Quasi-Monocrystalline Graphene Crystallization on Liquid Copper Matrix
Abstract
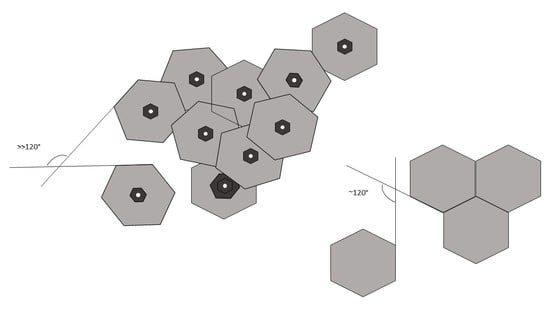
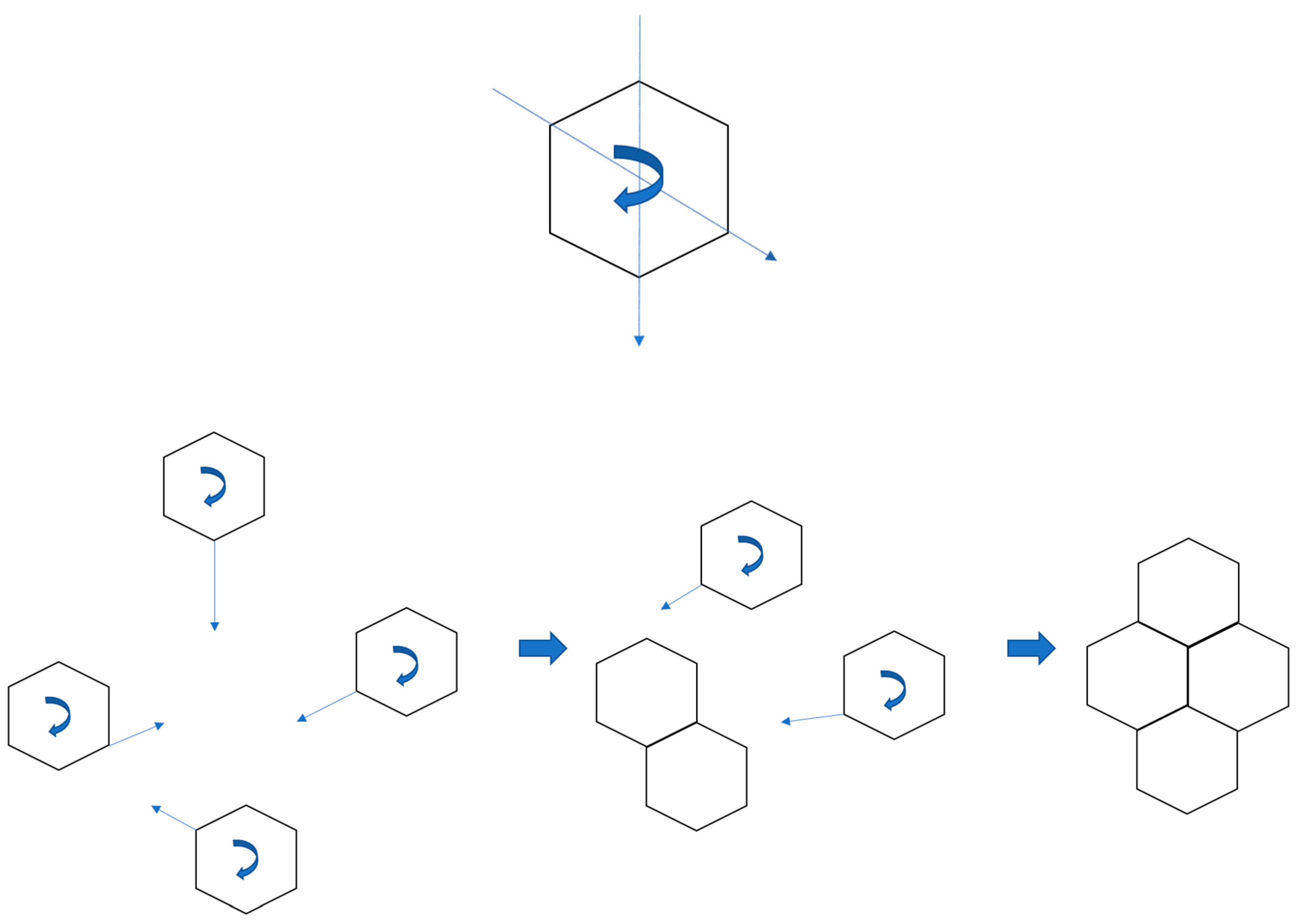
- Metallurgical graphene is grown on a liquid metal surface
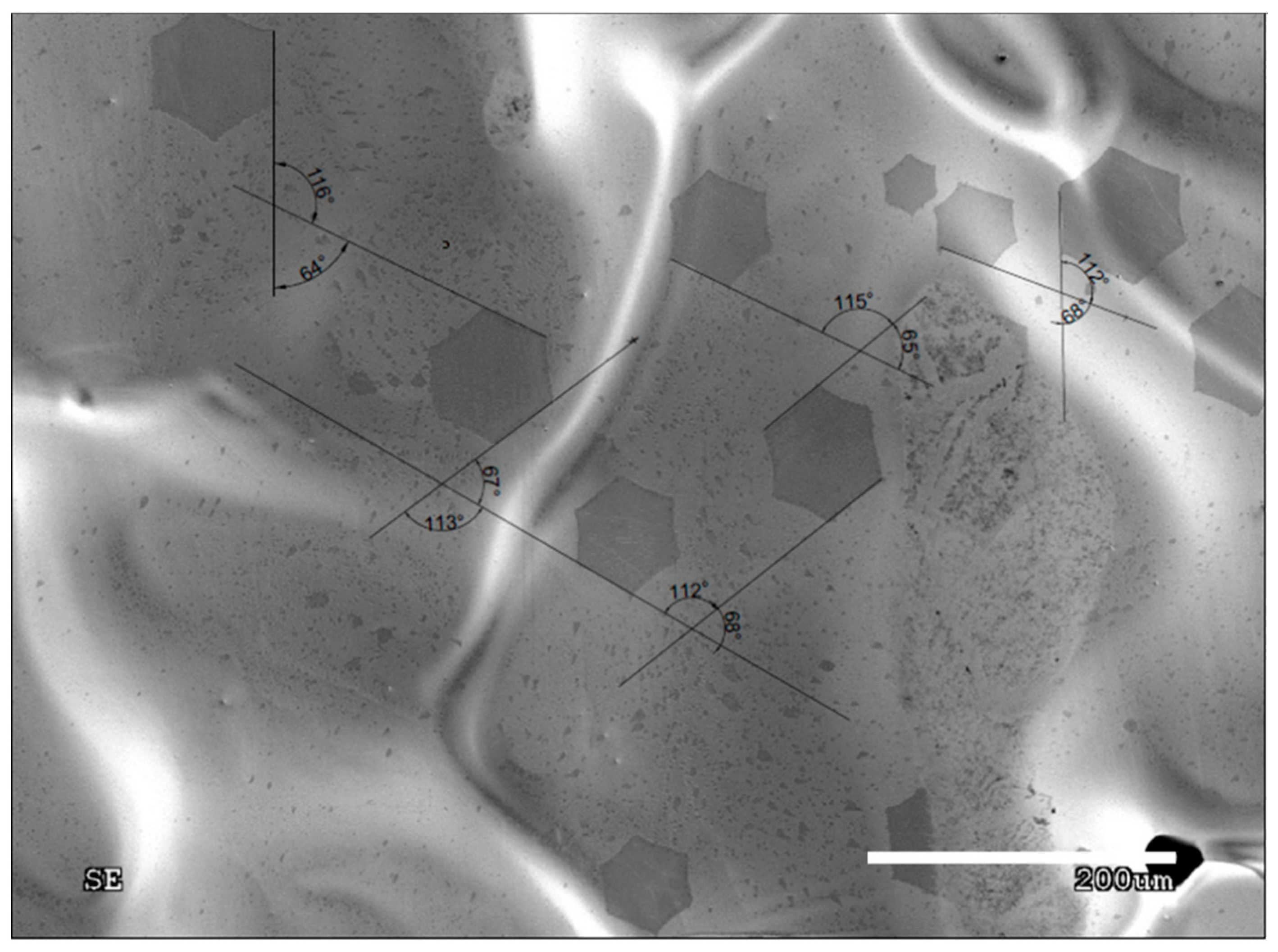
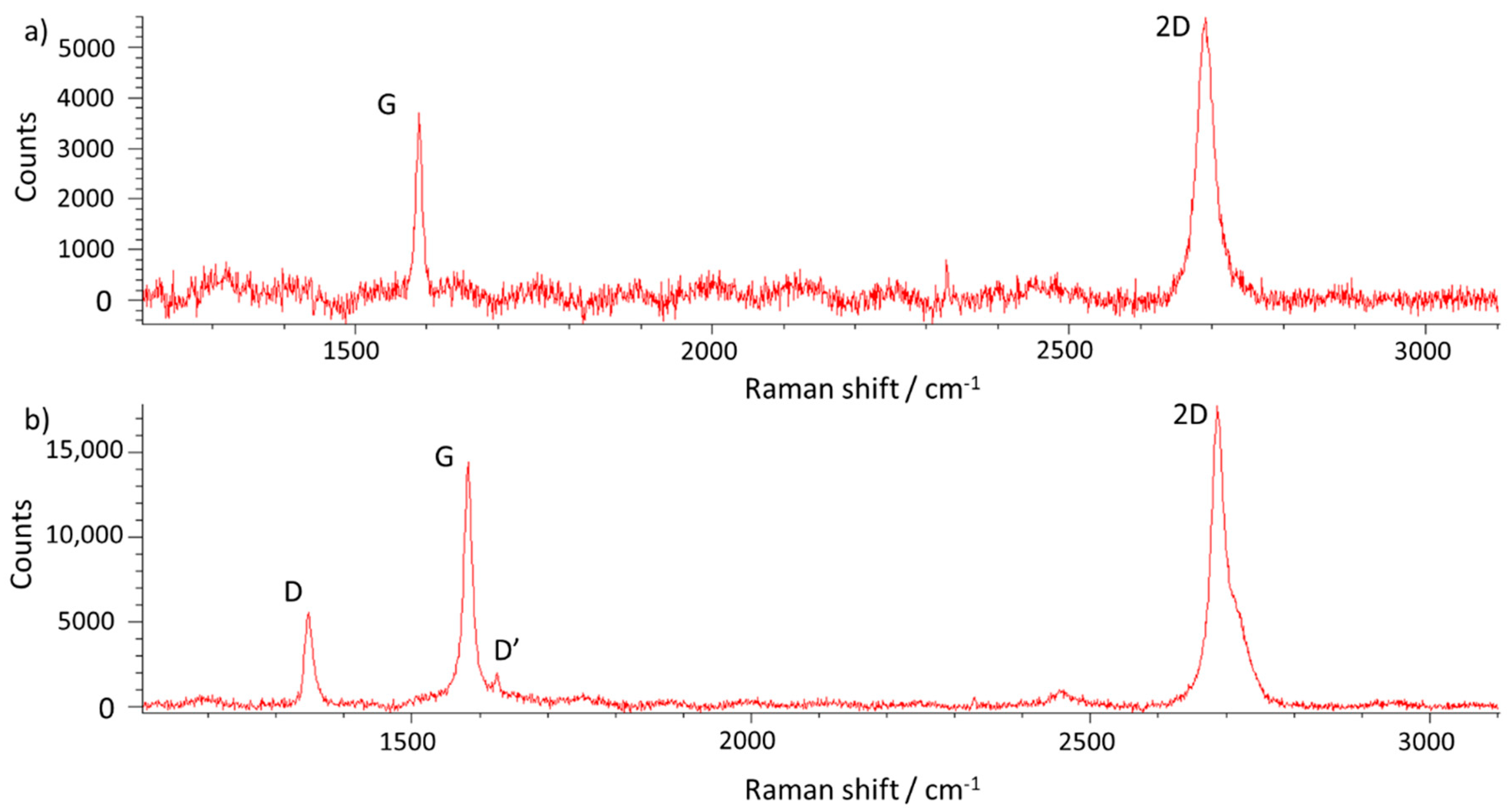
- Graphene grain boundaries obtained in the research are of low-angle type
- The lower the nucleation rate, the more ordered the quasi-monocrystalline structure
- A large number of impurities prevents the formation of an ordered (quasi-monocrystalline) structure
- Impurities initiate heterogeneous nucleation of graphene nuclei
1. Introduction
2. Materials and Methods
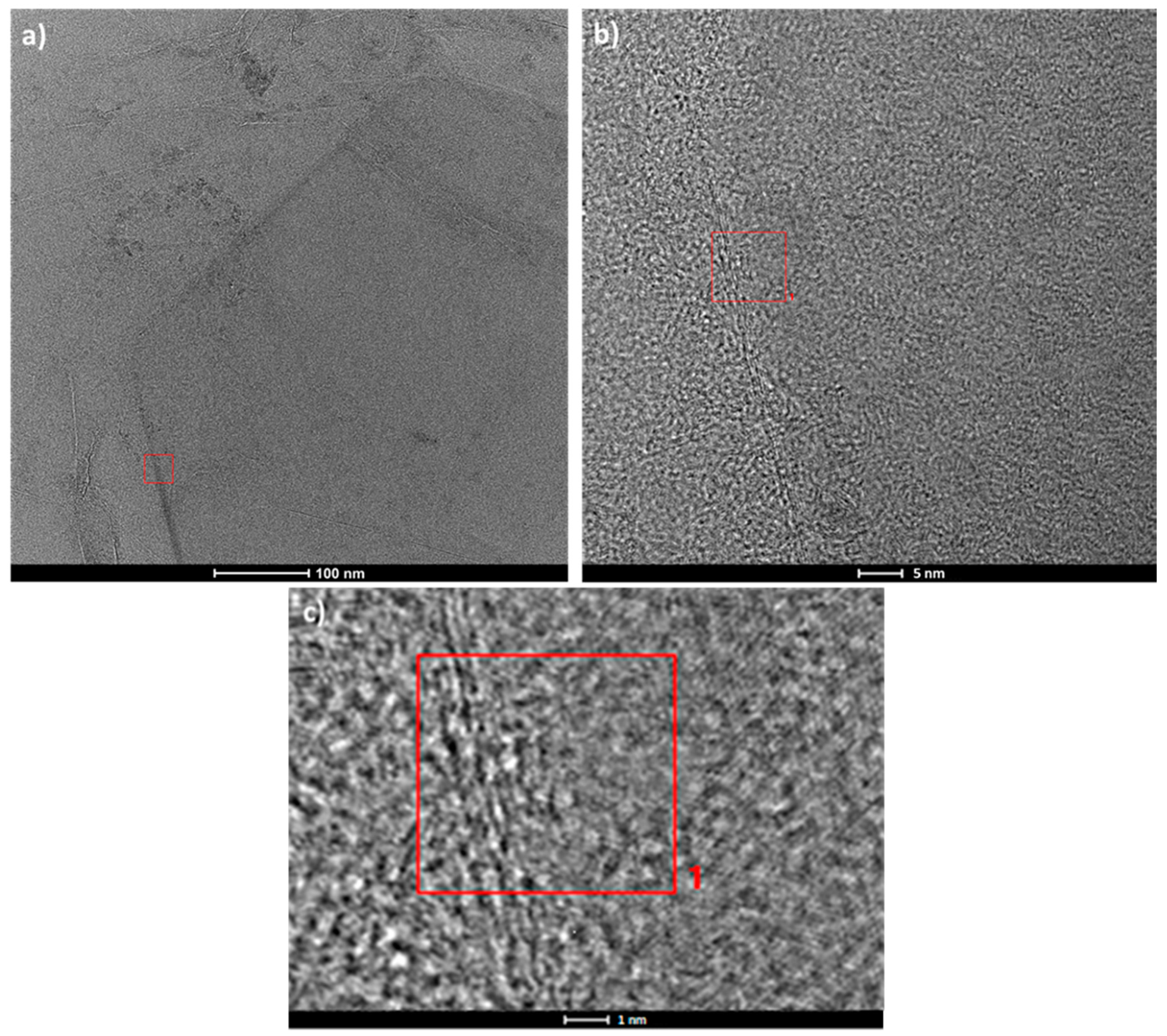
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mukanova, A.; Tussupbayev, R.; Sabitov, A.; Bondarenko, I.; Nemkaeva, R.; Aldamzharov, B.; Bakenov, Z. CVD graphene growth on a surface of liquid gallium. Mater. Today Proc. 2017, 4, 4548–4554. [Google Scholar] [CrossRef]
- Amini, S.; Garay, J.; Liu, G.; Balandin, A.A.; Abbaschian, R. Growth of large-area graphene films from metal-carbon melts. J. Appl. Phys. 2010, 108, 94321. [Google Scholar] [CrossRef]
- Ding, G.; Zhu, Y.; Wang, S.; Gong, Q.; Sun, L.; Wu, T.; Xie, X.; Jiang, M. Chemical vapor deposition of graphene on liquid metal catalysts. Carbon 2013, 53, 321–326. [Google Scholar] [CrossRef]
- Tan, L.; Zeng, M.; Zhang, T.; Fu, L. Design of catalytic substrates for uniform graphene films: From solid-metal to liquid-metal. Nanoscale 2015, 7, 9105–9121. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Wei, Q.; Zhang, G.; Zhong, Y.; Moghimian, N.; Tong, X.; Sun, S. LiFePO₄-Graphene Composites as High-Performance Cathodes for Lithium-Ion Batteries: The Impact of Size and Morphology of Graphene. Materials 2019, 12, 842. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liu, S.-K.; Lu, L.-H.; Lu, L.; Han, G. Preparation and Properties of Graphene/Nickel Composite Coating Based on Textured Surface of Aluminum Alloy. Materials 2019, 12, 3240. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Fu, J.; Liu, Y.; Wang, D.; Wang, X.; Dong, W.; Jiang, H. Recent Progress on Graphene/Polyaniline Composites for High-performance Supercapacitors. Materials 2019, 12, 1451. [Google Scholar] [CrossRef] [PubMed]
- Warheit, D. Hazard and risk assessment strategies for nanoparticle exposures: How far have we come in the past 10 years? F1000Research 2018, 7, 376. [Google Scholar] [CrossRef]
- Morgeneyer, M.; Aguerre-Chariol, O.; Bressot, C. STEM imaging to characterize nanoparticle emissions and help to design nanosafer paints. Chem. Eng. Res. Des. 2018, 136, 663–674. [Google Scholar] [CrossRef]
- Zaaba, N.; Foo, K.L.; Hashim, U.; Tan, S.; Liu, W.W.; Voon, C. Synthesis of Graphene Oxide using Modified Hummers Method: Solvent Influence. Procedia Eng. 2017, 184, 469–477. [Google Scholar] [CrossRef]
- Chen, M.; Haddon, R.; Yan, R.; Bekyarova, E. Advances in transferring chemical vapour deposition graphene: A review. Mater. Horizons 2017, 4, 1054–1063. [Google Scholar] [CrossRef]
- Guo, W.; Xu, C.; Xu, K.; Deng, J.; Guo, W.; Yurgens, A.; Sun, J. Rapid chemical vapor deposition of graphene on liquid copper. Synth. Met. 2016, 216, 93–97. [Google Scholar] [CrossRef]
- Wang, J.; Zeng, M.; Tan, L.; Dai, B.; Deng, Y.; Rümmeli, M.H.; Xu, H.; Li, Z.; Wang, S.; Peng, L.-M.; et al. High-mobility graphene on liquid p-block elements by ultra-low-loss CVD growth. Sci. Rep. 2013, 3, 2670. [Google Scholar] [CrossRef] [PubMed]
- Mattevi, C.; Kim, H.; Chhowalla, M. A review of chemical vapour deposition of graphene on copper. J. Mater. Chem. 2011, 21, 3324–3334. [Google Scholar] [CrossRef]
- Geng, D.; Wu, B.; Guo, Y.; Huang, L.; Xue, Y.; Chen, J.; Yu, G.; Jiang, L.; Hu, W.; Liu, Y. Uniform hexagonal graphene flakes and films grown on liquid copper surface. Proc. Natl. Acad. Sci. USA 2012, 109, 7992–7996. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, L.; Chen, S. Large area CVD growth of graphene. Synth. Met. 2015, 210, 95–108. [Google Scholar] [CrossRef]
- Yin, S.; Zhang, X.; Xu, C.; Wang, Y.; Wang, Y.; Li, P.; Sun, H.; Wang, M.; Xia, Y.; Lin, C.-T.; et al. Chemical vapor deposition growth of scalable monolayer polycrystalline graphene films with millimeter-sized domains. Mater. Lett. 2018, 215, 259–262. [Google Scholar] [CrossRef]
- Lavin-Lopez, M.P.; Sanchez-Silva, L.; Valverde, J.L.; Romero, A. CVD-graphene growth on different polycrystalline transition metals. AIMS Mater. Sci. 2017, 4, 194–208. [Google Scholar] [CrossRef]
- DiMarco, C.; Robillos, T.; Hone, J.; Kysar, J. Mechanisms and criteria for failure in polycrystalline graphene. Int. J. Solids Struct. 2018, 143, 232–244. [Google Scholar] [CrossRef]
- Yazyev, O.V.; Chen, Y.P. Polycrystalline graphene and other two-dimensional materials. Nat. Nanotechnol. 2014, 9, 755–767. [Google Scholar] [CrossRef]
- Nguyen, V.L.; Shin, B.G.; Duong, D.L.; Kim, S.T.; Perello, D.; Lim, Y.J.; Yuan, Q.; Ding, F.; Jeong, H.Y.; Shin, H.S.; et al. Seamless Stitching of Graphene Domains on Polished Copper (111) Foil. Adv. Mater. 2014, 27, 1376–1382. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, L.; Wu, N.; Kong, Z.; Zeng, M.; Zhang, T.; Zhuang, L.; Fu, L. Uniform graphene on liquid metal by chemical vapour deposition at reduced temperature. Carbon 2016, 96, 799–804. [Google Scholar] [CrossRef]
- Robertson, A.; Warner, J.H. Hexagonal Single Crystal Domains of Few-Layer Graphene on Copper Foils. Nano Lett. 2011, 11, 1182–1189. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhong, G.; D’Arsie’, L.; Sugime, H.; Esconjauregui, S.; Robertson, A.; Robertson, J. Growth of Continuous Monolayer Graphene with Millimeter-sized Domains Using Industrially Safe Conditions. Sci. Rep. 2016, 6, 21152. [Google Scholar] [CrossRef]
- Zhao, L.; Rim, K.; Zhou, H.; He, R.; Heinz, T.; Pinczuk, A.; Flynn, G.; Pasupathy, A. Influence of copper crystal surface on the CVD growth of large area monolayer graphene. Solid State Commun. 2011, 151, 509–513. [Google Scholar] [CrossRef]
- Ding, D.; Solís-Fernández, P.; Yunus, R.M.; Hibino, H.; Ago, H. Behavior and role of superficial oxygen in Cu for the growth of large single-crystalline graphene. Appl. Surf. Sci. 2017, 408, 142–149. [Google Scholar] [CrossRef]
- Chen, J.; Cui, M.; Wu, G.; Wang, T.; Mbengue, J.M.; Li, Y.; Li, M. Fast growth of large single-crystalline graphene assisted by sequential double oxygen passivation. Carbon 2017, 116, 133–138. [Google Scholar] [CrossRef]
- Zeng, M.; Tan, L.; Wang, L.; Mendes, R.G.; Qin, Z.; Huang, Y.; Zhang, T.; Fang, L.; Zhang, Y.; Yue, S.; et al. Isotropic Growth of Graphene toward Smoothing Stitching. ACS Nano 2016, 10, 7189–7196. [Google Scholar] [CrossRef]
- Geng, D.; Meng, L.; Chen, B.; Gao, E.; Yan, W.; Yan, H.; Luo, B.; Xu, J.; Wang, H.; Mao, Z.; et al. Controlled Growth of Single-Crystal Twelve-Pointed Graphene Grains on a Liquid Cu Surface. Adv. Mater. 2014, 26, 6423–6429. [Google Scholar] [CrossRef]
- Fujita, J.-I.; Miyazawa, Y.; Ueki, R.; Sasaki, M.; Saito, T. Fabrication of Large-Area Graphene Using Liquid Gallium and Its Electrical Properties. Jpn. J. Appl. Phys. 2010, 49, 06GC01. [Google Scholar] [CrossRef]
- Artyukhov, V.I.; Hao, Y.; Ruoff, R.S.; Yakobson, B.I. Breaking of Symmetry in Graphene Growth on Metal Substrates. Phys. Rev. Lett. 2015, 114, 115502. [Google Scholar] [CrossRef] [PubMed]
- Fujita, J.-I.; Ueki, R.; Nishijima, T.; Miyazawa, Y. Characteristics of graphene FET directly transformed from a resist pattern through interfacial graphitization of liquid gallium. Microelectron. Eng. 2011, 88, 2524–2526. [Google Scholar] [CrossRef]
- Yan, P.; Jeong, Y.J.; Islam, M.F.; Pistorius, P.C. Real time and in situ observation of graphene growth on liquid metal surfaces via a carbon segregation method using high-temperature confocal laser scanning microscopy. RSC Adv. 2016, 6, 101235–101241. [Google Scholar] [CrossRef]
- Hiyama, T.; Murakami, K.; Kuwajima, T.; Takeguchi, M.; Fujita, J.-I. Low-temperature growth of graphene using interfacial catalysis of molten gallium and diluted methane chemical vapor deposition. Appl. Phys. Express 2015, 8, 95102. [Google Scholar] [CrossRef]
- Wu, Y.A.; Fan, Y.; Speller, S.C.; Creeth, G.L.; Sadowski, J.T.; He, K.; Robertson, A.; Allen, C.S.; Warner, J.H. Large Single Crystals of Graphene on Melted Copper Using Chemical Vapor Deposition. ACS Nano 2012, 6, 5010–5017. [Google Scholar] [CrossRef]
- Zeng, M.; Tan, L.; Wang, J.; Chen, L.; Rümmeli, M.H.; Fu, L. Liquid Metal: An Innovative Solution to Uniform Graphene Films. Chem. Mater. 2014, 26, 3637–3643. [Google Scholar] [CrossRef]
- Kula, P.; Pietrasik, R.; Dybowski, K.; Atraszkiewicz, R.; Szymański, W.; Kolodziejczyk, L.; Niedzielski, P.; Nowak, D. Single and Multilayer Growth of Graphene from the Liquid Phase. Appl. Mech. Mater. 2014, 510, 8–12. [Google Scholar] [CrossRef]
- Kula, P.; Pietrasik, R.; Dybowski, K.; Atraszkiewicz, R.; Kaczmarek, L.; Kazimierski, D.; Niedzielski, P.; Modrzyk, W. The growth of a polycrystalline graphene from a liquid phase. In Nanotech, Technical Proceedings of the 2013 NSTI Nanotechnology Conference and Expo, NSTI-Nanotech 2013; CRC Press: Boca Raton, FL, USA, 2013; pp. 210–212. [Google Scholar]
- Kula, P.; Pietrasik, R.; Kazimierski, D.; Atraszkiewicz, R.; Dybowski, K.; Szymański, W.; Klimek, L.; Niedzielski, P.; Clapa, M. Resistance-temperature characteristics of CVD and high strength metallurgical graphene. Int. J. Nanotechnol. 2017, 14, 191. [Google Scholar] [CrossRef]
- Kula, P.; Szymański, W.; Kolodziejczyk, L.; Atraszkiewicz, R.; Dybowski, K.; Grabarczyk, J.; Pietrasik, R.; Niedzielski, P.; Kaczmarek, Ł.; Clapa, M. High Strength Metallurgical Graphene—Mechanisms of Growth and Properties/Grafen Metalurgiczny O Wysokiej Wytrzymałości—Mechanizmy Wzrostu I Właściwości. Arch. Met. Mater. 2015, 60, 2535–2542. [Google Scholar] [CrossRef]
- Kula, P.; Rzepkowski, A.; Pietrasik, R.; Atraszkiewicz, R.; Dybowski, K.; Modrzyk, W. Method of Producing Graphene from Liquid Metal. U.S. Patent 9284640B2, 15 March 2016. [Google Scholar]
- Dong, J.; Wang, H.; Peng, H.; Liu, Z.; Zhang, K.; Ding, F. Formation mechanism of overlapping grain boundaries in graphene chemical vapor deposition growth. Chem. Sci. 2016, 8, 2209–2214. [Google Scholar] [CrossRef] [PubMed]
- Min, S.Y.; Cho, C.; Shim, G.W.; Park, I.-J.; Jung, D.Y.; Woo, Y.; Lee, J.-Y.; Choi, S.-Y. Two-dimensional sheet resistance model for polycrystalline graphene with overlapped grain boundaries. FlatChem 2018, 7, 19–25. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Lee, J.-H.; Kim, M.J.; Dash, J.K.; Lee, C.-H.; Joshi, R.; Lee, S.; Hone, J.; Soon, A.; Lee, G.-H. Direct observation of grain boundaries in chemical vapor deposited graphene. Carbon 2017, 115, 147–153. [Google Scholar] [CrossRef]
- Park, S.; Shehzad, M.A.; Khan, M.F.; Nazir, G.; Eom, J.; Noh, H.; Seo, Y. Effect of grain boundaries on electrical properties of polycrystalline graphene. Carbon 2017, 112, 142–148. [Google Scholar] [CrossRef]
- Liu, Y.; Yakobson, B.I. Cones, Pringles, and Grain Boundary Landscapes in Graphene Topology. Nano Lett. 2010, 10, 2178–2183. [Google Scholar] [CrossRef]
- Nie, S.; Wofford, J.M.; Bartelt, N.C.; Dubon, O.D.; Mccarty, K. Origin of the mosaicity in graphene grown on Cu(111). Phys. Rev. B 2011, 84, 155425. [Google Scholar] [CrossRef]
- Biró, L.P.; Lambin, P. Grain boundaries in graphene grown by chemical vapor deposition. New J. Phys. 2013, 15, 35024. [Google Scholar] [CrossRef]
- Tsen, A.W.; Brown, L.; Levendorf, M.P.; Ghahari, F.; Huang, P.Y.; Havener, R.W.; Ruiz-Vargas, C.S.; Muller, D.A.; Kim, P.; Park, J. Tailoring Electrical Transport Across Grain Boundaries in Polycrystalline Graphene. Science 2012, 336, 1143–1146. [Google Scholar] [CrossRef]
- Lee, W.; Kihm, K.D.; Kim, H.G.; Shin, S.; Lee, C.; Park, J.S.; Cheon, S.; Kwon, O.M.; Lim, G.; Lee, W. In-Plane Thermal Conductivity of Polycrystalline Chemical Vapor Deposition Graphene with Controlled Grain Sizes. Nano Lett. 2017, 17, 2361–2366. [Google Scholar] [CrossRef]
- Lee, D.; Kwon, G.D.; Kim, J.H.; Moyen, E.; Lee, Y.H.; Baik, S.; Pribat, D. Significant enhancement of the electrical transport properties of graphene films by controlling the surface roughness of Cu foils before and during chemical vapor deposition. Nanoscale 2014, 6, 12943–12951. [Google Scholar] [CrossRef]
- Incze, P.N.-; Vancsó, P.; Osváth, Z.; Márk, G.I.; Jin, X.; Kim, Y.S.; Hwang, C.; Lambin, P.; Chapelier, C.; Péterbiró, L. Electronic states of disordered grain boundaries in graphene prepared by chemical vapor deposition. Carbon 2013, 64, 178–186. [Google Scholar] [CrossRef]
- Jhon, Y.I.; Zhu, S.-E.; Ahn, J.-H.; Jhon, M.S. The mechanical responses of tilted and non-tilted grain boundaries in graphene. Carbon 2012, 50, 3708–3716. [Google Scholar] [CrossRef]
- Grantab, R.; Shenoy, V.B.; Ruoff, R.S. Anomalous Strength Characteristics of Tilt Grain Boundaries in Graphene. Science 2010, 330, 946–948. [Google Scholar] [CrossRef] [PubMed]
- Tsen, A.W.; Brown, L.; Havener, R.W.; Park, J. Polycrystallinity and Stacking in CVD Graphene. Accounts Chem. Res. 2012, 46, 2286–2296. [Google Scholar] [CrossRef] [PubMed]
- Akinwande, D.; Brennan, C.J.; Bunch, J.S.; Egberts, P.; Felts, J.R.; Gao, H.; Huang, R.; Kim, J.; Li, T.; Li, Y.; et al. A review on mechanics and mechanical properties of 2D materials—Graphene and beyond. Extreme Mech. Lett. 2017, 13, 42–77. [Google Scholar] [CrossRef]
- Rao, R.; Pierce, N.; Xu, Q.; Harutyunyan, A.R. Probing inhomogeneous doping in overlapped graphene grain boundaries by Raman spectroscopy. Carbon 2014, 80, 513–522. [Google Scholar] [CrossRef]
- Lee, G.-H.; Cooper, R.; An, S.J.; Lee, S.; Van Der Zande, A.M.; Petrone, N.; Hammerberg, A.G.; Lee, C.; Crawford, B.; Oliver, W.; et al. High-Strength Chemical-Vapor-Deposited Graphene and Grain Boundaries. Science 2013, 340, 1073–1076. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, Z.; Yuan, Q.; Xin, J.H.; Ding, F. The favourable large misorientation angle grain boundaries in graphene. Nanoscale 2015, 7, 20082–20088. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S.; Cançado, L.G.; Jorio, A.; Saito, R. Studying disorder in graphite-based systems by Raman spectroscopy. Phys. Chem. Chem. Phys. 2007, 9, 1276–1290. [Google Scholar] [CrossRef]
- Malard, L.; Pimenta, M.; Dresselhaus, G.; Dresselhaus, M. Raman spectroscopy in graphene. Phys. Rep. 2009, 473, 51–87. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuten, D.; Dybowski, K.; Atraszkiewicz, R.; Kula, P. Quasi-Monocrystalline Graphene Crystallization on Liquid Copper Matrix. Materials 2020, 13, 2606. https://doi.org/10.3390/ma13112606
Kuten D, Dybowski K, Atraszkiewicz R, Kula P. Quasi-Monocrystalline Graphene Crystallization on Liquid Copper Matrix. Materials. 2020; 13(11):2606. https://doi.org/10.3390/ma13112606
Chicago/Turabian StyleKuten, Dominika, Konrad Dybowski, Radomir Atraszkiewicz, and Piotr Kula. 2020. "Quasi-Monocrystalline Graphene Crystallization on Liquid Copper Matrix" Materials 13, no. 11: 2606. https://doi.org/10.3390/ma13112606
APA StyleKuten, D., Dybowski, K., Atraszkiewicz, R., & Kula, P. (2020). Quasi-Monocrystalline Graphene Crystallization on Liquid Copper Matrix. Materials, 13(11), 2606. https://doi.org/10.3390/ma13112606