Energy Transfer Study on Tb3+/Eu3+ Co-Activated Sol-Gel Glass-Ceramic Materials Containing MF3 (M = Y, La) Nanocrystals for NUV Optoelectronic Devices
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Excitation Spectra of Fabricated Tb3+,Eu3+ Co-Doped Sol-Gel Materials
3.2. Influence of Controlled Heat-Treatment at 350 °C on Tb3+ → Eu3+ Energy Transfer
3.3. Luminescence Decay Analysis of the 5D4 (Tb3+) and the 5D0 (Eu3+) Levels in Sol-Gel Materials
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jamalaiah, B.C.; Venkatramaiah, N.; Rao, T.R.; Rasool, S.N.; Rao, B.N.; Ram, D.V.R.; Reddy, A.S.N. UV excited SrAl2O4: Tb3+ nanophosphors for photonic applications. Mat. Sci. Semicon. Proc. 2020, 105, 104722. [Google Scholar] [CrossRef]
- Dalmis, R.; Keskin, O.Y.; Azem, N.F.A.; Birlik, I. A new one-dimensional photonic crystal combination of TiO2/CuO for structural color applications. Ceram. Int. 2019, 45, 21333–21340. [Google Scholar] [CrossRef]
- Bian, W.; Lin, Y.; Wang, T.; Yu, X.; Qiu, J.; Zhou, M.; Luo, H.; Yu, S.F.; Xu, X. Direct Identification of surface defects and their influence on the optical characteristics of upconversion nanoparticles. ACS Nano. 2018, 12, 3623–3628. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.; Song, Y.; Tan, H.; Xie, S.; Zhang, C.; Xu, L.; Xu, J.; Zheng, J. Design of core/active-shell NaYF4:Ln3+@NaYF4:Yb3+ nanophosphors with enhanced red-green-blue upconversion luminescence for anti-counterfeiting printing. Compos. Part B—Eng. 2019, 179, 107504. [Google Scholar] [CrossRef]
- Wang, T.; Yu, H.; Siu, C.K.; Qiu, J.; Xu, X.; Yu, S.F. White-light whispering-gallery-mode lasing from lanthanide-doped upconversion NaYF4 hexagonal microrods. ACS Photonics 2017, 4, 1539–1543. [Google Scholar] [CrossRef]
- Chen, J.; Peng, Y.; Li, X.; Chen, W.; Huang, H.; Lin, L.; Chen, D. Near-infrared-laser-driven robust glass-ceramic-based upconverted solid-state lighting. J. Mater. Chem. C 2019, 7, 4109–4117. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, D.; Yang, Y.; Ren, P.; Qiu, J. Adjustable multicolor up-energy conversion in light-luminescence in Tb3+/Tm3+/Yb3+ co-doped oxyfluoride glass-ceramics containing Ba2LaF7 nanocrystals. Sci. Rep. 2017, 7, 6518. [Google Scholar] [CrossRef] [Green Version]
- do Carmo, F.F.; do Nascimento, J.P.C.; Façanha, M.X.; Sales, T.O.; Santos, W.Q.; Gouveia-Neto, A.S.; Jacinto, C.; Sombra, A.B.S. White light upconversion emission and color tunability in Er3+/Tm3+/Yb3+ tri-doped YNbO4 phosphor. J. Lumin. 2018, 204, 676–684. [Google Scholar] [CrossRef]
- Li, M.; Wang, L.; Ran, W.; Deng, Z.; Ren, C.; Shi, J. Tunable emission of single-phased NaY(WO4)2: Sm3+ phosphor based on energy transfer. Ceram. Int. 2017, 43, 6751–6757. [Google Scholar] [CrossRef]
- Li, Z.; Zhong, B.; Cao, Y.; Zhang, S.; Lv, Y.; Mu, Z.; Hu, Z.; Hu, Y. Energy transfer and tunable luminescence properties in Y3Al2Ga3O12:Tb3+,Eu3+ phosphors. J. Alloy. Compd. 2019, 787, 672–682. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, G.; Wang, J.; Dong, X.; Yu, W. Single-component and warm-white-emitting phosphor nagd(wo4)2:Tm3+,Dy3+,Eu3+: Synthesis, luminescence, energy transfer, and tunable color. Inorg. Chem. 2014, 53, 11457–11466. [Google Scholar] [CrossRef] [PubMed]
- Lü, Y.; Tang, X.; Yan, L.; Li, K.; Liu, X.; Shang, M.; Li, C.; Lin, J. Synthesis and luminescent properties of GdNdO4:RE3+ (RE = Tm, Dy) nanocrystalline phosphors via the sol-gel process. J. Phys. Chem. C 2013, 117, 21972–21980. [Google Scholar] [CrossRef]
- Baur, F.; Glocker, F.; Jüstel, T. Photoluminescence and energy transfer rates and efficiencies in Eu3+ activated Tb2Mo3O12. J. Mater. Chem. C 2015, 3, 2054–2064. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.; Ding, Y.; Luo, W.; Wang, L.; Li, S.; Liu, Y. Synthesis, luminescence properties and energy transfer behavior of color-tunable KAlP2O7:Tb3+,Eu3+ phosphors. Opt. Laser Technol. 2020, 121, 105829. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, X.; Huang, S.; Pan, Y. A novel tunable green-to-red emitting phosphor Ca4LaO(BO3)3:Tb,Eu via energy transfer with high quantum yield. Ceram. Int. 2016, 42, 13476–13484. [Google Scholar] [CrossRef]
- Kukku, T.; Dinu, A.; Sisira, S.; Gopi, S.; Biju, P.R.; Unnikrishnan, N.V.; Cryriac, J. Energy transfer driven tunable emission of Tb/Eu co-doped lanthanum molybdates nanophosphors. Opt. Mater. 2018, 80, 37–46. [Google Scholar]
- dos, S.; Bezerra, C.; Valerio, M.E.G. Structural and optical study of CaF2 nanoparticles produced by a microwave-assisted hydrothermal method. Phys. B Condens. Matter 2016, 501, 106–112. [Google Scholar]
- Rivera-López, F.; Babu, P.; Basavapoornima, C.; Jayasankar, C.K.; Lavín, V. Efficient Nd3+ → Yb3+ energy transfer processes in high phonon energy phosphate glasses for 1.0 μm Yb3+ ions. J. Appl. Phys. 2011, 109, 123514. [Google Scholar] [CrossRef]
- Cao, R.; Lu, Y.; Tian, Y.; Huang, F.; Xu, S.; Zhang, J. Spectroscopy of thulium and holmium co-doped silicate glasses. Opt. Mater. Express 2016, 6, 2252–2263. [Google Scholar] [CrossRef]
- Suo, H.; Guo, C.; Zheng, J.; Zhou, B.; Ma, C.; Zhao, X.; Li, T.; Guo, P.; Goldys, E.M. Sensitivity modulation of upconverting thermometry through engineering phonon energy of a matrix. ACS Appl. Mater. Interfaces 2016, 8, 32312–32319. [Google Scholar] [CrossRef]
- Biswas, A.; Maciel, G.S.; Friend, C.S.; Prasad, P.N. Upconversion properties of a transparent Er3+-Yb3+ co-doped LaF3-SiO2 glass-ceramics prepared by sol-gel method. J. Non-Cryst. Solids 2003, 316, 393–397. [Google Scholar] [CrossRef]
- Jacobsohn, L.G.; McPherson, C.L.; Oliveira, L.C.; Kucera, C.J.; Ballato, J.; Yukihara, E.G. Radioluminescence and thermoluminescence of rare earth doped and co-doped YF3. Radiat. Meas. 2017, 106, 79–83. [Google Scholar] [CrossRef]
- Milovanov, Y.S.; Skryshevsky, V.A.; Tolstoy, V.P.; Gulina, L.B.; Gavrilchenko, I.V.; Kuznetsov, G.V. Photoluminescence of porous silicon coated by SILD method with LaF3 nanolayers. Curr. Appl. Phys. 2013, 13, 1625–1629. [Google Scholar] [CrossRef]
- Du, Y.; Han, S.; Zou, Y.; Yuan, J.; Shao, C.; Jiang, X.; Chen, D. Luminescence properties of Ce3+-doped oxyfluoride aluminosilicate glass and glass ceramics. Opt. Mater. 2019, 89, 243–249. [Google Scholar] [CrossRef]
- Montoya-Quesada, E.; Villaquirán-Caicedo, M.A.; Mejía de Gutiérrez, R.; Muñoz-Saldaña, J. Effect of ZnO content on the physical, mechanical and chemical properties of glass-ceramics in the CaO-SiO2-Al2O3 system. Ceram. Int. 2020, 46, 4322–4328. [Google Scholar] [CrossRef]
- Wang, T.; Wang, S.; Wei, Y.; Zhou, X.; Zhang, H.; Wang, L.; Guo, Z.; Lv, H. Preparation and luminescence properties of Eu2O3 doped glass-ceramics containing NaY(MoO4)2. J. Eur. Ceram. Soc. 2020, 40, 1671–1676. [Google Scholar] [CrossRef]
- Marcondes, L.M.; Evangelista, R.O.; Gonçalves, R.R.; de Camargo, A.S.S.; Manzani, D.; Nalin, M.; Cassanjes, F.C.; Poirier, G.Y. Er3+-doped niobium alkali germanate glasses and glass-ceramics: NIR and visible luminescence properties. J. Non-Cryst. Solids 2019, 521, 119492. [Google Scholar] [CrossRef]
- Cheng, C.; Zeng, N.; Jiao, Q.; Zhang, X.; Liu, X. Tunable upconversion white photoemission in Yb3+/Mn2+/Tm3+ tri-doped transparent glass ceramics. Opt. Mater. 2020, 100, 109718. [Google Scholar] [CrossRef]
- Fedorov, P.P.; Luginina, A.A.; Popov, A.I. Transparent oxyfluoride glass ceramics. J. Fluor. Chem. 2015, 172, 22–50. [Google Scholar] [CrossRef]
- Yu, H.; Guo, H.; Zhang, M.; Liu, Y.; Liu, M.; Zhao, L. Distribution of Nd3+ ions in oxyfluoride glass ceramics. Nanoscale Res. Lett. 2012, 7, 275. [Google Scholar] [CrossRef] [Green Version]
- del-Castillo, J.; Yanes, A.C.; Méndez-Ramos, J.; Tikhomirov, V.K.; Moshchalkov, V.V.; Rodríguez, V.D. Sol-gel preparation and white up-conversion luminescence in rare-earth doped PbF2 nanocrystals dissolved in silica glass. J. Solgel Sci. Technol. 2010, 53, 509–514. [Google Scholar] [CrossRef] [Green Version]
- Leśniak, M.; Żmojda, J.; Kochanowicz, M.; Miluski, P.; Baranowska, A.; Mach, G.; Kuwik, M.; Pisarska, J.; Pisarski, W.A.; Dorosz, D. Spectroscopic properties of erbium-doped oxyfluoride phospho-tellurite glass and transparent glass-ceramic containing BaF2 nanocrystals. Materials 2019, 12, 3429. [Google Scholar] [CrossRef] [Green Version]
- Lisiecki, R.; Ryba-Romanowski, W. Silica-based oxyfluoride glass and glass-ceramic doped with Tm3+ and Yb3+-VUV-VIS-NIR spectroscopy and optical thermometry. J. Alloys Compd. 2020, 814, 152304. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, Y.; Bao, F.; Luo, W. Crystallization behavior and microstructure investigations on LaF3 containing oxyfluoride glass ceramics. J. Non-Cryst. Solids 2005, 351, 722–728. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, K.; Liu, C.; Yin, Q.; Han, J.; Heo, J. Intense up-conversion emission from Er3+/Yb3+ ion co-doped transparent oxyfluoride glass-ceramics containing Y5O4F7 nanorods for optical thermometry. J. Mater. Chem. C 2019, 7, 6134–6143. [Google Scholar]
- Zhang, X.; Hu, L.; Ren, J. Transparent aluminosilicate oxyfluoride glass ceramics containing upconversion luminescent caf2 nanocrystals: Glass-to-crystal structural evolution studied by the advanced solid-state nmr spectroscopy. J. Mater. Chem. C 2020, 124, 1594–1608. [Google Scholar] [CrossRef]
- Antuzevics, A.; Kemere, M.; Krieke, G.; Ignatans, R. Electron paramagnetic resonance and photoluminescence investigation of europium local structure in oxyfluoride glass ceramics containing SrF2 nanocrystals. Opt. Mater. 2017, 72, 749–755. [Google Scholar] [CrossRef]
- Zhou, B.; E, C.Q.; Bu, Y.Y.; Meng, L.; Yan, X.H.; Wang, X.F. Temperature-controlled down-conversion luminescence behavior of Eu3+-doped transparent MF2 (M = Ba, Ca, Sr) glass ceramics. Luminescence 2017, 32, 195–200. [Google Scholar] [CrossRef]
- Biswas, K.; Sontakke, A.D.; Sen, R.; Annapurna, K. Luminescence properties of dual valence Eu doped nano-crystalline BaF2 embedded glass-ceramics and observation of Eu2+ → Eu3+ energy transfer. J. Fluoresc. 2012, 22, 745–752. [Google Scholar] [CrossRef]
- Gorni, G.; Velázquez, J.J.; Mosa, J.; Balda, R.; Fernández, J.; Durán, A.; Castro, Y. Transparent glass-ceramics produced by sol-gel: A suitable alternative for photonic materials. Materials 2018, 11, 212. [Google Scholar] [CrossRef] [Green Version]
- Velázquez, J.J.; Mosa, J.; Gorni, G.; Balda, R.; Fernández, J.; Durán, A.; Castro, Y. Novel sol-gel SiO2-NaGdF4 transparent nano-glass-ceramics. J. Non-Cryst. Solids 2019, 520, 119447. [Google Scholar] [CrossRef]
- Gorni, G.; Velázquez, J.J.; Mosa, J.; Mather, G.C.; Serrano, A.; Vila, M.; Castro, G.R.; Bravo, D.; Balda, R.; Fernández, J.; et al. Transparent sol-gel oxyfluoride glass-ceramics with high crystalline fraction and study of re incorporation. Nanomaterials 2019, 9, 530. [Google Scholar] [CrossRef] [Green Version]
- Vinogradov, A.V.; Vinogradov, V.V. Low-temperature sol-gel synthesis of crystalline materials. RSC Adv. 2014, 4, 45903–45919. [Google Scholar] [CrossRef]
- Pawlik, N.; Szpikowska-Sroka, B.; Goryczka, T.; Pisarski, W.A. Photoluminescence investigation of sol-gel glass-ceramic materials containing SrF2:Eu3+ nanocrystals. J. Alloys Compd. 2019, 810, 151935. [Google Scholar] [CrossRef]
- Chen, D.; Wang, Z.; Zhou, Y.; Huang, P.; Ji, Z. Tb3+/Eu3+:YF3 nanophase embedded glass ceramics: Structural characterization, tunable luminescence and temperature sensing behavior. J. Alloys Compd. 2015, 646, 339–344. [Google Scholar] [CrossRef]
- Pawlik, N.; Szpikowska-Sroka, B.; Goryczka, T.; Zubko, M.; Lelątko, J.; Pisarski, W.A. Structure and luminescent properties of oxyfluoride glass-ceramics with YF3:Eu3+ nanocrystals derived by sol-gel method. J. Eur. Ceram. Soc. 2019, 39, 5010–5017. [Google Scholar] [CrossRef]
- Pawlik, N.; Szpikowska-Sroka, B.; Pietrasik, E.; Goryczka, T.; Pisarski, W.A. Structural and luminescence properties of silica powders and transparent glass-ceramics containing LaF3:Eu3+ nanocrystals. J. Am. Ceram. Soc. 2018, 101, 4654–4668. [Google Scholar] [CrossRef]
- Yanes, A.C.; del-Castillo, J.; Méndez-Ramos, J.; Rodríguez, V.D.; Torres, M.E.; Arbiol, J. Luminescence and structural characterization of transparent nanostructures Eu3+-doped LaF3-SiO2 glass-ceramics prepared by sol-gel method. Opt. Mater. 2007, 29, 999–1003. [Google Scholar] [CrossRef]
- Wu, X.; Zheng, J.; Ren, Q.; Zhu, J.; Ren, Y.; Hai, O. Luminescent properties and energy transfer in novel single-phase multicolor tunable Sr3Y(BO3)3:Tb3+,Eu3+ phosphors. J. Alloys Compd. 2019, 805, 12–18. [Google Scholar] [CrossRef]
- Yanes, A.C.; del-Castillo, J.; Ortiz, E. Energy transfer and tunable emission in BaGdF5:RE3+ (RE = Ce, Tb, Eu) nano-glass-ceramics. J. Alloys Compd. 2019, 773, 1099–1107. [Google Scholar] [CrossRef]
- Wang, B.; Ren, Q.; Hai, O.; Wu, X. Luminescence properties and energy transfer in Tb3+ and Eu3+ co-doped Ba2P2O7 phosphors. RSC Adv. 2017, 7, 15222–15227. [Google Scholar] [CrossRef] [Green Version]
- Xie, F.; Li, J.; Dong, Z.; Wen, D.; Shi, J.; Yan, J.; Wu, M. Energy transfer and luminescent properties of Ca8MgLu(PO4)7:Tb3+/Eu3+ as a green-to-red color tunable phosphor under NUV excitation. RSC Adv. 2015, 5, 59830–59836. [Google Scholar] [CrossRef]
- Caldiño, U.; Speghini, A.; Berneschi, S.; Bettinelli, M.; Brenci, M.; Pasquini, E.; Pelli, S.; Righini, G.C. Optical spectroscopy and optical waveguide fabrication in Eu3+ and Eu3+/Tb3+ doped zinc-sodium-aluminosilicate glasses. J. Lumin. 2014, 147, 336–340. [Google Scholar] [CrossRef]
- Gopi, S.; Jose, S.K.; Sreeja, E.; Manasa, P.; Unnikrishnan, N.V.; Cyriac, J.; Biju, P.R. Tunable green to red emission via Tb sensitized energy transfer in Tb/Eu co-doped alkali fluoroborate glass. J. Lumin. 2017, 192, 1288–1294. [Google Scholar] [CrossRef]
- Liu, H.-G.; Zheng, W.-C. Theoretical investigations of the optical and EPR spectra for trivalent cerium and ytterbium ions in orthorhombic YF3 crystal. Phys. B 2016, 496, 15–19. [Google Scholar] [CrossRef]
- Rast, H.E.; Caspers, H.H.; Miller, S.A. Infrared Dispersion and Lattice Vibrations of LaF3. Phys. Rev. 1968, 171, 1051–1057. [Google Scholar] [CrossRef]
- Binnemans, K. Interpretation of europium(III) spectra. Coord. Chem. Rev. 2015, 295, 1–45. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Huang, X.; Guo, H.; Zeng, Y. Energy transfer and tunable photoluminescence of LaBWO6:Tb3+,Eu3+ phosphors for near-UV white LEDs. Dye. Pigment. 2018, 150, 67–72. [Google Scholar] [CrossRef]
- Xia, Z.; Zhuang, J.; Liao, L. Novel red-emitting Ba2Tb(BO3)2Cl:Eu phosphor with efficient energy transfer for potential application in white light-emitting diodes. Inorg. Chem. 2012, 51, 7202–7209. [Google Scholar] [CrossRef]
- Chen, J.; Meng, Q.; May, P.S.; Berry, M.T.; Lin, C. Sensitization of Eu3+ luminescence in Eu:YPO4 nanocrystals. J. Phys. Chem. C 2013, 117, 5953–5962. [Google Scholar] [CrossRef]
- Werts, M.H.V.; Jukes, R.T.F.; Verhoeven, J.W. The emission spectrum and the radiative lifetime of Eu3+ in luminescent lanthanide complexes. Phys. Chem. Chem. Phys. 2002, 4, 1542–1548. [Google Scholar] [CrossRef]
- Zhang, S.-T.; Modreanu, M.; Roussel, H.; Jiménez, C.; Deschanvres, J.-L. Exploring the optical properties of Vernier phase yttrium oxyfluoride thin films grown by pulsed liquid injection MOCVD. Dalton Trans. 2018, 47, 2655–2661. [Google Scholar] [CrossRef] [PubMed]
- Gorni, G.; Balda, R.; Fernández, J.; Velázquez, J.J.; Pascual, L.; Mosa, J.; Durán, A.; Castro, Y. 80SiO2-20LaF3 oxyfluoride glass ceramic coatings doped with Nd3+ for optical applications. Int. J. Appl. Glass Sci. 2018, 9, 208–217. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Wang, N.; Wang, D.; Tang, Z.; Yu, K.; Wei, W. Method for refractive index measurement of nanoparticles. Opt. Lett. 2014, 39, 4251–4254. [Google Scholar] [CrossRef]
- Lu, P.; Wang, Y.; Huang, L.; Lian, S.; Wang, Y.; Tang, J.; Belfiore, L.A.; Kipper, M.J. Tb3+/Eu3+ complex-doped rigid nanoparticles in transparent nanofibrous membranes exhibit high quantum yield fluorescence. Nanomaterial 2020, 10, 694. [Google Scholar] [CrossRef] [Green Version]
- Shrivastava, N.; Khan, L.U.; Vergas, J.M.; Ospina, C.; Coaquira, J.A.Q.; Zoppellaro, G.; Brito, H.F.; Javed, Y.; Shukla, D.K.; Felinto, M.C.F.C.; et al. Efficient multicolor tunability of ultrasmall ternary-doped LaF3 nanoparticles: Energy conversion and magnetic behavior. Phys. Chem. Chem. Phys. 2017, 19, 18660–18670. [Google Scholar] [CrossRef]
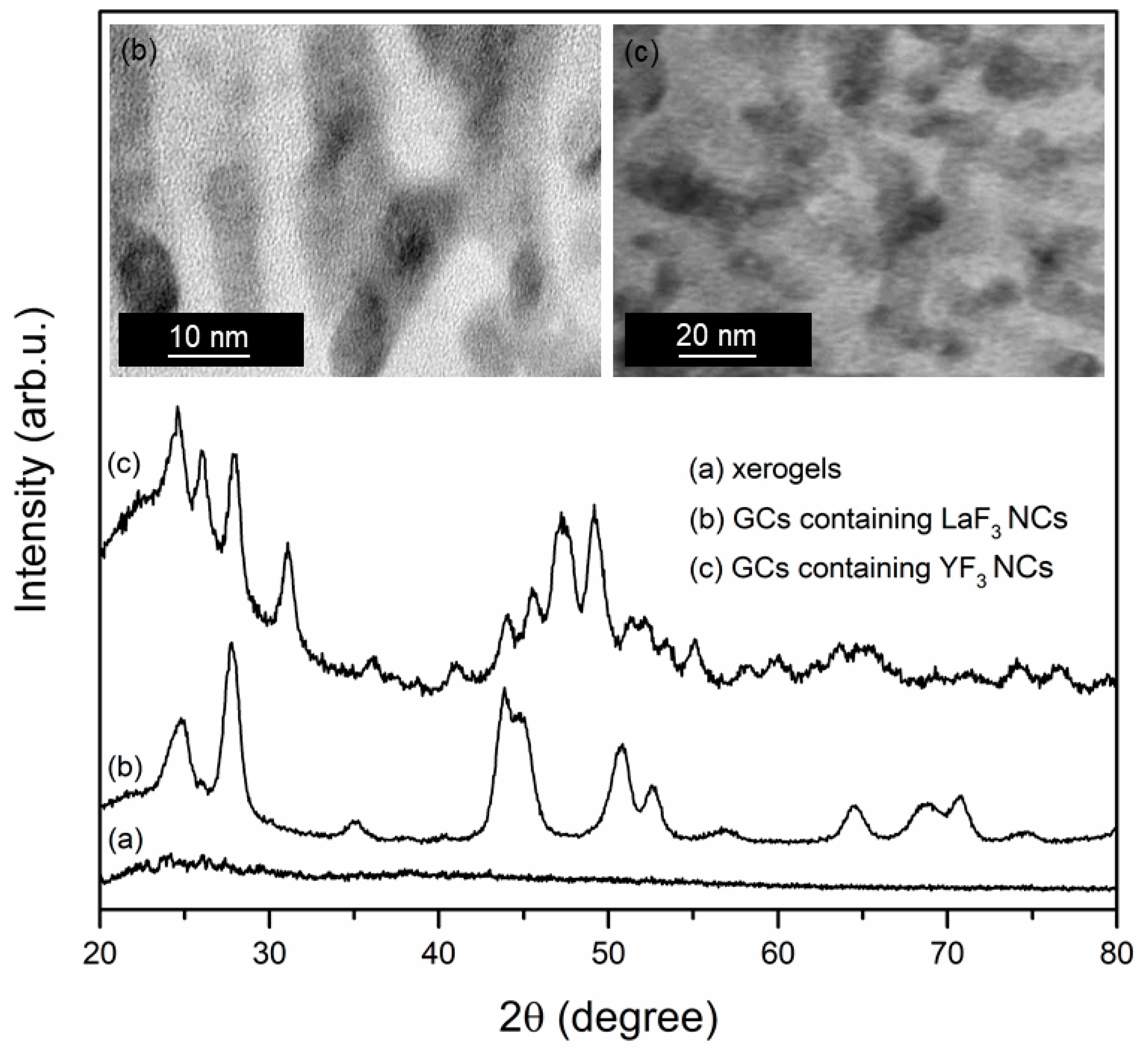
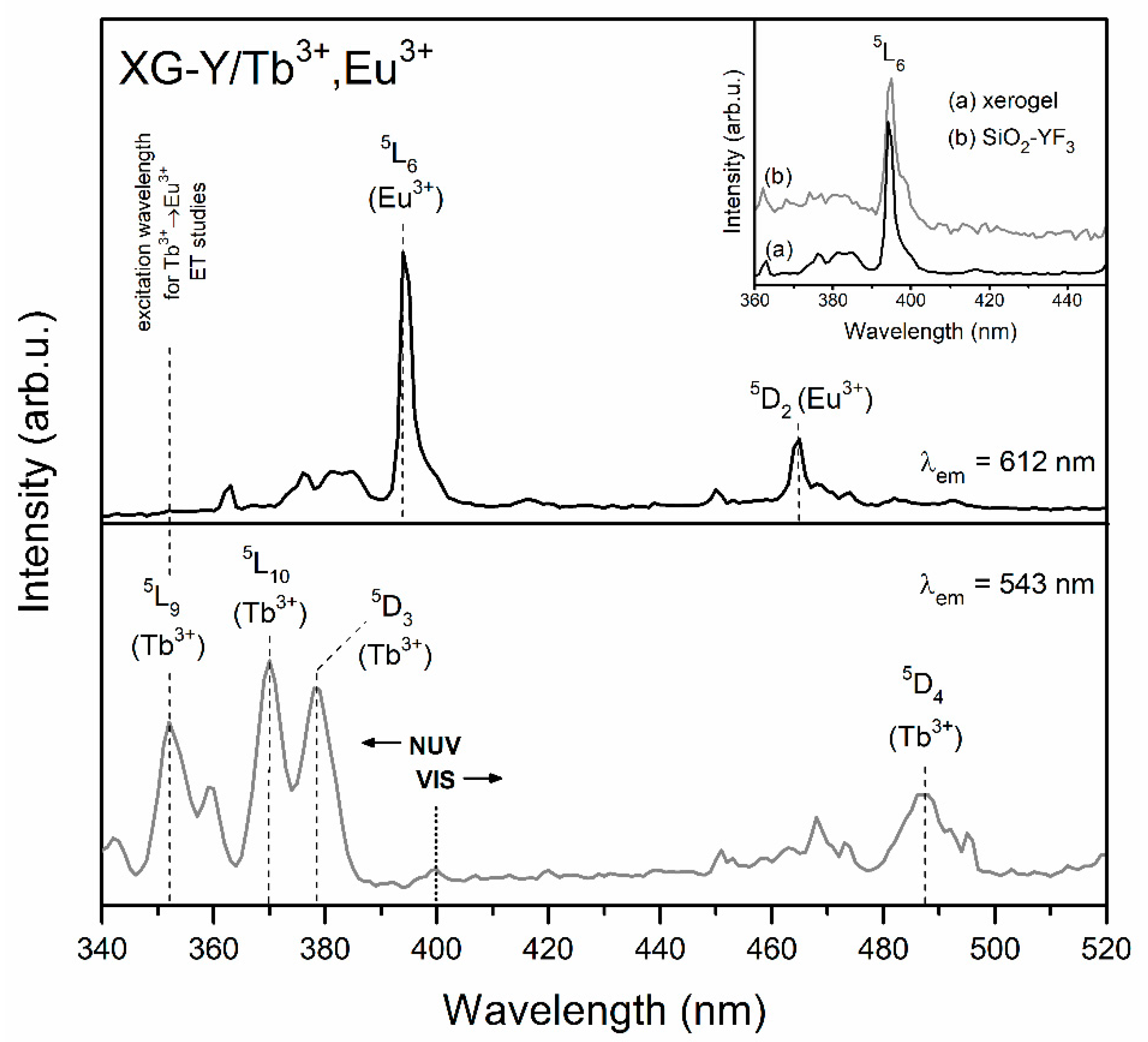
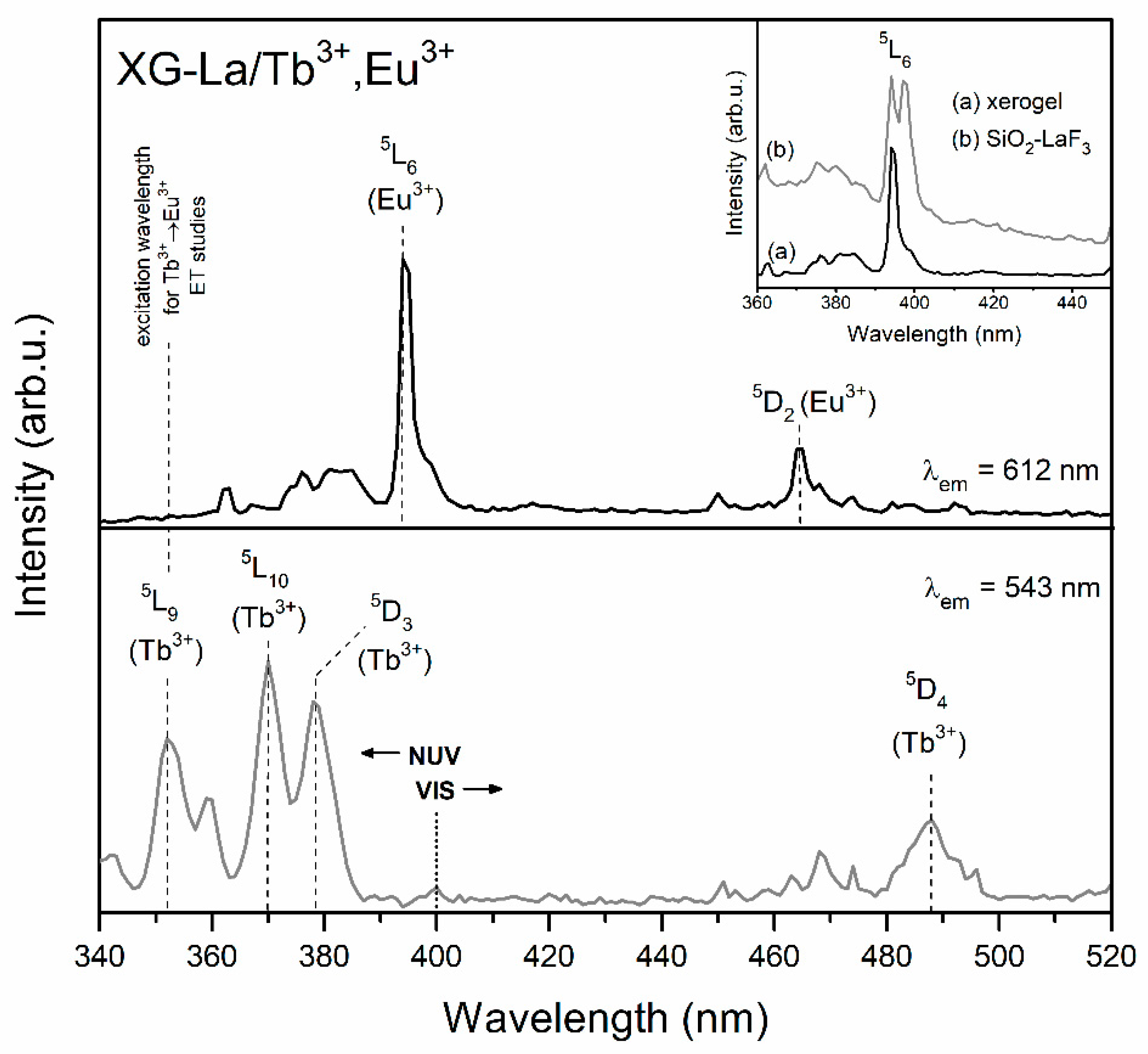
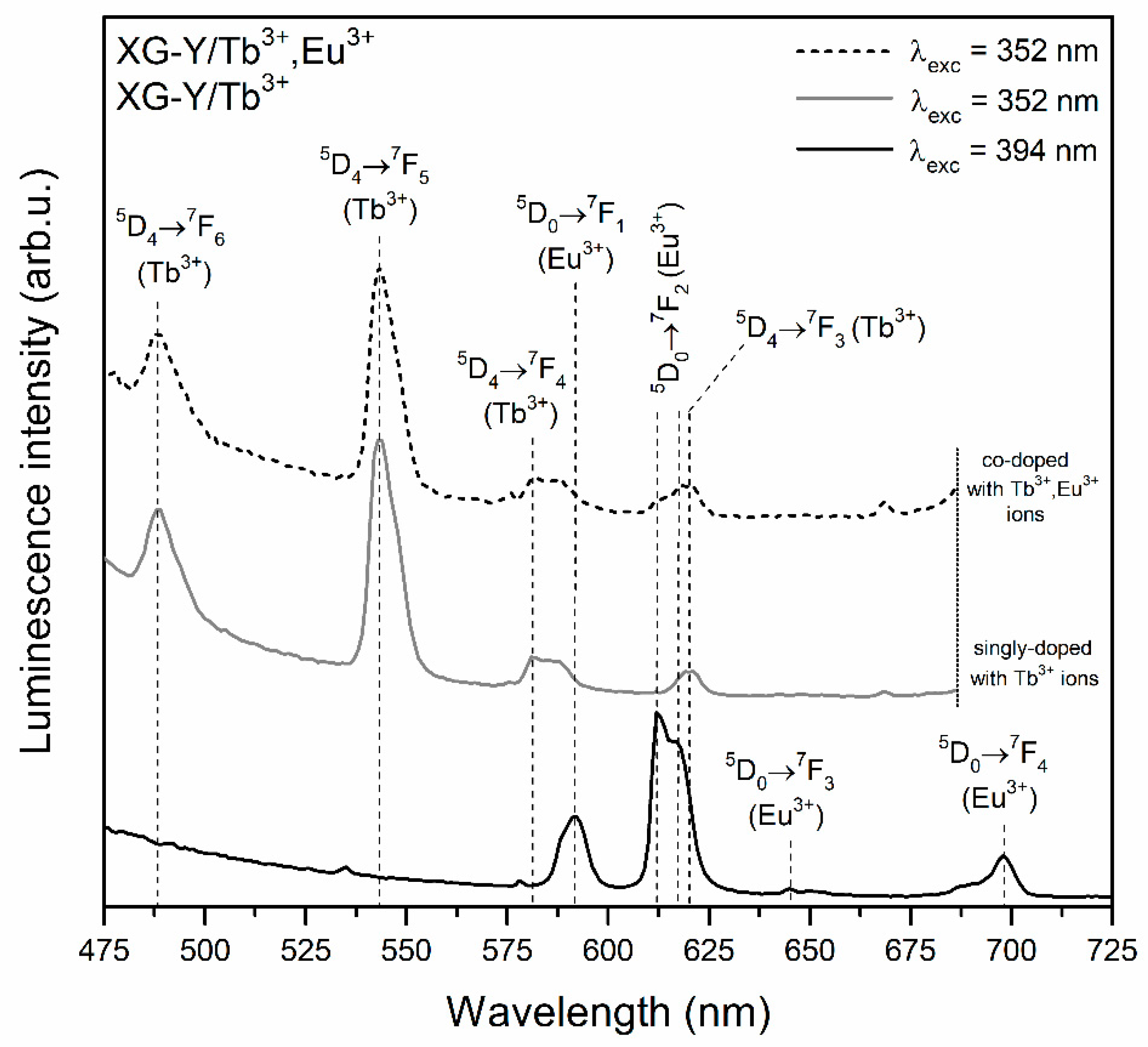
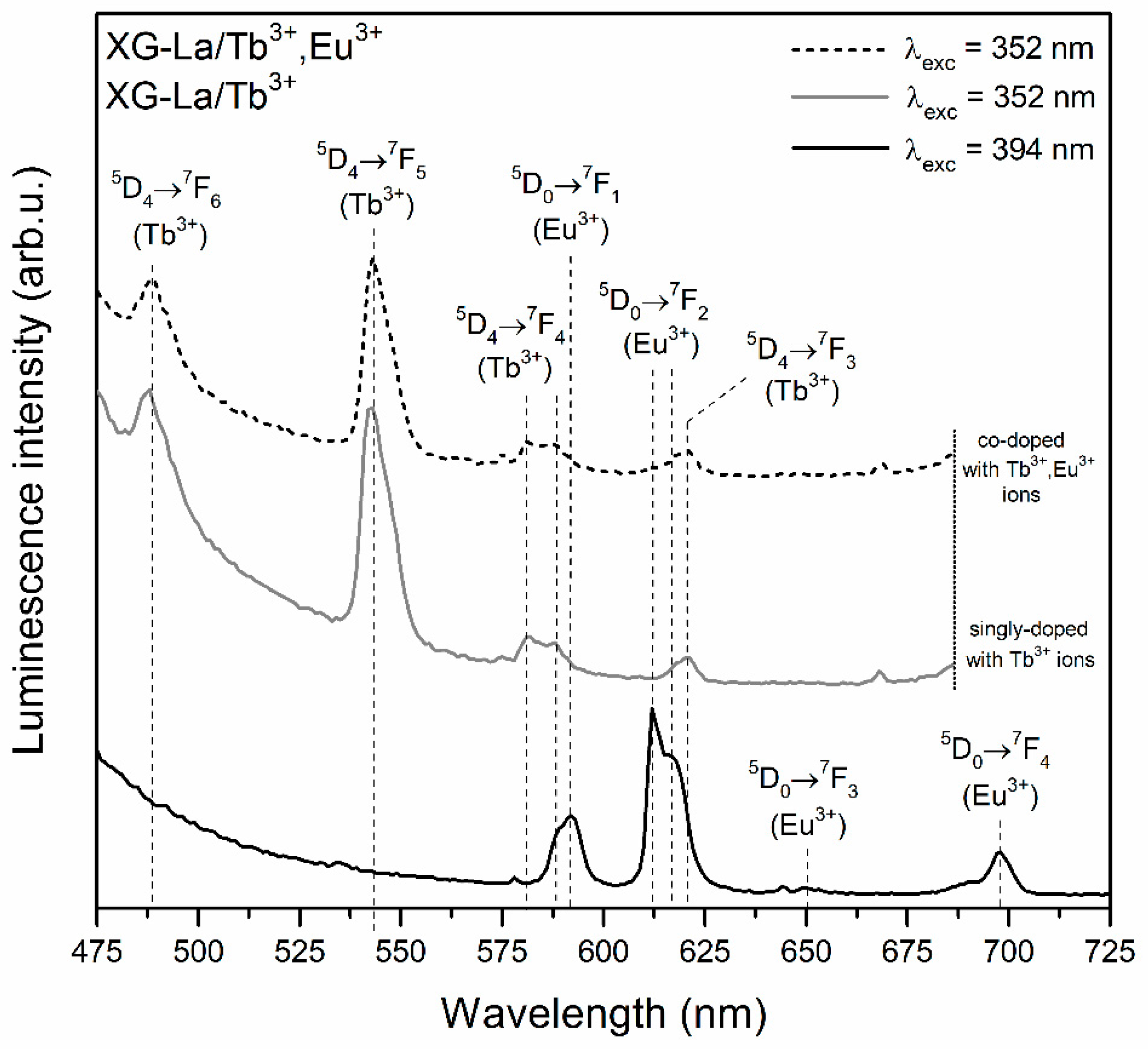
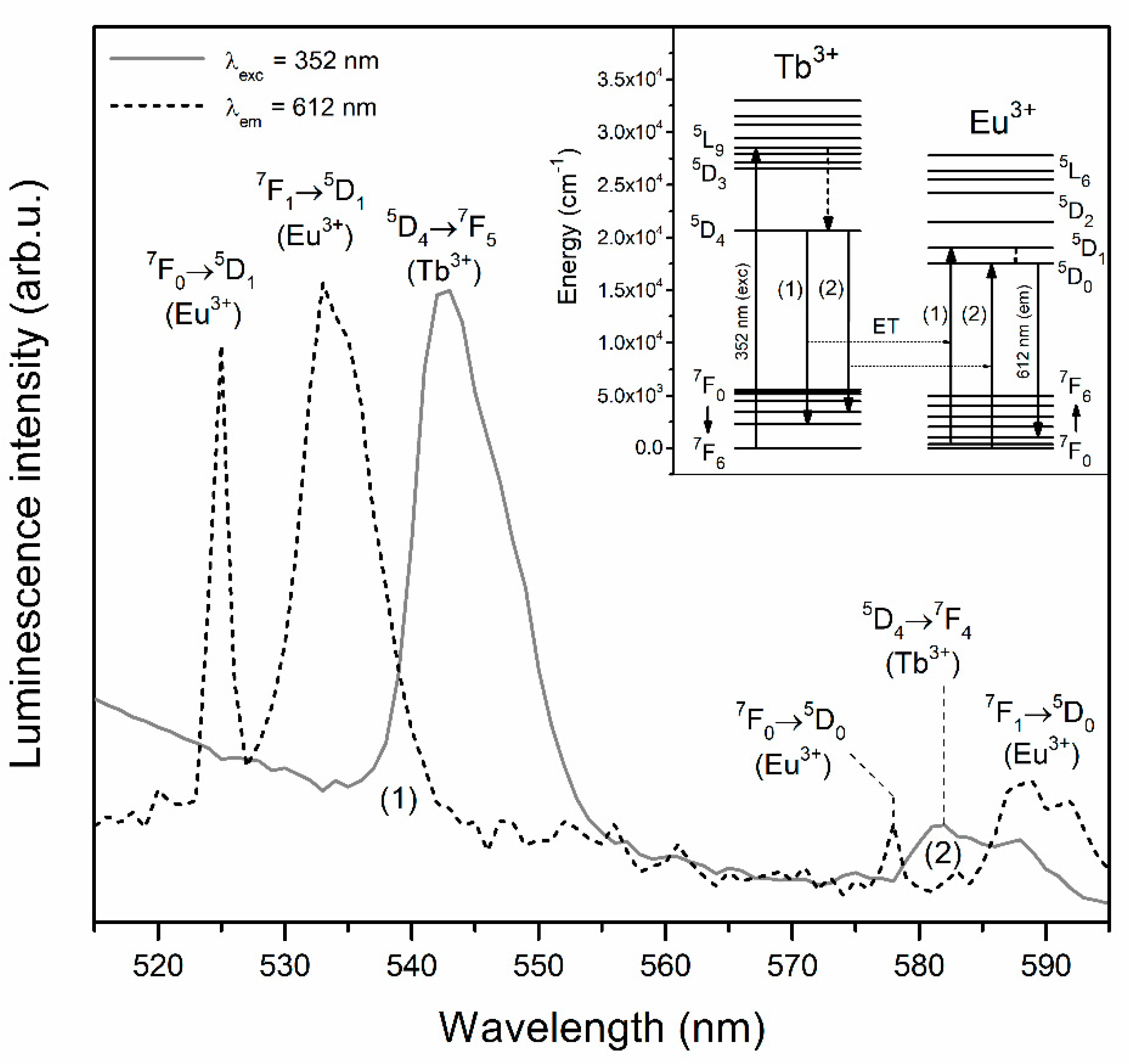
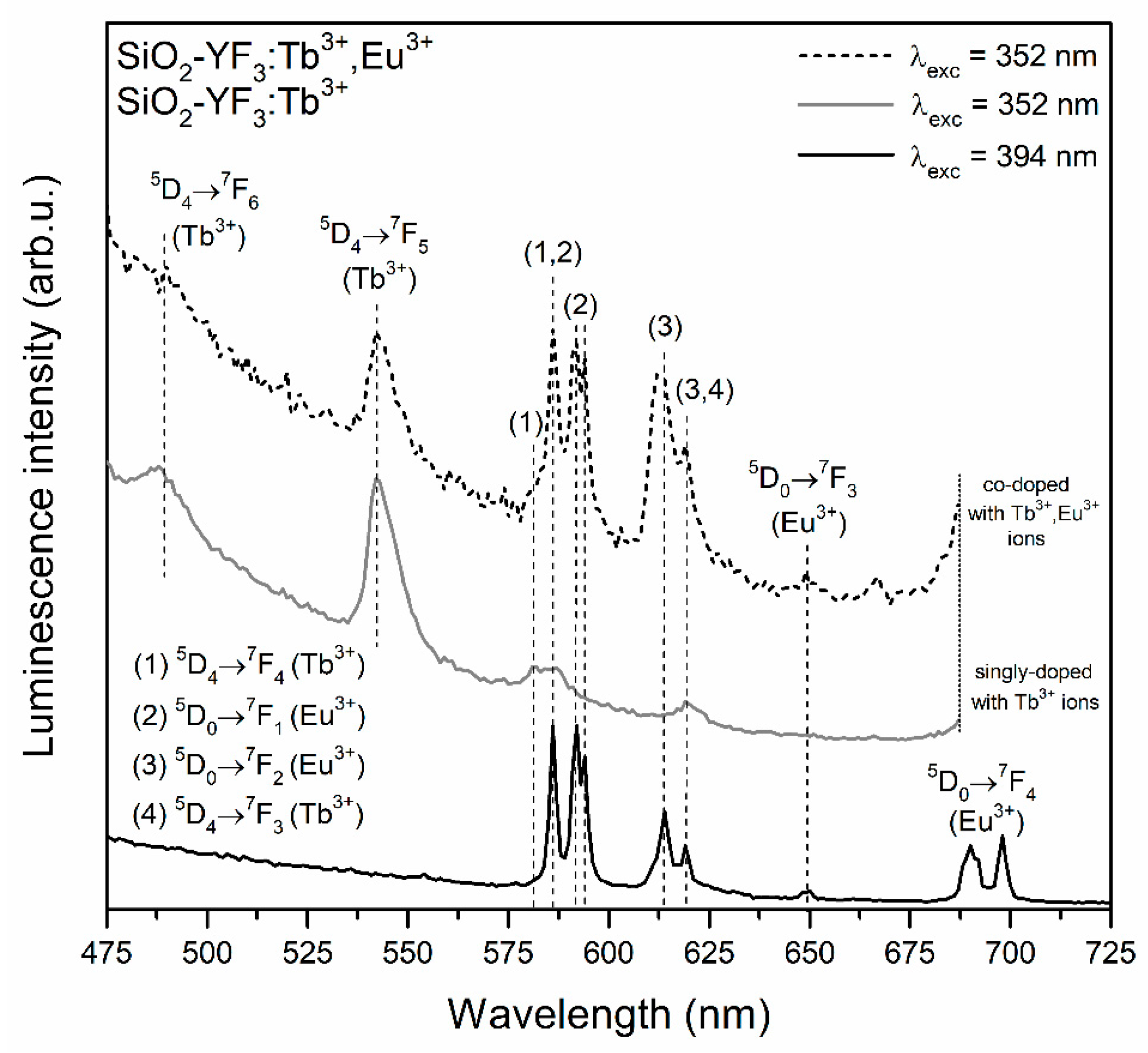
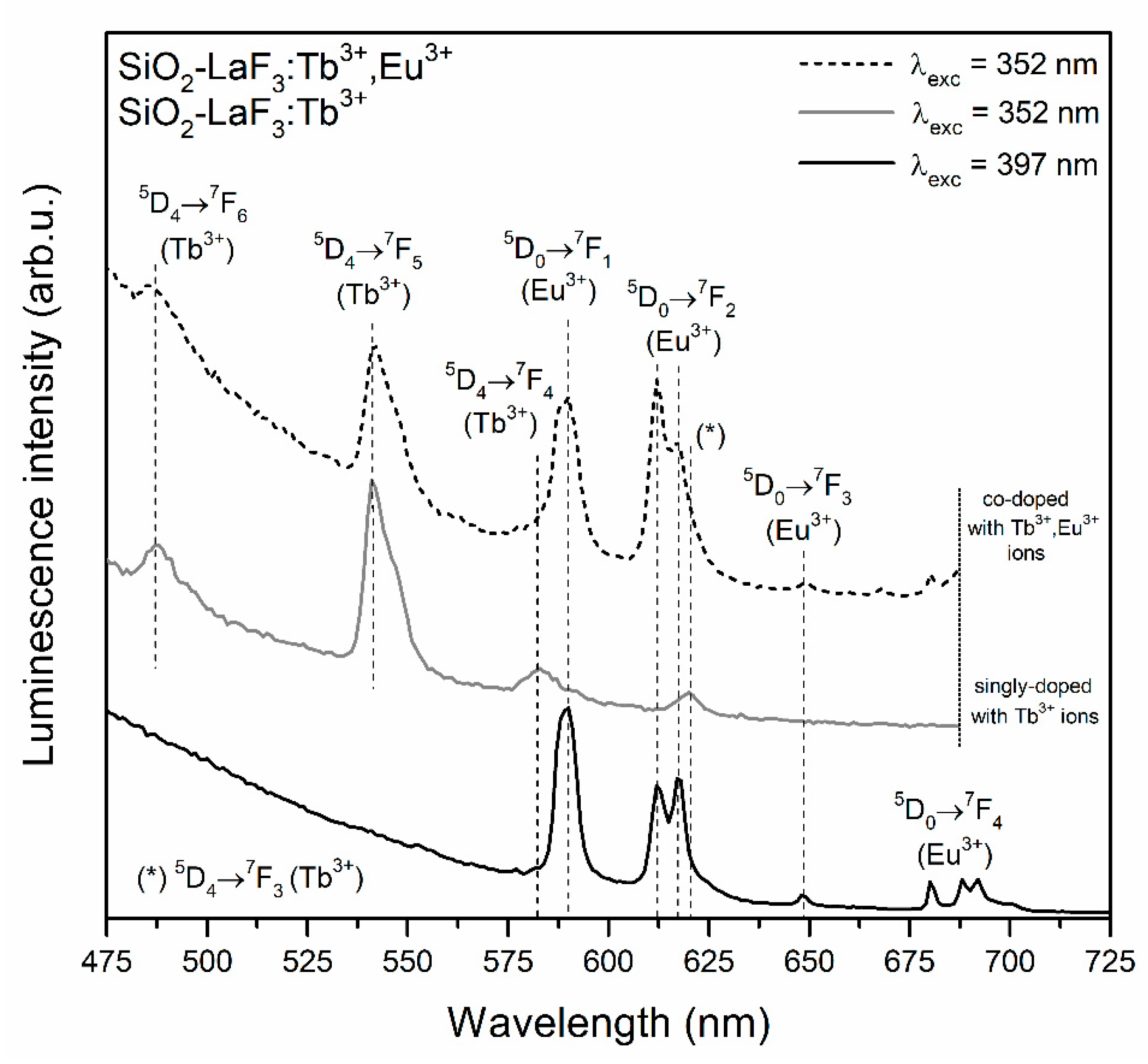
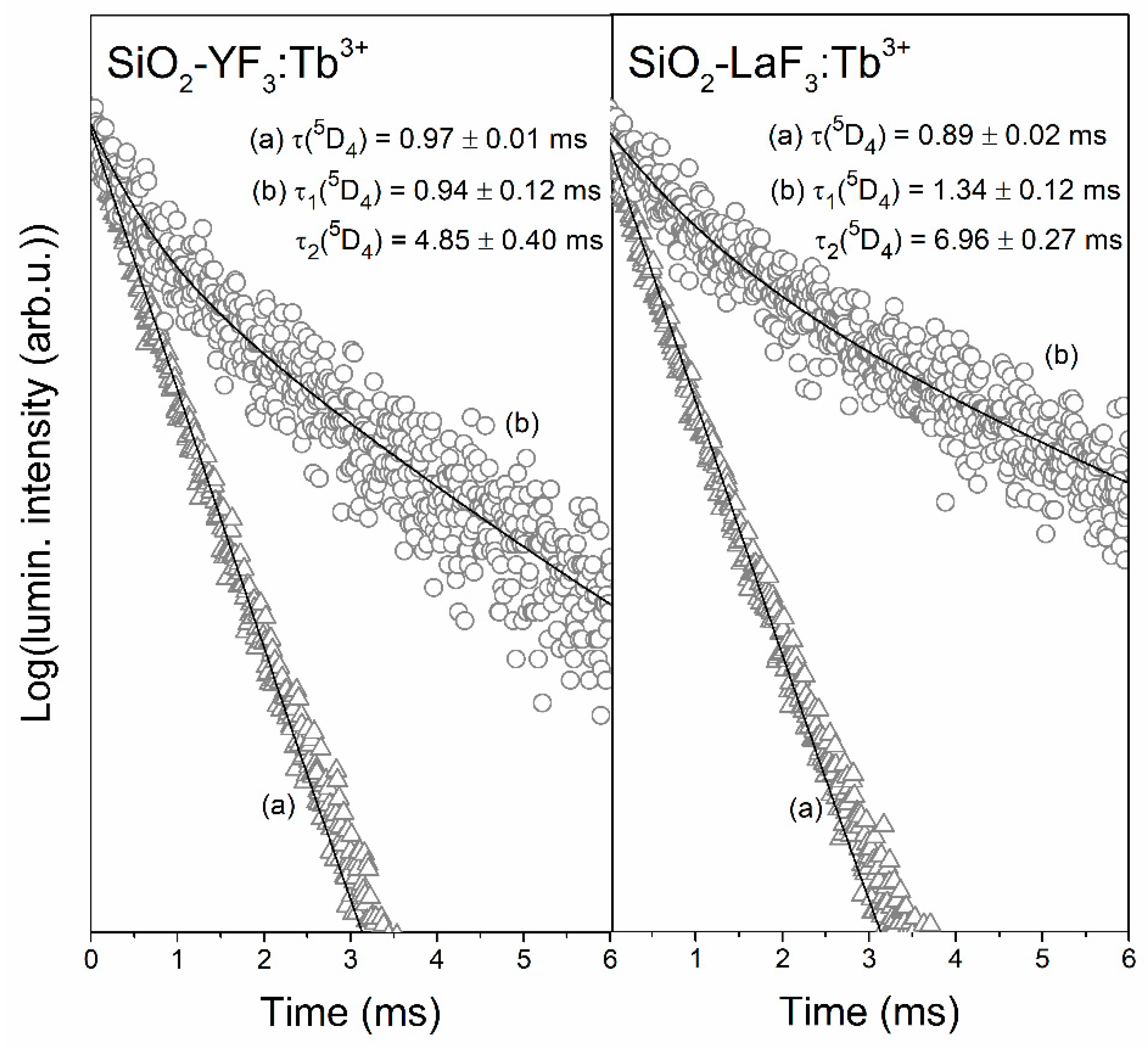
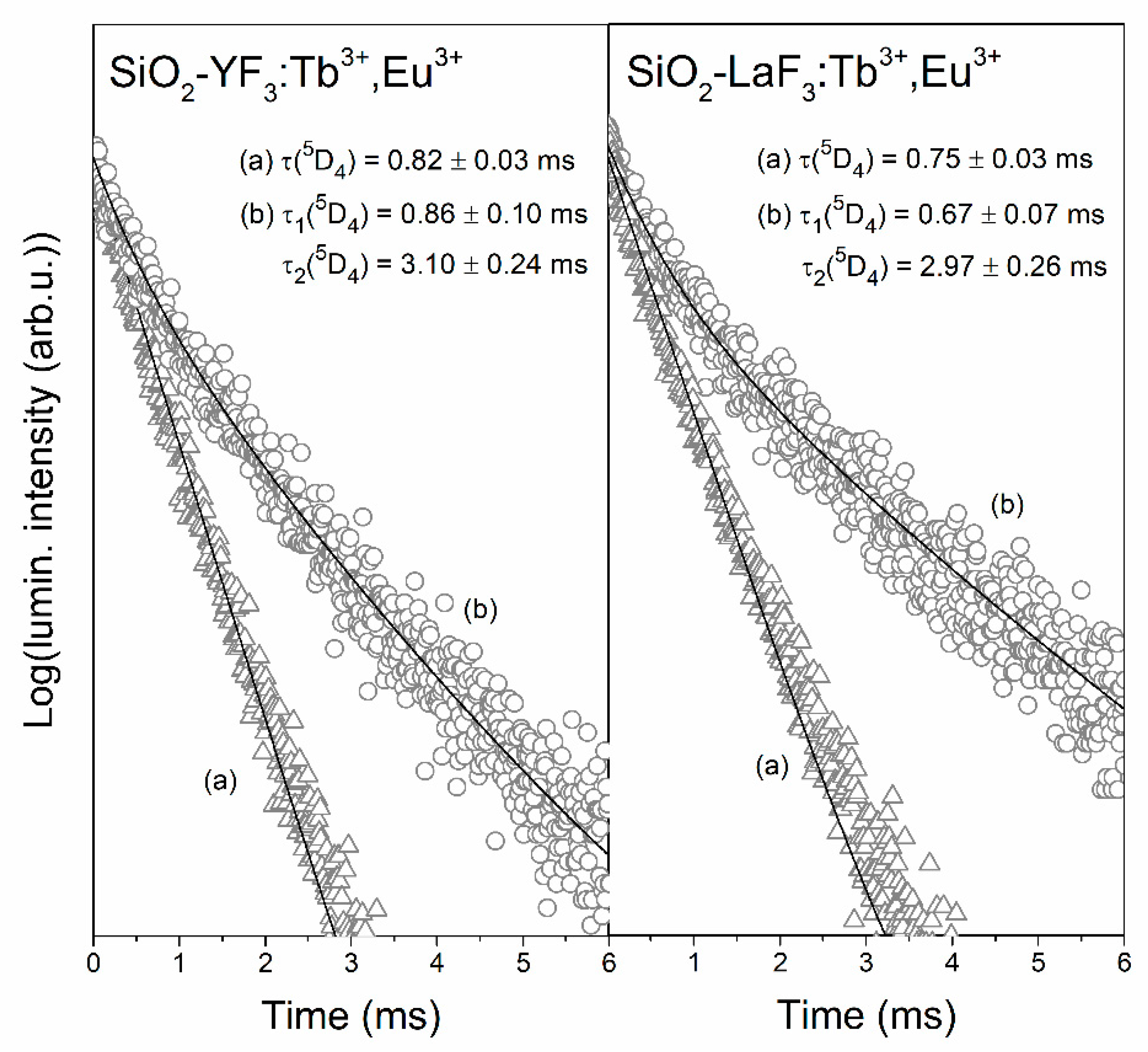
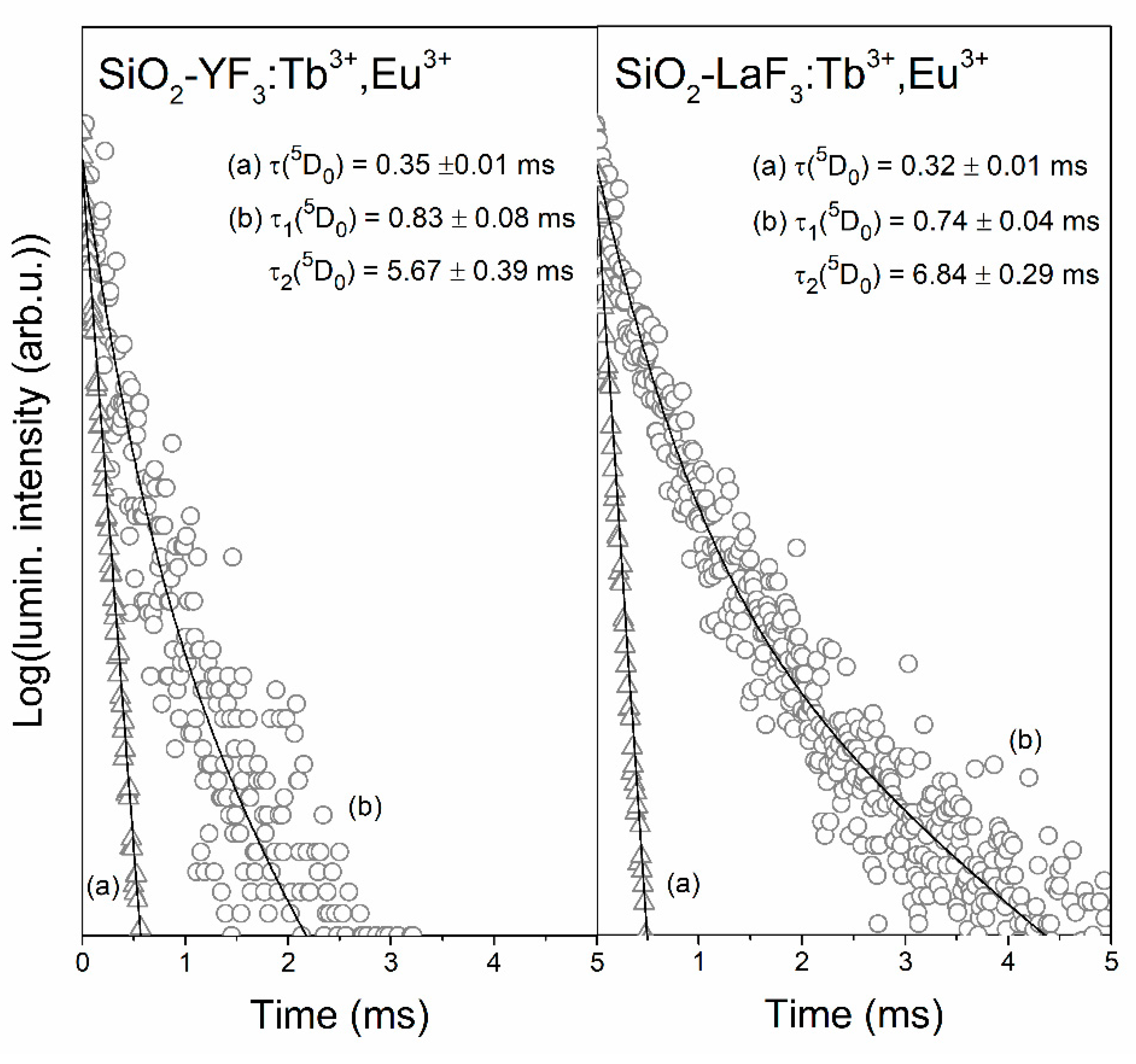
| Sample | τ(5D4) (ms) | ηET (%) |
|---|---|---|
| XG-Y/Tb3+,Eu3+ | 0.82 ± 0.03 | 15.5 |
| XG-Y/Tb3+ | 0.97 ± 0.01 | - |
| XG-La/Tb3+,Eu3+ | 0.75 ± 0.03 | 15.7 |
| XG-La/Tb3+ | 0.89 ± 0.02 | - |
| Sample | τ(5D4) (ms) | %,τn(5D4) | τavg(5D4) (ms) | ηET (%) |
|---|---|---|---|---|
| SiO2-YF3:Tb3+,Eu3+ | 0.86 ± 0.10 (τ1) 3.10 ± 0.24 (τ2) | 49.79% (τ1) 50.21% (τ2) | 2.62 | 37.3 |
| SiO2-YF3:Tb3+ | 0.94 ± 0.12 (τ1) 4.85 ± 0.40 (τ2) | 51.50% (τ1) 48.50% (τ2) | 4.18 | - |
| SiO2-LaF3:Tb3+,Eu3+ | 0.67 ± 0.07 (τ1) 2.97 ± 0.26 (τ2) | 57.16% (τ1) 42.84% (τ2) | 2.44 | 60.8 |
| SiO2-LaF3:Tb3+ | 1.34 ± 0.12 (τ1) 6.96 ± 0.27 (τ2) | 43.58% (τ1) 56.42% (τ2) | 6.23 | - |
| Sample | τ(5D0) (ms) | %, τn(5D0) | τavg(5D0) (ms) |
|---|---|---|---|
| SiO2-YF3:Tb3+,Eu3+ | 0.83 ± 0.08 (τ1) 5.67 ± 0.39 (τ2) | 66.30% (τ1) 33.70% (τ2) | 4.59 |
| SiO2-LaF3:Tb3+,Eu3+ | 0.74 ± 0.04 (τ1) 6.84 ± 0.29 (τ2) | 63.58% (τ1) 36.42% (τ2) | 5.87 |
| Sample | ФEu (%) |
|---|---|
| XG-Y/Tb3+,Eu3+ | 9.2 |
| XG-La/Tb3+,Eu3+ | 8.1 |
| SiO2-YF3:Tb3+,Eu3+ | 73.0 |
| SiO2-LaF3:Tb3+,Eu3+ | 49.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawlik, N.; Szpikowska-Sroka, B.; Pisarski, W.A. Energy Transfer Study on Tb3+/Eu3+ Co-Activated Sol-Gel Glass-Ceramic Materials Containing MF3 (M = Y, La) Nanocrystals for NUV Optoelectronic Devices. Materials 2020, 13, 2522. https://doi.org/10.3390/ma13112522
Pawlik N, Szpikowska-Sroka B, Pisarski WA. Energy Transfer Study on Tb3+/Eu3+ Co-Activated Sol-Gel Glass-Ceramic Materials Containing MF3 (M = Y, La) Nanocrystals for NUV Optoelectronic Devices. Materials. 2020; 13(11):2522. https://doi.org/10.3390/ma13112522
Chicago/Turabian StylePawlik, Natalia, Barbara Szpikowska-Sroka, and Wojciech A. Pisarski. 2020. "Energy Transfer Study on Tb3+/Eu3+ Co-Activated Sol-Gel Glass-Ceramic Materials Containing MF3 (M = Y, La) Nanocrystals for NUV Optoelectronic Devices" Materials 13, no. 11: 2522. https://doi.org/10.3390/ma13112522





