Evaluation of Effective Condyle Positioning Assisted by 3D Surgical Guide in Mandibular Reconstruction Using Osteocutaneous Free Flap
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Fabrication of 3D Surgical Guide
2.3. Surgical Technique
2.4. 3D Analysis for Reproducibility of 3D Surgical Guide and Stability of Condylar Position
2.5. Statistical Analysis
3. Results
3.1. Reproducibility of 3D Surgical Guide
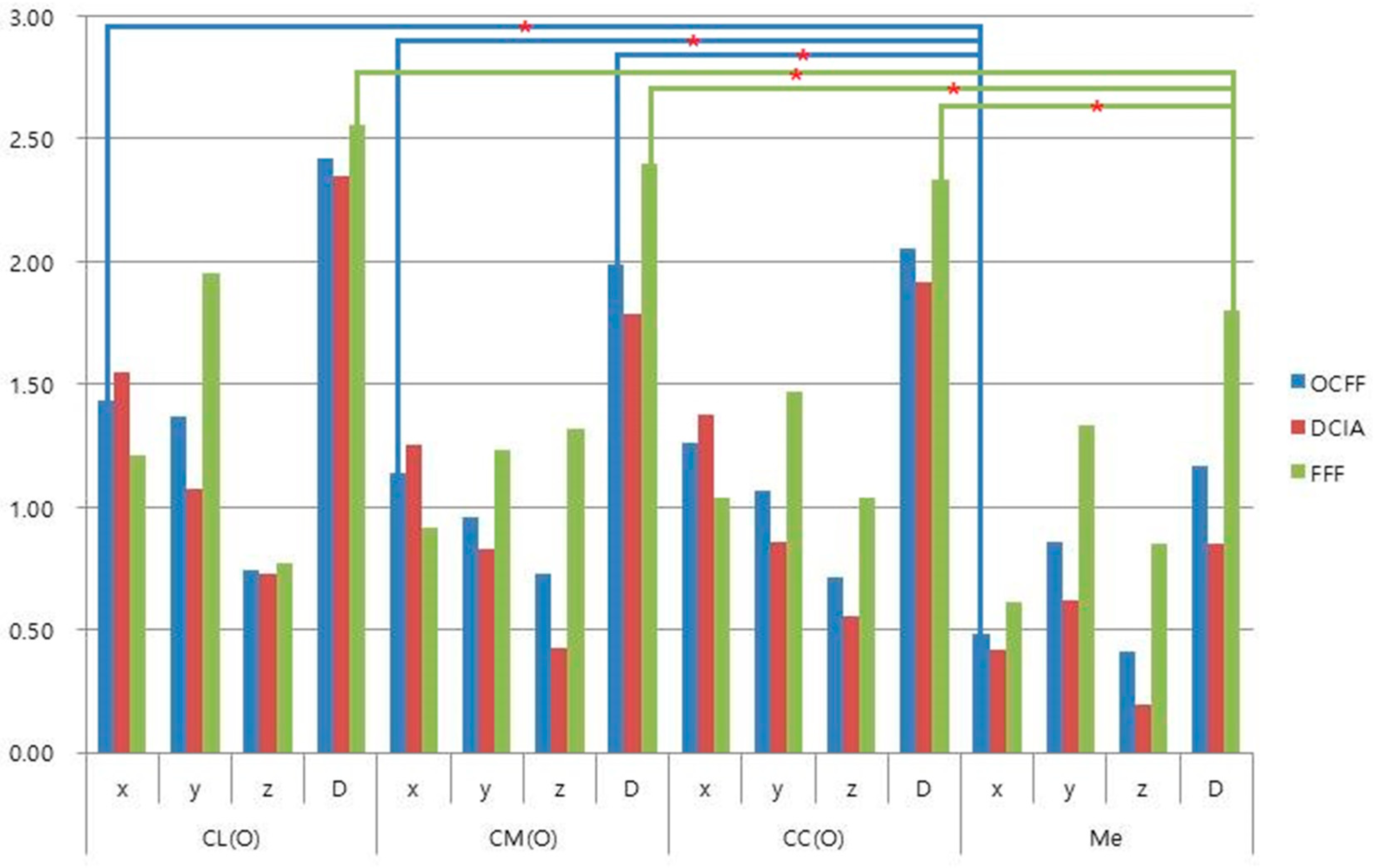
3.2. Stability of Condylar Position
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Urken, M.L.; Buchbinder, D.; Weinberg, H.; Vickery, C.; Sheiner, A.; Parker, R.; Schaefer, J.; Som, P.; Shapiro, A.; Lawson, W.; et al. Functional evaluation following microvascular oromandibular reconstruction of the oral cancer patient: A comparative study of reconstructed and nonreconstructed patients. Laryngoscope 1991, 101, 935–950. [Google Scholar] [CrossRef]
- Lonie, S.; Herle, P.; Paddle, A.; Pradhan, N.; Birch, T.; Shayan, R. Mandibular reconstruction: Meta-analysis of iliac-versus fibula-free flaps. ANZ J. Surg. 2016, 86, 337–342. [Google Scholar] [CrossRef]
- Chen, S.H.; Chen, H.C.; Horng, S.Y.; Tai, H.C.; Hsieh, J.H.; Yeong, E.K.; Cheng, N.C.; Hsieh, T.M.; Chien, H.F.; Tang, Y.B. Reconstruction for osteoradionecrosis of the mandible: Superiority of free iliac bone flap to fibula flap in postoperative infection and healing. Ann. Plast. Surg. 2014, 73, S18–S26. [Google Scholar] [CrossRef]
- Powcharoen, W.; Yang, W.F.; Yan Li, K.; Zhu, W.; Su, Y.X. Computer-Assisted versus Conventional Freehand Mandibular Reconstruction with Fibula Free Flap: A Systematic Review and Meta-Analysis. Plast. Reconstr. Surg. 2019, 144, 1417–1428. [Google Scholar] [CrossRef]
- Goh, B.T.; Lee, S.; Tideman, H.; Stoelinga, P.J. Mandibular reconstruction in adults: A review. Int. J. Oral Maxillofac. Surg. 2008, 37, 597–605. [Google Scholar] [CrossRef]
- van Gemert, J.T.; van Es, R.J.; Rosenberg, A.J.; van der Bilt, A.; Koole, R.; Van Cann, E.M. Free vascularized flaps for reconstruction of the mandible: Complications, success, and dental rehabilitation. J. Oral Maxillofac. Surg. 2012, 70, 1692–1698. [Google Scholar] [CrossRef]
- Bisase, B.; Sloane, J.; Coombes, D.M.; Norris, P.M. The deep circumflex iliac artery perforator flap (DCIAP)--a reconstructive option for the large composite oro-mandibular cutaneous defect. Br. J. Oral Maxillofac. Surg. 2013, 51, 962–964. [Google Scholar] [CrossRef]
- Cordeiro, P.G.; Disa, J.J.; Hidalgo, D.A.; Hu, Q.Y. Reconstruction of the mandible with osseous free flaps: A 10-year experience with 150 consecutive patients. Plast. Reconstr. Surg. 1999, 104, 1314–1320. [Google Scholar] [CrossRef]
- Strackee, S.D.; Kroon, F.H.; Jaspers, J.E.; Bos, K.E. Modeling a fibula transplant in mandibular reconstructions: Evaluation of the effects of a minimal number of osteotomies on the contour of the jaw. Plast. Reconstr. Surg. 2001, 108, 1915–1921; discussion 1922-3. [Google Scholar] [CrossRef]
- Takushima, A.; Harii, K.; Asato, H.; Nakatsuka, T.; Kimata, Y. Mandibular reconstruction using microvascular free flaps: A statistical analysis of 178 cases. Plast. Reconstr. Surg. 2001, 108, 1555–1563. [Google Scholar] [CrossRef]
- Kang, S.H.; Lee, S.; Nam, W. Condyle dislocation following mandibular reconstruction using a fibula free flap: Complication cases. Maxillofac. Plast. Reconstr. Surg. 2019, 41, 14. [Google Scholar] [CrossRef] [PubMed]
- Chao, A.H.; Weimer, K.; Raczkowsky, J.; Zhang, Y.; Kunze, M.; Cody, D.; Selber, J.C.; Hanasono, M.M.; Skoracki, R.J. Pre-programmed robotic osteotomies for fibula free flap mandible reconstruction: A preclinical investigation. Microsurgery 2016, 36, 246–249. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, D.A. Reply: Long-Term Operative Outcomes of Preoperative Computed Tomography-Guided Virtual Surgical Planning for Osteocutaneous Free Flap Mandible Reconstruction. Plast. Reconstr. Surg. 2016, 138, 560e. [Google Scholar] [CrossRef]
- Ritschl, L.M.; Mucke, T.; Fichter, A.; Gull, F.D.; Schmid, C.; Duc, J.M.P.; Kesting, M.R.; Wolff, K.D.; Loeffelbein, D.J. Functional Outcome of CAD/CAM-Assisted versus Conventional Microvascular, Fibular Free Flap Reconstruction of the Mandible: A Retrospective Study of 30 Cases. J. Reconstr. Microsurg. 2017, 33, 281–291. [Google Scholar] [PubMed]
- Pauchet, D.; Pigot, J.L.; Chabolle, F.; Bach, C.A. Prefabricated fibula free flap with dental implants for mandibular reconstruction. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2018, 135, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Sawh-Martinez, R.; Parsaei, Y.; Wu, R.; Lin, A.; Metzler, P.; DeSesa, C.; Steinbacher, D.M. Improved Temporomandibular Joint Position after 3-Dimensional Planned Mandibular Reconstruction. J. Oral Maxillofac. Surg. 2017, 75, 197–206. [Google Scholar] [CrossRef]
- De Maesschalck, T.; Courvoisier, D.S.; Scolozzi, P. Computer-assisted versus traditional freehand technique in fibular free flap mandibular reconstruction: A morphological comparative study. Eur. Arch. Otorhinolaryngol. 2017, 274, 517–526. [Google Scholar] [CrossRef]
- Yuan, X.; Xuan, M.; Tian, W.; Long, J. Application of digital surgical guides in mandibular resection and reconstruction with fibula flaps. Int. J. Oral Maxillofac. Surg. 2016, 45, 1406–1409. [Google Scholar] [CrossRef]
- Nahabedian, M.Y.; Tufaro, A.; Manson, P.N. Improved mandible function after hemimandibulectomy, condylar head preservation, and vascularized fibular reconstruction. Ann. Plast. Surg. 2001, 46, 506–510. [Google Scholar] [CrossRef]
- Antony, A.K.; Chen, W.F.; Kolokythas, A.; Weimer, K.A.; Cohen, M.N. Use of virtual surgery and stereolithography-guided osteotomy for mandibular reconstruction with the free fibula. Plast. Reconstr. Surg. 2011, 128, 1080–1084. [Google Scholar] [CrossRef]
- Avraham, T.; Franco, P.; Brecht, L.E.; Ceradini, D.J.; Saadeh, P.B.; Hirsch, D.L.; Levine, J.P. Functional outcomes of virtually planned free fibula flap reconstruction of the mandible. Plast. Reconstr. Surg. 2014, 134, 628e–634e. [Google Scholar] [CrossRef] [PubMed]
- Eckardt, A.; Swennen, G.R. Virtual planning of composite mandibular reconstruction with free fibula bone graft. J. Craniofac. Surg. 2005, 16, 1137–1140. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, D.L.; Garfein, E.S.; Christensen, A.M.; Weimer, K.A.; Saddeh, P.B.; Levine, J.P. Use of computer-aided design and computer-aided manufacturing to produce orthognathically ideal surgical outcomes: A paradigm shift in head and Neck. reconstruction. J. Oral Maxillofac. Surg. 2009, 67, 2115–2122. [Google Scholar] [CrossRef] [PubMed]
- Toto, J.M.; Chang, E.I.; Agag, R.; Devarajan, K.; Patel, S.A.; Topham, N.S. Improved operative efficiency of free fibula flap mandible reconstruction with patient-specific, computer-guided preoperative planning. Head Neck 2015, 37, 1660–1664. [Google Scholar] [CrossRef] [PubMed]
- Ueki, K.; Moroi, A.; Sotobori, M.; Ishihara, Y.; Marukawa, K.; Yoshizawa, K.; Kato, K.; Kawashiri, S. Changes in temporomandibular joint and ramus after sagittal split ramus osteotomy in mandibular prognathism patients with and without asymmetry. J. CranioMaxillofac. Surg. 2012, 40, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Han, J.J.; Hwang, S.J. Three-dimensional analysis of postoperative returning movement of perioperative condylar displacement after bilateral sagittal split ramus osteotomy for mandibular setback with different fixation methods. J. CranioMaxillofac. Surg. 2015, 43, 1918–1925. [Google Scholar] [CrossRef]
- Mendez-Manjon, I.; Guijarro-Martinez, R.; Valls-Ontanon, A.; Hernandez-Alfaro, F. Early changes in condylar position after mandibular advancement: A three-dimensional analysis. Int. J. Oral Maxillofac. Surg. 2016, 45, 787–792. [Google Scholar] [CrossRef]
- Geusens, J.; Sun, Y.; Luebbers, H.T.; Bila, M.; Darche, V.; Politis, C. Accuracy of Computer-Aided Design/Computer-Aided Manufacturing-Assisted Mandibular Reconstruction with a Fibula Free Flap. J. Craniofac. Surg. 2019, 30, 2319–2323. [Google Scholar] [CrossRef]
- Goormans, F.; Sun, Y.; Bila, M.; Schoenaers, J.; Geusens, J.; Lubbers, H.T.; Coucke, W.; Politis, C. Accuracy of computer-assisted mandibular reconstructions with free fibula flap: Results of a single-center series. Oral Oncol. 2019, 97, 69–75. [Google Scholar] [CrossRef]
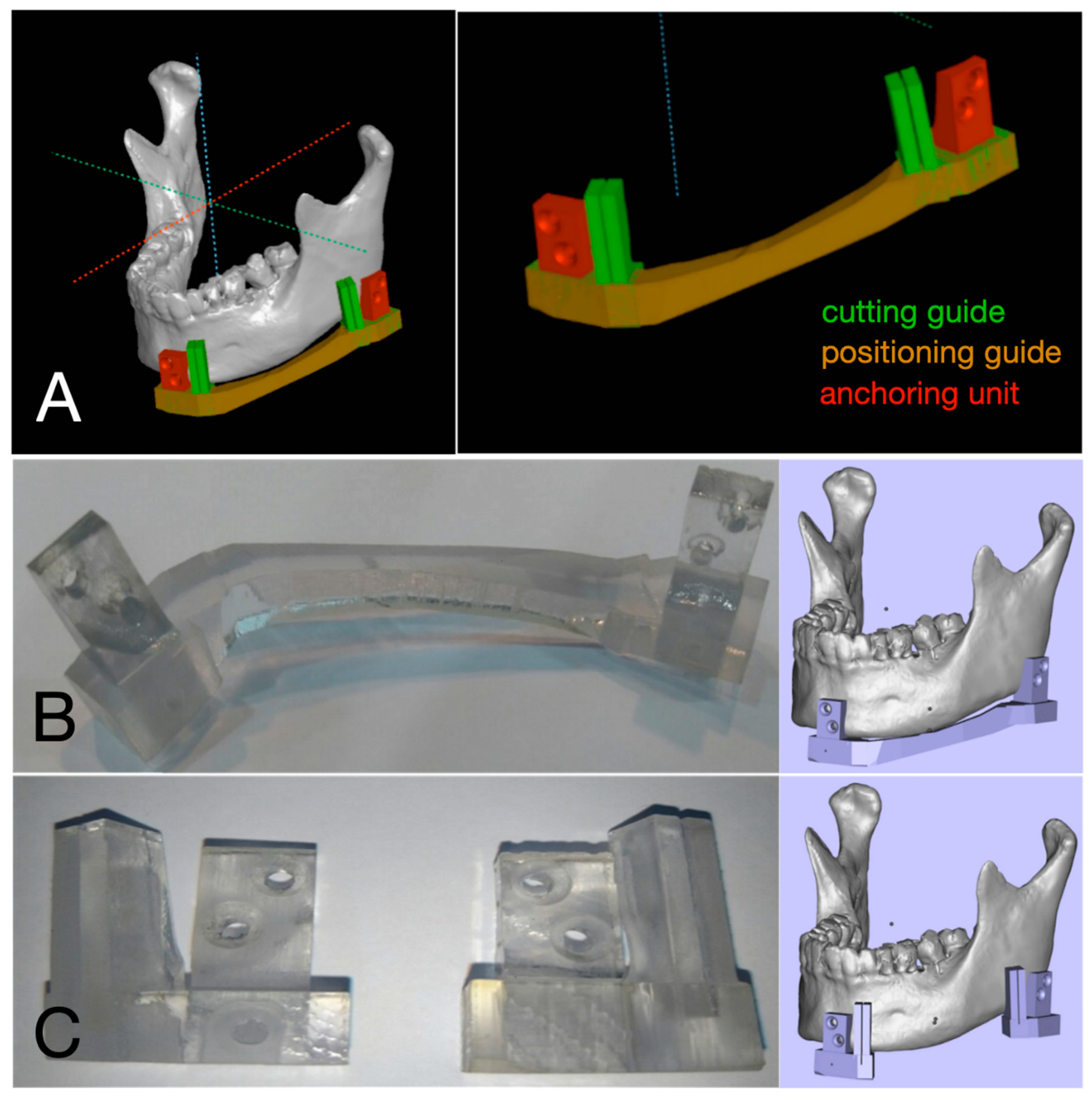
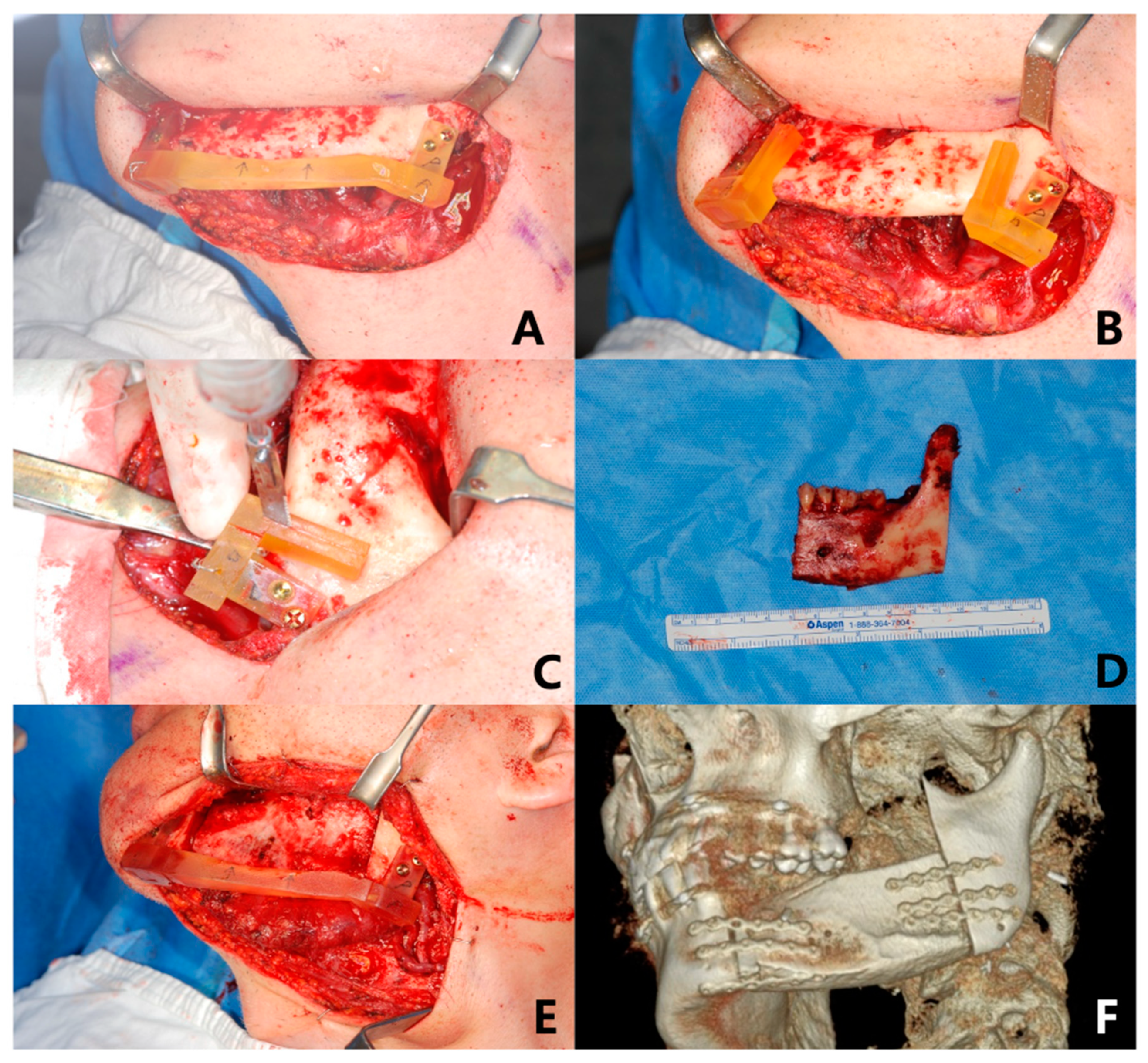
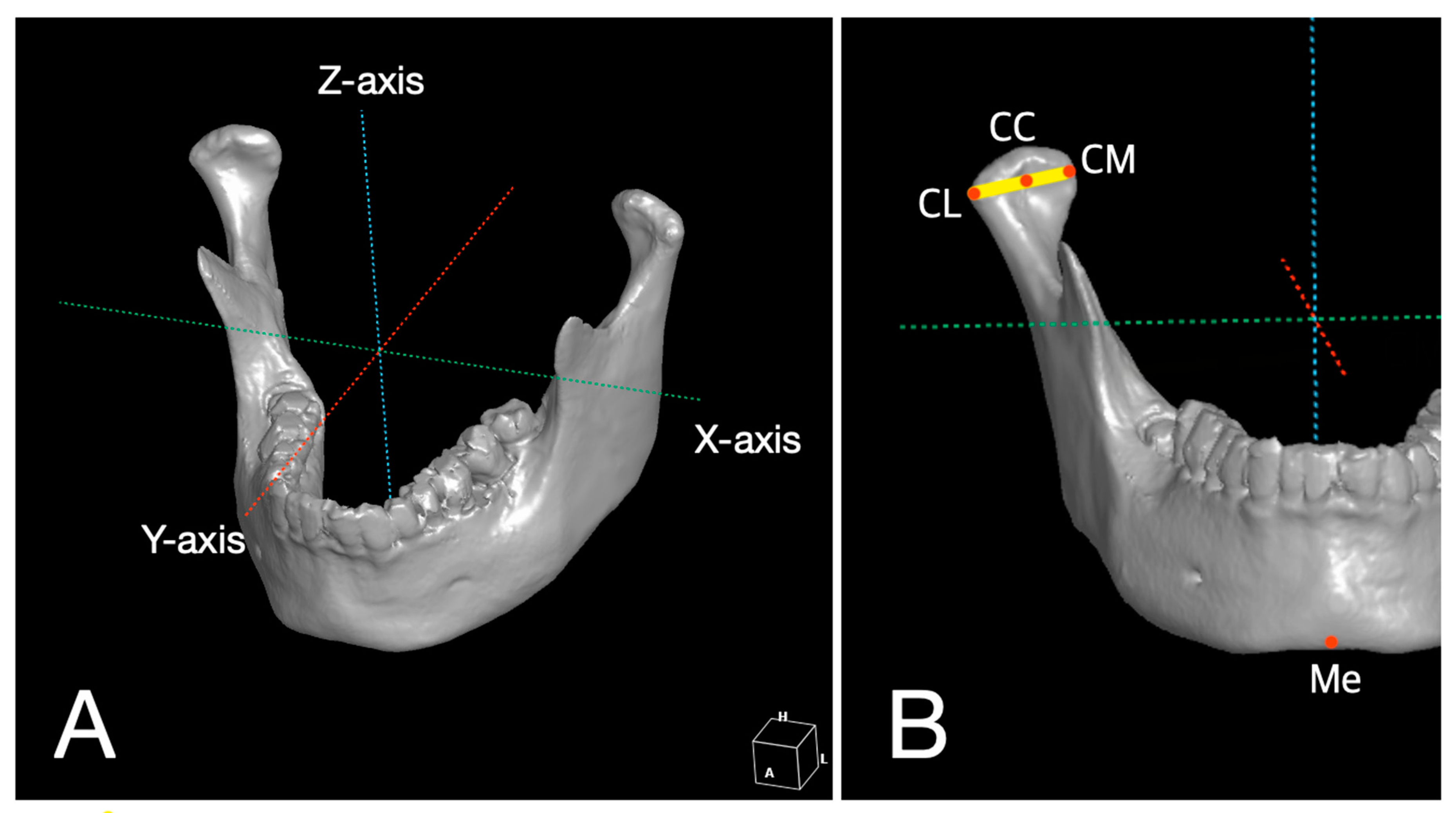
| No. | Age | Gender | Diagnosis | Type of OCFF |
|---|---|---|---|---|
| 1 | 26 | F 1 | Ameloblastoma | DCIA |
| 2 | 27 | M 2 | Keratocystic odontogenic tumor | DCIA |
| 3 | 40 | M | Osteosarcoma | FFF |
| 4 | 41 | F | Ameloblastoma | DCIA |
| 5 | 49 | M | Osteomyelitis | DCIA |
| 6 | 51 | M | Ameloblastoma | DCIA |
| 7 | 52 | M | Ondontogenic myxoma | DCIA |
| 8 | 57 | M | Ameloblastoma | DCIA |
| 9 | 59 | M | Keratocystic odontogenic tumor | DCIA |
| 10 | 65 | M | MRONJ 3 | FFF |
| 11 | 71 | F | Squamous cell carcinoma | FFF |
| 12 | 75 | F | MRONJ | FFF |
| 13 | 80 | F | Osteomyelitis | DCIA |
| 14 | 83 | M | Squamous cell carcinoma | FFF |
| 15 | 89 | F | MRONJ | DCIA |
| Landmark | OCFF | DCIA | FFF |
|---|---|---|---|
| n = 15 | n = 10 | n = 5 | |
| Mean (SD) | Mean (SD) | Mean (SD) | |
| CL | |||
| x | 1.44 (0.98) | 1.55 (1.09) | 1.21 (0.76) |
| y | 1.37 (1.54) | 1.08 (1.45) | 1.95 (1.72) |
| z | 0.74 (0.71) | 0.73 (0.73) | 0.77 (0.76) |
| D | 2.42 (1.55) | 2.35 (1.50) | 2.56 (1.81) |
| CM | |||
| x | 1.14 (0.95) | 1.25 (0.93) | 0.92 (1.07) |
| y | 0.96 (0.77) | 0.83 (0.59) | 1.23 (1.07) |
| z | 0.73 (1.32) | 0.43 (0.54) | 1.32 (2.20) |
| D | 1.99 (1.40) | 1.79 (0.82) | 2.40 (2.25) |
| CC | |||
| x | 1.26 (0.89) | 1.38 (0.90) | 1.04 (0.93) |
| y | 1.06 (1.02) | 0.86 (0.75) | 1.47 (1.44) |
| z | 0.71 (0.84) | 0.55 (0.43) | 1.04 (1.36) |
| D | 2.05 (1.22) | 1.91 (0.86) | 2.33 (1.83) |
| Me | |||
| x | 0.48 (0.47) | 0.42 (0.41) | 0.61 (0.60) |
| y | 0.86 (1.20) | 0.62 (0.98) | 1.33 (1.58) |
| z | 0.41 (0.58) | 0.19 (0.22) | 0.85 (0.83) |
| D | 1.17 (1.32) | 0.85 (1.02) | 1.80 (1.74) |
| Landmark | OCFF | DCIA | FFF |
|---|---|---|---|
| n = 15 | n = 10 | n = 5 | |
| Mean (SD) | Mean (SD) | Mean (SD) | |
| CL | |||
| x | 1.44 (1.87) * | 1.07 (1.43) | 2.18 (2.57) |
| y | 0.98 (1.25) | 0.52 (0.74) | 1.90 (1.63) |
| z | 0.27 (0.33) | 0.24 (0.33) | 0.35 (0.34) |
| D | 2.03 (2.02) | 1.33 (1.54) | 3.42 (2.31) |
| CM | |||
| x | 1.56 (1.70) | 1.25 (1.31) | 2.20 (2.35) * |
| y | 0.60 (0.62) | 0.56 (0.44) | 0.69 (0.94) |
| z | 0.38 (0.48) | 0.35 (0.52) | 0.42 (0.42) |
| D | 1.95 (1.61) | 1.56 (1.29) | 2.71 (2.06) |
| CC | |||
| x | 1.45 (1.8) | 1.08 (1.39) | 2.18 (2.44) * |
| y | 0.68 (0.91) | 0.48 (0.56) | 1.08 (1.39) |
| z | 0.3 (0.28) | 0.27 (0.28) | 0.36 (0.31) |
| D | 1.82 (1.85) * | 1.31 (1.43) | 2.85 (2.32) * |
| Me | |||
| x | 0.36 (0.45) | 0.23 (0.19) | 0.61 (0.70) |
| y | 0.99 (1.77) | 0.47 (0.39) | 2.02 (2.95) |
| z | 1.12 (1.53) | 0.91 (1.07) | 1.56 (2.30) * |
| D | 1.74 (2.23) | 1.13 (1.07) | 2.97 (3.47) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.R.; Jang, S.; Ahn, K.-M.; Lee, J.-H. Evaluation of Effective Condyle Positioning Assisted by 3D Surgical Guide in Mandibular Reconstruction Using Osteocutaneous Free Flap. Materials 2020, 13, 2333. https://doi.org/10.3390/ma13102333
Kim SR, Jang S, Ahn K-M, Lee J-H. Evaluation of Effective Condyle Positioning Assisted by 3D Surgical Guide in Mandibular Reconstruction Using Osteocutaneous Free Flap. Materials. 2020; 13(10):2333. https://doi.org/10.3390/ma13102333
Chicago/Turabian StyleKim, Seong Ryoung, Sam Jang, Kang-Min Ahn, and Jee-Ho Lee. 2020. "Evaluation of Effective Condyle Positioning Assisted by 3D Surgical Guide in Mandibular Reconstruction Using Osteocutaneous Free Flap" Materials 13, no. 10: 2333. https://doi.org/10.3390/ma13102333
APA StyleKim, S. R., Jang, S., Ahn, K.-M., & Lee, J.-H. (2020). Evaluation of Effective Condyle Positioning Assisted by 3D Surgical Guide in Mandibular Reconstruction Using Osteocutaneous Free Flap. Materials, 13(10), 2333. https://doi.org/10.3390/ma13102333





