin. Oral Implant. Res. 2002, 13, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Sena, K.; Morotome, Y.; Baba, O.; Terashima, T.; Takano, Y.; Ishikawa, I. Gene expression of growth differentiation factors in the developing periodontium of rat molars. J. Dent. Res. 2003, 82, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Moore, Y.R.; Dickinson, D.P.; Wikesjö, U.M.E. Growth/differentiation factor-5: A candidate therapeutic agent for periodontal regeneration? A review of pre-clinical data. J. Clin. Periodontol. 2010, 37, 288–298. [Google Scholar] [CrossRef]
- Emerton, K.B.; Drapeau, S.J.; Prasad, H.; Rohrer, M.; Roffe, P.; Hopper, K.; Schoolfield, J.; Jones, A.; Cochran, D.L. Regeneration of periodontal tissues in non-human primates with rhGDF-5 and beta-tricalcium phosphate. J. Dent. Res. 2011, 90, 1416–1421. [Google Scholar] [CrossRef]
- Gruber, R.M.; Ludwig, A.; Merten, H.-A.; Achilles, M.; Poehling, S.; Schliephake, H. Sinus floor augmentation with recombinant human growth and differentiation factor-5 (rhGDF-5): A histological and histomorphometric study in the Goettingen miniature pig. Clin. Oral Implant. Res. 2008, 19, 522–529. [Google Scholar] [CrossRef]
- Schwarz, F.; Rothamel, D.; Herten, M.; Ferrari, D.; Sager, M.; Becker, J. Lateral ridge augmentation using particulated or block bone substitutes biocoated with rhGDF-5 and rhBMP-2: An immunohistochemical study in dogs. Clin. Oral Implant. Res. 2008, 19, 642–652. [Google Scholar] [CrossRef]
- Weng, D.; Poehling, S.; Pippig, S.; Bell, M.; Richter, E.J.; Zuhr, O.; Hürzeler, M.B. The effects of recombinant human growth/differentiation factor-5 (rhGDF-5) on bone regeneration around titanium dental implants in barrier membrane-protected defects: A pilot study in the mandible of beagle dogs. Int. J. Oral Maxillofac. Implant. 2009, 24, 31–37. [Google Scholar]
- Stavropoulos, A.; Windisch, P.; Gera, I.; Capsius, B.; Sculean, A.; Wikesjö, U.M. A phase IIa randomized controlled clinical and histological pilot study evaluating rhGDF-5/β-TCP for periodontal regeneration. J. Clin. Periodontol. 2011, 38, 1044–1054. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.R.; Wikesjö, U.M.; Park, J.C.; Kim, Y.T.; Bastone, P.; Pippig, S.D.; Kim, C.K. Growth/differentiation factor-5 significantly enhances periodontal wound healing/regeneration compared with platelet-derived growth factor-BB in dogs. J. Clin. Periodontol. 2010, 37, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Bowen-Pope, D.F.; Malpass, T.W.; Foster, D.M.; Ross, R. Platelet-derived growth factor in vivo: Levels, activity, and rate of clearance. Blood 1984, 64, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.C.; Briquez, P.S.; Hubbell, J.A.; Cochran, J.R. Engineering growth factors for regenerative medicine applications. Acta Biomater. 2016, 30, 1–12. [Google Scholar] [CrossRef]
- Cochran, D.L.; Oh, T.J.; Mills, M.P.; Clem, D.S.; McClain, P.K.; Schallhorn, R.A.; McGuire, M.K.; Scheyer, E.T.; Giannobile, W.V.; Reddy, M.S.; et al. A randomized clinical trial evaluating rh-FGF-2/β-TCP in periodontal defects. J. Dent. Res. 2016, 95, 523–530. [Google Scholar] [CrossRef]
- Sangiorgio, J.P.M.; Neves, F.; Rocha Dos Santos, M.; França-Grohmann, I.L.; Casarin, R.C.V.; Casati, M.Z.; Santamaria, M.P.; Sallum, E.A. Xenogenous collagen matrix and/or enamel matrix derivative for treatment of localized gingival recessions: A randomized clinical trial. Part I: Clinical outcomes. J. Periodontol. 2017, 88, 1309–1318. [Google Scholar] [CrossRef]
- Stähli, A.; Bosshardt, D.; Sculean, A.; Gruber, R. Emdogain-regulated gene expression in palatal fibroblasts requires TGF-βRI kinase signaling. PLoS ONE 2014, 9, e105672. [Google Scholar] [CrossRef]
- Neligan, P.C.; Gullane, P.J.; Gilbert, R.W. Functional reconstruction of the oral cavity. World J. Surg. 2003, 27, 856–862. [Google Scholar] [CrossRef]
- Lee, C.H.; Singla, A.; Lee, Y. Biomedical applications of collagen. Int. J. Pharm. 2001, 221, 1–22. [Google Scholar] [CrossRef]
- Trabulsi, M.; Oh, T.J.; Eber, R.; Weber, D.; Wang, H.L. Effect of enamel matrix derivative on collagen guided tissue regeneration-based root coverage procedure. J. Periodontol. 2004, 75, 1446–1457. [Google Scholar] [CrossRef]
- Nevins, M.; Camelo, M.; Nevins, M.L.; Schenk, R.K.; Lynch, S.E. Periodontal regeneration in humans using recombinant human platelet-derived growth factor-BB (rhPDGF-BB) and allogenic bone. J. Periodontol. 2003, 74, 1282–1292. [Google Scholar] [CrossRef] [PubMed]
- Shirakata, Y.; Sculean, A.; Shinohara, Y.; Sena, K.; Takeuchi, N.; Bosshardt, D.D.; Noguchi, K. Healing of localized gingival recessions treated with a coronally advanced flap alone or combined with an enamel matrix derivative and a porcine acellular dermal matrix: A preclinical study. Clin. Oral Investig. 2016, 20, 1791–1800. [Google Scholar] [CrossRef] [PubMed]
- Suárez-López Del Amo, F.; Rodriguez, J.C.; Asa’ad, F.; Wang, H.L. Comparison of two soft tissue substitutes for the treatment of gingival recession defects: An animal histological study. J. Appl. Oral Sci. 2019, 27, e20180584. [Google Scholar] [CrossRef] [PubMed]
- Giannobile, W.V. Periodontal tissue engineering by growth factors. Bone 1996, 19, S23–S37. [Google Scholar] [CrossRef]
- Yang, D.H.; Lee, D.W.; Kwon, Y.D.; Kim, H.J.; Chun, H.J.; Jang, J.W.; Khang, G. Surface modification of titanium with hydroxyapatite-heparin-BMP-2 enhances the efficacy of bone formation and osseointegration in vitro and in vivo. J. Tissue Eng. Regen. Med. 2015, 9, 1067–1077. [Google Scholar] [CrossRef]
- Sculean, A.; Chapple, I.L.C.; Giannobile, W.V. Wound models for periodontal and bone regeneration: The role of biologic research. Periodontol. 2000 2015, 68, 7–20. [Google Scholar] [CrossRef]
- Collin-Osdoby, P.; Rothe, L.; Bekker, S.; Anderson, F.; Huang, Y.; Osdoby, P. Basic fibroblast growth factor stimulates osteoclast recruitment, development, and bone pit resorption in association with angiogenesis in vivo on the chick chorioallantoic membrane and activates isolated avian osteoclast resorption in vitro. J. Bone Min. Res. 2002, 17, 1859–1871. [Google Scholar] [CrossRef]
- Cowan, C.M.; Aalami, O.O.; Shi, Y.Y.; Chou, Y.F.; Mari, C.; Thomas, R.; Quarto, N.; Nacamuli, R.P.; Contag, C.H.; Wu, B.; et al. Bone morphogenetic protein 2 and retinoic acid accelerate in vivo bone formation, osteoclast recruitment, and bone turnover. Tissue Eng. 2005, 11, 645–658. [Google Scholar] [CrossRef]
- Turri, A.; Elgali, I.; Vazirisani, F.; Johansson, A.; Emanuelsson, L.; Dahlin, C.; Thomsen, P.; Omar, O. Guided bone regeneration is promoted by the molecular events in the membrane compartment. Biomaterials 2016, 84, 167–183. [Google Scholar] [CrossRef]
- Rubert, M.; Monjo, M.; Lyngstadaas, S.P.; Ramis, J.M. Effect of alginate hydrogel containing polyproline-rich peptides on osteoblast differentiation. Biomed. Mater. 2012, 7, 055003. [Google Scholar] [CrossRef]
- Ramis, J.M.; Rubert, M.; Vondrasek, J.; Gayà, A.; Lyngstadaas, S.P.; Monjo, M. Effect of enamel matrix derivative and of proline-rich synthetic peptides on the differentiation of human mesenchymal stem cells toward the osteogenic lineage. Tissue Eng. Part A 2012, 18, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Stakkestad, Ø.; Lyngstadaas, S.P.; Vondrasek, J.; Gordeladze, J.O.; Reseland, J.E. Ameloblastin Peptides Modulates the Osteogenic Capacity of Human Mesenchymal Stem Cells. Front. Physiol. 2017, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Perale, G.; Monjo, M.; Ramis, J.M.; Øvrebø, Ø.; Betge, F.; Lyngstadaas, P.; Haugen, H.J. Biomimetic Biomolecules in Next Generation Xeno-Hybrid Bone Graft Material Show Enhanced In Vitro Bone Cells Response. J. Clin. Med. 2019, 8, 2159. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Gomez, M.; Xiao, J.; Perale, G.; Betge, F.; Lyngstadaas, S.P.; Haugen, H.J. Xenohybrid bone graft containing intrinsically disordered proteins shows enhanced in vitro bone formation. ACS Appl. Bio Mater. 2020, 3, 2263–2274. [Google Scholar] [CrossRef]
- Petzold, C.; Monjo, M.; Rubert, M.; Reinholt, F.P.; Gomez-Florit, M.; Ramis, J.M.; Ellingsen, J.E.; Lyngstadaas, S.P. Effect of proline-rich synthetic peptide-coated titanium implants on bone healing in a rabbit model. Int. J. Oral Maxillofac. Implant. 2013, 28, e547–e555. [Google Scholar] [CrossRef]
- Pieper, J.S.; Hafmans, T.; van Wachem, P.B.; van Luyn, M.J.; Brouwer, L.A.; Veerkamp, J.H.; van Kuppevelt, T.H. Loading of collagen-heparan sulfate matrices with bFGF promotes angiogenesis and tissue generation in rats. J. Biomed. Mater. Res. 2002, 62, 185–194. [Google Scholar] [CrossRef]
- Yoon, J.J.; Chung, H.J.; Lee, H.J.; Park, T.G. Heparin-immobilized biodegradable scaffolds for local and sustained release of angiogenic growth factor. J. Biomed. Mater. Res. A 2006, 79, 934–942. [Google Scholar] [CrossRef]
- Nillesen, S.T.; Geutjes, P.J.; Wismans, R.; Schalkwijk, J.; Daamen, W.F.; van Kuppevelt, T.H. Increased angiogenesis and blood vessel maturation in acellular collagen-heparin scaffolds containing both FGF2 and VEGF. Biomaterials 2007, 28, 1123–1131. [Google Scholar] [CrossRef]
- Ho, Y.C.; Mi, F.L.; Sung, H.W.; Kuo, P.L. Heparin-functionalized chitosan-alginate scaffolds for controlled release of growth factor. Int. J. Pharm. 2009, 376, 69–75. [Google Scholar] [CrossRef]
- Sommer, A.; Rifkin, D.B. Interaction of heparin with human basic fibroblast growth factor: Protection of the angiogenic protein from proteolytic degradation by a glycosaminoglycan. J. Cell Physiol. 1989, 138, 215–220. [Google Scholar] [CrossRef]
- Werner, S.; Grose, R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 2003, 83, 835–870. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Romanos, G.E.; Geurs, N.C.; Sullivan, A.; Suárez-López Del Amo, F.; Eber, R.M. Comparison of two differently processed acellular dermal matrix products for root coverage procedures: A prospective, randomized multicenter study. J. Periodontol. 2014, 85, 1693–1701. [Google Scholar] [CrossRef] [PubMed]
- Ahmedbeyli, C.; Ipçi Ş, D.; Cakar, G.; Kuru, B.E.; Yılmaz, S. Clinical evaluation of coronally advanced flap with or without acellular dermal matrix graft on complete defect coverage for the treatment of multiple gingival recessions with thin tissue biotype. J. Clin. Periodontol. 2014, 41, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Yamano, S.; Haku, K.; Yamanaka, T.; Dai, J.; Takayama, T.; Shohara, R.; Tachi, K.; Ishioka, M.; Hanatani, S.; Karunagaran, S.; et al. The effect of a bioactive collagen membrane releasing PDGF or GDF-5 on bone regeneration. Biomaterials 2014, 35, 2446–2453. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nica, C.; Lin, Z.; Sculean, A.; Asparuhova, M.B. Adsorption and Release of Growth Factors from Four Different Porcine-Derived Collagen Matrices. Materials 2020, 13, 2635. https://doi.org/10.3390/ma13112635
Nica C, Lin Z, Sculean A, Asparuhova MB. Adsorption and Release of Growth Factors from Four Different Porcine-Derived Collagen Matrices. Materials. 2020; 13(11):2635. https://doi.org/10.3390/ma13112635
Chicago/Turabian StyleNica, Cristina, Zhikai Lin, Anton Sculean, and Maria B. Asparuhova. 2020. "Adsorption and Release of Growth Factors from Four Different Porcine-Derived Collagen Matrices" Materials 13, no. 11: 2635. https://doi.org/10.3390/ma13112635
APA StyleNica, C., Lin, Z., Sculean, A., & Asparuhova, M. B. (2020). Adsorption and Release of Growth Factors from Four Different Porcine-Derived Collagen Matrices. Materials, 13(11), 2635. https://doi.org/10.3390/ma13112635






