The Effects of Cyclic Loading and Motion on the Implant–Cement Interface and Cement Mantle of PEEK and Cobalt–Chromium Femoral Total Knee Arthroplasty Implants: A Preliminary Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
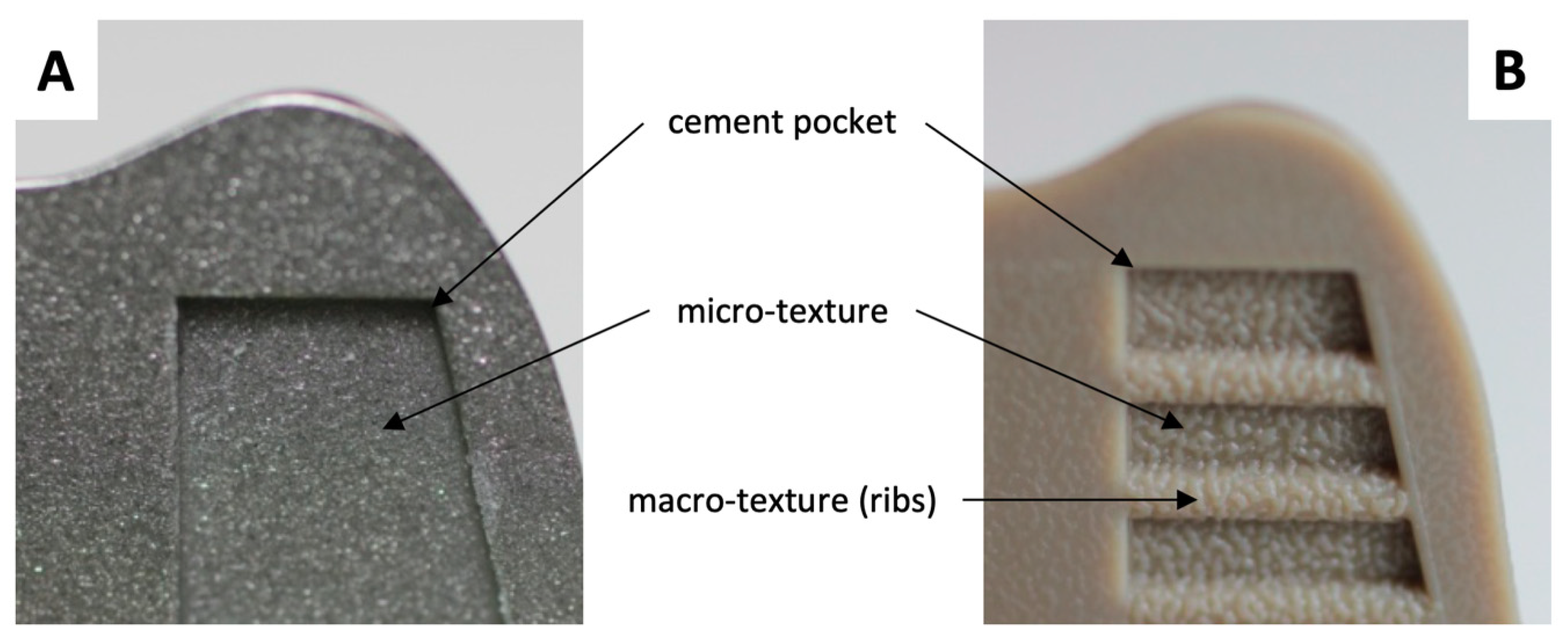
2.2. Experimental Design
2.3. Analysis of Dye Penetration (Fluorescence)
2.4. Analysis of Cement Damage (Full-Thickness Cracks)
2.5. Statistics
3. Results
3.1. Analysis of Dye Penetration (Fluorescence)
3.2. Analysis of Cement Damage
4. Discussion
4.1. Analysis of Dye Penetration
4.2. Analysis of Cement Cracks
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR). Hip, Knee & Shoulder Arthroplasty: 2017 Annual Report; AOA: Adelaide, Australia, 2017. [Google Scholar]
- Gandhi, R.; Tsvetkov, D.; Davey, J.R.; Mahomed, N.N. Survival and clinical function of cemented and uncemented prostheses in total knee replacement: A meta-analysis. J. Bone Jt. Surg. Br. 2009, 91, 889–895. [Google Scholar] [CrossRef] [PubMed]
- National Joint Registry for England, Wales, Northern Ireland and the Isle of Man. 2017 Annual Report; NJR: Hemel Hempstead, UK, 2017. [Google Scholar]
- Hopley, C.D.J.; Dalury, D.F. A systematic review of clinical outcomes and survivorship after total knee arthroplasty with a contemporary modular knee system. J. Arthroplast. 2014, 29, 1398–1411. [Google Scholar] [CrossRef] [PubMed]
- Erli, H.J.; Marx, R.; Paar, O.; Niethard, F.U.; Weber, M.; Wirtz, D.C. Surface pretreatments for medical application of adhesion. Biomed. Eng. Online 2003, 2, 15. [Google Scholar] [CrossRef] [PubMed]
- Marx, R.; Qunaibi, M.; Wirtz, D.C.; Niethard, F.U.; Mumme, T. Surface pretreatment for prolonged survival of cemented tibial prosthesis components: Full- vs. surface-cementation technique. Biomed. Eng. Online 2005, 4, 61. [Google Scholar] [CrossRef]
- Yerby, S.A.; Paal, A.F.; Young, P.M.; Beaupre, G.S.; Ohashi, K.L.; Goodman, S.B. The effect of a silane coupling agent on the bond strength of bone cement and cobalt—Chrome alloy. J. Biomed. Mater. Res. 2000, 49, 127–133. [Google Scholar] [CrossRef]
- Ohashi, K.L.; Yerby, S.A.; Dauskardt, R.H. Effects of an adhesion promoter on the debond resistance of a metal–polymethylmethacrylate interface. J. Biomed. Mater. Res. 2001, 54, 419–427. [Google Scholar] [CrossRef]
- Mann, K.; Bhashyam, S. Mixed-mode fracture toughness of the cobalt-chromium alloy/polymethlymethacrylate cement interface. J. Orthop. Res. 1999, 17, 321–328. [Google Scholar] [CrossRef]
- Ong, A.; Wong, K.L.; Lai, M.; Garino, J.; Steinberg, M.E. Early failure of precoated femoral components in primary total hip arthroplasty. J. Bone Jt. Surg. Am. 2002, 84, 786–792. [Google Scholar] [CrossRef]
- Bini, S.A.; Chen, Y.; Khatod, M.; Paxton, E.W. Does pre-coating total knee tibial implants affect the risk of aseptic revision? Bone Jt. J. 2013, 95, 367–370. [Google Scholar] [CrossRef]
- Ohashi, K.; Romero, A.C.; McGowan, P.D.; Maloney, W.; Dauskardt, R. Adhesion and reliability of interfaces in cemented total hip arthroplasties. J. Orthop. Res. 1998, 16, 705–714. [Google Scholar] [CrossRef]
- Goodheart, J.R.; Miller, M.A.; Mann, K.A. In Vivo Loss of Cement-Bone Interlock Reduces Fixation Strength in Total Knee Arthroplasties. J. Orthop. Res. 2014, 32, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, P.; Miller, M.A.; Verdonschot, N.; Mann, K.A.; Janssen, D. Experimental and computational micromechanics at the tibial cement-trabeculae interface. J. Biomech. 2016, 49, 1641–1648. [Google Scholar] [CrossRef] [PubMed]
- Van de Groes, S.; de Waal-Malefijt, M.; Verdonschot, N. Probability of mechanical loosening of the femoral component in high flexion total knee arthroplasty can be reduced by rather simple surgical techniques. Knee 2014, 21, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Bollars, P.; Luyckx, J.; Innocenti, B.; Labey, L.; Victor, J.; Bellemans, J. Femoral component loosening in high-flexion total knee replacement. J. Bone Jt. Surg. Br. 2011, 93, 1355–1361. [Google Scholar] [CrossRef]
- Bergschmidt, P.; Dammer, R.; Zietz, C.; Finze, S.; Mittelmeier, W.; Bader, R. Adhesive strength of total knee endoprostheses to bone cement—Analysis of metallic and ceramic femoral components under worst-case conditions. Biomed. Tech. 2016, 61, 281–289. [Google Scholar] [CrossRef]
- Zelle, J.; Janssen, D.; Peeters, S.; Brouwer, C.; Verdonschot, N. Mixed-mode failure strength of implant-cement interface specimens with varying surface roughness. J. Biomech. 2011, 44, 780–783. [Google Scholar] [CrossRef]
- Arnholt, C.M.; MacDonald, D.W.; Malkani, A.L.; Klein, G.R.; Rimnac, C.M.; Kurtz, S.M.; Kocagoz, S.B.; Gilbert, J.L. Corrosion Damage and Wear Mechanisms in Long-Term Retrieved CoCr Femoral Components for Total Knee Arthroplasty. J. Arthroplast. Arthroplast. 2016, 31, 2900–2906. [Google Scholar] [CrossRef]
- Zelle, J.; van de Groes, S.; de Waal Malefijt, M.; Verdonschot, N. Femoral loosening of high-flexion total knee arthroplasty: The effect of posterior cruciate ligament retention and bone quality reduction. Med. Eng. Phys. 2014, 36, 318–324. [Google Scholar] [CrossRef]
- Han, H.S.; Kang, S.-B.; Yoon, K.S. High incidence of loosening of the femoral component in legacy posterior stabilised-flex total knee replacement. J. Bone Jt. Surg. Br. 2007, 89, 1457–1461. [Google Scholar] [CrossRef]
- Rankin, K.E.; Dickinson, A.S.; Briscoe, A.; Browne, M. Does a PEEK Femoral TKA Implant Preserve Intact Femoral Surface Strains Compared With CoCr? A Preliminary Laboratory Study. Clin. Orthop. Relat. Res. 2016, 474, 2405–2413. [Google Scholar] [CrossRef]
- Cowie, R.M.; Briscoe, A.; Fisher, J.; Jennings, L.M. PEEK-OPTIMATM as an alternative to cobalt chrome in the femoral component of total knee replacement: A preliminary study. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2016, 230, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- De Ruiter, L.; Janssen, D.; Briscoe, A.; Verdonschot, N. The mechanical response of a polyetheretherketone femoral knee implant under a deep squatting loading condition. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2017, 231, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- De Ruiter, L.; Janssen, D.; Briscoe, A.; Verdonschot, N. A preclinical numerical assessment of a polyetheretherketone femoral component in total knee arthroplasty during gait. J. Exp. Orthop. 2017, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- De Ruiter, L.; Janssen, D.; Briscoe, A.; Verdonschot, N. Fixation strength of a polyetheretherketone femoral component in total knee arthroplasty. Med. Eng. Phys. 2017, 49, 157–162. [Google Scholar] [CrossRef]
- Du, Z.; Zhu, Z.; Yue, B.; Li, Z.; Wang, Y. Feasibility and Safety of a Cemented PEEK-on-PE Knee Replacement in a Goat Model: A Preliminary Study. Artif. Organs 2018, 42, E204–E214. [Google Scholar] [CrossRef]
- Meng, X.; Du, Z.; Wang, Y. Characteristics of wear particles and wear behavior of retrieved PEEK-on-HXLPE total knee implants: A preliminary study. RSC Adv. 2018, 8, 30330–30339. [Google Scholar] [CrossRef]
- Gallo, J.; Goodman, S.B.; Konttinen, Y.T.; Wimmer, M.A.; Holinka, M. Osteolysis around total knee arthroplasty: A review of pathogenetic mechanisms. Acta Biomater. 2013, 9, 8046–8058. [Google Scholar] [CrossRef]
- Cowie, R.M.; Pallem, N.M.; Briscoe, A.; Jennings, L.M. Third Body Wear of UHMWPE-on-PEEK-OPTIMATM. Materials 2020, 13, 1264. [Google Scholar] [CrossRef]
- McEwen, H.M.J.; Barnett, P.I.; Bell, C.J.; Farrar, R.; Auger, D.D.; Stone, M.H.; Fisher, J. The influence of design, materials and kinematics on the in vitro wear of total knee replacements. J. Biomech. 2005, 38, 357–365. [Google Scholar] [CrossRef]
- Ranawat, A.S.; Mohanty, S.S.; Goldsmith, S.E.; Rasquinha, V.J.; Rodriguez, J.A.; Ranawat, C.S. Experience with an all-polyethylene total knee arthroplasty in younger, active patients with follow-up from 2 to 11 years. J. Arthroplast. 2005, 20, 7–11. [Google Scholar] [CrossRef]
- Gioe, T.J.; Stroemer, E.S.; Santos, E.R.G. All-polyethylene and metal-backed tibias have similar outcomes at 10 years: A randomized level II evidence study. Clin. Orthop. Relat. Res. 2007, 455, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Gioe, T.J.; Bowman, K.R. A randomized comparison of all-polyethylene and metal-backed tibial components. Clin. Orthop. Relat. Res. 2000, 380, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Haft, G.F.; Heiner, A.D.; Dorr, L.D.; Brown, T.D.; Callaghan, J.J. A biomechanical analysis of polyethylene liner cementation into a fixed metal acetabular shell. J. Bone Jt. Surg. Am. 2003, 85, 1100–1110. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.; Freeman, M.; Revell, P.; Bradley, G.; Tuke, M. Can a total knee replacement prosthesis be made entirely of polymers? J. Arthroplast. 1998, 13, 388–395. [Google Scholar] [CrossRef]
- Zelle, J.; Janssen, D.; Van Eijden, J.; de Waal Malefijt, M.; Verdonschot, N. Does high-flexion total knee arthroplasty promote early loosening of the femoral component? J. Orthop. Res. 2011, 29, 976–983. [Google Scholar] [CrossRef]
- Schmalzried, T.P.; Szuszczewicz, E.S.; Northfield, M.R.; Akizuki, K.H.; Frankel, R.E.; Belcher, G.; Amstutz, H.C. Quantitative assessment of walking activity after total hip or knee replacement. J. Bone Jt. Surg. Am. 1998, 80, 54–59. [Google Scholar] [CrossRef]
- Race, A.; Miller, M.A.; Ayers, D.C.; Mann, K.A. Early cement damage around a femoral stem is concentrated at the cement/bone interface. J. Biomech. 2003, 36, 489–496. [Google Scholar] [CrossRef]
- Berahmani, S.; Janssen, D.; Wolfson, D.; De Waal Malefijt, M.; Fitzpatrick, C.K.; Rullkoetter, P.J.; Verdonschot, N. FE analysis of the effects of simplifications in experimental testing on micromotions of uncemented femoral knee implants. J. Orthop. Res. 2016, 34, 812–819. [Google Scholar] [CrossRef]
- Lorber, V.; Paulus, A.C.; Buschmann, A.; Schmitt, B.; Grupp, T.M.; Jansson, V.; Utzschneider, S. Elevated cytokine expression of different PEEK wear particles compared to UHMWPE in vivo. J. Mater. Sci. Mater. Med. 2014, 25, 141–149. [Google Scholar] [CrossRef]
- Paulus, A.C.; Haßelt, S.; Jansson, V.; Giurea, A.; Neuhaus, H.; Grupp, T.M.; Utzschneider, S. Histopathological Analysis of PEEK Wear Particle Effects on the Synovial Tissue of Patients. Biomed. Res. Int. 2016, 2, 2198914. [Google Scholar] [CrossRef]







| Group 1 | Group 2 | Group 3 | |
|---|---|---|---|
| Unloaded control | Gait control | 10 MC gait | |
| PEEK | 3 | 3 | 3 |
| CoCr | - | 3 | 3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Ruiter, L.; Cowie, R.M.; Jennings, L.M.; Briscoe, A.; Janssen, D.; Verdonschot, N. The Effects of Cyclic Loading and Motion on the Implant–Cement Interface and Cement Mantle of PEEK and Cobalt–Chromium Femoral Total Knee Arthroplasty Implants: A Preliminary Study. Materials 2020, 13, 3323. https://doi.org/10.3390/ma13153323
de Ruiter L, Cowie RM, Jennings LM, Briscoe A, Janssen D, Verdonschot N. The Effects of Cyclic Loading and Motion on the Implant–Cement Interface and Cement Mantle of PEEK and Cobalt–Chromium Femoral Total Knee Arthroplasty Implants: A Preliminary Study. Materials. 2020; 13(15):3323. https://doi.org/10.3390/ma13153323
Chicago/Turabian Stylede Ruiter, Lennert, Raelene M. Cowie, Louise M. Jennings, Adam Briscoe, Dennis Janssen, and Nico Verdonschot. 2020. "The Effects of Cyclic Loading and Motion on the Implant–Cement Interface and Cement Mantle of PEEK and Cobalt–Chromium Femoral Total Knee Arthroplasty Implants: A Preliminary Study" Materials 13, no. 15: 3323. https://doi.org/10.3390/ma13153323
APA Stylede Ruiter, L., Cowie, R. M., Jennings, L. M., Briscoe, A., Janssen, D., & Verdonschot, N. (2020). The Effects of Cyclic Loading and Motion on the Implant–Cement Interface and Cement Mantle of PEEK and Cobalt–Chromium Femoral Total Knee Arthroplasty Implants: A Preliminary Study. Materials, 13(15), 3323. https://doi.org/10.3390/ma13153323






