NiO/Carbon Aerogel Microspheres with Plum-Pudding Structure as Anode Materials for Lithium Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
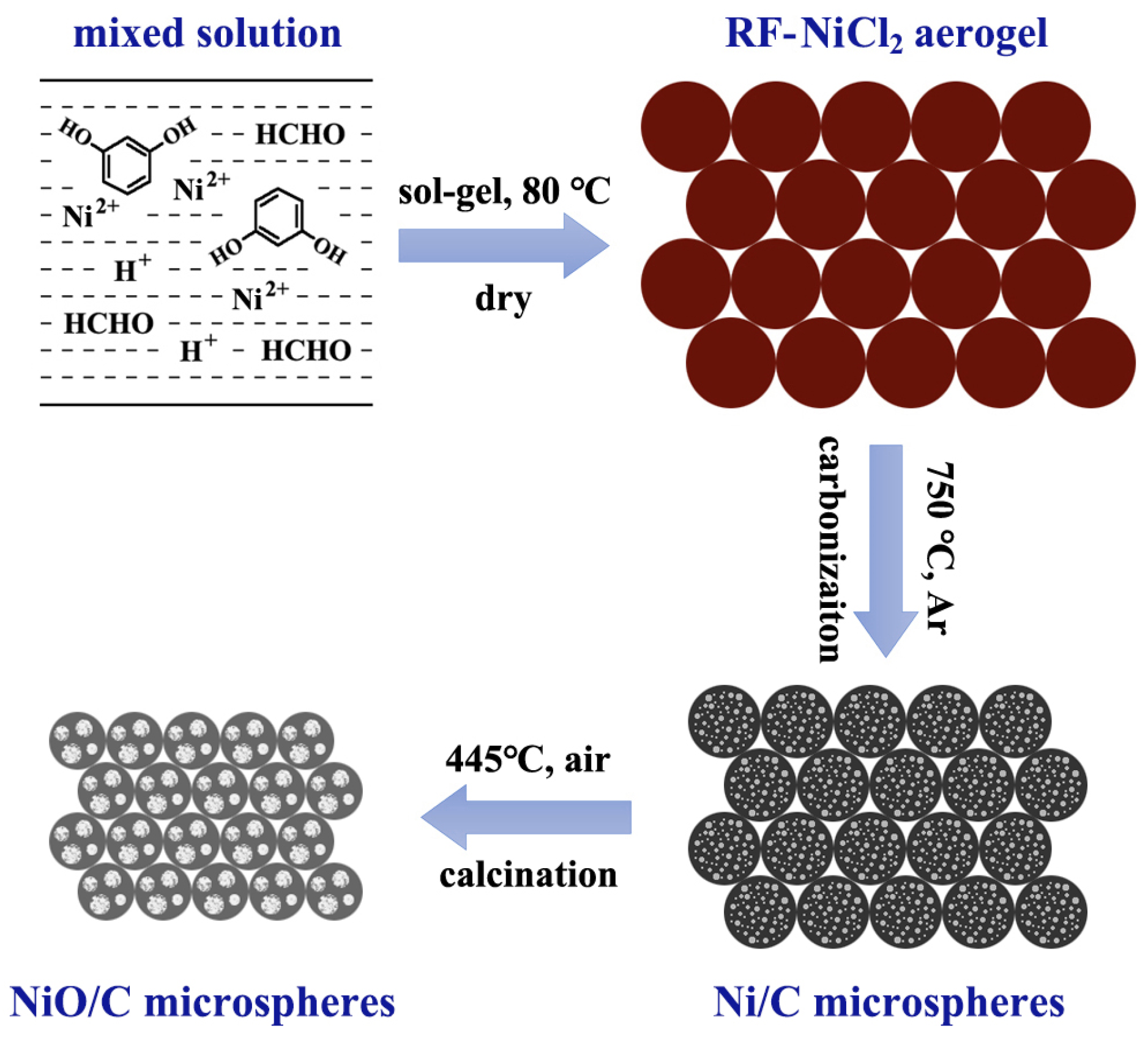
2.1. Sample Preparation
2.2. Material Characterization
2.3. Electrochemical Measurements
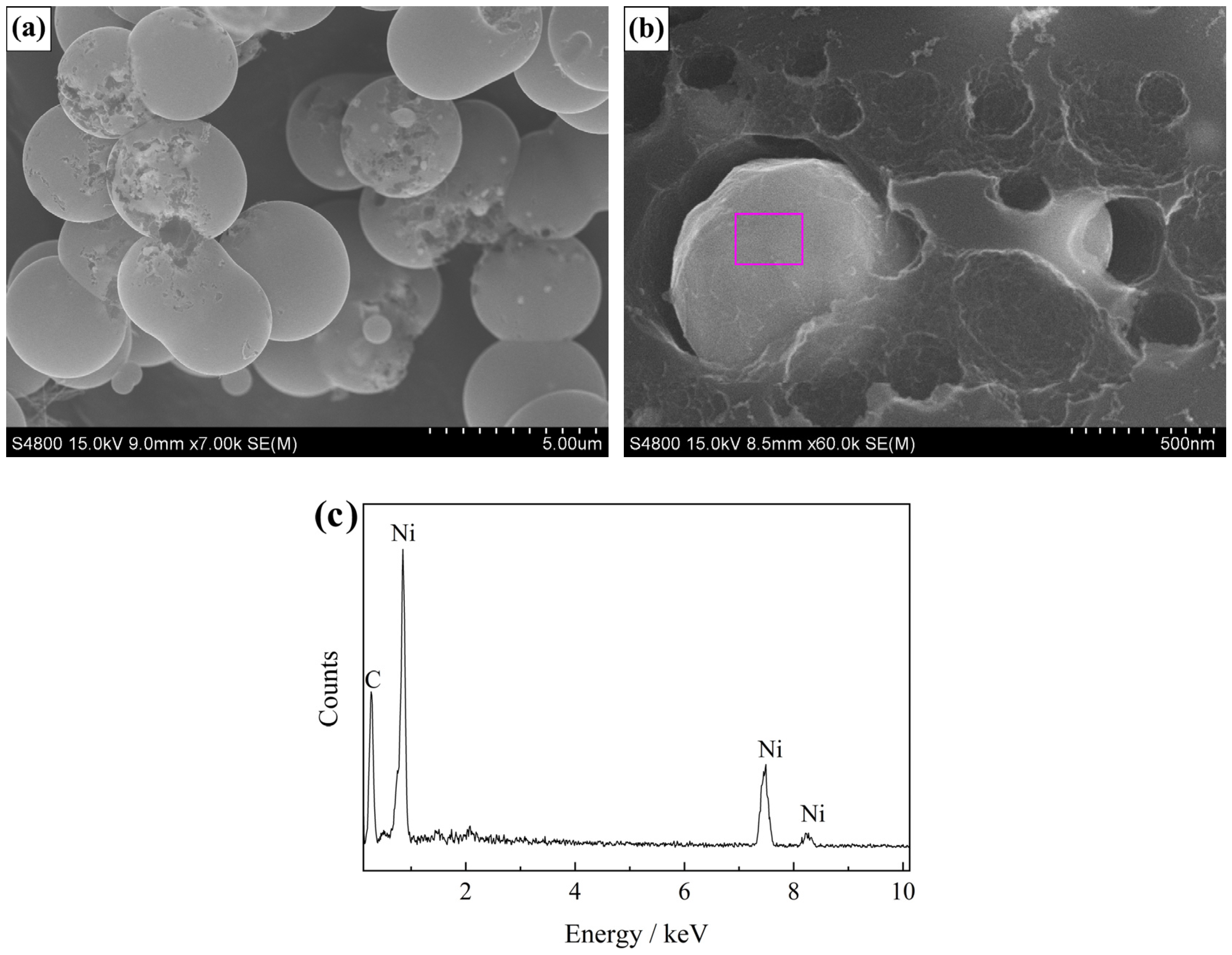
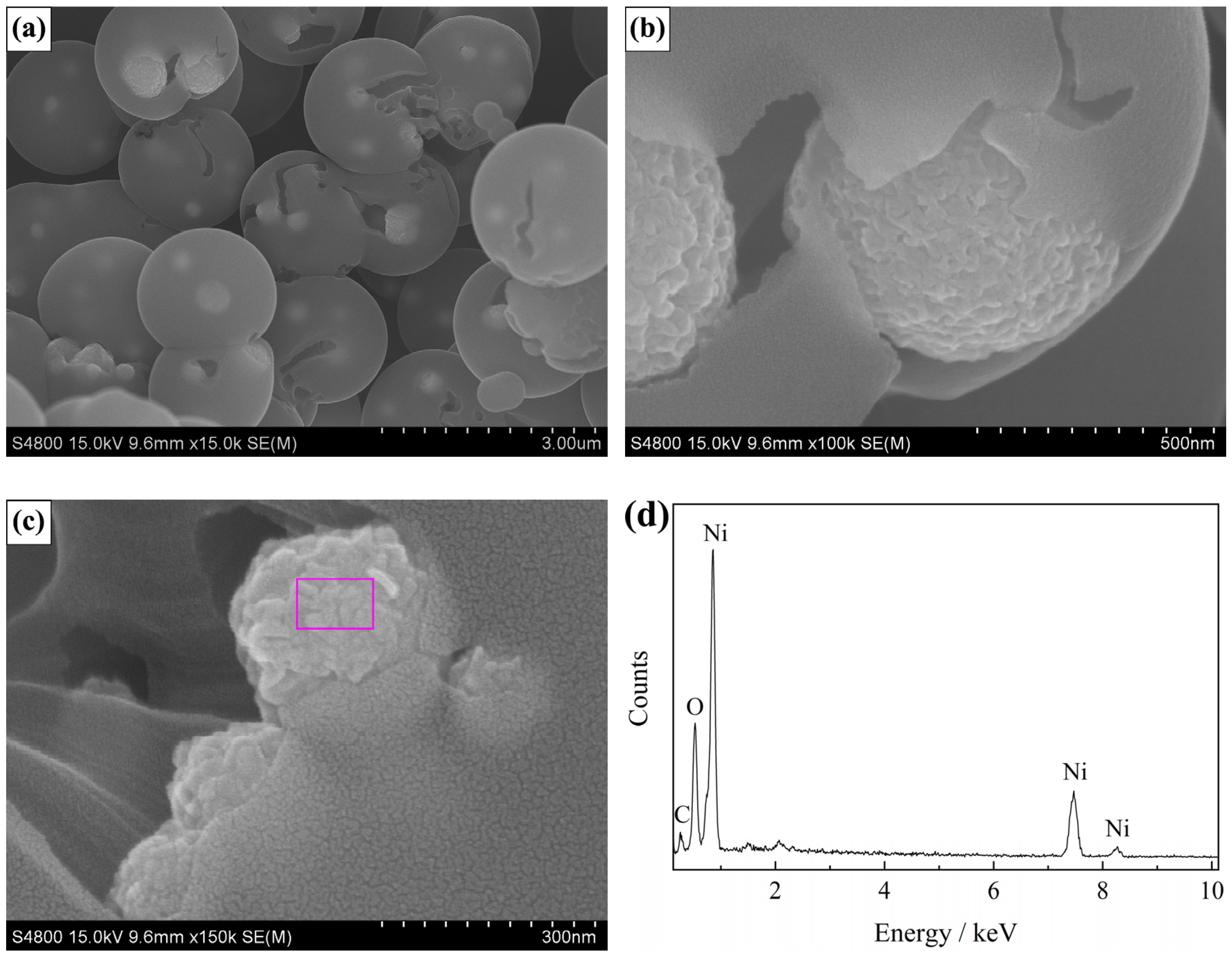
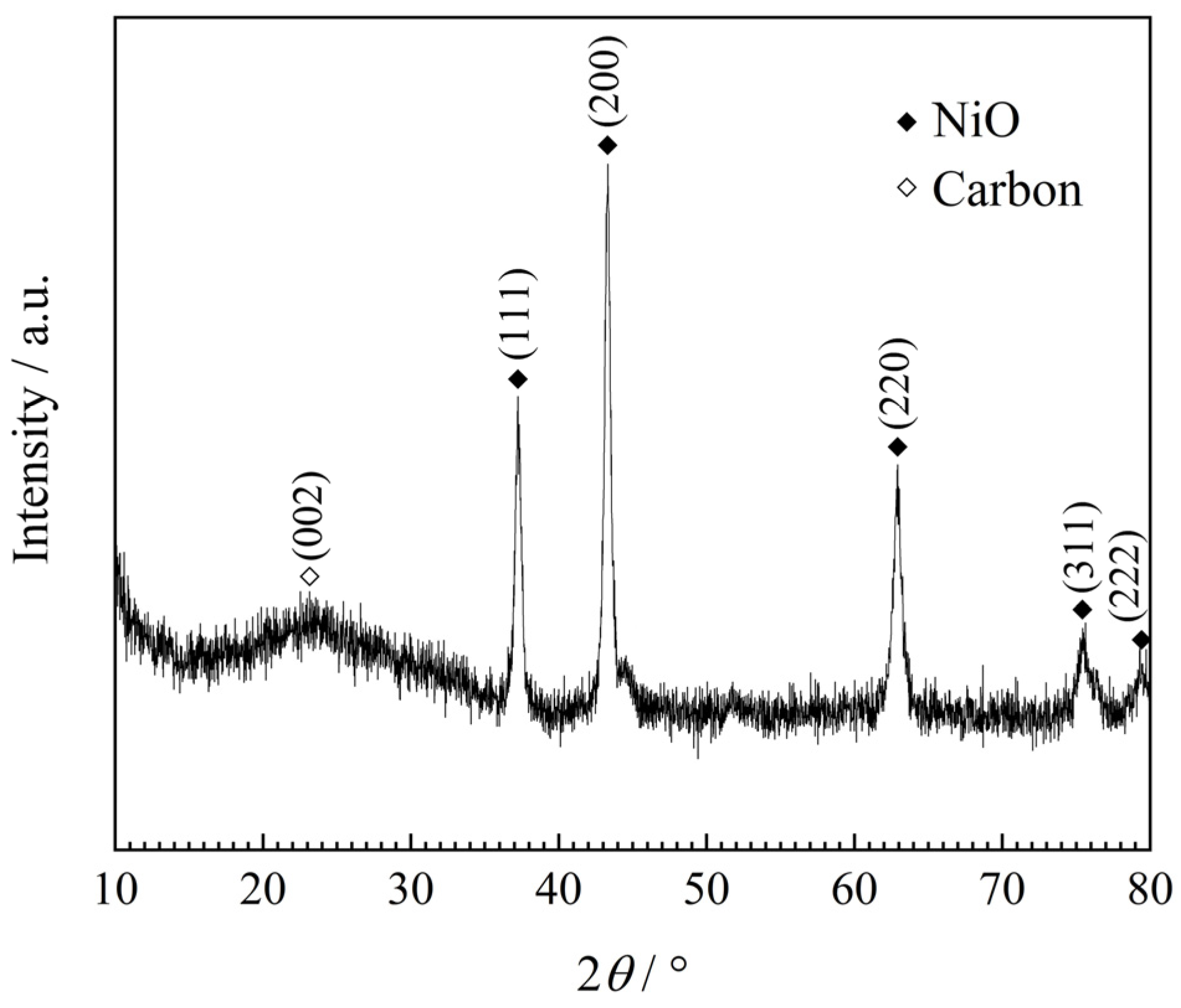
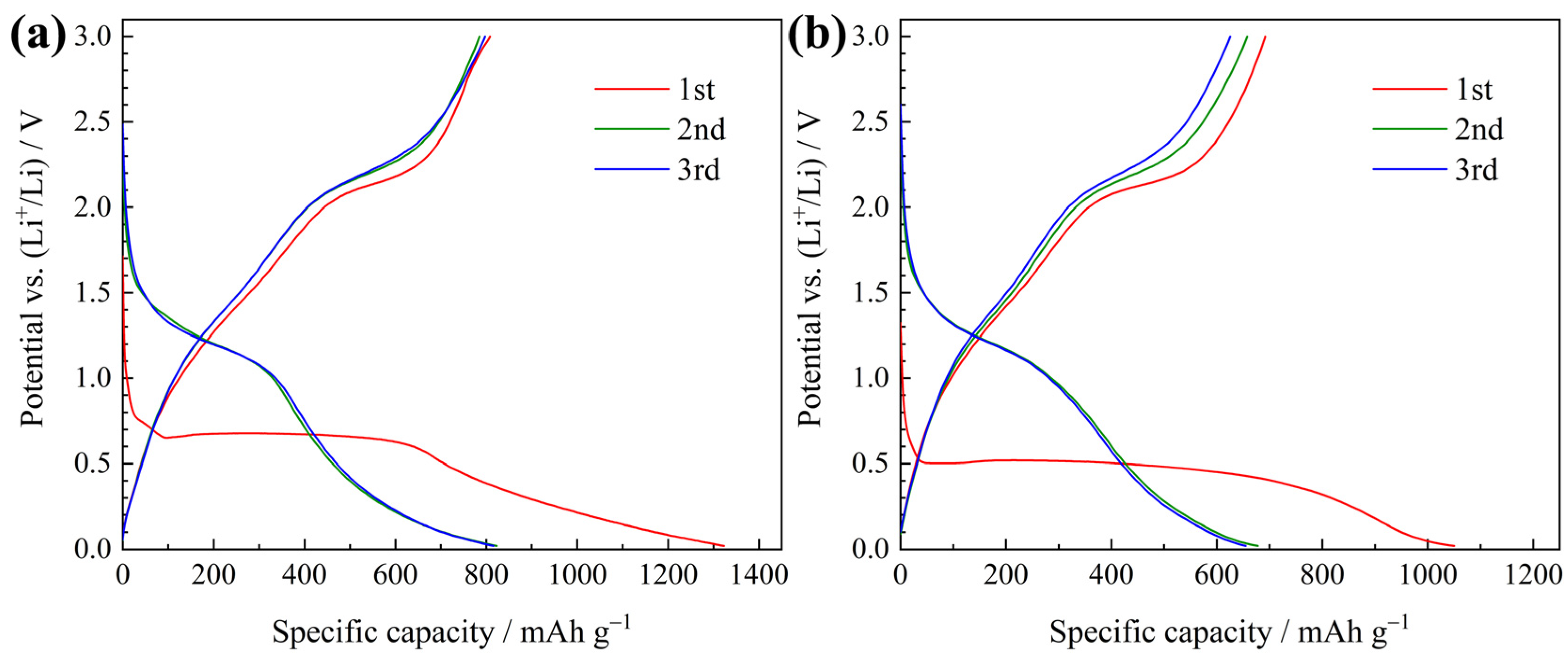
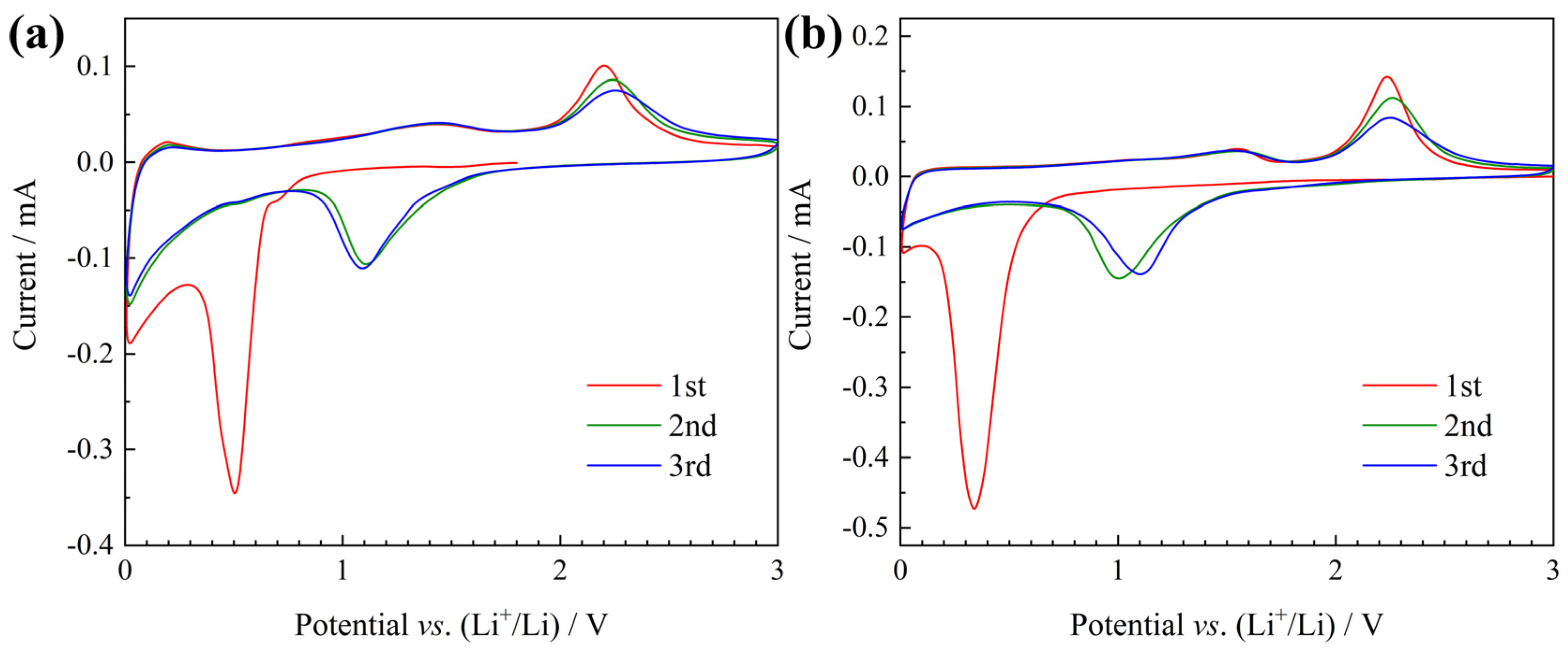
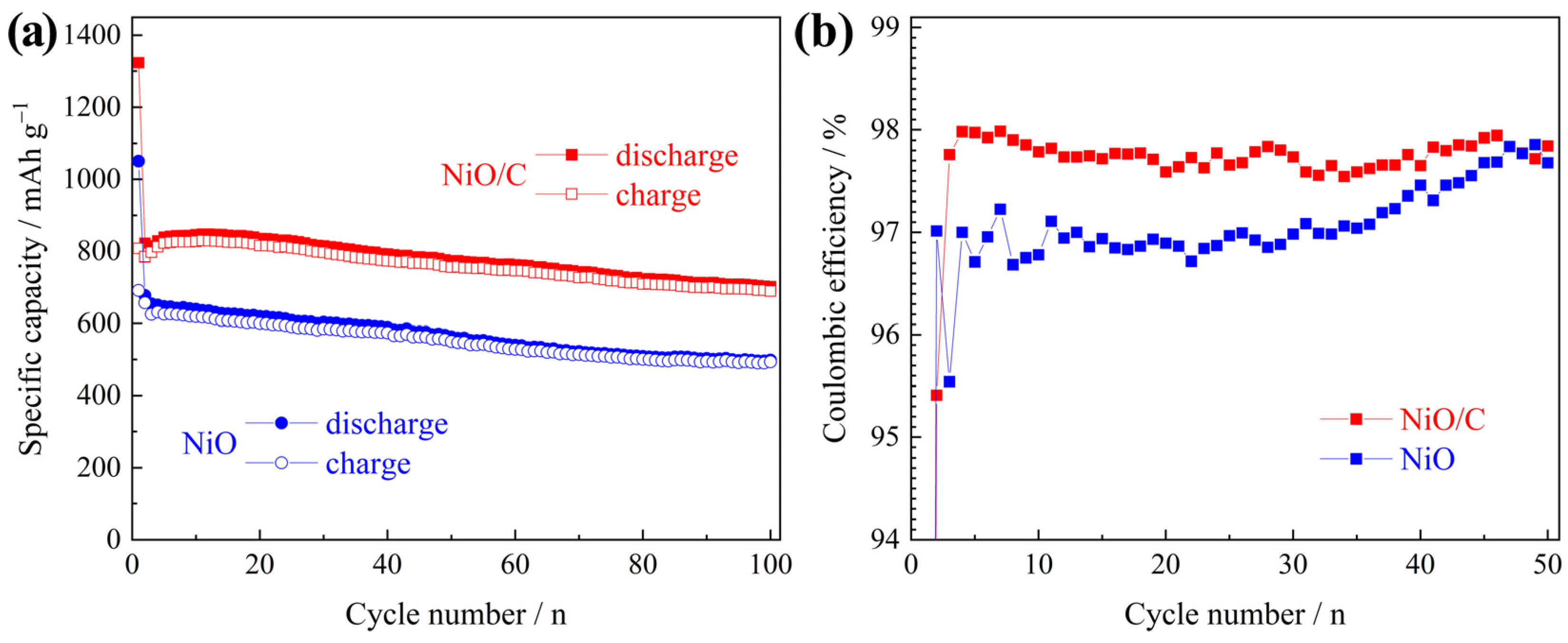
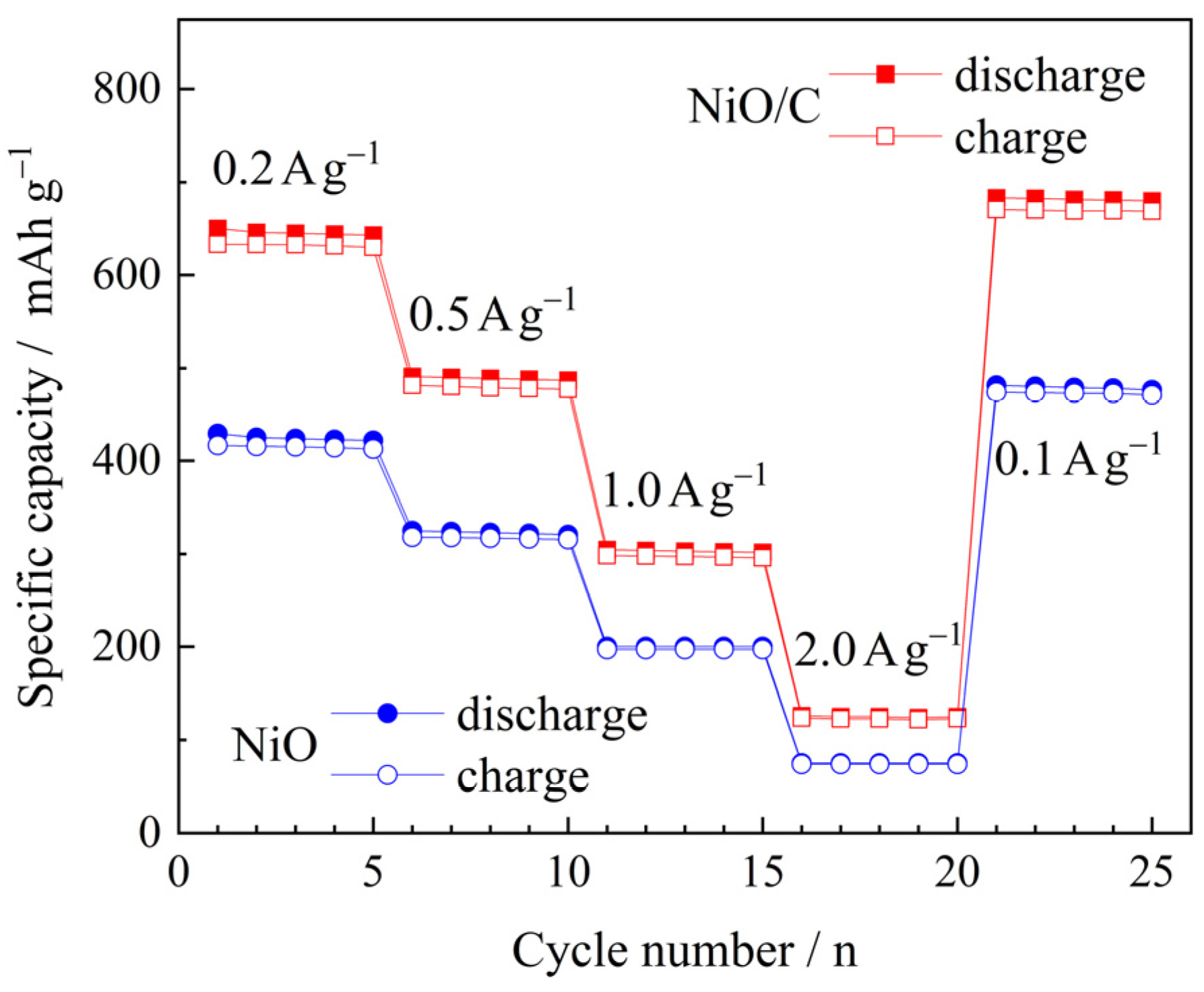
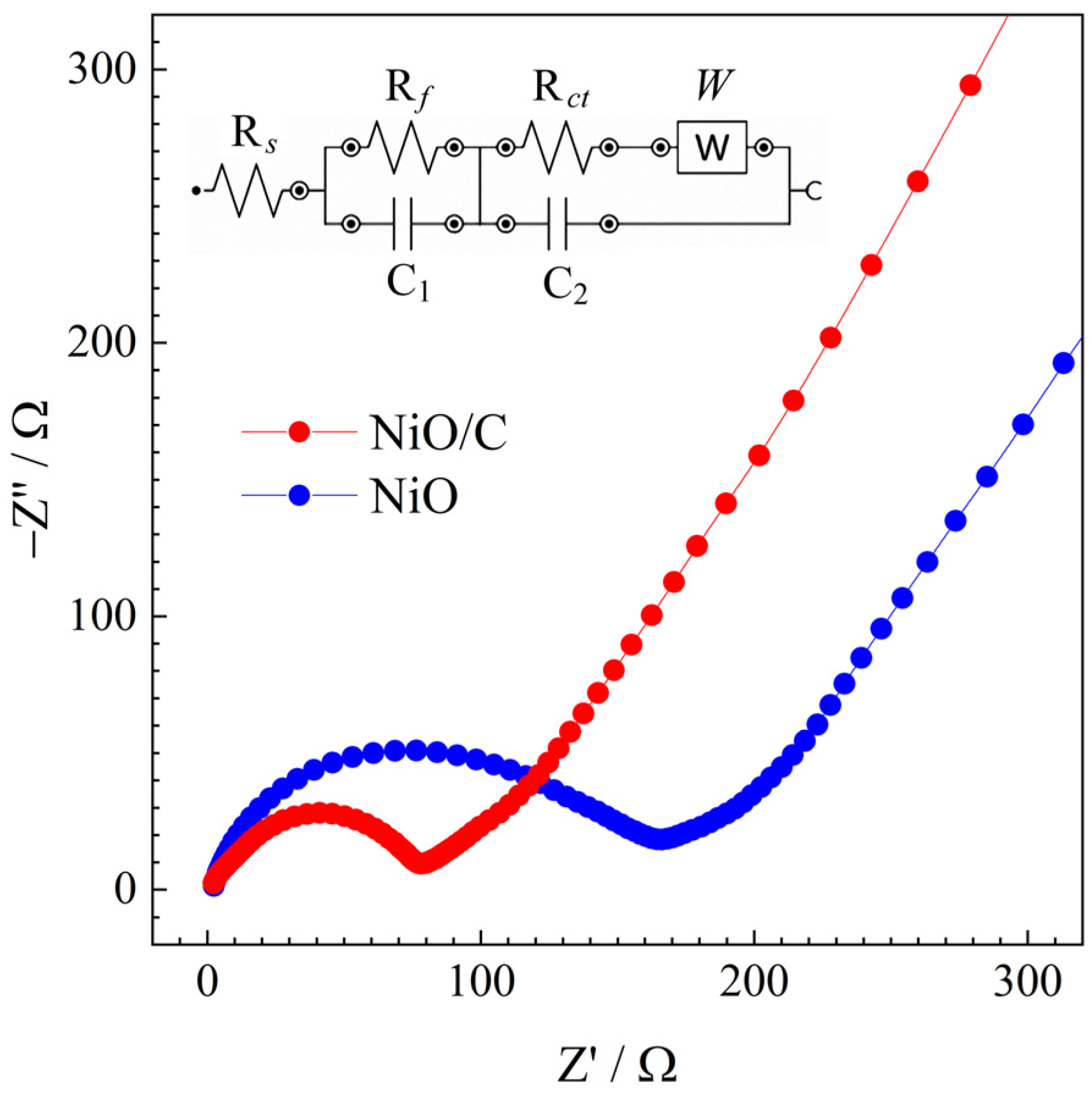
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Poizot, P.; Laruelle, S.; Grugeon, S.; Dupont, L.; Tarascon, J.M. Nano-sized transition-metal oxides as negative-electrode materials for lithium-ion batteries. Nature 2000, 407, 496–499. [Google Scholar] [CrossRef]
- Poizot, P.; Laruelle, S.; Grugeon, S.; Tarascon, J.M. Rationalization of the low-potential reactivity of 3d-metal-based inorganic compounds toward Li. J. Electrochem. Soc. 2002, 149, A1212–A1217. [Google Scholar] [CrossRef]
- Huang, X.H.; Wu, J.B.; Guo, R.Q.; Lin, Y.; Zhang, P. Aligned nickel-cobalt oxide nanosheet arrays for lithium ion battery applications. Int. J. Hydrog. Energy 2014, 39, 21399–21404. [Google Scholar] [CrossRef]
- Huang, X.H.; Wu, J.B.; Cao, Y.Q.; Zhang, P.; Lin, Y.; Guo, R.Q. Cobalt nanosheet arrays supported silicon film as anode materials for lithium ion batteries. Electrochim. Acta 2016, 203, 213–220. [Google Scholar] [CrossRef]
- Zhong, W.W.; Huang, J.D.; Liang, S.Q.; Liu, J.; Li, Y.J.; Cai, G.M.; Jiang, Y.; Liu, J. New prelithiated V2O5 superstructure for lithium-ion batteries with long cycle life and high power. ACS Energy Lett. 2020, 5, 31–38. [Google Scholar] [CrossRef]
- Wang, L.; Gu, C.; Ge, X.; Zhang, J.; Zhu, H.; Tu, J.P. A NiCo2O4 shell on a hollow Ni nanorod array core for water splitting with enhanced electrocatalytic performance. ChemNanoMat 2018, 4, 124–131. [Google Scholar] [CrossRef]
- Shen, S.J.; Zhong, W.W.; Huang, X.H.; Lin, Y.; Wang, T.L. Ordered ZnO/Ni hollow microsphere arrays as anode materials for lithium ion batteries. Materials 2019, 12, 1193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Yuan, Y.F.; Zhang, T.; Chen, Q.; Guo, S.Y. Co3O4 hollow nanospheres/carbon-assembled mesoporous polyhedron with internal bubbles encapsulating TiO2 nanosphere for high-performance lithium ion batteries. Nanotechnology 2019, 30, 355401. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.H.; Park, J.S.; Jo, M.S.; Kang, Y.C.; Cho, J.S. Design and synthesis of tube-in-tube structured NiO nanobelts with superior electrochemical properties for lithium-ion storage. Chem. Eng. J. 2018, 347, 889–899. [Google Scholar]
- Gu, L.L.; Xie, W.H.; Bai, S.; Liu, B.L.; Xue, S.; Li, Q.; He, D.Y. Facile fabrication of binder-free NiO electrodes with high rate capacity for lithium-ion batteries. Appl. Surf. Sci. 2016, 368, 298–302. [Google Scholar] [CrossRef]
- Jiang, T.C.; Bu, F.X.; Feng, X.X.; Shakir, I.; Hao, G.L.; Xu, Y.X. Porous Fe2O3 nanoframeworks encapsulated within three-dimensional graphene as high-performance flexible anode for lithium-ion battery. ACS Nano 2017, 11, 5140–5147. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Geng, B.J.; Zhang, C.; Shen, W.W.; Yang, D.W.; Li, Z.; Yang, Z.B.; Pan, D.Y. Hierarchical porous arrays of mesoporous Co3O4 nanosheets grown on graphene skin for high-rate and high-capacity energy storage. J. Alloy. Compd. 2020, 820, 153296. [Google Scholar] [CrossRef]
- Bai, Z.C.; Ju, Z.C.; Guo, C.L.; Qian, Y.T.; Tang, B.; Xiong, S.L. Direct large-scale synthesis of 3D hierarchical mesoporous NiO microspheres as high-performance anode materials for lithium ion batteries. Nanoscale 2014, 6, 3268–3273. [Google Scholar] [CrossRef] [PubMed]
- Han, W.J.; Qin, X.Y.; Wu, J.X.; Li, Q.; Liu, M.; Xia, Y.; Du, H.D.; Li, B.H.; Kang, F.Y. Electrosprayed porous Fe3O4/carbon microspheres as anode materials for high-performance lithium-ion batteries. Nano Res. 2018, 11, 892–904. [Google Scholar] [CrossRef]
- Lv, P.P.; Zhao, H.L.; Zeng, Z.P.; Gao, C.H.; Liu, X.; Zhang, T.H. Self-assembled three-dimensional hierarchical NiO nano/microspheres as high-performance anode material for lithium ion batteries. App. Surf. Sci. 2015, 329, 301–305. [Google Scholar] [CrossRef]
- Li, J.B.; Yan, D.; Hou, S.J.; Lu, T.; Yao, Y.F.; Chua, D.H.C.; Pan, L.K. Metal-organic frameworks derived yolk-shell ZnO/NiO microspheres as high-performance anode materials for lithium-ion batteries. Chem. Eng. J. 2018, 335, 579–589. [Google Scholar] [CrossRef]
- Tian, J.Y.; Shao, Q.; Dong, X.J.; Zheng, J.L.; Pan, D.; Zhang, X.Y.; Cao, H.L.; Hao, L.H.; Liu, J.R.; Mai, X.M.; et al. Bio-template synthesized NiO/C hollow microspheres with enhanced Li-ion battery electrochemical performance. Electrochim. Acta 2017, 261, 236–245. [Google Scholar] [CrossRef]
- Mao, Y.Q.; Shen, X.Y.; Wu, Z.H.; Zhu, L.P.; Liao, G.H. Preparation of Co3O4 hollow microspheres by recycling spent lithium-ion batteries and their application in electrochemical supercapacitors. J. Alloy. Compd. 2020, 816, 152604. [Google Scholar] [CrossRef]
- Wang, B.B.; Wang, G.; Cheng, X.M.; Wang, H. Synthesis and electrochemical investigation of core-shell ultrathin NiO nanosheets grown on hollow carbon microspheres composite for high performance lithium and sodium ion batteries. Chem. Eng. J. 2016, 306, 1193–1202. [Google Scholar] [CrossRef]
- Li, G.M.; Li, Y.; Chen, J.; Zhao, P.P.; Li, D.G.; Dong, Y.H.; Zhang, L.P. Synthesis and research of egg shell-yolk NiO/C porous composites as lithium-ion battery anode material. Electrochim. Acta 2017, 245, 941–948. [Google Scholar] [CrossRef]
- Wang, X.H.; Wang, J.Y.; Chen, Z.H.; Yang, K.; Zhang, Z.X.; Shi, Z.X.; Mei, T.; Qian, J.W.; Li, J.H.; Wang, X.B. Yolk-double shell Fe3O4@C@C composite as high-performance anode materials for lithium-ion batteries. J. Alloys Compd. 2020, 822, 153656. [Google Scholar] [CrossRef]
- Bell, J.; Ye, R.; Ahmed, K.; Liu, C.; Ozkan, M.; Ozkan, C.S. Free-standing Ni-NiO nanofiber cloth anode for high capacity and high rate Li-ion batteries. Nano Energy 2015, 18, 47–56. [Google Scholar] [CrossRef]
- Zhang, C.W.; Song, Y.; Xu, L.B.; Yin, F.X. In situ encapsulation of Co/Co3O4 nanoparticles in nitrogen-doped hierarchically ordered porous carbon as high performance anode for lithium-ion batteries. Chem. Eng. J. 2020, 380, 122545. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, W.X.; Chen, Y.; Fan, H.B.; Su, D.W.; Wang, G.X. MOF-derived porous N-Co3O4@N-C nanododecahedral wrapped with reduced graphene oxide as high capacity cathodes for lithium-sulfur batteries. J. Mater. Chem. A 2018, 6, 2729–2807. [Google Scholar]
- Shi, W.P.; Zhang, Y.M.; Key, J.L.; Shen, P.K. Three-dimensional graphene sheets with NiO nanobelt outgrowths for enhanced capacity and long term high rate cycling Li-ion battery anode material. J. Power Sources 2018, 379, 362–370. [Google Scholar] [CrossRef]
- Chen, X.L.; Xiao, T.; Wang, S.L.; Li, J.; Xiang, P.; Jiang, L.H.; Tan, X.Y. Superior Li-ion storage performance of graphene decorated NiO nanowalls on Ni as anode for lithium ion batteries. Mater. Chem. Phys. 2019, 222, 31–36. [Google Scholar] [CrossRef]
- Elkhatat, A.M.; Al-Muhtaseb, S.A. Advances in tailoring resorcinol-formaldehyde organic and carbon gels. Adv. Mater. 2011, 23, 2887–2903. [Google Scholar] [CrossRef]
- Leventis, N.; Chandrasekaran, N.; Sadekar, A.G.; Mulik, S.; Sotiriou-Leventis, C. The effect of compactness on the carbothermal conversion of interpenetrating metal oxide/resorcinol-formaldehyde nanoparticle networks to porous metals and carbides. J. Mater. Chem. 2010, 20, 7456–7471. [Google Scholar] [CrossRef]
- Yang, X.Q.; Huang, H.; Zhang, G.Q.; Li, X.X.; Wu, D.C.; Fu, R.W. Carbon aerogel with 3-D continuous skeleton and mesopore structure for lithium-ion batteries application. Mater. Chem. Phys. 2015, 149, 657–662. [Google Scholar] [CrossRef]
- Liu, N.P.; Shen, J.; Liu, D. A Fe2O3 nanoparticle/carbon aerogel composite for use as an anode material for lithium ion batteries. Electrochim. Acta 2013, 97, 271–277. [Google Scholar] [CrossRef]
- Hao, F.B.; Zhang, Z.W.; Yin, L.W. Co3O4/carbon aerogel hybrids as anode materials for lithium-ion batteries with enhanced electrochemical properties. ACS Appl. Mater. Interfaces 2013, 5, 8337–8344. [Google Scholar] [CrossRef] [PubMed]
- Khodabakhshi, S.; Fulvio, P.F.; Andreoli, E. Carbon black reborn: Structure and chemistry for renewable energy harnessing. Carbon 2020, 162, 604–649. [Google Scholar] [CrossRef] [Green Version]
- Pan, Y.; Zeng, W.J.; Hu, R.; Li, B.; Wang, G.L.; Li, Q.T. Investigation of Cu doped flake-NiO as an anode material for lithium ion batteries. RSC Adv. 2019, 9, 35948–35956. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Y.; Huang, H.; Song, W.L.; Gan, Y.P.; Wang, K.; Zhang, J.; Xia, Y.; Liang, C.; He, X.P.; Zhang, W.K. In-situ electrolytic synthesis and superior lithium storage capability of Ni-NiO/C nanocomposite by sacrificial nickel anode in molten carbonates. J. Alloy. Compd. 2020, 834, 155111. [Google Scholar] [CrossRef]
- Li, X.J.; Fan, L.L.; Li, X.F.; Shan, H.; Chen, C.; Yan, B.; Xiong, D.B.; Li, D.J. Enhanced anode performance of flower-like NiO/RGO nanocomposites for lithium-ion batteries. Mater. Chem. Phys. 2018, 217, 547–552. [Google Scholar] [CrossRef]
- Wu, H.J.; Wang, Y.Q.; Zheng, C.H.; Zhu, J.M.; Wu, G.L.; Li, X.H. Multi-shelled NiO hollow spheres: Easy hydrothermal synthesis and lithium storage performances. J. Alloy. Compd. 2016, 685, 8–14. [Google Scholar] [CrossRef]
- Pang, H.C.; Guan, B.Q.; Sun, W.W.; Wang, Y. Metal-organic-frameworks derivation of mesoporous NiO nanorod for high-performance lithium ion batteries. Electrochim. Acta 2016, 213, 351–357. [Google Scholar] [CrossRef]

| Material | Initial Reversible Capacity mAh g−1 | Capacity Retention % (Nth) | Current Density mA g−1 | Reference |
|---|---|---|---|---|
| NiO nano/microspheres | 735 | 96 (100) | 100 | [15] |
| NiO/C hollow microspheres | 760 | 83 (100) | 100 | [17] |
| Cu-doped NiO nanoflakes | 1108.9 | 59 (50) | 100 | [33] |
| Ni−NiO/C nanocomposite | 914.11 | 70 (300) | 100 | [34] |
| NiO/rGO nanoflowers | 996.9 | 70 (100) | 100 | [35] |
| NiO double-shelled hollow spheres | 964.3 | 14 (100) | 200 | [36] |
| NiO mesoporous nanorods | 737 | 39 (100) | 100 | [37] |
| NiO/C aerogel microspheres | 808 | 85 (100) | 100 | This work |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, R.; Huang, X.; Lin, Y.; Cao, Y. NiO/Carbon Aerogel Microspheres with Plum-Pudding Structure as Anode Materials for Lithium Ion Batteries. Materials 2020, 13, 2363. https://doi.org/10.3390/ma13102363
Guo R, Huang X, Lin Y, Cao Y. NiO/Carbon Aerogel Microspheres with Plum-Pudding Structure as Anode Materials for Lithium Ion Batteries. Materials. 2020; 13(10):2363. https://doi.org/10.3390/ma13102363
Chicago/Turabian StyleGuo, Renqing, Xiaohua Huang, Yan Lin, and Yiqi Cao. 2020. "NiO/Carbon Aerogel Microspheres with Plum-Pudding Structure as Anode Materials for Lithium Ion Batteries" Materials 13, no. 10: 2363. https://doi.org/10.3390/ma13102363
APA StyleGuo, R., Huang, X., Lin, Y., & Cao, Y. (2020). NiO/Carbon Aerogel Microspheres with Plum-Pudding Structure as Anode Materials for Lithium Ion Batteries. Materials, 13(10), 2363. https://doi.org/10.3390/ma13102363





