Comparison of the Hydration Characteristics of Ultra-High-Performance and Normal Cementitious Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Measurement Methods
2.2.1. Preparation of UHPC
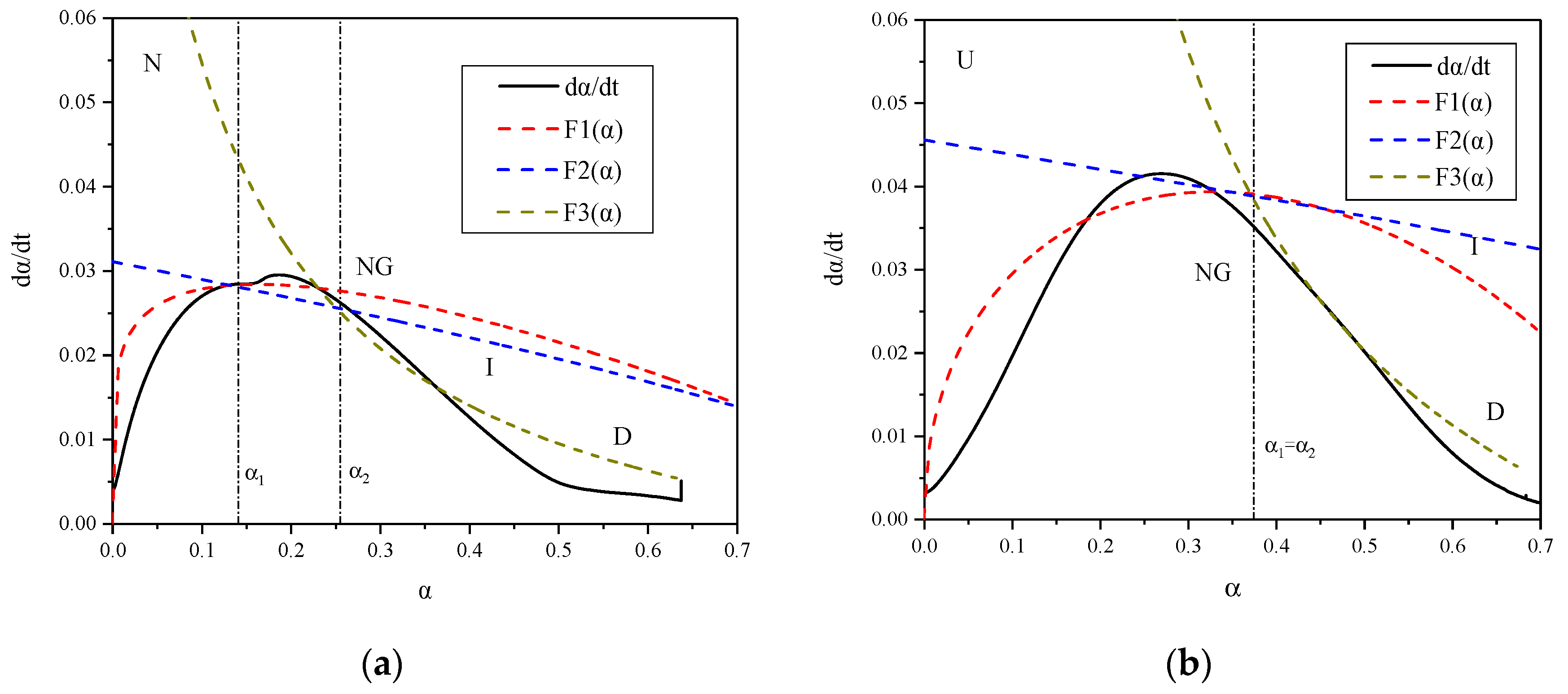
2.2.2. Krstulovic-Dabic Model
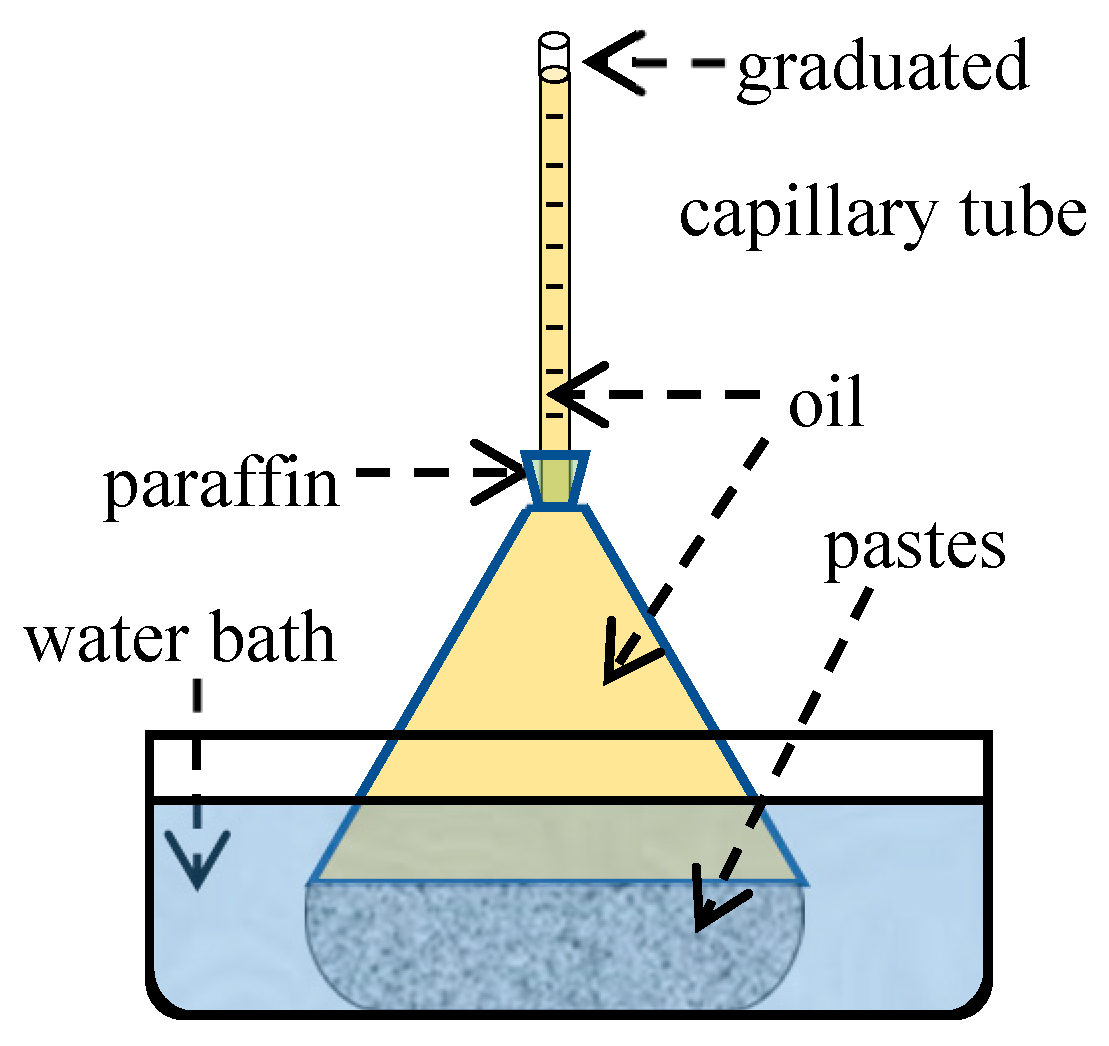
2.2.3. Chemical Shrinkage Test
- (1)
- Firstly, put the weighed material powders, including the water reducer, into a pycnometer, and add water. A small electric whisk was used to mix the powder and water into a uniform slurry.
- (2)
- Secondly, vibrate the pycnometer slightly to remove air bubbles, and pour oil into the bottle until its neck.
- (3)
- Then, insert the graduated tube into the pycnometer and seal it with paraffin. Pour oil from the top of the graduated tube to raise the liquid level until it reaches the scale line.
- (4)
- Finally, place the pycnometer in a water bath at 20 °C, and record the descent height of the liquid level at specific intervals.
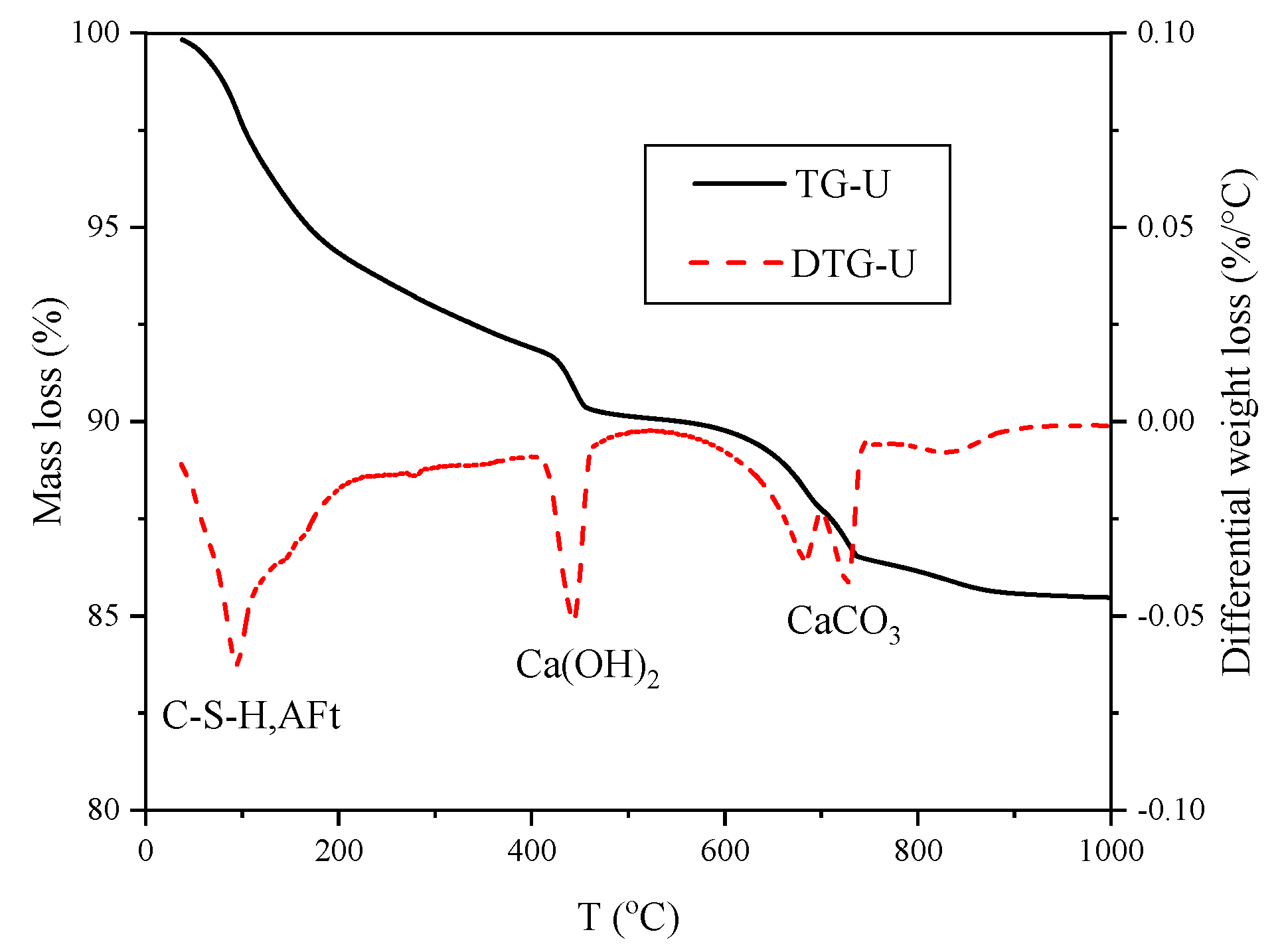
2.2.4. TG and XRD
3. Results and Discussion
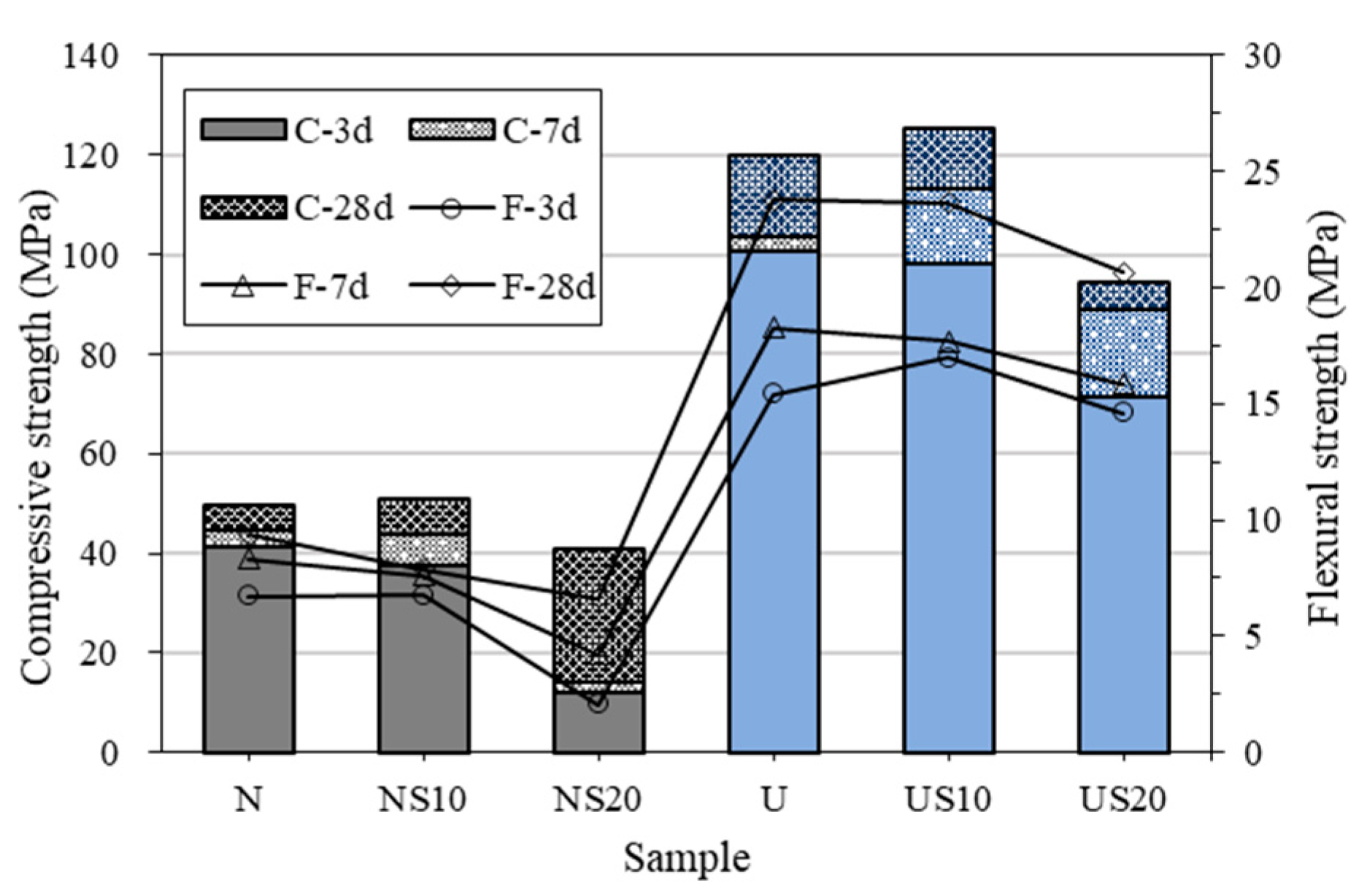
3.1. Mechanical Property
3.2. Analysis of Calorimetric Data
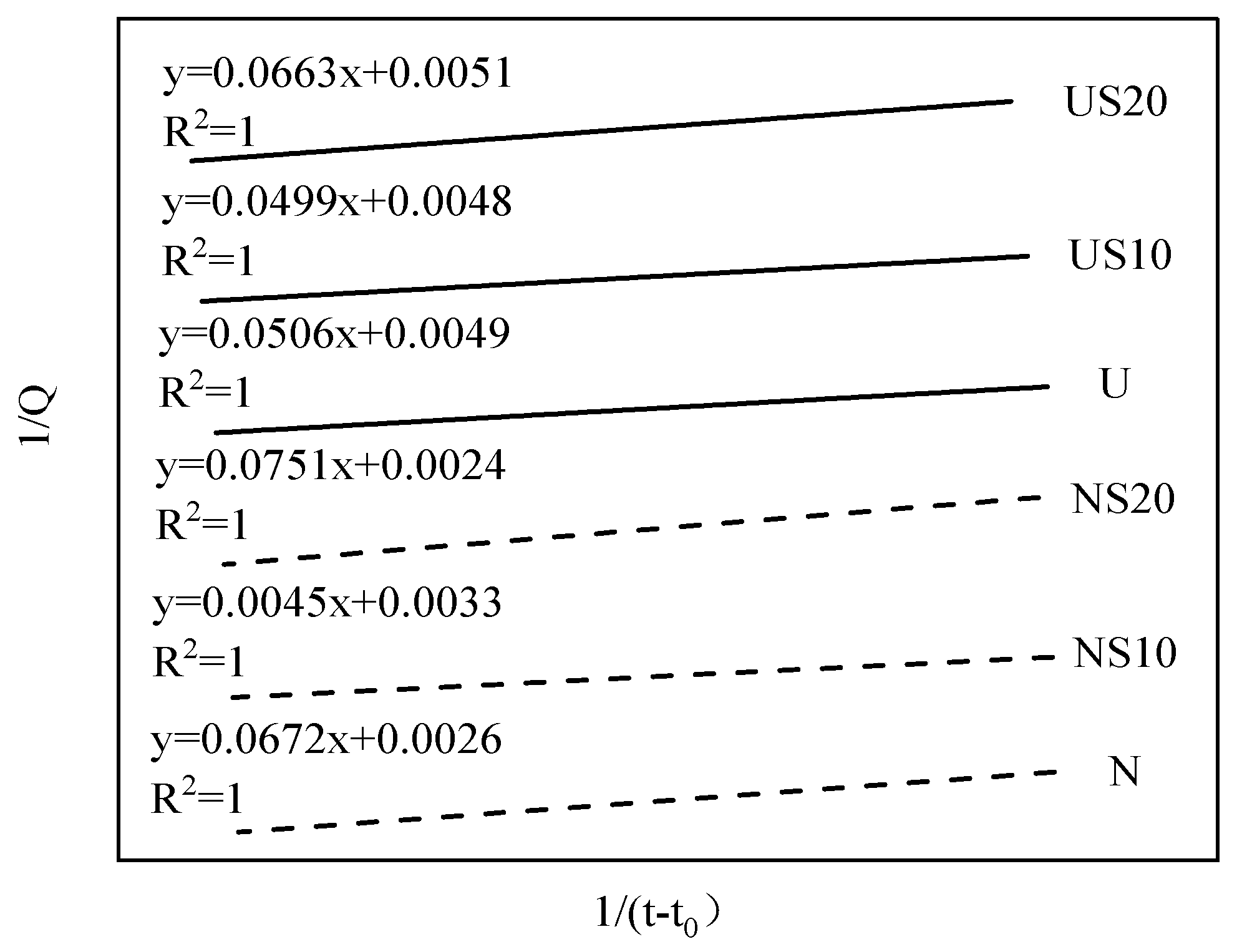
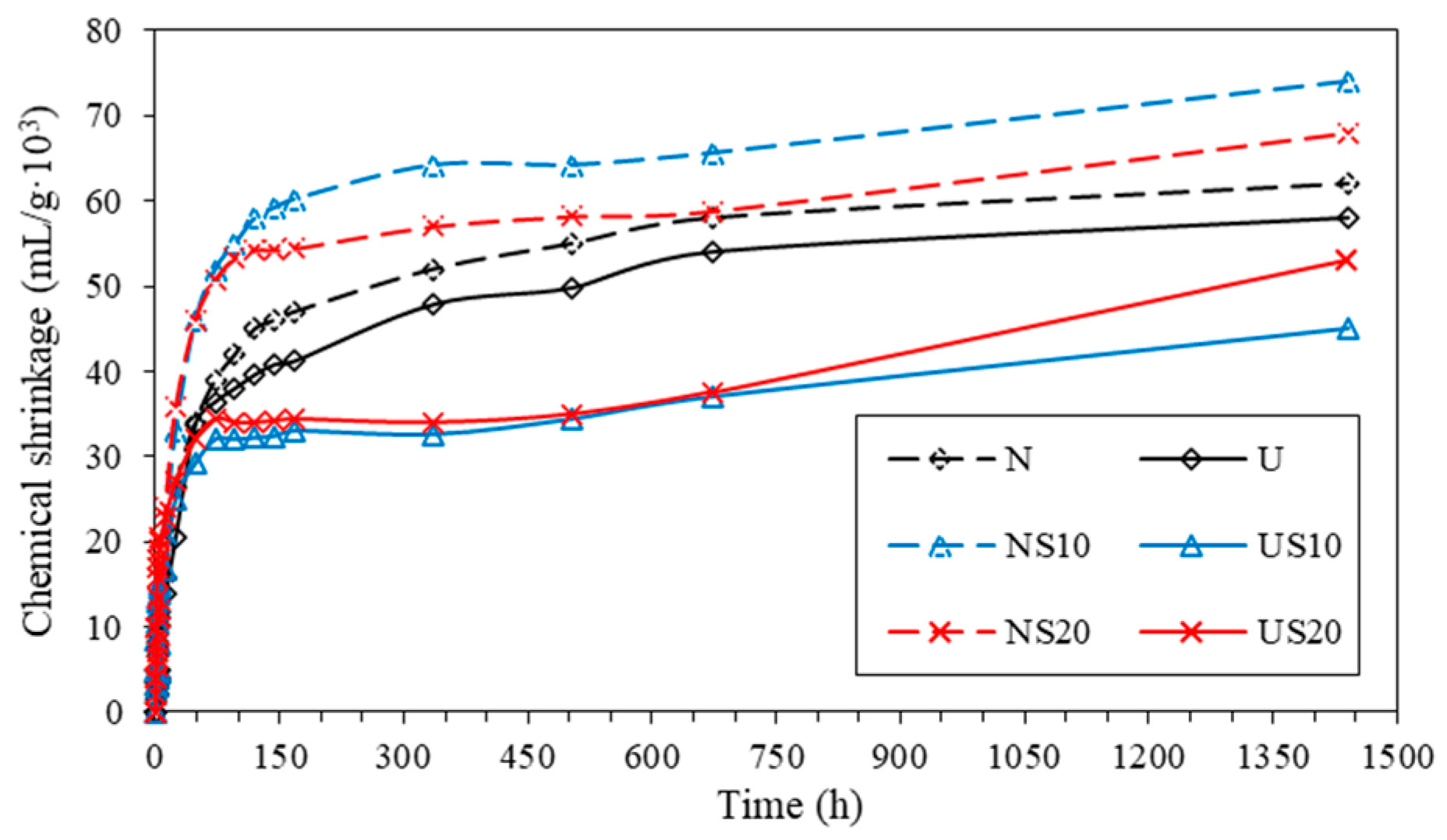
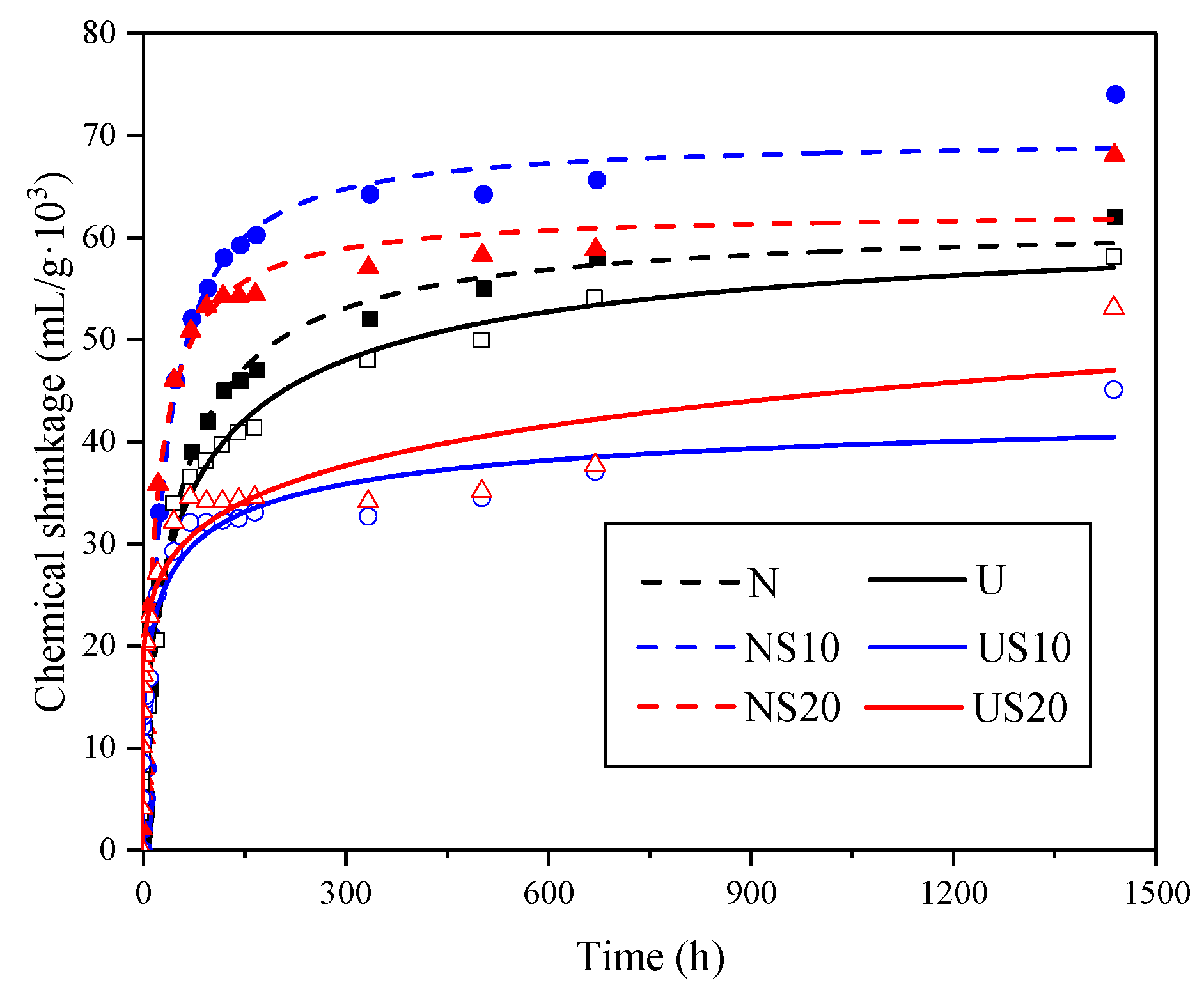
3.3. Prediction of Ultimate Chemical Shrinkage
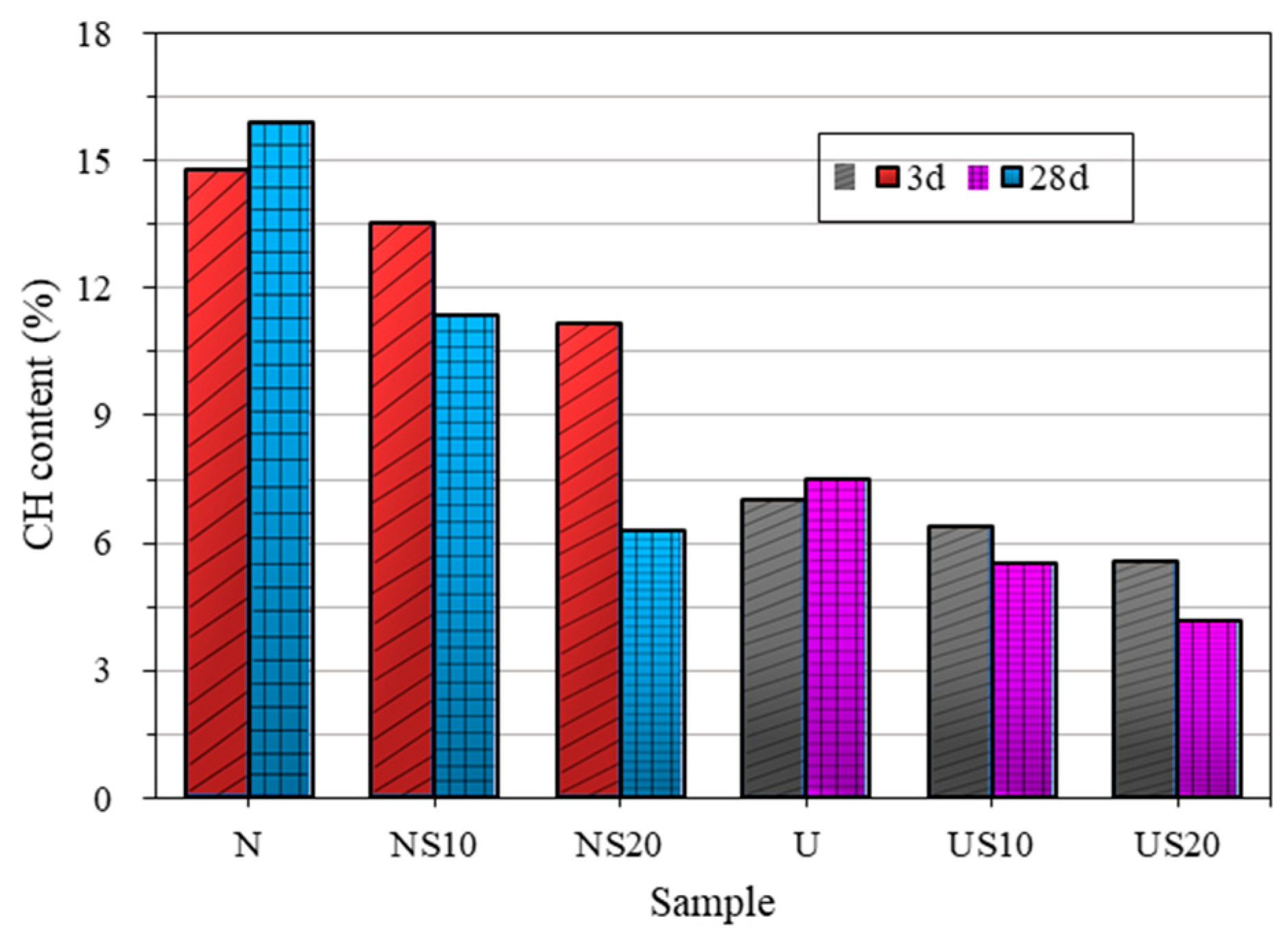
3.4. Calculation of Phase Content from Combined TG /XRD
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, D.; Shi, C.; Wu, Z.; Xiao, J.; Huang, Z.; Fang, Z. A review on ultra high performance concrete: Part ii. hydration, microstructure and properties. Constr. Build. Mater. 2015, 96, 368–377. [Google Scholar] [CrossRef]
- Huang, H.; Gao, X.; Li, L.; Saleem, M.A. A review on ultra high performance concrete on mechanical performance of UHPC. Constr. Build. Mater. 2018, 188, 709–721. [Google Scholar] [CrossRef]
- Singh, M.; Sheikh, A.H.; Ali, M.S.M.; Visintin, P.; Griffith, M.C. Experimental and numerical study of the flexural behaviour of ultra-high performance fibre reinforced concrete beams. Constr. Build. Mater. 2017, 138, 12–25. [Google Scholar] [CrossRef]
- Zhang, Y.; Bouillona, C.; Vlasopoulos, N. Measuring and modeling hydration kinetics of well cements under elevated temperature and pressure using chemical shrinkage test method. Cem. Concr. Res. 2019, 123, 105768. [Google Scholar] [CrossRef]
- Zhang, J.; Weissinger, E.A.; Peethamparan, S.; Scherer, G.W. Early hydration and setting of oil well cement. Cem. Concr. Res. 2010, 40, 1023–1033. [Google Scholar] [CrossRef]
- Pang, X.; Meyer, C.; Darbe, R.; Funkhouser, G.P. Modeling the effect of curing temperature and pressure on cement hydration kinetics. ACI Mater. J. 2013, 110, 137–147. [Google Scholar]
- Lam, L.; Wong, Y.L.; Poon, C.S. Degree of hydration and gel/space ratio of high-volume fly ash/cement systems. Cem. Concr. Res. 2000, 30, 747–756. [Google Scholar] [CrossRef]
- Huang, W.; Kazemi-Kamyab, H.; Sun, W.; Scrivener, K. Effect of cement substitution by limestone on the hydration and microstructural development of ultra-high performance concrete (UHPC). Cem. Concr. Compos. 2017, 77, 86–101. [Google Scholar] [CrossRef]
- Yan, P.Y.; Zheng, F. Kinetics model for the hydration mechanism of cementitious materials. Ku Suan Jen Hsueh Pao/J. Chin. Ceram. Soc. 2006, 34, 555–559. [Google Scholar]
- Zhang, X. Thermokinetic Study on Hydration of Cementitious Materials. Master’s Thesis, Wuhan University of Technology, Wuhan, China, April 2011. (In Chinese). [Google Scholar]
- Korpa, A.; Kowald, T.; Trettin, R. Phase development in normal and ultra high performance cementitious systems by quantitative X-ray analysis and thermoanalytical methods. Cem. Concr. Res. 2009, 39, 69–76. [Google Scholar] [CrossRef]
- Pang, X.; Bentz, D.P.; Meyer, C.; Funkhouser, G.P.; Darbe, R. A comparison study of portland cement hydration kinetics as measured by chemical shrinkage and isothermal calorimetry. Cem. Concr. Compos. 2013, 39, 23–32. [Google Scholar] [CrossRef]
- Shen, P.; Lu, L.; He, Y.; Rao, M.; Fu, Z.; Wang, F.; Hu, S. Experimental investigation on the autogenous shrinkage of steam cured ultra-high performance concrete. Constr. Build. Mater. 2018, 162, 512–522. [Google Scholar] [CrossRef]
- Ali, S.; Sama, T.; Behzad, T. Effect of materials proportion on rheology and mechanical strength and microstructure of ultra-high performance concrete (UHPC). Constr. Build. Mater. 2018, 187, 1103–1112. [Google Scholar]
- GB/T 17671–1999. Method of Testing Cements-Determination of Strength; The State Bureau of Quality and Technical Supervision: Beijing, China, 1999.
- Krstulovic, R.; Dabic, P. A conceptual model of the cement hydration process. Cem. Concr. Res. 2000, 30, 693–698. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C. Study on hydration heat evolution model of fly ash-slag-cement ternary system. Bull. Chin. Ceram. Soc. 2019, 38, 2695–2700. (In Chinese) [Google Scholar]
- ASTM C1608. Standard Test Method for Chemical Shrinkage of Hydraulic Cement Paste; ASTM International: West Conshocken, PA, USA, 2017. [Google Scholar]
- Lin, Y.; Yan, J.; Wang, Z.; Fan, F.; Zou, C. Effect of silica fumes on fluidity of UHPC: Experiments, influence mechanism and evaluation methods. Constr. Build. Mater. 2019, 210, 451–460. [Google Scholar] [CrossRef]
- Shi, C.; Xiao, J.; Cao, Z.; Wang, D.; Wu, Z.; Qu, Z.H. Effects of UHPC constituents on its properties. Bull. Chin. Ceram. Soc. 2013, 32, 1005–1011. (In Chinese) [Google Scholar]
- Chong, W.; Xin-Cheng, P.U.; Ke, C.; Fang, L.; Jian-Hua, W.U.; Xiao-Qin, P. Measurement of hydration progress of cement paste materials with extreme-low w/b. J. Mater. Sci. Eng. 2008, 26, 852–857. [Google Scholar]
- Xu, J.; Xu, Y.; Wang, S. Experimental study on the influence of steel fiber and silica fume on the properties of UHPC. In Fly Ash Comprehensive Utilization; Hebei building materials industry Office: Baoding, China, 2017; pp. 8–10. (In Chinese) [Google Scholar]
- Powers, T.C. Properties of cement paste and concrete. In Proceedings of the Fourth International Symposium on the Chemical of Cement, Washington, DC, USA, 2–7 October 1960; Volume 2, p. 577. [Google Scholar]
- Wille, K.; Naaman, A.E.; Parra-Montesinos, G.J. Ultra-high performance concrete with compressive strength exceeding 150 MPa (22 ksi) a simpler way. ACI Mater. J. 2011, 108, 46–54. [Google Scholar]
- ASTM C1679-17. Standard Practice for Measuring Hydration Kinetics of Hydraulic Cementitious Mixtures Using Isothermal Calorimetry; ASTM International: West Conshocken, PA, USA, 2017. [Google Scholar]
- Lothenbach, B.; Rentsch, D.; Wieland, E. Hydration of a silica fume blended low-alkali shotcrete cement. Phys. Chem. Earth. Parts A/B/C. 2014, 70, 3–16. [Google Scholar] [CrossRef]
- Weerdt, K.D.; Haha, M.B.; Saout, G.L.; Kjellsen, K.O.; Justnes, H.; Lothenbach, B. Hydration mechanisms of ternary portland cements containing limestone powder and fly ash. Cem. Concr. Res. 2011, 41, 279–291. [Google Scholar] [CrossRef]
- Mitchell, D.R.G.; Hinczak, I.; Day, R.A. Interaction of silica fume with calcium hydroxide solutions and hydrated cement paste. Cem. Concr. Res. 1998, 28, 1571–1584. [Google Scholar] [CrossRef]
- Sánchez de Rojas, M.I.; Luxán, M.P.D.; Frías, M.; García, N. The influence of different additions on portland cement hydration heat. Cem. Concr. Res. 1993, 23, 46–54. [Google Scholar]
- Larbi, J.A.; Fraay, A.L.A.; Bijen, J.M. Chemical of the porefluid of silica fume-blended cement system. Cem. Concr. Res. 1990, 20, 506–516. [Google Scholar] [CrossRef]
- Johann, P.; Christof, S.; Mirko, G.; Matthias, L.; Roland, S. Effectiveness of polycarboxylate superplasticizers in ultra-high strength concrete: The importance of PCE compatibility with silica fume. J. Adv. Concr. Technol. 2009, 7, 5–12. [Google Scholar]
- GUO, Y.; Wang, Q.; Wang, Y.; Xie, S. Pozzolanic effect of composite mineral admixture under different water cement ratio. Concrete 2017, 8, 42–45. (In Chinese) [Google Scholar]
- Wei, Y.; Yao, W.; Xing, X.; Wu, M. Quantitative evaluation of hydrated cement modified by silica fume using QXRD, 27Al MAS NMR, TG-DSC and selective dissolution techniques. Constr. Build. Mater. 2012, 36, 925–932. [Google Scholar] [CrossRef]
- Geiker, M.; Knudsen, T. Chemical shrinkage of Portland cement pastes. Cem. Concr. Res. 1982, 12, 603–610. [Google Scholar] [CrossRef]
- Mounanga, P.; Khelidj, A.; Loukili, A.; Baroghel-Bouny, V. Erratum to “Predicting Ca(OH)2 content and chemical shrinkage of hydrating cement pastes using analytical approach”. Cem. Concr. Res. 2005, 35, 423–424. [Google Scholar] [CrossRef]
- Mounanga, P.; Khelidj, A.; Loukili, A.; Baroghel-Bouny, V. Predicting Ca(OH)2 content and chemical shrinkage of hydrating cement pastes using analytical approach. Cem. Concr. Res. 2004, 34, 255–265. [Google Scholar] [CrossRef]
- Xiao, K.; Yang, H.; Dong, Y. Study on the Influence of Admixture on Chemical Shrinkage of Cement Based Materials. Key Eng. Mater. 2009, 405, 226–233. [Google Scholar] [CrossRef]
- Bouasker, M.; Mounanga, P.; Turcry, P.; Loukili, A.; Khelidj, A. Chemical shrinkage of cement pastes and mortars at very early age: Effect of limestone filler and granular inclusions. Cem. Concr. Compos. 2008, 30, 13–22. [Google Scholar] [CrossRef]
- Singh, N.B.; Sarvahi, R.; Singh, N.P.; Shukla, A.K. Effect of temperature on the hydration of ordinary Portland cement in the presence of a superplasticizer. Thermochim. Acta 1994, 247, 381–388. [Google Scholar] [CrossRef]
- Zhu, H.; Li, C.; Cheng, Y.; Wu, K.; Jiang, Z. Pozzolanicity of fly ash modified by fluidized bed reactor-vapor deposition. Constr. Build. Mater. 2017, 156, 719–727. [Google Scholar] [CrossRef]








| Materials | CaO | SiO2 | Al2O3 | Fe2O3 | SO3 | MgO | K2O | Na2O |
|---|---|---|---|---|---|---|---|---|
| C | 65.00 | 20.90 | 4.56 | 3.23 | 2.65 | 0.87 | 0.71 | 0.10 |
| SF | 0.46 | 97.77 | 0.22 | 0.07 | 0.16 | 0.44 | 0.42 | 0.15 |
| NC | Cement | SF | UHPC | Cement | SF |
|---|---|---|---|---|---|
| N | 1 | - | U | 1 | - |
| NS10 | 0.9 | 0.1 | US10 | 0.9 | 0.1 |
| NS20 | 0.8 | 0.2 | US20 | 0.8 | 0.2 |
| Sample | Q3d (J/g) | t0 (h) | t50 (h) | Qmax (J/g) | Knudsen’s Extrapolation Formula |
|---|---|---|---|---|---|
| N | 282.17 | 1.33 | 25.85 | 384.62 | y = 0.0672x + 0.0026 |
| NS10 | 252.47 | 1.47 | 13.64 | 303.03 | y = 0.0045x + 0.0033 |
| NS20 | 292.29 | 1.24 | 31.29 | 416.67 | y = 0.0751x + 0.0024 |
| U | 176.83 | 3.53 | 10.33 | 204.08 | y = 0.0506x + 0.0049 |
| US10 | 181.45 | 3.28 | 10.40 | 208.33 | y = 0.0499x + 0.0048 |
| US20 | 166.33 | 3.02 | 13.00 | 196.08 | y = 0.0663x + 0.0051 |
| Sample | CSU (mL/g·103) | t0.5 (h) | R2 |
|---|---|---|---|
| N | 62.46 ± 1.47 | 40.49 | 1.00 |
| NS10 | 68.87 ± 1.21 | 27.81 | 0.99 |
| NS20 | 62.35 ± 1.32 | 19.37 | 0.99 |
| U | 60.46 ± 2.51 | 55.95 | 1.00 |
| US10 | 45.57 ± 3.97 | 20.83 | 0.97 |
| US20 | 52.29 ± 0.00 | 22.51 | 1.00 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, H.; Zhu, H.; Gou, H.; Yang, Z. Comparison of the Hydration Characteristics of Ultra-High-Performance and Normal Cementitious Materials. Materials 2020, 13, 2594. https://doi.org/10.3390/ma13112594
Zhou H, Zhu H, Gou H, Yang Z. Comparison of the Hydration Characteristics of Ultra-High-Performance and Normal Cementitious Materials. Materials. 2020; 13(11):2594. https://doi.org/10.3390/ma13112594
Chicago/Turabian StyleZhou, Haiyun, Hongbo Zhu, Hongxiang Gou, and Zhenghong Yang. 2020. "Comparison of the Hydration Characteristics of Ultra-High-Performance and Normal Cementitious Materials" Materials 13, no. 11: 2594. https://doi.org/10.3390/ma13112594
APA StyleZhou, H., Zhu, H., Gou, H., & Yang, Z. (2020). Comparison of the Hydration Characteristics of Ultra-High-Performance and Normal Cementitious Materials. Materials, 13(11), 2594. https://doi.org/10.3390/ma13112594





