Distributions of Potential and Contact-Induced Charges in Conventional Organic Photovoltaics
Abstract
1. Introduction
2. Charged Molecules at Electrode/Organic Interfaces in the Ground State
3. Distributions of Potential and Charge Density in Organic Heterojunctions
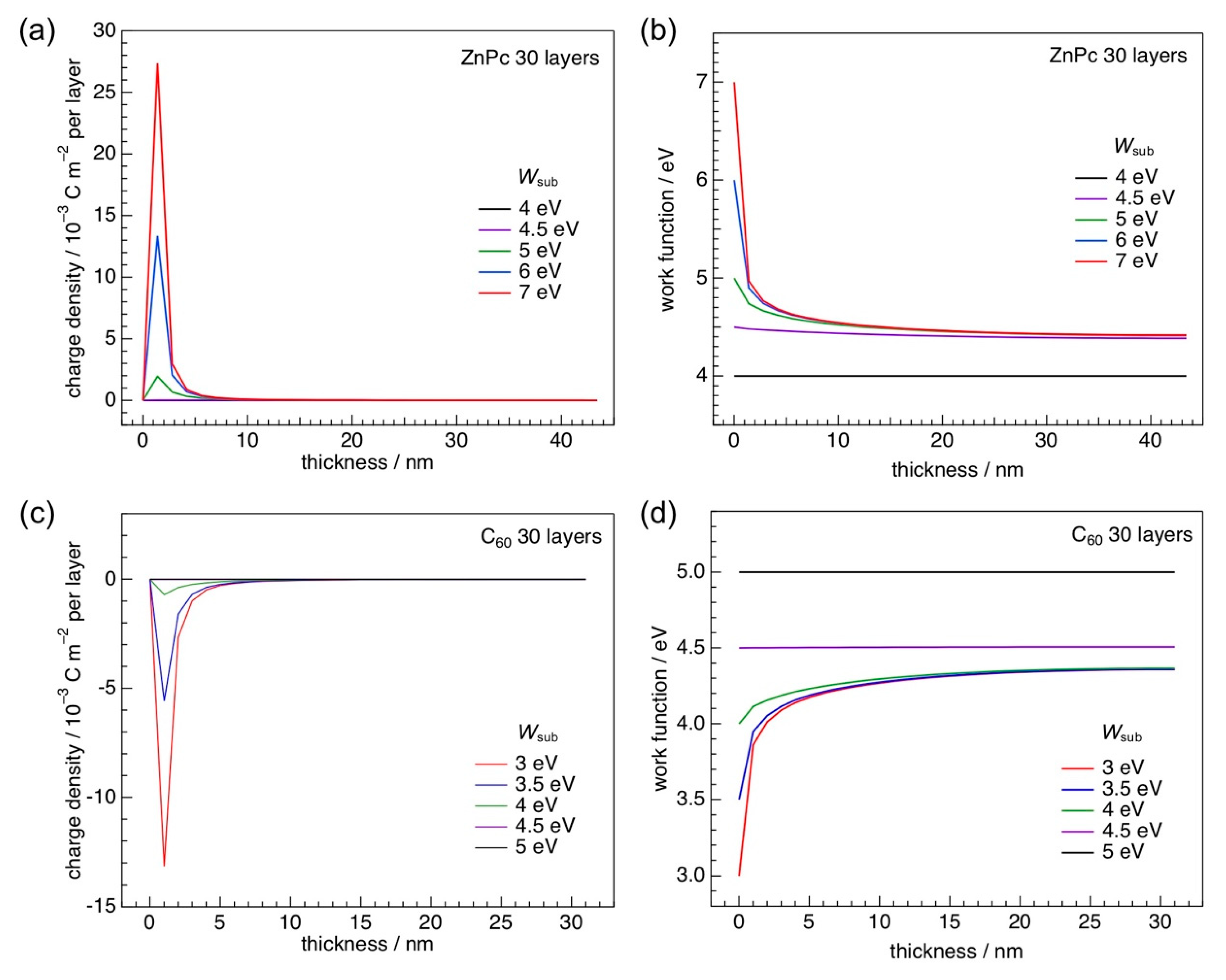
3.1. Fermi-level Pinning at Organic Heterointerfaces
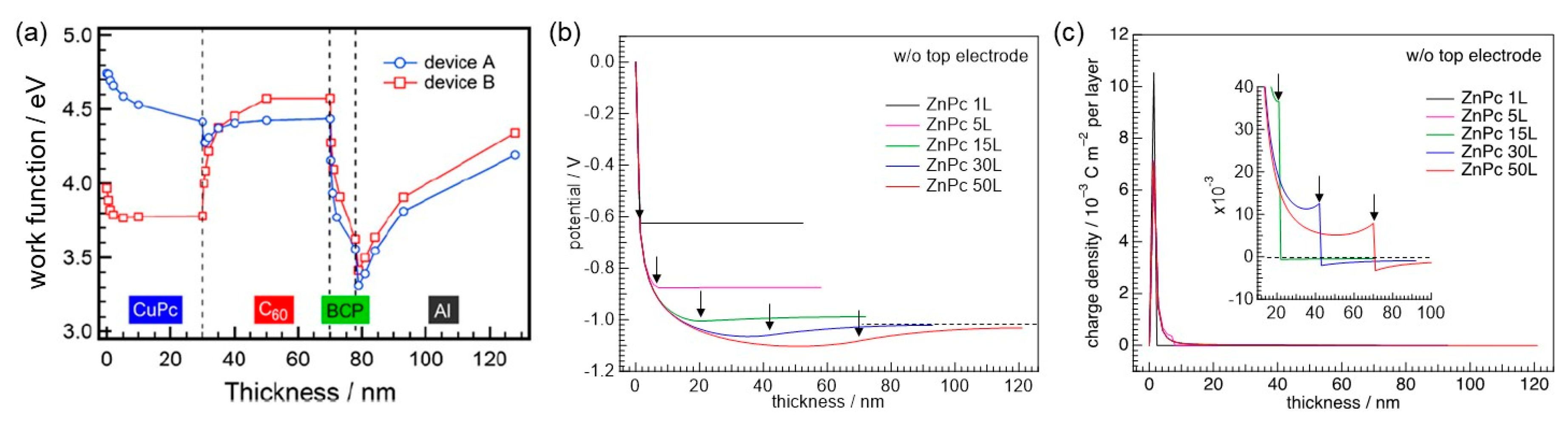
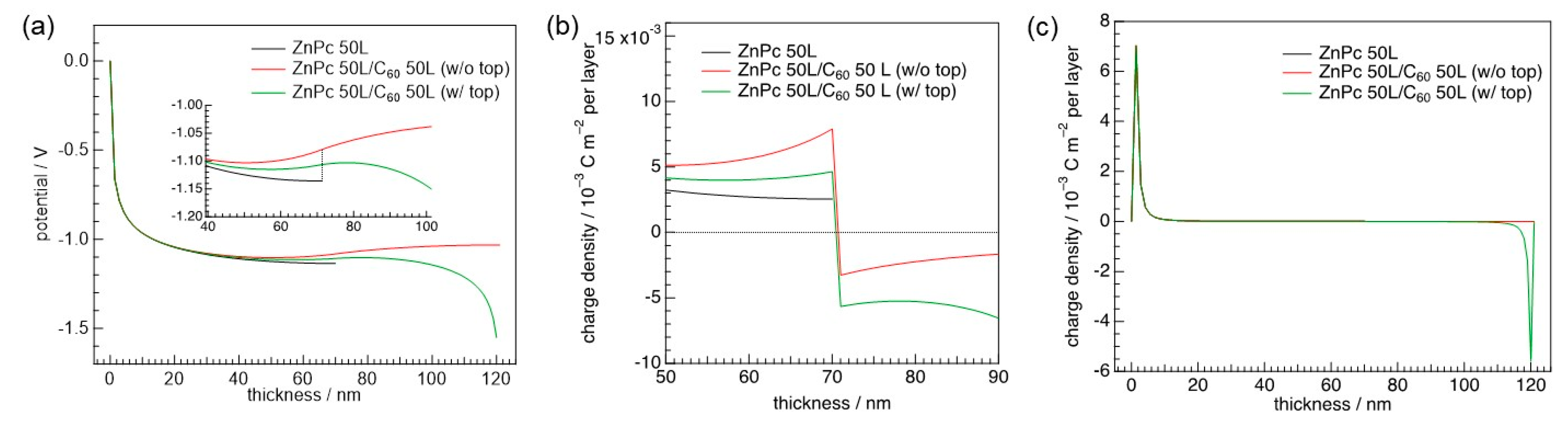
3.2. Consideration of Top Electrodes
3.3. Structural Disorder at Donor/Acceptor Interface
3.4. Energy-Level Alignment in Reversed Stacking
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Jinno, H.; Fukuda, K.; Xu, X.; Park, S.; Suzuki, Y.; Koizumi, M.; Yokota, T.; Osaka, I.; Takimiya, K.; Someya, T. Stretchable and waterproof elastomer-coated organic photovoltaics for washable electronic textile applications. Nat. Energy 2017, 2, 780–785. [Google Scholar] [CrossRef]
- Tai, Q.; Yan, F. Emerging Semitransparent Solar Cells: Materials and Device Design. Adv. Mater. 2017, 29, 1700192. [Google Scholar] [CrossRef] [PubMed]
- Brus, V.V.; Lee, J.; Luginbuhl, B.; Ko, S.; Bazan, G.C.; Nguyen, T. Solution-Processed Semitransparent Organic Photovoltaics: From Molecular Design to Device Performance. Adv. Mater. 2019, 1900904. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Li, G.; Zhan, X.; Yang, Y. Next-generation organic photovoltaics based on non-fullerene acceptors. Nat. Photonics 2018, 12, 131–142. [Google Scholar] [CrossRef]
- Meng, L.; Zhang, Y.; Wan, X.; Li, C.; Zhang, X.; Wang, Y.; Ke, X.; Xiao, Z.; Ding, L.; Xia, R.; et al. Organic and solution-processed tandem solar cells with 17.3% efficiency. Science 2018, eaat2612. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Jiang, Y.; Jin, K.; Qin, J.; Xu, J.; Li, W.; Xiong, J.; Liu, J.; Xiao, Z.; Sun, K.; et al. 18% Efficiency organic solar cells. Sci. Bull. 2020, 65, 272–275. [Google Scholar] [CrossRef]
- Lu, L.; Zheng, T.; Wu, Q.; Schneider, A.M.; Zhao, D.; Yu, L. Recent Advances in Bulk Heterojunction Polymer Solar Cells. Chem. Rev. 2015, 115, 12666–12731. [Google Scholar] [CrossRef]
- Yan, C.; Barlow, S.; Wang, Z.; Yan, H.; Jen, A.K.-Y.; Marder, S.R.; Zhan, X. Non-fullerene acceptors for organic solar cells. Nat. Rev. Mater. 2018, 3, 1–19. [Google Scholar] [CrossRef]
- Kim, D.Y.; Subbiah, J.; Sarasqueta, G.; So, F.; Ding, H.; Irfan; Gao, Y. The effect of molybdenum oxide interlayer on organic photovoltaic cells. Appl. Phys. Lett. 2009, 95, 093304. [Google Scholar] [CrossRef]
- Irfan; Ding, H.; Gao, Y.; Kim, D.Y.; Subbiah, J.; So, F. Energy level evolution of molybdenum trioxide interlayer between indium tin oxide and organic semiconductor. Appl. Phys. Lett. 2010, 96, 073304. [Google Scholar] [CrossRef]
- Akaike, K.; Kanai, K.; Ouchi, Y.; Seki, K. Impact of Ground-State Charge Transfer and Polarization Energy Change on Energy Band Offsets at Donor/Acceptor Interface in Organic Photovoltaics. Adv. Funct. Mater. 2010, 20, 715–721. [Google Scholar] [CrossRef]
- Osikowicz, W.; de Jong, M.P.; Salaneck, W.R. Formation of the Interfacial Dipole at Organic-Organic Interfaces: C60/Polymer Interfaces. Adv. Mater. 2007, 19, 4213–4217. [Google Scholar] [CrossRef]
- Wilke, A.; Mizokuro, T.; Blum, R.-P.; Rabe, J.P.; Koch, N. Electronic Properties of Cu-Phthalocyanine/Fullerene Planar and Bulk Hetereojunctions on PEDOT:PSS. IEEE J. Sel. Top. Quantum Electron. 2010, 16, 1732–1737. [Google Scholar] [CrossRef]
- Wilke, A.; Endres, J.; Hörmann, U.; Niederhausen, J.; Schlesinger, R.; Frisch, J.; Amsalem, P.; Wagner, J.; Gruber, M.; Opitz, A.; et al. Correlation between interface energetics and open circuit voltage in organic photovoltaic cells. Appl. Phys. Lett. 2012, 101, 233301. [Google Scholar] [CrossRef]
- Tang, J.X.; Lee, C.S.; Lee, S.T. Electronic structures of organic/organic heterojunctions: From vacuum level alignment to Fermi level pinning. J. Appl. Phys. 2007, 101, 064504. [Google Scholar] [CrossRef]
- Nakayama, Y.; Nguyen, T.L.; Ozawa, Y.; Machida, S.; Sato, T.; Tokairin, H.; Noguchi, Y.; Ishii, H. Complete Demonstration of the Valence Electronic Structure Inside a Practical Organic Solar Cell Probed by Low Energy Photoemission. Adv. Energy Mater. 2014, 4, 1301354. [Google Scholar] [CrossRef]
- Frisch, J.; Schubert, M.; Preis, E.; Rabe, J.P.; Neher, D.; Scherf, U.; Koch, N. Full electronic structure across a polymer heterojunction solar cell. J. Mater. Chem. 2012, 22, 4418. [Google Scholar] [CrossRef]
- Ishii, H.; Sugiyama, K.; Ito, E.; Seki, K. Energy Level Alignment and Interfacial Electronic Structures at Organic/Metal and Organic/Organic Interfaces. Adv. Mater. 1999, 11, 605–625. [Google Scholar] [CrossRef]
- Braun, S.; de Jong, M.P.; Osikowicz, W.; Salaneck, W.R. Influence of the electrode work function on the energy level alignment at organic-organic interfaces. Appl. Phys. Lett. 2007, 91, 202108. [Google Scholar] [CrossRef]
- Zhao, W.; Salomon, E.; Zhang, Q.; Barlow, S.; Marder, S.R.; Kahn, A. Substrate-dependent electronic structure of an organic heterojunction. Phys. Rev. 2008, 77. [Google Scholar] [CrossRef]
- Akaike, K.; Koch, N.; Heimel, G.; Oehzelt, M. The Impact of Disorder on the Energy Level Alignment at Molecular Donor-Acceptor Interfaces. Adv. Mater. Interfaces 2015, 2, 1500232. [Google Scholar] [CrossRef]
- Oehzelt, M.; Koch, N.; Heimel, G. Organic semiconductor density of states controls the energy level alignment at electrode interfaces. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Akaike, K.; Koch, N.; Oehzelt, M. Fermi level pinning induced electrostatic fields and band bending at organic heterojunctions. Appl. Phys. Lett. 2014, 105, 223303. [Google Scholar] [CrossRef]
- Oehzelt, M.; Akaike, K.; Koch, N.; Heimel, G. Energy-level alignment at organic heterointerfaces. Sci. Adv. 2015, 1, e1501127. [Google Scholar] [CrossRef] [PubMed]
- Peisert, H.; Knupfer, M.; Schwieger, T.; Auerhammer, J.M.; Golden, M.S.; Fink, J. Full characterization of the interface between the organic semiconductor copper phthalocyanine and gold. J. Appl. Phys. 2002, 91, 4872. [Google Scholar] [CrossRef]
- Kröger, I.; Stadtmüller, B.; Stadler, C.; Ziroff, J.; Kochler, M.; Andreas, S.; Pollinger, F.; Lee, T.-L.; Zegenhagen, J.; Reinert, F.; et al. Submonolayer growth of copper-phthalocyanine on Ag(111). New J. Phys. 2010, 12, 083038. [Google Scholar] [CrossRef]
- Tanaka, Y.; Takahashi, K.; Kuzumaki, T.; Yamamoto, Y.; Hotta, K.; Harasawa, A.; Miyoshi, Y.; Yoshikawa, H.; Ouchi, Y.; Ueno, N.; et al. Intermolecular band dispersion in a self-assembled phthalocyanine derivative film: The case of tetrakis(thiadiazole)porhyrazine. Phys. Rev. 2010, 82. [Google Scholar] [CrossRef]
- Hayashi, N.; Ishii, H.; Ouchi, Y.; Seki, K. Examination of band bending at buckminsterfullerene (C60)/metal interfaces by the Kelvin probe method. J. Appl. Phys. 2002, 92, 3784–3793. [Google Scholar] [CrossRef]
- Perdigón-Toro, L.; Zhang, H.; Markina, A.; Yuan, J.; Hosseini, S.M.; Wolff, C.M.; Zuo, G.; Stolterfoht, M.; Zou, Y.; Gao, F.; et al. Barrierless Free Charge Generation in the High-Performance PM6:Y6 Bulk Heterojunction Non-Fullerene Solar Cell. Adv. Mater. 2020, 32, 1906763. [Google Scholar] [CrossRef]
- Akaike, K. Advanced understanding on electronic structure of molecular semiconductors and their interfaces. Jpn. J. Appl. Phys. 2018, 57, 03EA03. [Google Scholar] [CrossRef]
- Heilmeier, G.H.; Warfield, G. Investigation of Bulk Currents in Metal-Free Phthalocyanine Crystals. J. Chem. Phys. 1963, 38, 163–168. [Google Scholar] [CrossRef]
- Wang, H.; Amsalem, P.; Heimel, G.; Salzmann, I.; Koch, N.; Oehzelt, M. Band-Bending in Organic Semiconductors: The Role of Alkali-Halide Interlayers. Adv. Mater. 2014, 26, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Amsalem, P.; Niederhausen, J.; Wilke, A.; Heimel, G.; Schlesinger, R.; Winkler, S.; Vollmer, A.; Rabe, J.P.; Koch, N. Role of charge transfer, dipole-dipole interactions, and electrostatics in Fermi-level pinning at a molecular heterojunction on a metal surface. Phys. Rev. 2013, 87. [Google Scholar] [CrossRef]
- Niederhausen, J.; Amsalem, P.; Wilke, A.; Schlesinger, R.; Winkler, S.; Vollmer, A.; Rabe, J.P.; Koch, N. Doping of C60 (sub)monolayers by Fermi-level pinning induced electron transfer. Phys. Rev. 2012, 86. [Google Scholar] [CrossRef]
- Winkler, S.; Amsalem, P.; Frisch, J.; Oehzelt, M.; Heimel, G.; Koch, N. Probing the energy levels in hole-doped molecular semiconductors. Mater. Horiz. 2015, 2, 427–433. [Google Scholar] [CrossRef]
- Shallcross, R.C.; Stubhan, T.; Ratcliff, E.L.; Kahn, A.; Brabec, C.J.; Armstrong, N.R. Quantifying the Extent of Contact Doping at the Interface between High Work Function Electrical Contacts and Poly(3-hexylthiophene) (P3HT). J. Phys. Chem. Lett. 2015, 6, 1303–1309. [Google Scholar] [CrossRef]
- Rand, B.P.; Cheyns, D.; Vasseur, K.; Giebink, N.C.; Mothy, S.; Yi, Y.; Coropceanu, V.; Beljonne, D.; Cornil, J.; Brédas, J.-L.; et al. The Impact of Molecular Orientation on the Photovoltaic Properties of a Phthalocyanine/Fullerene Heterojunction. Adv. Funct. Mater. 2012, 22, 2987–2995. [Google Scholar] [CrossRef]
- Chen, W.; Qi, D.-C.; Huang, H.; Gao, X.; Wee, A.T.S. Organic-Organic Heterojunction Interfaces: Effect of Molecular Orientation. Adv. Funct. Mater. 2011, 21, 410–424. [Google Scholar] [CrossRef]
- Ngongang Ndjawa, G.O.; Graham, K.R.; Li, R.; Conron, S.M.; Erwin, P.; Chou, K.W.; Burkhard, G.F.; Zhao, K.; Hoke, E.T.; Thompson, M.E.; et al. Impact of Molecular Orientation and Spontaneous Interfacial Mixing on the Performance of Organic Solar Cells. Chem. Mater. 2015, 27, 5597–5604. [Google Scholar] [CrossRef]
- Opitz, A.; Wilke, A.; Amsalem, P.; Oehzelt, M.; Blum, R.-P.; Rabe, J.P.; Mizokuro, T.; Hörmann, U.; Hansson, R.; Moons, E.; et al. Organic heterojunctions: Contact-induced molecular reorientation, interface states, and charge re-distribution. Sci. Rep. 2016, 6, 21291. [Google Scholar] [CrossRef]
- Tengstedt, C.; Osikowicz, W.; Salaneck, W.R.; Parker, I.D.; Hsu, C.-H.; Fahlman, M. Fermi-level pinning at conjugated polymer interfaces. Appl. Phys. Lett. 2006, 88, 053502. [Google Scholar] [CrossRef]
- Yang, J.-P.; Bussolotti, F.; Kera, S.; Ueno, N. Origin and role of gap states in organic semiconductor: As the nature of organic molecular crystals. J. Phys. Appl. Phys. 2017. [Google Scholar] [CrossRef]
- Yoshida, H. Low-Energy Inverse Photoemission Study on the Electron Affinities of Fullerene Derivatives for Organic Photovoltaic Cells. J. Phys. Chem. 2014, 118, 24377–24382. [Google Scholar] [CrossRef]
- Akaike, K.; Kubozono, Y. Correlation between energy level alignment and device performance in planar heterojunction organic photovoltaics. Org. Electron. 2013, 14, 1–7. [Google Scholar] [CrossRef]
- Würfel, U.; Perdigón-Toro, L.; Kurpiers, J.; Wolff, C.M.; Caprioglio, P.; Rech, J.J.; Zhu, J.; Zhan, X.; You, W.; Shoaee, S.; et al. Recombination between Photogenerated and Electrode-Induced Charges Dominates the Fill Factor Losses in Optimized Organic Solar Cells. J. Phys. Chem. Lett. 2019, 10, 3473–3480. [Google Scholar] [CrossRef]
- Wang, J.; Xu, L.; Lee, Y.-J.; De Anda Villa, M.; Malko, A.V.; Hsu, J.W.P. Effects of Contact-Induced Doping on the Behaviors of Organic Photovoltaic Devices. Nano Lett. 2015, 15, 7627–7632. [Google Scholar] [CrossRef]
- Izawa, S.; Shintaku, N.; Hiramoto, M. Effect of Band Bending and Energy Level Alignment at the Donor/Acceptor Interface on Open-Circuit Voltage in Organic Solar Cells. J. Phys. Chem. Lett. 2018, 9, 2914–2918. [Google Scholar] [CrossRef]
- Breuer, T.; Karthäuser, A.; Witte, G. Effects of Molecular Orientation in Acceptor-Donor Interfaces between Pentacene and C60 and Diels-Alder Adduct Formation at the Molecular Interface. Adv. Mater. Interfaces 2016, 3, 1500452. [Google Scholar] [CrossRef]
- Sweetnam, S.; Graham, K.R.; Ngongang Ndjawa, G.O.; Heumüller, T.; Bartelt, J.A.; Burke, T.M.; Li, W.; You, W.; Amassian, A.; McGehee, M.D. Characterization of the Polymer Energy Landscape in Polymer: Fullerene Bulk Heterojunctions with Pure and Mixed Phases. J. Am. Chem. Soc. 2014, 136, 14078–14088. [Google Scholar] [CrossRef]
- Sai, N.; Gearba, R.; Dolocan, A.; Tritsch, J.R.; Chan, W.-L.; Chelikowsky, J.R.; Leung, K.; Zhu, X. Understanding the Interface Dipole of Copper Phthalocyanine (CuPc)/C60: Theory and Experiment. J. Phys. Chem. Lett. 2012, 3, 2173–2177. [Google Scholar] [CrossRef]
- Lin, X.; Purdum, G.E.; Zhang, Y.; Barlow, S.; Marder, S.R.; Loo, Y.-L.; Kahn, A. Impact of a Low Concentration of Dopants on the Distribution of Gap States in a Molecular Semiconductor. Chem. Mater. 2016, 28, 2677–2684. [Google Scholar] [CrossRef]
- Stadtmüller, B.; Gruenewald, M.; Peuker, J.; Forker, R.; Fritz, T.; Kumpf, C. Molecular Exchange in a Heteromolecular PTCDA/CuPc Bilayer Film on Ag(111). J. Phys. Chem. 2014, 118, 28592–28602. [Google Scholar] [CrossRef]
- Wang, Q.; Franco-Cañellas, A.; Ji, P.; Bürker, C.; Wang, R.-B.; Broch, K.; Thakur, P.K.; Lee, T.-L.; Zhang, H.; Gerlach, A.; et al. Bilayer Formation vs Molecular Exchange in Organic Heterostructures: Strong Impact of Subtle Changes in Molecular Structure. J. Phys. Chem. 2018, 122, 9480–9490. [Google Scholar] [CrossRef]
- Akaike, K.; Onishi, A.; Wakayama, Y.; Kanai, K. Structural Disordering upon Formation of Molecular Heterointerfaces. J. Phys. Chem. 2019, 123, 12242–12248. [Google Scholar] [CrossRef]
- Arkhipov, V.I.; Heremans, P.; Bässler, H. Why is exciton dissociation so efficient at the interface between a conjugated polymer and an electron acceptor? Appl. Phys. Lett. 2003, 82, 4605–4607. [Google Scholar] [CrossRef]
- Guan, Z.-L.; Kim, J.B.; Wang, H.; Jaye, C.; Fischer, D.A.; Loo, Y.-L.; Kahn, A. Direct determination of the electronic structure of the poly(3-hexylthiophene):phenyl-[6,6]-C61 butyric acid methyl ester blend. Org. Electron. 2010, 11, 1779–1785. [Google Scholar] [CrossRef]
- Poelking, C.; Andrienko, D. Design Rules for Organic Donor–Acceptor Heterojunctions: Pathway for Charge Splitting and Detrapping. J. Am. Chem. Soc. 2015, 137, 6320–6326. [Google Scholar] [CrossRef]
- Schwarze, M.; Tress, W.; Beyer, B.; Gao, F.; Scholz, R.; Poelking, C.; Ortstein, K.; Gunther, A.A.; Kasemann, D.; Andrienko, D.; et al. Band structure engineering in organic semiconductors. Science 2016, 352, 1446–1449. [Google Scholar] [CrossRef]
- Poelking, C.; Tietze, M.; Elschner, C.; Olthof, S.; Hertel, D.; Baumeier, B.; Würthner, F.; Meerholz, K.; Leo, K.; Andrienko, D. Impact of mesoscale order on open-circuit voltage in organic solar cells. Nat. Mater. 2014, 14, 434–439. [Google Scholar] [CrossRef]
- Liu, J.; Chen, S.; Qian, D.; Gautam, B.; Yang, G.; Zhao, J.; Bergqvist, J.; Zhang, F.; Ma, W.; Ade, H.; et al. Fast charge separation in a non-fullerene organic solar cell with a small driving force. Nat. Energy 2016, 1, 16089. [Google Scholar] [CrossRef]
- Chen, S.; Wang, Y.; Zhang, L.; Zhao, J.; Chen, Y.; Zhu, D.; Yao, H.; Zhang, G.; Ma, W.; Friend, R.H.; et al. Efficient Nonfullerene Organic Solar Cells with Small Driving Forces for Both Hole and Electron Transfer. Adv. Mater. 2018, 30, 1804215. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Zhang, Y.; Zhou, L.; Zhang, G.; Yip, H.-L.; Lau, T.-K.; Lu, X.; Zhu, C.; Peng, H.; Johnson, P.A.; et al. Single-Junction Organic Solar Cell with over 15% Efficiency Using Fused-Ring Acceptor with Electron-Deficient Core. Joule 2019, 3, 1140–1151. [Google Scholar] [CrossRef]



© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akaike, K. Distributions of Potential and Contact-Induced Charges in Conventional Organic Photovoltaics. Materials 2020, 13, 2411. https://doi.org/10.3390/ma13102411
Akaike K. Distributions of Potential and Contact-Induced Charges in Conventional Organic Photovoltaics. Materials. 2020; 13(10):2411. https://doi.org/10.3390/ma13102411
Chicago/Turabian StyleAkaike, Kouki. 2020. "Distributions of Potential and Contact-Induced Charges in Conventional Organic Photovoltaics" Materials 13, no. 10: 2411. https://doi.org/10.3390/ma13102411
APA StyleAkaike, K. (2020). Distributions of Potential and Contact-Induced Charges in Conventional Organic Photovoltaics. Materials, 13(10), 2411. https://doi.org/10.3390/ma13102411



