Pulsed Laser Deposition Derived Bioactive Glass-Ceramic Coatings for Enhancing the Biocompatibility of Scaffolding Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Target Fabrication
2.2. Thin Films Deposition
2.3. Physicochemical Characterization
2.4. Biological Evaluation
3. Results and Discussion
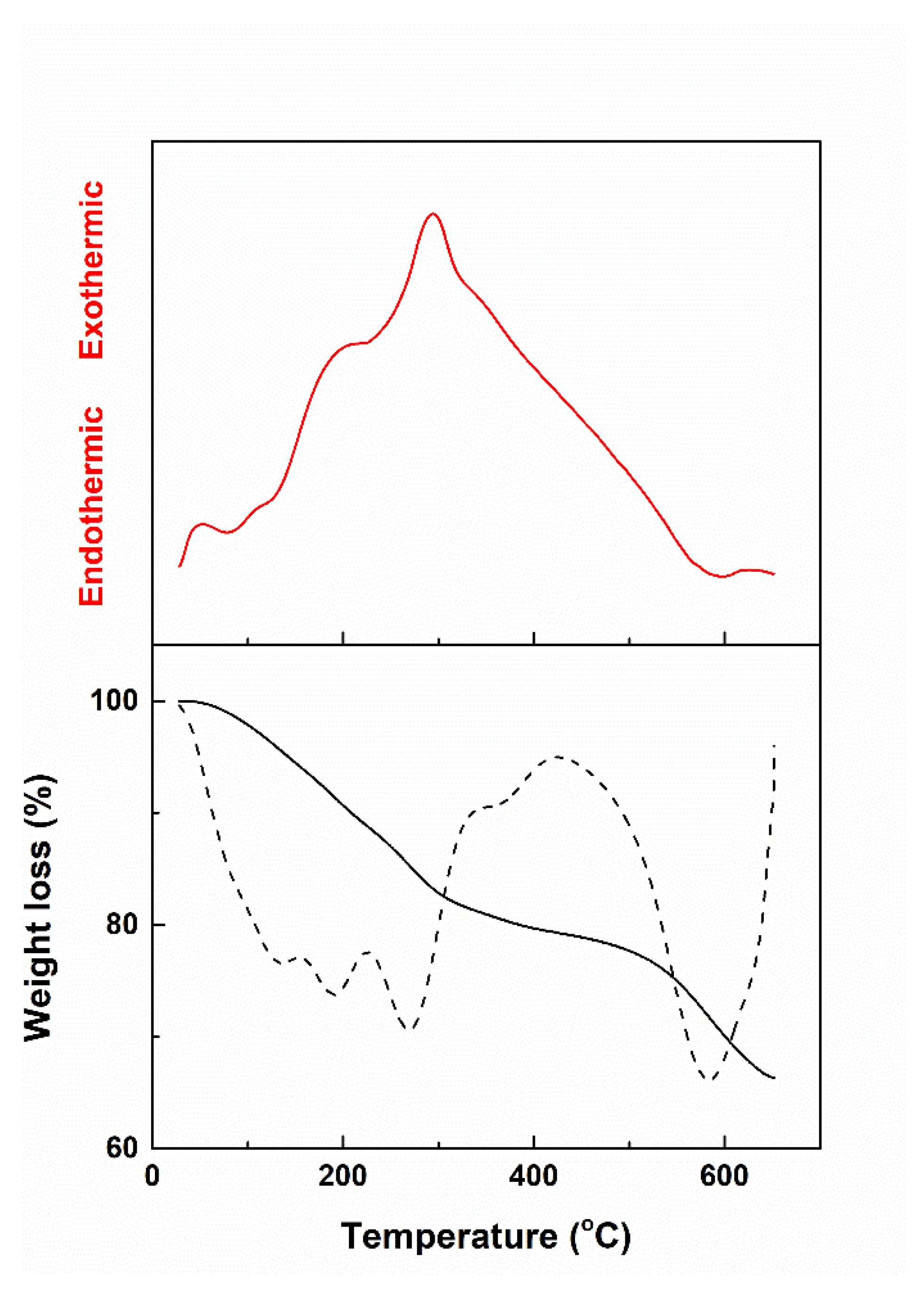
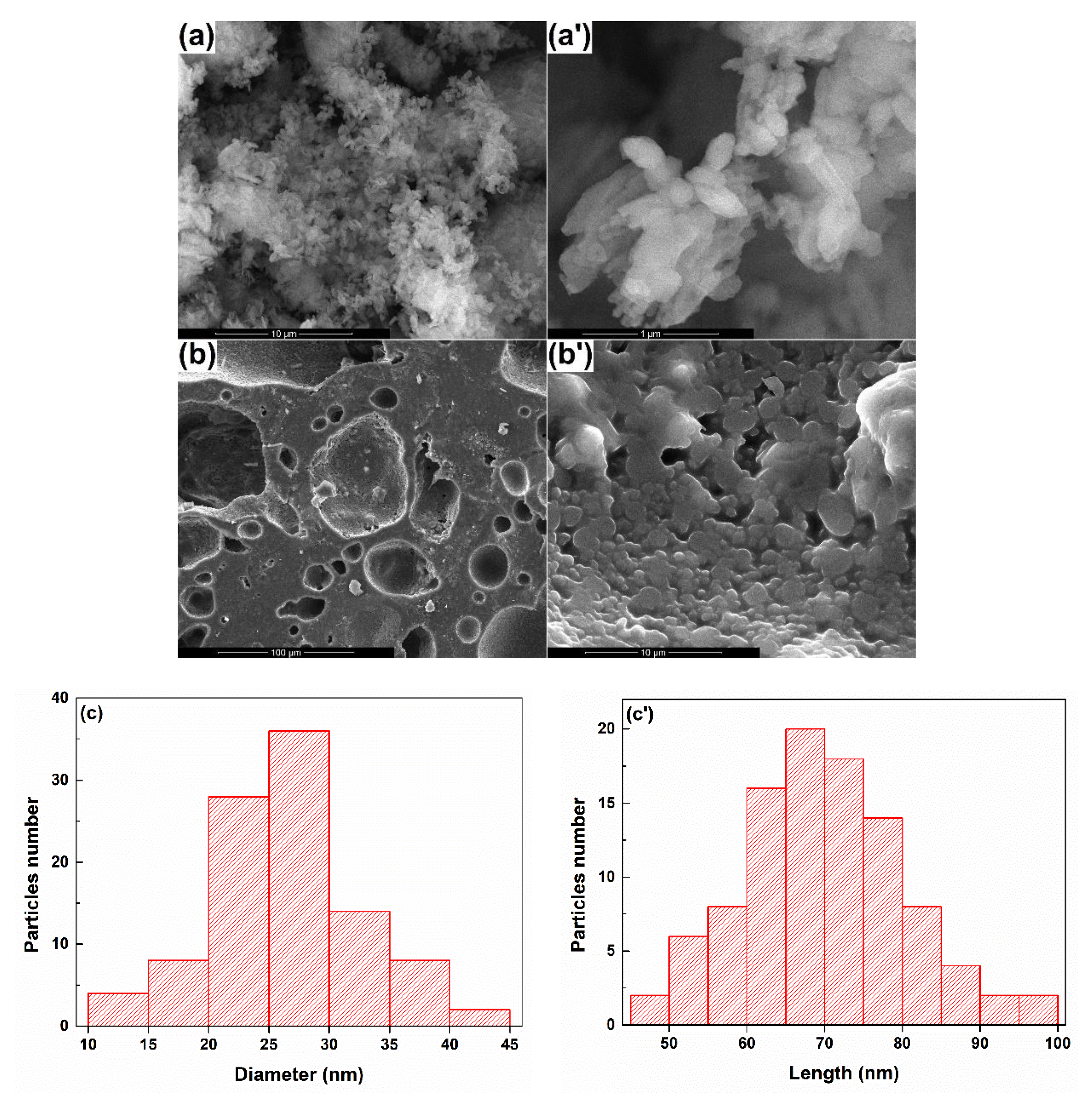
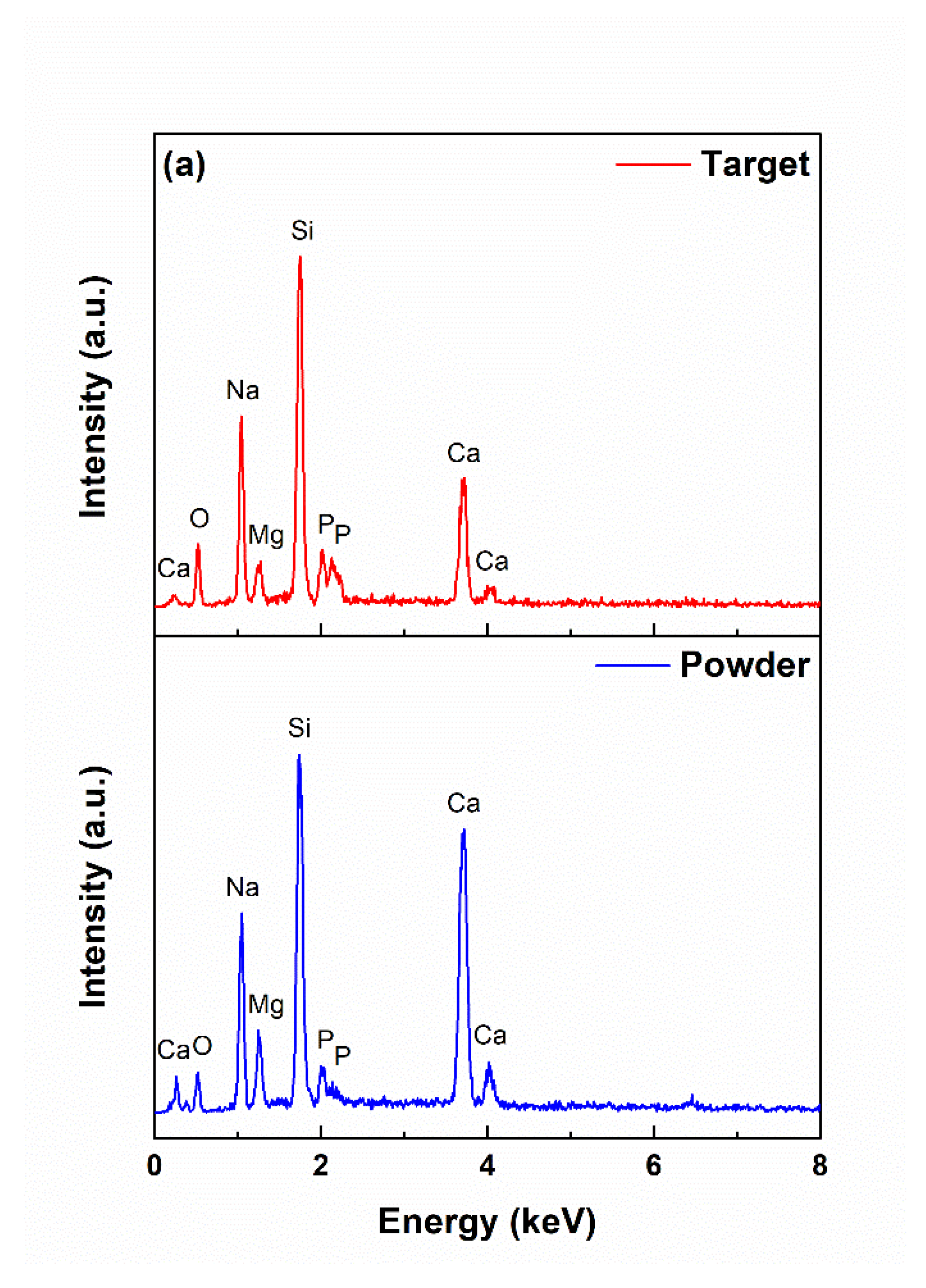
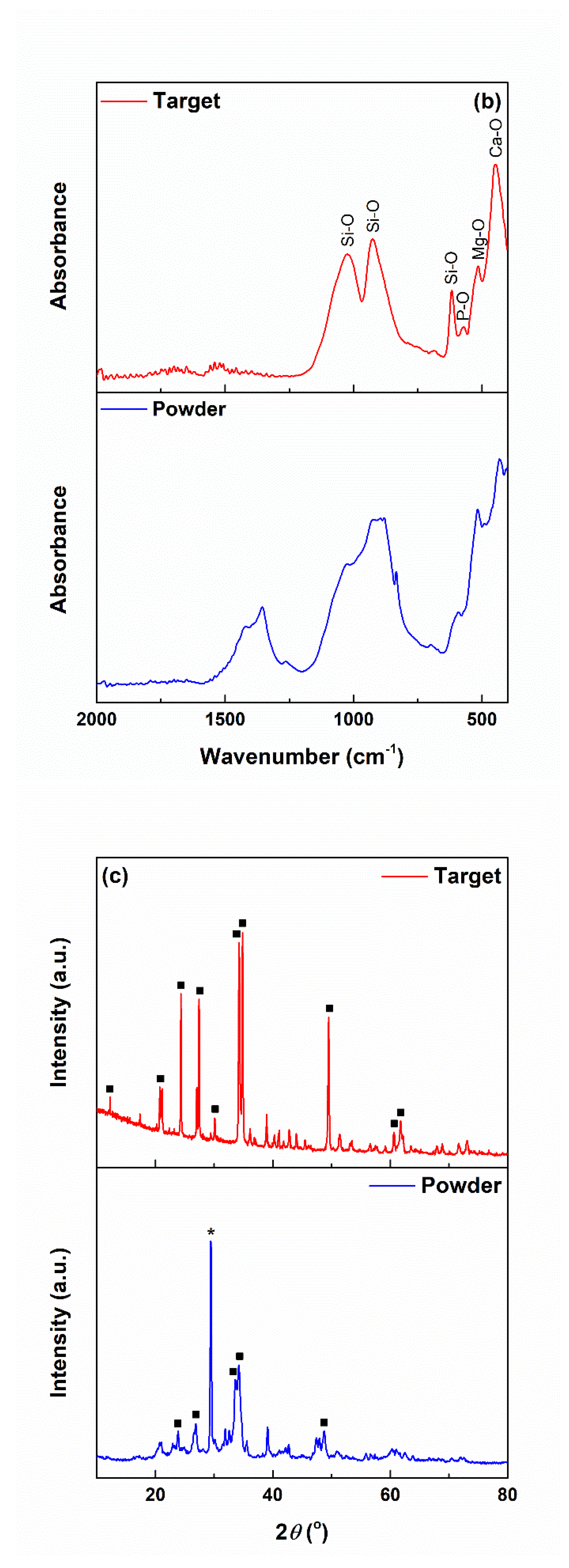
3.1. Powder and Target Characterization
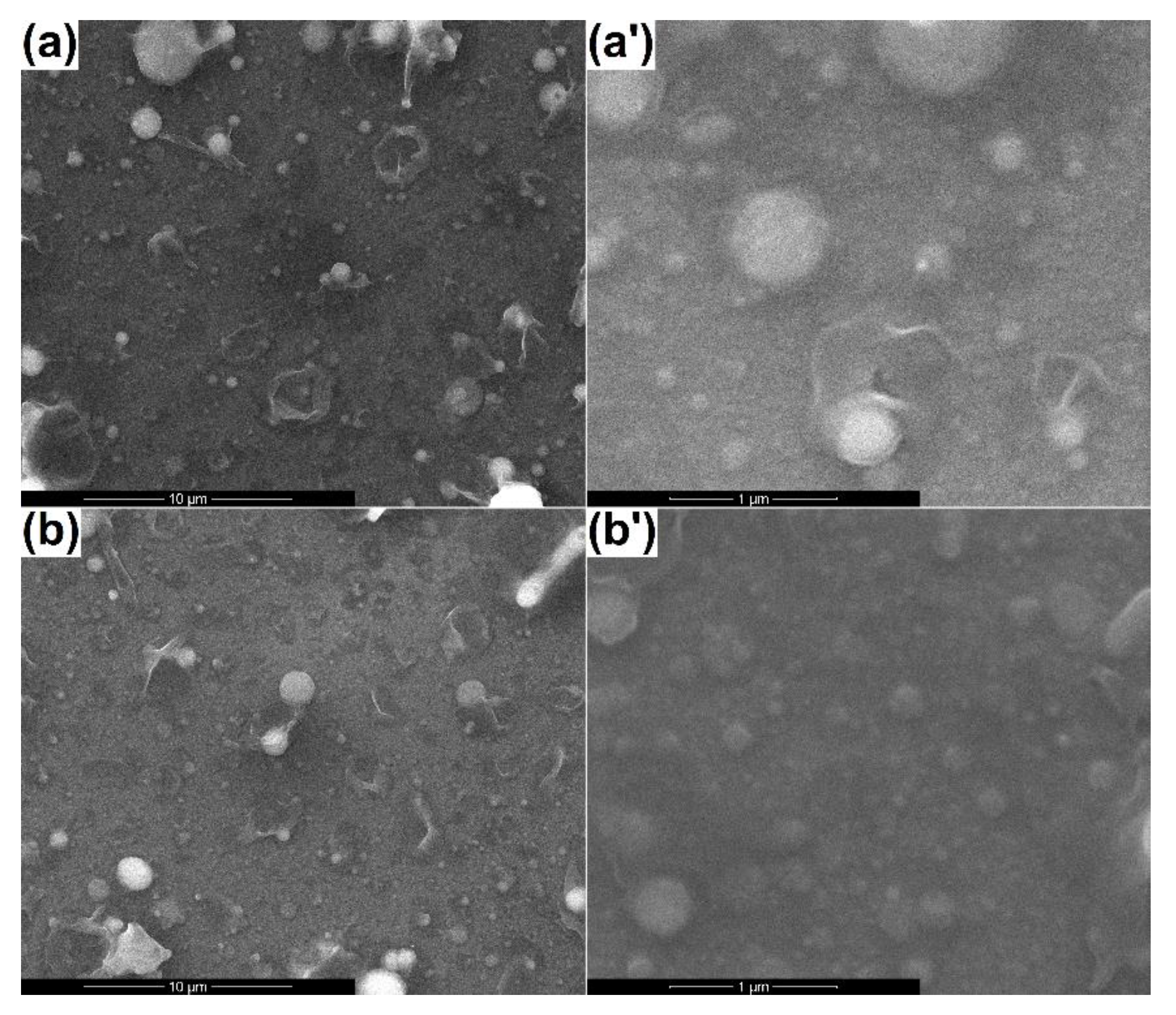
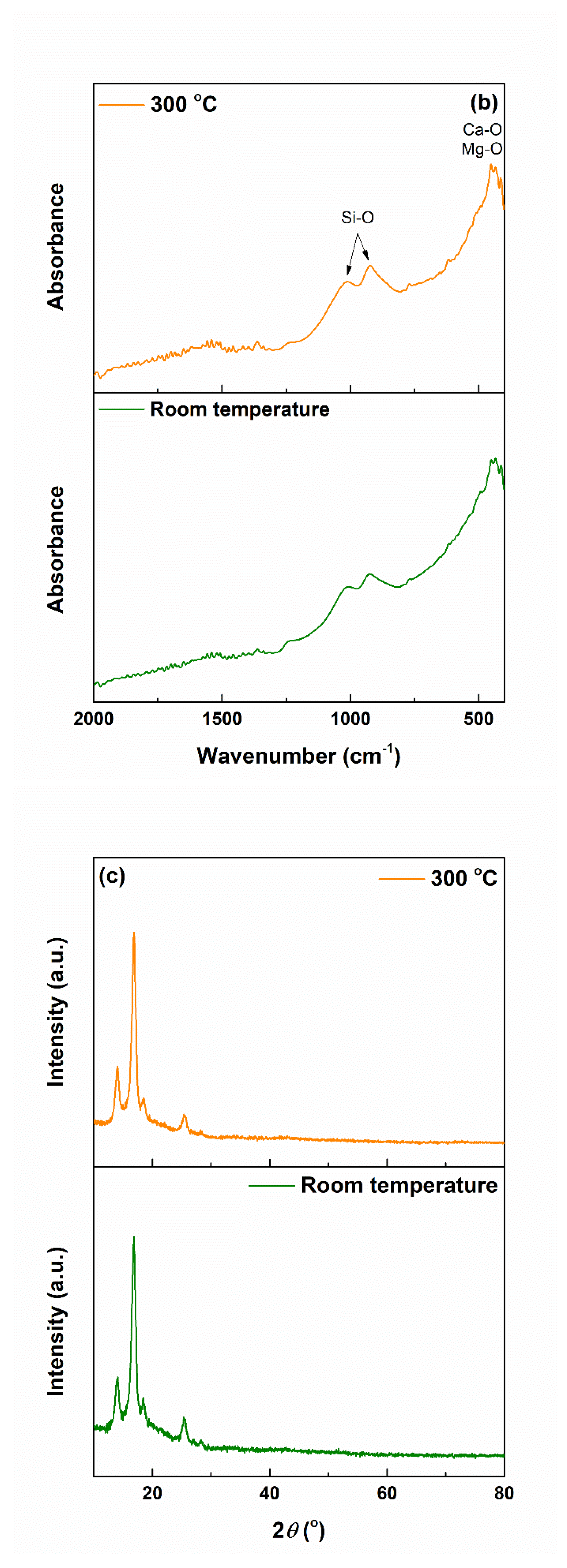
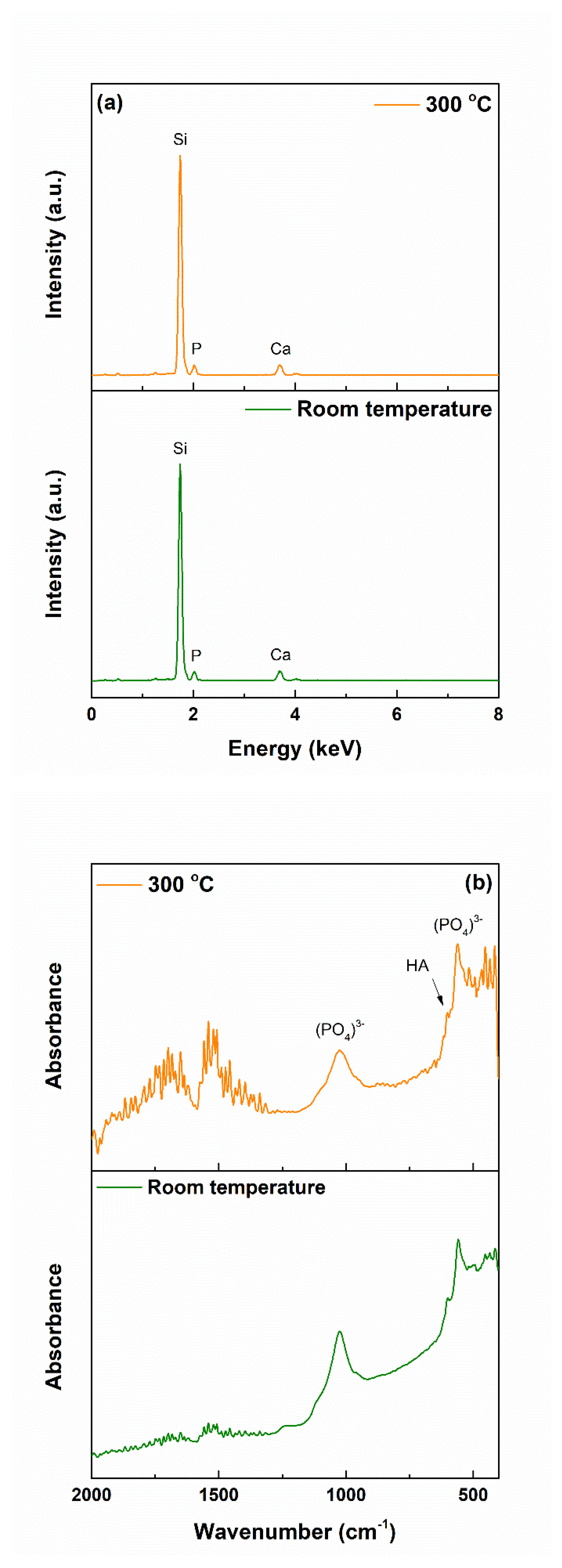
3.2. Coatings’ Physicochemical Characterization
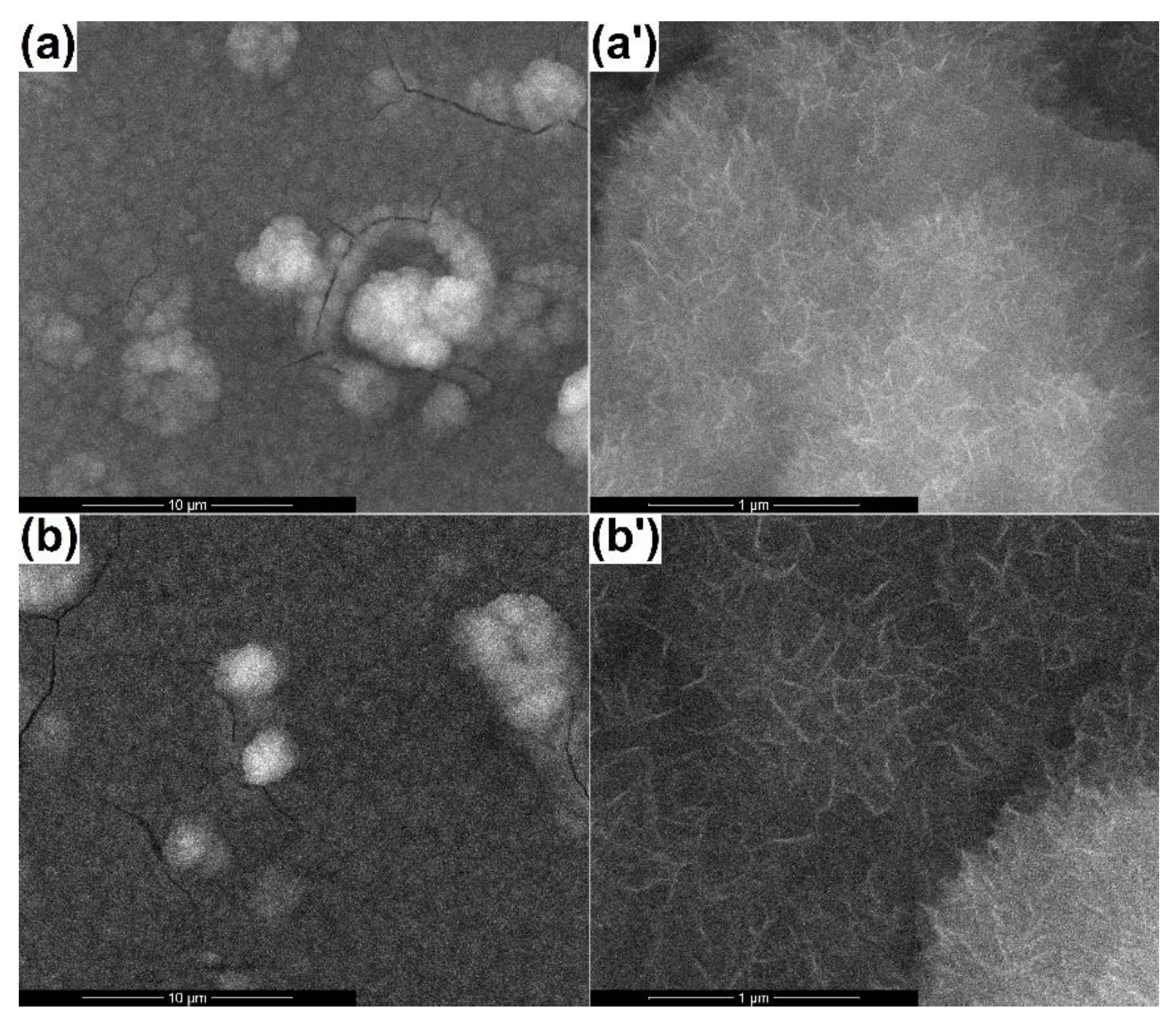
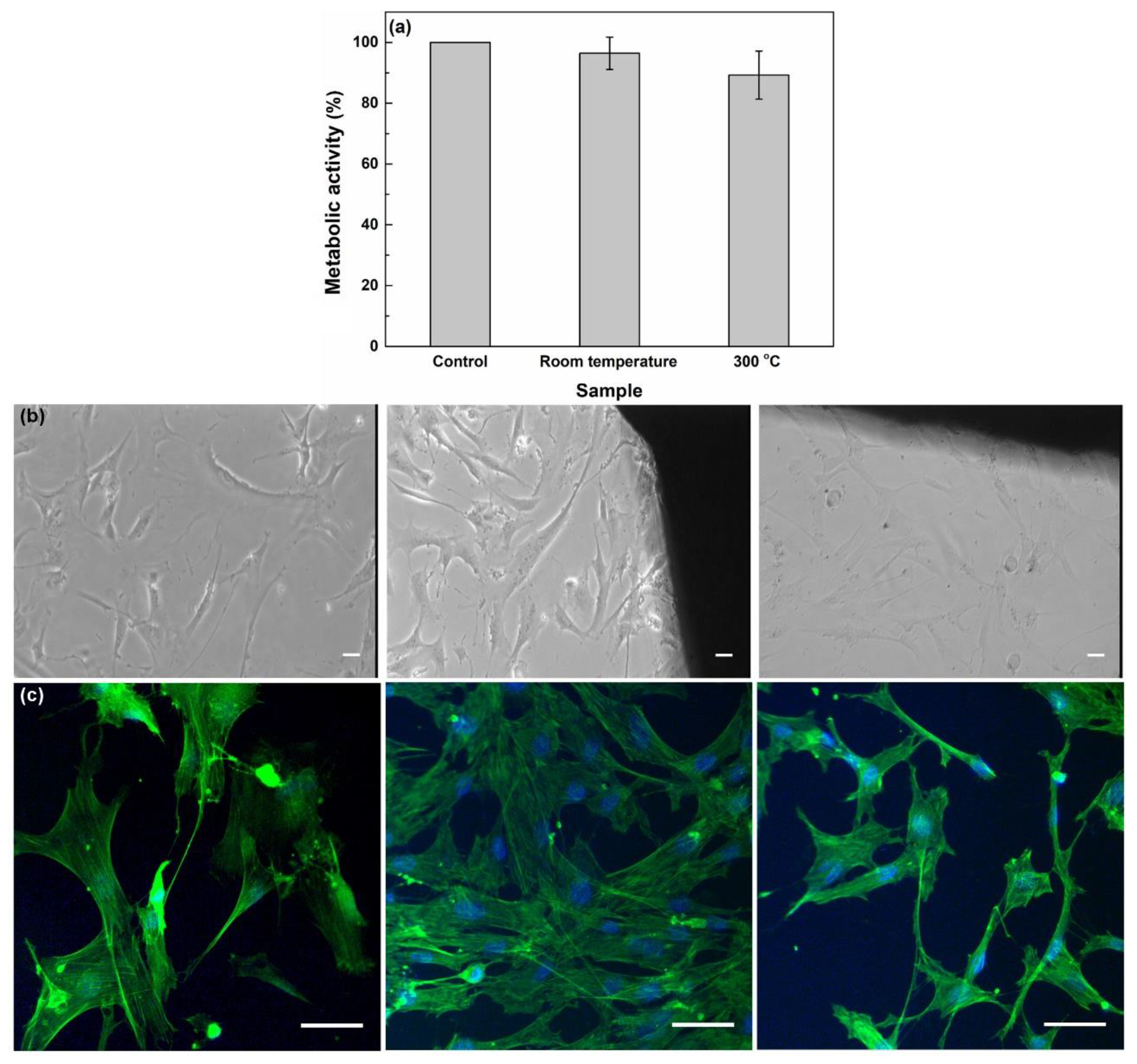
3.3. Coatings’ Biological Evaluation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kim, Y.S.; Smoak, M.M.; Melchiorri, A.J.; Mikos, A.G. An overview of the tissue engineering market in the United States from 2011 to 2018. Tissue Eng. A 2019, 25, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tsao, N. Tissue Engineering 2018–2028: Technologies, Markets, Forecasts. 2017. Available online: www.idtechex.com (accessed on 8 May 2020).
- Global Tissue Engineering Market Analysis, Drivers, Restraints, Opportunities, Threats, Trends, Applications, and Growth Forecast to 2026. 2018. Available online: www/marketresearch.biz (accessed on 8 May 2020).
- Jones, J.R. Review of bioactive glass: From Hench to hybrids. Acta Biomater. 2013, 9, 4457–4486. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, M.N.; Day, D.E.; Sonny Bal, B.; Fu, Q.; Jung, S.B.; Bonewald, L.F.; Tomsia, A.P. Bioactive glass in tissue engineering. Acta Biomater. 2011, 7, 2355–2373. [Google Scholar] [CrossRef] [PubMed]
- Karimi, A.Z.; Rezabeigi, E.; Drew, R.A.L. Glass ionomer cements with enhanced mechanical and remineralizing properties containing 45S5 bioglass-ceramic particles. J. Mech. Behav. Biomed. Mater. 2019, 97, 396–405. [Google Scholar] [CrossRef]
- Sousa, E.A.; Silva, M.J.; Sanches, A.O.; Soares, V.O.; Job, A.E.; Malmonge, J.A. Mechanical, thermal, and morphological properties of natural rubber/45S5 Bioglass® fibrous mat with ribbon-like morphology produced by solution blow spinning. Eur. Polym. J. 2019, 119, 1–7. [Google Scholar] [CrossRef]
- Machado Lopez, M.M.; Faure, J.; Espitia Cabrera, M.I.; Contreras Garcia, M.E. Structural characterization and electrochemical behavior of 45S5 bioglass coating on Ti6Al4V alloy for dental applications. Mater. Sci. Eng. B 2016, 206, 30–38. [Google Scholar] [CrossRef]
- Dittler, M.L.; Unalan, I.; Grunewald, A.; Beltran, A.M.; Grillo, C.A.; Destch, R.; Gonzalez, M.C.; Boccaccini, A.R. Bioactive glass (45S5)-based 3D scaffolds coated with magnesium and zinc-loaded hydroxyapatite nanoparticles for tissue engineering applications. Colloids Surf. B 2019, 182, 110346. [Google Scholar] [CrossRef]
- Khorami, M.; Hesaraki, S.; Behnamghader, A.; Nazarian, H.; Shahrabi, S. In vitro bioactivity and biocompatibility of lithium substituted 45S5 bioglass. Mater. Sci. Eng. C 2011, 31, 1584–1592. [Google Scholar] [CrossRef]
- Fiume, E.; Barberi, J.; Verne, E.; Baino, F. Bioactive glasses: From parent 45S5 composition to scaffold-assisted tissue-healing therapies. J. Funct. Biomater. 2018, 9, 24. [Google Scholar] [CrossRef]
- Hench, L.L.; Hench, J.W.; Greenspan, D.C. Bioglass: A short history and bibliography. J. Aust. Ceram. Soc. 2004, 40, 1–42. [Google Scholar]
- Porter, A.E.; Taak, P.; Hobbs, L.W.; Coathup, M.J.; Blunn, G.W.; Spector, M. Bone bonding to hydroxyapatite and titanium surfaces on femoral stems retrieved from human subjects at autopsy. Biomaterials 2004, 25, 5199–5208. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.L.; Jiang, Q.H.; He, F.M.; Yang, G.L.; Liu, L. Fluorescence microscopic analysis of bone osseointegration of strontium-substituted hydroxyapatite implants. J. Zhejiang Univ. Sci. B 2012, 13, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Stulajterova, R.; Medvecky, L.; Giretova, M.; Sopcak, T.; Kovalcikova, A. Effect of bioglass 45S5 addition on properties, microstructure and cellular response of tetracalcium phosphate/monetite cements. Mater. Charact. 2017, 126, 104–115. [Google Scholar] [CrossRef]
- Haro Durand, L.A.; Vargas, G.E.; Romero, N.M.; Vera-Mesones, R.; Porto-Lopez, J.M.; Boccaccini, A.R.; Zago, M.P.; Baldi, A.; Gorustovich, A. Angiogenic effects of ionic dissolution products released from a boron-doped 45S5 bioactive glass. J. Mater. Chem. B 2015, 3, 1142–1148. [Google Scholar] [CrossRef]
- Jodati, H.; Guner, B.; Evis, Z.; Keskin, D.; Tezcaner, A. Synthesis and characterization of magnesium-lanthanum dual doped bioactive glasses. Ceram. Int. 2020, 46, 10503–10511. [Google Scholar] [CrossRef]
- Ma, J.; Chen, C.Z.; Wang, D.G.; Hu, J.H. Synthesis, characterization and in vitro bioactivity of magnesium-doped sol-gel glass and glass-ceramics. Ceram. Int. 2011, 37, 1637–1644. [Google Scholar] [CrossRef]
- Banerjee, S.S.; Tarafder, S.; Davies, N.M.; Bandyopadhyay, A.; Bose, S. Understanding the influence of MgO and SrO binary doping on the mechanical and biological properties of β-TCP ceramics. Acta Biomater. 2010, 6, 4167–4174. [Google Scholar] [CrossRef]
- Oudadesse, H.; Dietrich, E.; Bui, X.V.; Le Gal, Y.; Pellen, P.; Cathelineau, G. Enhancement of cells proliferation and control of bioactivity of strontium doped glass. Appl. Surf. Sci. 2011, 257, 8587–8593. [Google Scholar] [CrossRef]
- Yin, H.; Yang, C.; Gao, Y.; Wang, C.; Li, M.; Guo, H.; Tong, Q. Fabrication and characterization of strontium-doped borate-based bioactive glass scaffolds for bone tissue engineering. J. Alloys Compd. 2018, 743, 564–569. [Google Scholar] [CrossRef]
- Nicolini, V.; Malavasi, G.; Lusvardi, G.; Zambon, A.; Benedetti, F.; Cerrato, G.; Valeri, S.; Luches, P. Mesoporous bioactive glasses doped with cerium : Investigation over enzymatic-like mimetic activities and bioactivity. Ceram. Int. 2019, 45, 20910–20920. [Google Scholar] [CrossRef]
- Deliormanli, A.M.; Seda Vatansever, H.; Yesil, H.; Ozdal-Kurt, F. In vivo evaluation of cerium, gallium and vanadium-doped borate-based bioactive glass scaffolds using rat subcutaneous implantation model. Ceram. Int. 2016, 42, 11574–11583. [Google Scholar] [CrossRef]
- Sakka, S. Sol-gel process and applications. In Handbook of Advanced Ceramics: Materials, Applications, Processing, and Properties, 2nd ed.; Somiya, S., Ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 883–910. [Google Scholar]
- Oubaha, M.; Gorin, A.; McDonagh, C.; Duffy, B.; Copperwhite, R. Development of a multianalyte optical sol-gel biosensor for medical diagnostic. Sens. Actuators B 2015, 221, 96–103. [Google Scholar] [CrossRef]
- Salahinejad, E.; Hadianfard, M.J.; Macdonald, D.D.; Mozafari, M.; Vashaee, D.; Tayebi, L. A new double-layer sol–gel coating to improve the corrosion resistance of a medical-grade stainless steel in a simulated body fluid. Mater. Lett. 2013, 97, 162–165. [Google Scholar] [CrossRef]
- Podbielska, H.; Ulatowska-Jarza, A.; Muller, G.; Holowacz, I.; Bauer, J.; Bindig, U. Silica sol-gel matrix doped with Photolon molecules for sensing and medical therapy purposes. Biomol. Eng. 2007, 24, 425–433. [Google Scholar] [CrossRef]
- Socol, G.; Macovei, A.M.; Miroiu, F.; Stefan, N.; Duta, L.; Dorcioman, G.; Mihailescu, I.N.; Petrescu, S.M.; Stan, G.E.; Marcov, D.A.; et al. Hydroxyapatite thin films synthesized by pulsed laser deposition and magnetron sputtering on PMMA substrates for medical applications. Mater. Sci. Eng. B 2010, 169, 159–168. [Google Scholar] [CrossRef]
- Pisarik, P.; Jelinek, M.; Remsa, J.; Miksovsky, J.; Zemek, J.; Jurek, K.; Kubinova, S.; Lukea, J.; Sepitka, J. Antibacterial, mechanical and surface properties of Ag-DLC films prepared by dual PLD for medical applications. Mater. Sci. Eng. C 2017, 77, 955–962. [Google Scholar] [CrossRef]
- Abdelrehman, M.H.M.; Craciun, V.; Kroon, R.E.; Yousif, A.; Seed Ahmed, H.A.A.; Swart, H.C. Effect of background atmosphere and substrate temperature on SrO:Bi3+ (0.2 mol%) thin films produced using pulsed laser deposition with different lasers. Phys. B 2020, 581, 411757. [Google Scholar] [CrossRef]
- Negrea, R.; Busuioc, C.; Constantinoiu, I.; Miu, D.; Enache, C.; Iordache, F.; Jinga, S.I. Akermanite based coatings grown by pulsed laser deposition for metallic implants employed in orthopaedics. Surf. Coat. Technol. 2019, 357, 1015–1026. [Google Scholar] [CrossRef]
- Voicu, G.; Miu, D.; Dogaru, I.; Jinga, S.I.; Busuioc, C. Vitroceramic interface deposited on titanium substrate by pulsed laser deposition method. Int. J. Pharm. 2016, 510, 449–456. [Google Scholar] [CrossRef]
- Kokubo, T. Surface chemistry of bioactive glass-ceramics. J. Non-Cryst. Solids 1990, 120, 138–151. [Google Scholar] [CrossRef]
- Jinga, S.I.; Skokin, M.; Vasile, B.S.; Constantinoiu, I.; Miu, D.; Bacalum, M.; Busuioc, C. Development of vitroceramic coatings and analysis of their suitability for biomedical applications. Coatings 2019, 9, 671. [Google Scholar] [CrossRef]
- Thomas, A.; Bera, J. Preparation and characterization of gelatin-bioactive glass ceramic scaffolds for bone tissue engineering. J. Biomater. Sci. Polym. Ed. 2019, 30, 561–579. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.A.; Essien, E.R. Bioactivity of quaternary glass prepared from bentonite clay. J. Adv. Ceram. 2016, 5, 47–53. [Google Scholar] [CrossRef][Green Version]
- Chen, Q.Z.; Thompson, I.D.; Boccaccini, A.R. 45S5 Bioglass®-derived glass-ceramic scaffolds for bone tissue engineering. Biomaterials 2006, 27, 2414–2425. [Google Scholar] [CrossRef]
- Karimi, A.Z.; Rezabeigi, E.; Drew, R.A.L. Crystallization behavior of combeite in 45S5 Bioglass® via controlled heat treatment. J. Non Cryst. Solids 2018, 502, 176–183. [Google Scholar] [CrossRef]
- Du, R.; Chang, J. Preparation and characterization of bioactive sol-gel-derived Na2Ca2Si3O9. J. Mater. Sci. 2004, 15, 1285–1289. [Google Scholar] [CrossRef]
- Cabal, B.; Alou, L.; Cafini, F.; Couceiro, R.; Sevillano, D.; Esteban-Tejeda, L.; Guitian, F.; Torrecillas, R.; Moya, J.S. A new biocompatible and antibacterial phosphate free glass-ceramic for medical applications. Sci. Rep. 2015, 4, 5440. [Google Scholar] [CrossRef]
- Busuioc, C.; Olaret, E.; Stancu, I.C.; Nicoara, A.I.; Jinga, S.I. Electrospun fibre webs templated synthesis of mineral scaffolds based on calcium phosphates and barium titanate. Nanomaterials 2020, 10, 772. [Google Scholar] [CrossRef]
- An, J.; Leeuwenburgh, S.; Wolke, J.; Jansen, J. Mineralization processes in hard tissue: Bone. In Biomineralization and Biomaterials: Fundamentals and Applications; Aparicio, C., Ginebra, M.P., Eds.; Woodhead Publishing: Sawston, UK, 2016; pp. 129–146. [Google Scholar]
- Ohtsuki, C.; Kokubo, T.; Yamamurob, T. Mechanism of apatite formation on CaO‒SiO2‒P2O5 glasses in a simulated body fluid. J. Non Cryst. Solids 1992, 143, 84–92. [Google Scholar] [CrossRef]
- Schmitz, S.I.; Widholz, B.; Essers, C.; Becker, M.; Tulyaganov, D.U.; Moghaddam, A.; Gonzalo de Juan, I.; Westhauser, F. Superior biocompatibility and comparable osteoinductive properties: Sodium-reduced fluoride-containing bioactive glass belonging to the CaO-MgO-SiO2 system as a promising alternative to 45S5 bioactive glass. Bioact. Mater. 2020, 5, 55–65. [Google Scholar] [CrossRef]
- Agathopoulos, S.; Tulyaganov, D.U. Bioglasses and glass-ceramics in the Na2O–CaO–MgO–SiO2–P2O5–CaF2 system. In Bioceramics and Biocomposites: From Research to Clinical Practice; Antoniac, I., Ed.; John Wiley and Sons: Hoboken, NJ, USA, 2019; pp. 123–148. [Google Scholar]
- Kaur, G.; Kumar, V.; Baino, F.; Mauro, J.C.; Pickrell, G.; Evans, I.; Bretcanu, O. Mechanical properties of bioactive glasses, ceramics, glass-ceramics and composites: State-of-the-art review and future challenges. Mater. Sci. Eng. C 2019, 104, 109895. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.H.; Byun, K.M.; Hwang, S.; Jun, S.B. In vitro biocompatibility test of multi-layered plasmonic substrates with flint glasses and adhesion films. J. Opt. Soc. Korea 2014, 18, 174–179. [Google Scholar] [CrossRef][Green Version]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schitea, R.-I.; Nitu, A.; Ciobota, A.-A.; Munteanu, A.-L.; David, I.-M.; Miu, D.; Raileanu, M.; Bacalum, M.; Busuioc, C. Pulsed Laser Deposition Derived Bioactive Glass-Ceramic Coatings for Enhancing the Biocompatibility of Scaffolding Materials. Materials 2020, 13, 2615. https://doi.org/10.3390/ma13112615
Schitea R-I, Nitu A, Ciobota A-A, Munteanu A-L, David I-M, Miu D, Raileanu M, Bacalum M, Busuioc C. Pulsed Laser Deposition Derived Bioactive Glass-Ceramic Coatings for Enhancing the Biocompatibility of Scaffolding Materials. Materials. 2020; 13(11):2615. https://doi.org/10.3390/ma13112615
Chicago/Turabian StyleSchitea, Ruxandra-Ioana, Alexandru Nitu, Andreea-Aurelia Ciobota, Andrei-Lucian Munteanu, Irina-Madalina David, Dana Miu, Mina Raileanu, Mihaela Bacalum, and Cristina Busuioc. 2020. "Pulsed Laser Deposition Derived Bioactive Glass-Ceramic Coatings for Enhancing the Biocompatibility of Scaffolding Materials" Materials 13, no. 11: 2615. https://doi.org/10.3390/ma13112615
APA StyleSchitea, R.-I., Nitu, A., Ciobota, A.-A., Munteanu, A.-L., David, I.-M., Miu, D., Raileanu, M., Bacalum, M., & Busuioc, C. (2020). Pulsed Laser Deposition Derived Bioactive Glass-Ceramic Coatings for Enhancing the Biocompatibility of Scaffolding Materials. Materials, 13(11), 2615. https://doi.org/10.3390/ma13112615




