Rheological Study of Phenol Formaldehyde Resole Resin Synthesized for Laminate Application
Abstract
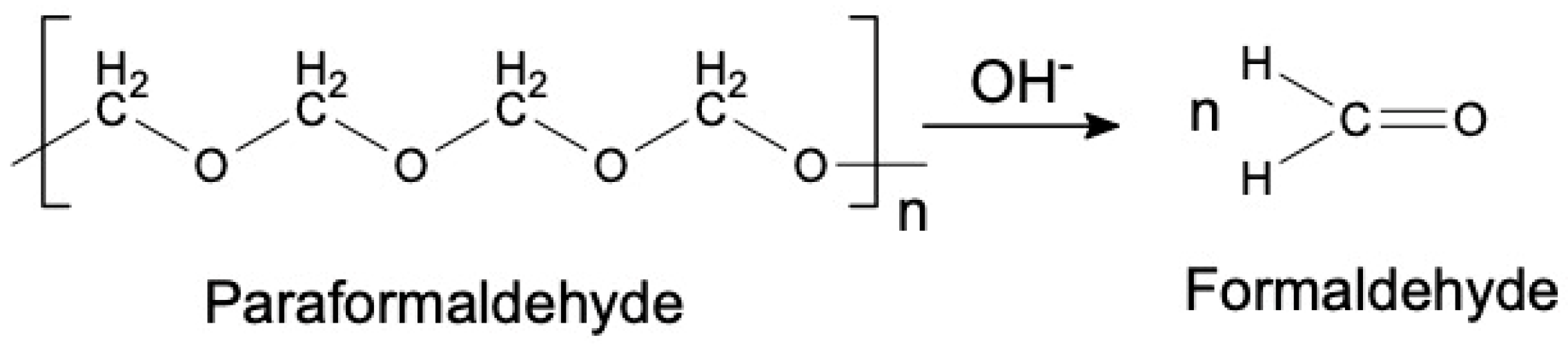
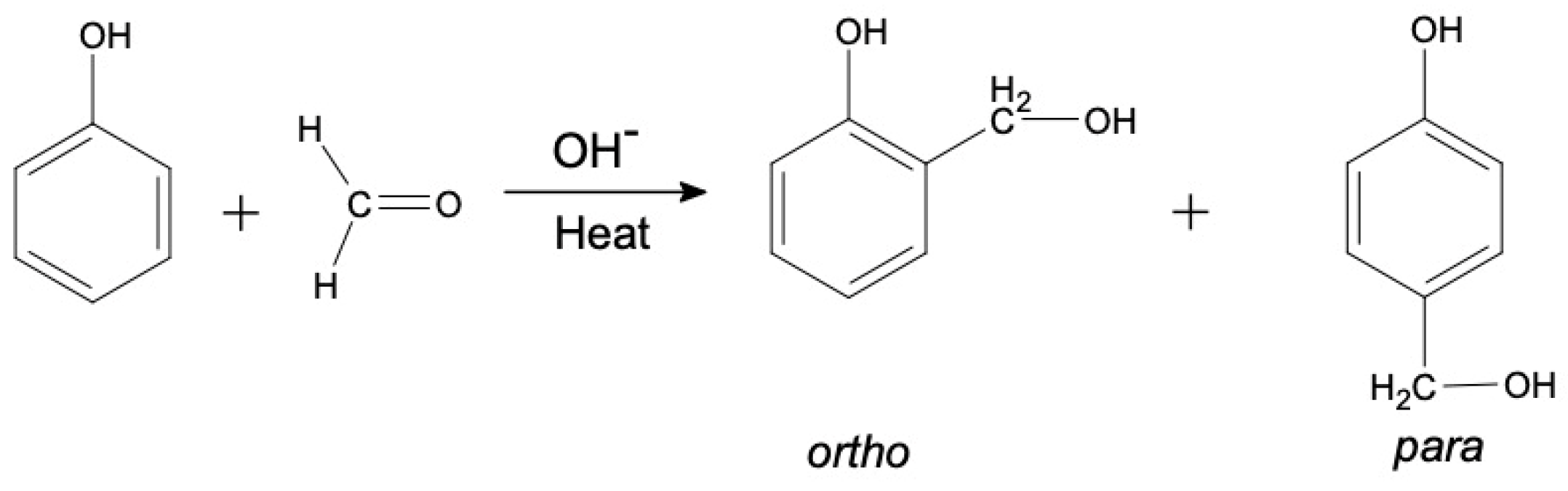
1. Introduction
2. Materials and Methods
2.1. Synthesis of Phenol Formaldehyde (PF) Resin
2.2. Rheological Behavior of Phenol Formaldehyde (PF) Resin
2.3. Viscosity Measurement
2.4. Viscoelasticity Analysis
3. Results and Discussions
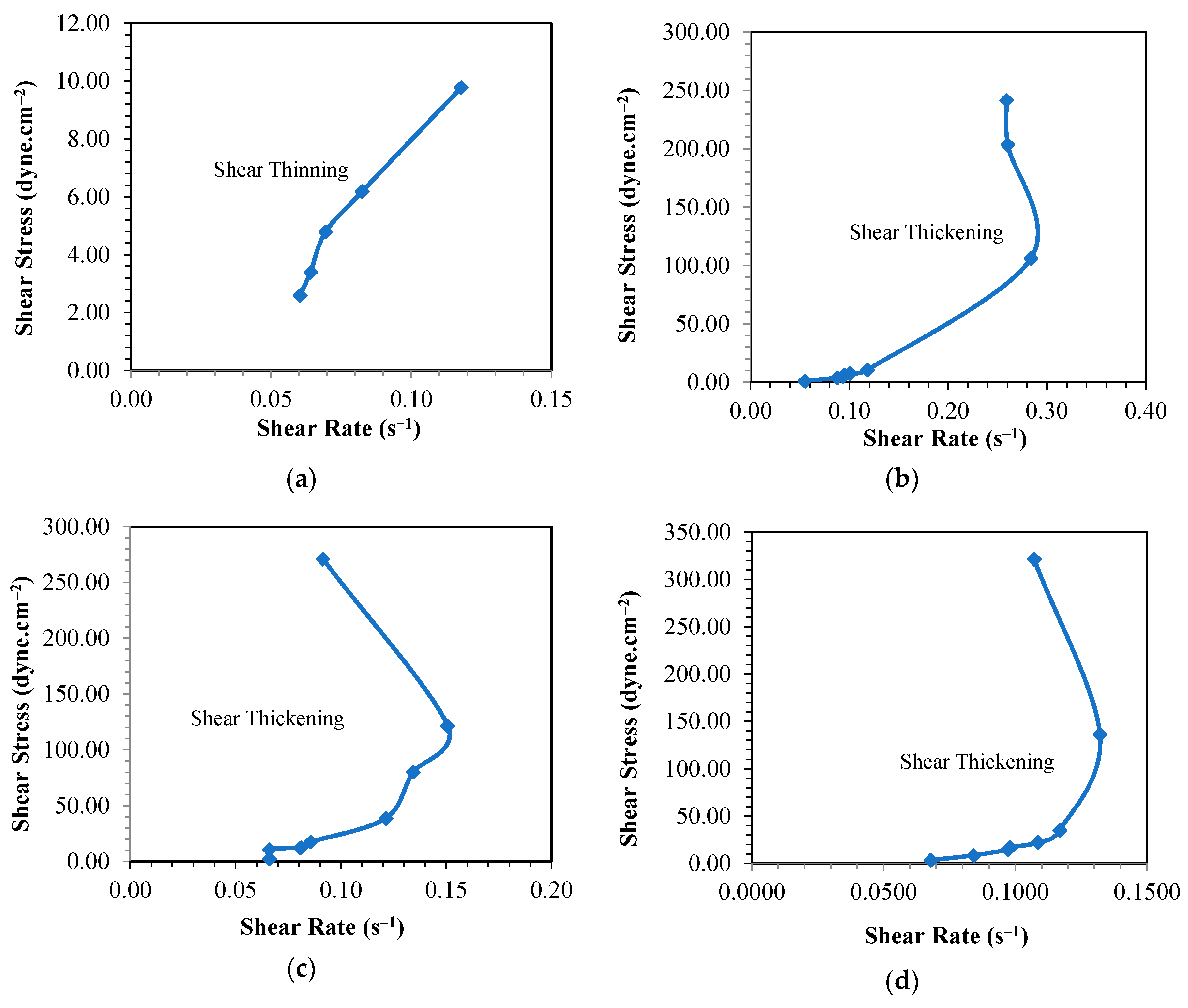
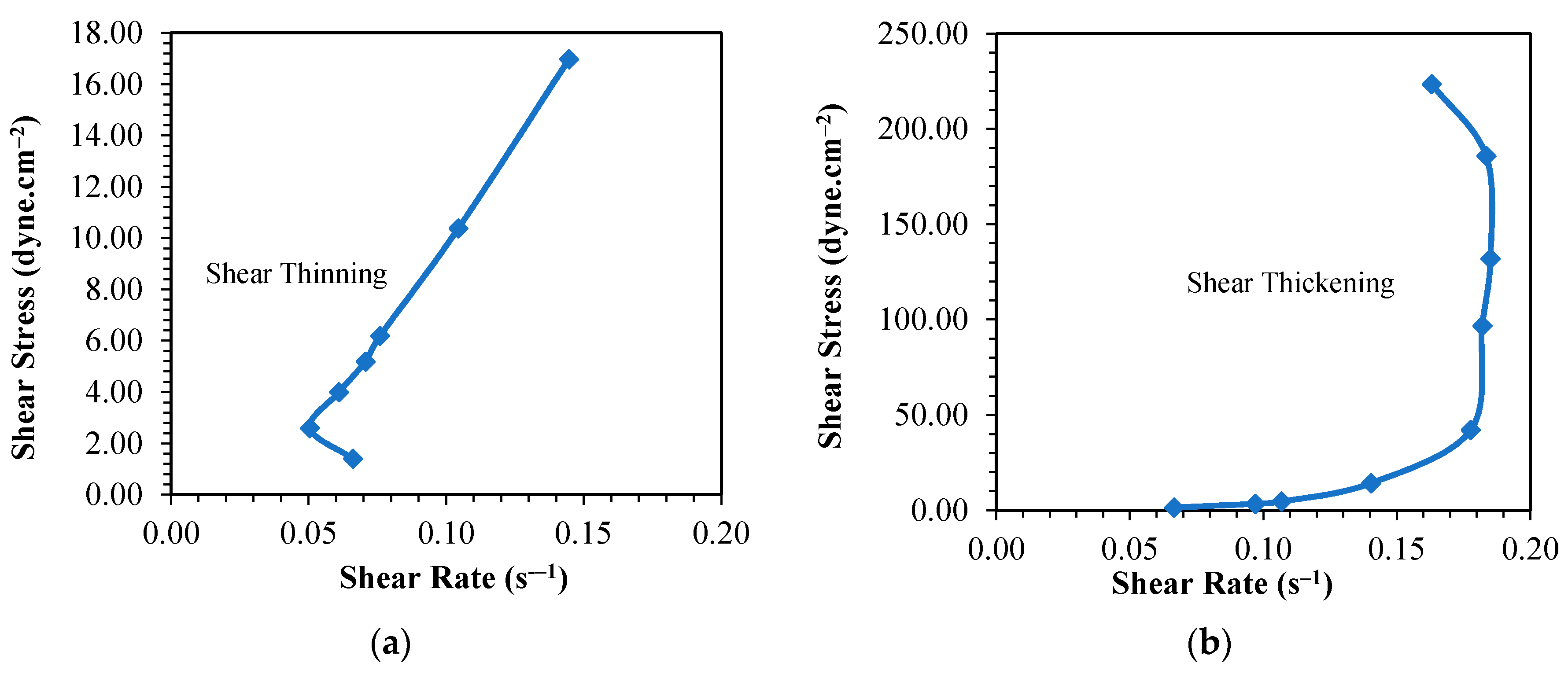
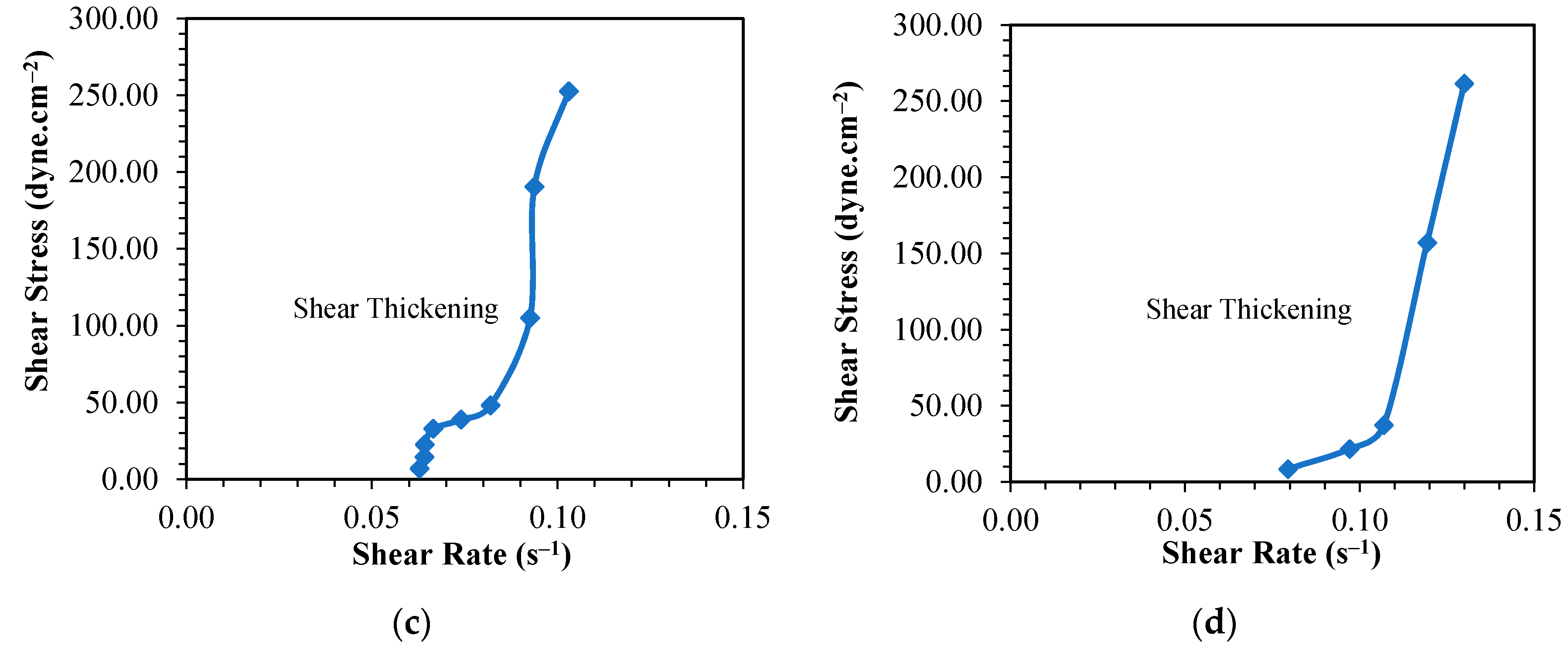
3.1. Rheological Behavior of Phenol Formaldehyde (PF) Resin
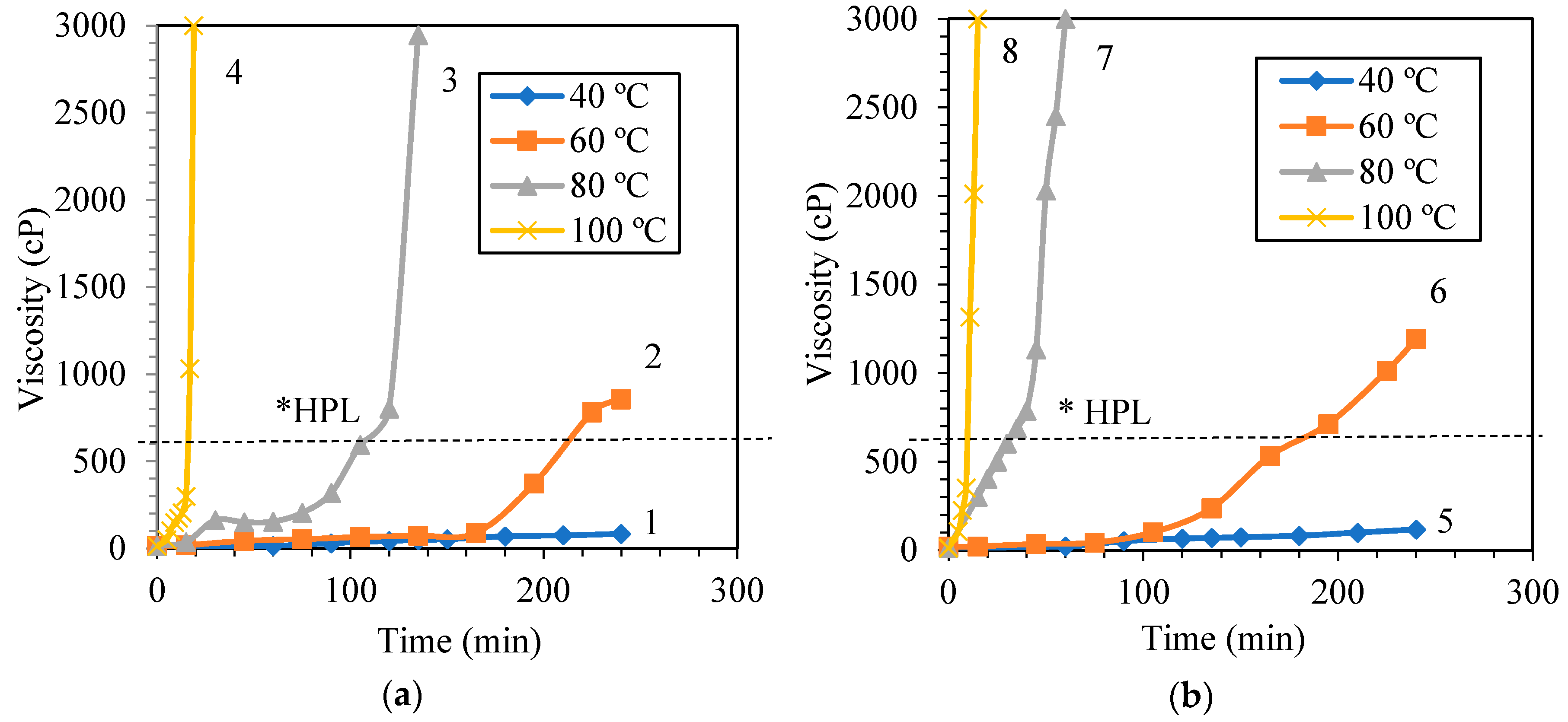
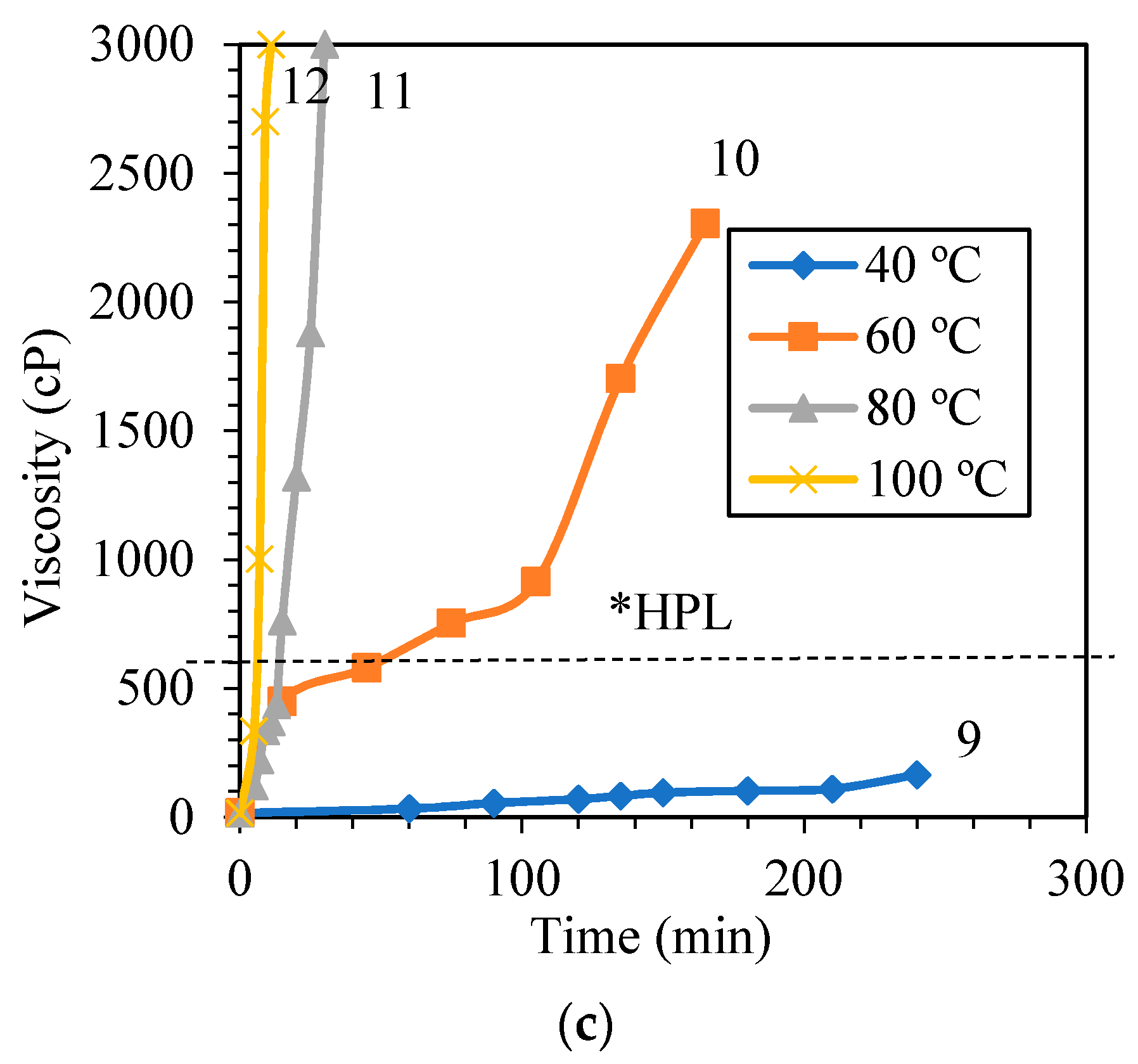
3.1.1. Influence of Synthesis Temperature on the Viscosity of PF Resin
3.1.2. Influence of Formaldehyde Molar Ratio
3.2. Viscoelasticity of Phenol Formaldehyde (PF) Resin
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nair, C.P.R. Non-conventional Phenolic Resins -An Overview on Recent Advances. J. Sci. Ind. Res. 2002, 61, 17–33. [Google Scholar]
- Wang, J.; Laborie, M.-P.G.; Wolcott, M.P. Comparison of model-free kinetic methods for modeling the cure kinetics of commercial phenol–formaldehyde resins. Thermochim. Acta 2005, 439, 68–73. [Google Scholar] [CrossRef]
- Bader, S. Crystic Composites Handbook; Scott Bader Comp: Wollaston, MA, USA, 2002; Volume 45. [Google Scholar]
- Jahanshaei, S.; Tabarsa, T.; Asghari, J. Eco-friendly tannin-phenol formaldehyde resin for producing wood composites. Pigment Resin Technol. 2012, 41, 296–301. [Google Scholar] [CrossRef]
- Ku, H.; Rogers, D.; Davey, R.; Cardona, F.; Trada, M. Fracture Toughness of Phenol Formaldehyde Composites: Pilot Study. J. Mater. Eng. Perform. 2008, 17, 85–90. [Google Scholar] [CrossRef][Green Version]
- Sulaiman, S.; Yunus, R.; Ibrahim, N.A.; Rezaei, F. Effect of Hardener on Mechanical Properties of Carbon Fibre Reinforced Phenolic Resin Composites. J. Eng. Sci. Technol. 2008, 3, 8. [Google Scholar]
- Ganeshram, V.; Achudhan, M. Synthesis and Characterization of Phenol Formaldehyde Resin as a Binder used for Coated Abrasives. Indian J. Sci. Technol. 2013, 6, 10. [Google Scholar]
- Cardona, F.; bin Hamid Sultan, M.T. Characterization of Environmentally Sustainable Resole Phenolic Resins Synthesized with Plant-based Bioresources. BioResources 2015, 11, 965–983. [Google Scholar] [CrossRef][Green Version]
- Kopf, P.W. Phenolic Resins. Encyclopedia of Polymer Science and Technology; John Wiley & Sons: Hoboken, NJ, USA, 2002; Volume 7. [Google Scholar]
- Poljanšek, I.; Krajnc, M. Characterization of Phenol-Formaldehyde Prepolymer Resins by In Line FT-IR Spectroscopy. Acta Chim. Slov. 2005, 52, 238–244. [Google Scholar]
- Cardona, F.; Moscou, C. Synthesis and characterization of modified Phenolic resins for Composites with enhanced mechanical performance. In Proceedings of the 20th Australasian Conference on the Mechanics of Structures and Materials (ACMSM20): Futures in Mechanics of Structures and Materials, Toowoomba, Australia, 2–5 December 2008; pp. 317–322. [Google Scholar]
- Gardziella, A.; Pilato, L.A.; Knop, A. Phenolic Resins: Chemistry, Applications, Standardization, Safety and Ecology, 2nd ed.; Softcover version of original hardcover edition 2000; Springer: Berlin, Germany, 2010; ISBN 978-3-642-08484-3. [Google Scholar]
- Asim, M.; Saba, N.; Jawaid, M.; Nasir, M.; Pervaiz, M.; Alothman, O.Y. A Review on Phenolic Resin and its Composites. CAC 2018, 14, 185–197. [Google Scholar] [CrossRef]
- Younesi-Kordkheili, H.; Pizzi, A. Improving the physical and mechanical properties of particleboards made from urea–glyoxal resin by addition of pMDI. Eur. J. Wood Prod. 2018, 76, 871–876. [Google Scholar] [CrossRef]
- Shafizadeh, J.E.; Guionnet, S.; Tillman, M.S.; Seferis, J.C. Synthesis and characterization of phenolic resole resins for composite applications. J. Appl. Polym. Sci. 1999, 73, 505–514. [Google Scholar] [CrossRef]
- Bajia, S.C.; Swarnkar, P.; Kumar, S.; Bajia, B. Microwave Assisted Synthesis of Phenol-Formaldehyde Resole. J. Chem. 2007, 4, 457–460. [Google Scholar] [CrossRef][Green Version]
- Christjanson, P.; Pehk, T.; Paju, J. Structure and curing mechanism of resol phenol-formaldehyde prepolymer resins. Proc. Est. Acad. Sci. 2010, 59, 225. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, Y.; Wang, C.; Li, S.; Zhang, M.; Chu, F. Preparation and properties of lignin–phenol–formaldehyde resins based on different biorefinery residues of agricultural biomass. Ind. Crop. Prod. 2013, 43, 326–333. [Google Scholar] [CrossRef]
- Lin, W.-S.; Lee, W.-J. Influence of curing temperature on the bonding strength of heat-treated plywood made with melamine-urea-formaldehyde and phenol–formaldehyde resins. Eur. J. Wood Prod. 2018, 76, 297–303. [Google Scholar] [CrossRef]
- Pilato, L. (Ed.) Phenolic Resins: A Century of Progress; Springer: Heidelberg, Germany; New York, NY, USA, 2010; ISBN 978-3-642-04713-8. [Google Scholar]
- Gillern, M.F.; Oita, K.; Teng, R.J.; Tiedeman, G.T. Phenol Formaldehyde Resoles and Laminates. U.S. Patent 4,264,671, 28 April 1981. [Google Scholar]
- Taverna, M.E.; Ollearo, R.; Morán, J.; Nicolau, V.; Estenoz, D.; Frontini, P. Mechanical Evaluation of Laminates Based on Phenolic Resins using Lignins as Partial Substitutes for Phenol. BioResources 2015, 10, 8325–8338. [Google Scholar] [CrossRef]
- Cui, Y.; Hou, X.; Wang, W.; Chang, J. Synthesis and Characterization of Bio-Oil Phenol Formaldehyde Resin Used to Fabricate Phenolic Based Materials. Materials 2017, 10, 668. [Google Scholar] [CrossRef]
- Fleckenstein, M.; Biziks, V.; Mai, C.; Militz, H. Modification of beech veneers with lignin phenol formaldehyde resins in the production of laminated veneer lumber (LVL). Eur. J. Wood Prod. 2018, 76, 843–851. [Google Scholar] [CrossRef]
- Kiernan, J.A. Formaldehyde, Formalin, Paraformaldehyde and Glutaraldehyde: What they are and what they do. Micros Today 2000, 8, 8–13. [Google Scholar] [CrossRef]
- Bhattacharjee, G.; Neogi, S.; Das, S.K. Phenol–formaldehyde runaway reaction: A case study. Int. J. Ind. Chem. 2014, 5, 13. [Google Scholar] [CrossRef]
- Hu, X.-M.; Zhao, Y.-Y.; Cheng, W.-M. Effect of formaldehyde/phenol ratio (F/P) on the properties of phenolic resins and foams synthesized at room temperature. Polym. Compos. 2015, 36, 1531–1540. [Google Scholar] [CrossRef]
- Gabilondo, N.; Larrañaga, M.; Peña, C.; Corcuera, M.A.; Echeverría, J.M.; Mondragon, I. Polymerization of resole resins with several formaldehyde/phenol molar ratios: Amine catalysts against sodium hydroxide catalysts. J. Appl. Polym. Sci. 2006, 102, 2623–2631. [Google Scholar] [CrossRef]
- Niederhauser, W.; Miller, M. Phenol Formaldehyde Adhesives. U.S. Patent 2,357,798, 12 September 1944. [Google Scholar]
- Shafizadeh, J.E.; Seferis, J.C. Characterization of Phenolic Resins for Composite Honeycomb Applications; Society of Manufacturing Engineers: Dearborn, MI, USA, 2000; pp. 1–10. [Google Scholar]
- Turunen, M.; Alvila, L.; Pakkanen, T.T.; Rainio, J. Modification of phenol-formaldehyde resol resins by lignin, starch, and urea. J. Appl. Polym. Sci. 2003, 88, 582–588. [Google Scholar] [CrossRef]
- Barnes, H.A. A Handbook of Elementary Rheology; Univ. of Wales, Institute of Non-Newtonian Fluid Mechanics: Aberystwyth, UK, 2000; ISBN 978-0-9538032-0-0. [Google Scholar]
- Shaghaghi, S.; Beheshty, M.H.; Rahimi, H. Preparation and Rheological Characterization of Phenolic/Glass Prepregs. Iran. Polym. J. 2011, 20, 969–977. [Google Scholar]
- Zhao, C.; Zhang, G.; Zhao, L. Effect of Curing Agent and Temperature on the Rheological Behavior of Epoxy Resin Systems. Molecules 2012, 17, 8587–8594. [Google Scholar] [CrossRef] [PubMed]
- Kordani, N.; Vanini, A.S. Different method to make laminates by shear thickening fluid. Sci. Eng. Compos. Mater. 2014, 21, 421–425. [Google Scholar] [CrossRef]
- Gürgen, S. An investigation on composite laminates including shear thickening fluid under stab condition. J. Compos. Mater. 2019, 53, 1111–1122. [Google Scholar] [CrossRef]
- Parameswaran, P.S. Modification of Phenol Formaldehyde Resin for Improved Mechanical Properties. Ph.D. Thesis, Cochin University of Science and Technology, Kochi, India, 2009. [Google Scholar]
- Park, B.-D.; Riedl, B.; Kim, Y.S.; So, W.T. Effect of synthesis parameters on thermal behavior of phenol-formaldehyde resol resin. J. Appl. Polym. Sci. 2002, 83, 1415–1424. [Google Scholar] [CrossRef]
- Żihorska-gotfryd, A. Phenol-formaldehyde resols modified by boric acid. Polymer 2006, 51, 386–388. [Google Scholar]
- Pizzi, A.; Ibeh, C.C. Phenol–Formaldehydes. In Handbook of Thermoset Plastics; Elsevier: The Boulevard, Oxford, UK, 2014; pp. 13–44. ISBN 978-1-4557-3107-7. [Google Scholar]
- Lorenzo, G.; Zaritzky, N.E.; Califano, A.N. Viscoelastic characterization of fluid and gel like food emulsions stabilized with hydrocolloids. Procedia Food Sci. 2011, 1, 281–286. [Google Scholar] [CrossRef]
- Hashemnejad, S.M.; Kundu, S. Rheological properties and failure of alginate hydrogels with ionic and covalent crosslinks. Soft Matter 2017, 13, 8368–8378. [Google Scholar] [CrossRef]
- Moubarik, A. Rheology Study of Sugar Cane Bagasse Lignin-Added Phenol–Formaldehyde Adhesives. J. Adhes. 2015, 91, 347–355. [Google Scholar] [CrossRef]
- Guseva, D.; Rudyak, V.; Komarov, P.; Bulgakov, B.; Babkin, A.; Chertovich, A. Dynamic and Static Mechanical Properties of Crosslinked Polymer Matrices: Multiscale Simulations and Experiments. Polymers 2018, 10, 792. [Google Scholar] [CrossRef]





| Sample | Molar Ratio of Phenol to Formaldehyde (P:F) | Synthesis Temperature (°C) | Synthesis Period (min) | Sampling Intervals (min) |
|---|---|---|---|---|
| PF I | 1.00:1.25 | 40 | 240 | 30 |
| 1.00:1.25 | 60 | 240 | 30 | |
| 1.00:1.25 | 80 | 240 | 15 | |
| 1.00:1.25 | 100 | 240 | 2 | |
| PF II | 1.00:1.50 | 40 | 240 | 30 |
| 1.00:1.50 | 60 | 240 | 30 | |
| 1.00:1.50 | 80 | 240 | 5 | |
| 1.00:1.50 | 100 | 240 | 2 | |
| PF III | 1.00:1.75 | 40 | 240 | 30 |
| 1.00:1.75 | 60 | 240 | 30 | |
| 1.00:1.75 | 80 | 240 | 2 | |
| 1.00:1.75 | 100 | 240 | 2 |
| Molar Ratio of Phenol to Formaldehyde (PF) Resin | Synthesis Temperature (°C) | Synthesis Time (min) | PF Resin Viscosity (cP) | Remarks |
|---|---|---|---|---|
| PF I (1.00:1.25) | 40 | 240 | 83 | Runny |
| 60 | 195 | 373 * | Slightly viscous | |
| 240 | 850 | Very viscous | ||
| 80 | 105 | 595 * | Viscous | |
| 135 | 2946 | Almost Solid | ||
| 100 | 15 | 299 | Runny | |
| 20 | 3000 | Solid | ||
| PF II (1.00:1.50) | 40 | 240 | 117 | Runny |
| 60 | 165 | 532 * | Viscous | |
| 240 | 1192 | Highly viscous | ||
| 80 | 30 | 492 * | Viscous | |
| 60 | 3000 | Solid | ||
| 100 | 9 | 351 * | Slightly viscous | |
| 15 | 3000 | Solid | ||
| PF III (1.00:1.75) | 40 | 240 | 165 | Runny |
| 60 | 15 | 450 * | Viscous | |
| 165 | 2305 | Highly viscous | ||
| 80 | 13 | 436 * | Viscous | |
| 30 | 3000 | Solid | ||
| 100 | 5 | 335 * | Slightly viscous | |
| 12 | 3000 | Solid |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamarudin, N.; Awang Biak, D.R.; Zainal Abidin, Z.; Cardona, F.; Sapuan, S.M. Rheological Study of Phenol Formaldehyde Resole Resin Synthesized for Laminate Application. Materials 2020, 13, 2578. https://doi.org/10.3390/ma13112578
Kamarudin N, Awang Biak DR, Zainal Abidin Z, Cardona F, Sapuan SM. Rheological Study of Phenol Formaldehyde Resole Resin Synthesized for Laminate Application. Materials. 2020; 13(11):2578. https://doi.org/10.3390/ma13112578
Chicago/Turabian StyleKamarudin, Nuruldiyanah, Dayang Radiah Awang Biak, Zurina Zainal Abidin, Francisco Cardona, and Salit Mohd Sapuan. 2020. "Rheological Study of Phenol Formaldehyde Resole Resin Synthesized for Laminate Application" Materials 13, no. 11: 2578. https://doi.org/10.3390/ma13112578
APA StyleKamarudin, N., Awang Biak, D. R., Zainal Abidin, Z., Cardona, F., & Sapuan, S. M. (2020). Rheological Study of Phenol Formaldehyde Resole Resin Synthesized for Laminate Application. Materials, 13(11), 2578. https://doi.org/10.3390/ma13112578






