1. Introduction
The global environmental problems of climate change as a result of human impacts on nature have been firmly established as the most internationally discussed issues (Conference of Parties (COPs) in Warsaw (COP-19 in 2013), in Lima (COP-20 in 2014), in Paris (COP-21 in 2015), and most recently in Marrakech (COP-22 in November of 2016). The increasing concentration of greenhouse gases in the atmosphere at the current stage of economic development requires the development of new products and production methods characterized by lower CO2 emissions than traditional approaches.
One of the branches of industry that emits a significant amount of anthropogenic CO
2 into the atmosphere is the construction materials industry, which is in second place after the energy sector in terms of global CO
2 emissions. Thus, the production of Portland cement and other binders alone accounts for up to 8% of the world’s anthropogenic CO
2 emissions [
1,
2,
3,
4]. In this sense, CO
2 sequestration is one of the most important technical challenges of our time [
5].
It is noteworthy that at the same time, it is the construction industry that has significant potential in terms of creating technologies for the production of various materials and products that use carbon dioxide in their manufacturing process. It is shown in [
6,
7] that CO
2 can be used both as an effective additive of cement concrete curing accelerator and as the main raw material component [
8,
9,
10]. Recently, there has been a steady and growing interest in various alternative binders and materials with binder properties, containing minerals that can interact with CO
2 and bind it into stable compounds.
A significant reserve of raw materials in the production of building materials and products hardening due to carbon dioxide are various wastes and industrial waste products, which at certain technological processing can exhibit bindering properties and enter into chemical interaction with carbon dioxide, forming an artificial stone. As a result of numerous research studies [
11,
12,
13,
14,
15], a number of kinds of the secondary raw materials possessing the essential potential for СО
2 binding have been revealed, scientific and technological bases for introduction of the received results in the industry have been developed [
13,
14,
15,
16], and moreover, pilot batches of the building products hardened in the environment of the raised concentration of СО
2 have been issued [
13,
14,
15,
16].
One of the promising secondary raw materials for the production of carbonate hardening building materials is the waste generated in the production of soda ash. The chemical process of soda ash production using ammonia technology is based on five reactions, one of which is the production of calcium oxide and carbon dioxide as a result of carbonate rock cooking in lime kilns [
17]. As a result of cleaning the carbon dioxide for its further use, a by-product is obtained, the so-called lime dust, which has low activity and does not find a proper further application. In several of the conducted scientific research studies, the directions for the use of by-products of soda manufacturing for the production of building materials and products are defined [
18,
19], including low-active lime. However, in practice, it does not occur, and does not allow one to leave the problem of accumulation of the given by-product, in dumps and in its negative influence on the environment. The research carried out in [
20] has established the possibility of using lime dust for production of building materials by the method of forced carbonation, which has high physical and mechanical characteristics. The filler in these materials was finely dispersed limestone, which is also a by-product of the sorting of limestone crushed to produce lime and carbon dioxide. The analysis of utilization directions of soda production wastes in construction materials of forced carbonate hardening has shown that carbonate technology allows one to fully utilize all formed solid by-products, as well as carbon dioxide emitted into the atmosphere, and does not require special preparation, so one can directly use it without any cleaning. Such a solution is inexpensive, quite simple from the technical and technological point of view and allows one to significantly reduce the formation of waste during the production of soda, and due to the reduction of CO
2 emissions into the atmosphere to significantly increase the environmental friendliness of the technology, it is transferred to the "low-carbon" class.
Forced carbonation is an active curing process and allows the production of materials with high physical and mechanical characteristics within a short time period (1–3 h) [
20,
21,
22]. However, issues related to the increase of productivity of technologies of the manufacturing of artificially carbonated building materials and products at the expense of applications of more intensive methods of their forced carbonation and regularities of formation of the properties of received materials thus cause certain scientific and practical interest.
In this regard, the main idea of the performed research work is to research and develop intensive methods of forced carbonation of the systems consisting of soda production wastes—lime dust and finely dispersed limestone, and their influence on the formation of the basic properties corresponding for structural building materials and products, depending on the various formulation and technological factors. For this purpose, a series of experiments have been conducted, the main results of which are presented in this article.
2. Materials and Methods
2.1. Materials
The materials used for scientific research were lime dust mechanically precipitated in cyclones and bag filters of mine lime kilns that calcin limestone. Finely dispersed marble limestone with fraction up to 5 mm, formed in the process of crushing and sorting the initial rock into a working fraction sent to the shaft kiln for cooking, was also used.
The activity of lime dust, determined by standard methods of lime testing, was 45%, humidity 0.6% wt., temperature and quenching time are 41 °С and 47 min, respectively.
The phase composition was determined using Perkin Elmer’s STA 8000 (PerkinElmer Inc., Waltham, Massachusetts, USA) high-temperature synchronous analyzer (USA), in the temperature range of 30–1000 °С, at a heating rate of 10 °С/min in a nitrogen environment. The particle size of the investigated materials was determined on the laser analyzer HORIBA LA-960 (HORIBA, Ltd. Kyoto, Japan).
The lime dust is factory stored and stored in bulk in an open fenced area. It is natural to believe that under such storage conditions, a portion of the free СаО contained in it is extinguished and transferred to Ca(OH)
2 and CaCO
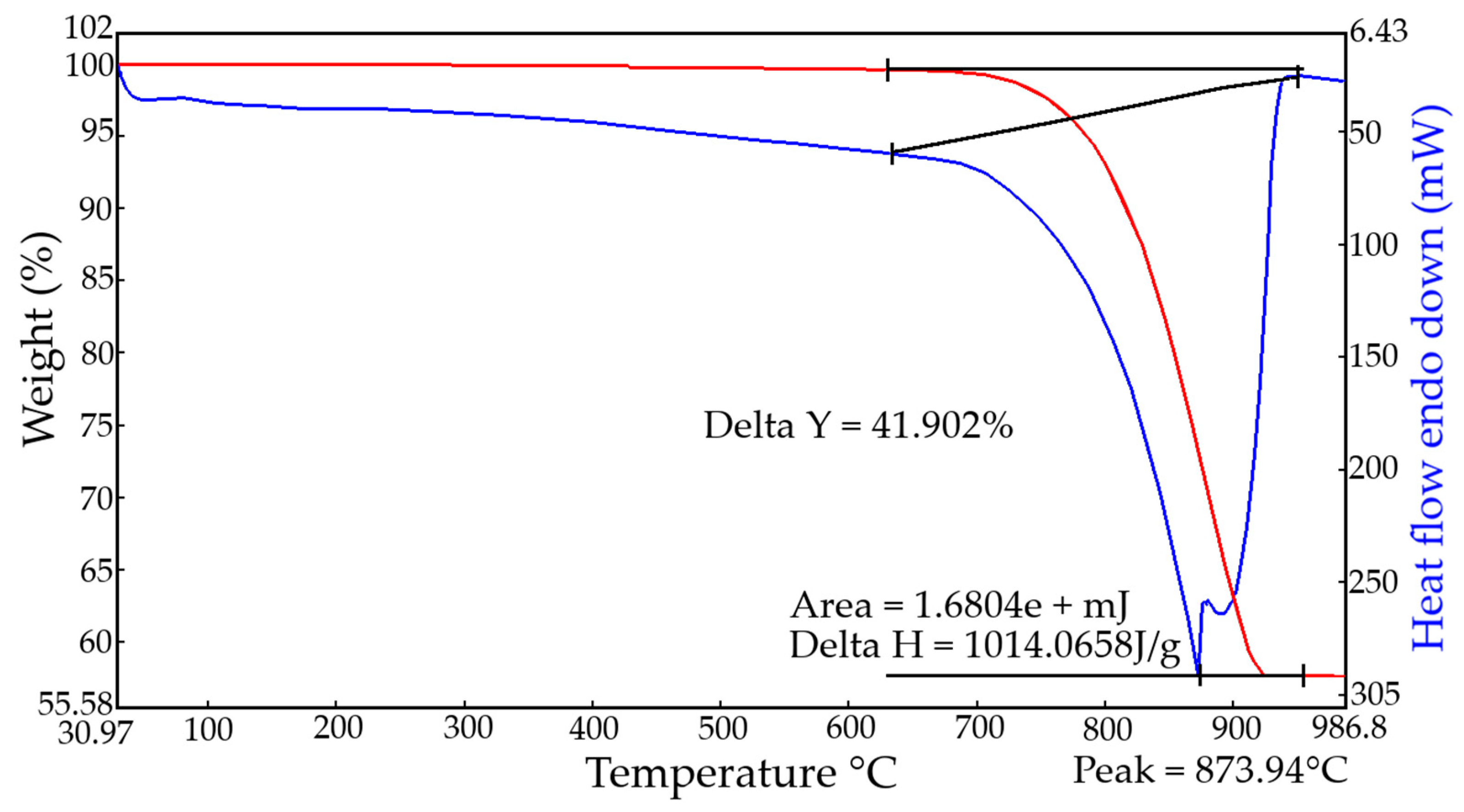
3. Quantitative changes in the phase composition of lime dust depending on the conditions of its pre-preparation were investigated using synchronous Thermogravimetric and Differential thermal analysis (TG-DTA) analysis under a dynamic nitrogen atmosphere. Samples were tested in their natural state and after quenching under different conditions, in order to determine the presence of calcinated CaO particles. Samples were dried before testing. Changes in the content of the main phases in lime dust under various conditions of its preliminary preparation are presented in
Table 1.
The analysis of experimental data on changes in the mineralogical composition of lime dust depending on the conditions of its preparation show that in its natural state lime dust is in a hydrated state and contains about 40% Ca(OH)2. Equivalent to CaO, this is 30.5%. TG-curves of freshly slaked lime dust samples show that the Ca(OH)2 content is increasing, however, it can be seen from the CaO content that there is still a significant amount of free calcinated CaO particles in the composition. After quenching and the additional maintenance of products of quenching of lime dust during 120 and 420 min, and also after boiling and maintenance during 420 min, according to the data of TG-DTA curves, the quantity of Ca(OH)2 with increase in time of maintenance increases to 55.1%, and after boiling, increases up to 59.5%. Based on the results of the thermal analysis, the amount of calcinated CaO particles in the lime dust is about 7%. According to the analysis, lime dust is found to be one-third composed of calcium carbonate—uncalcinated limestone particles. In addition, lime dust in its composition contains admixtures of silica (SiO2) and uncalcinated hard coal, manifested on thermograms in the form of exothermic effects in the temperature range of 350–400 °C.
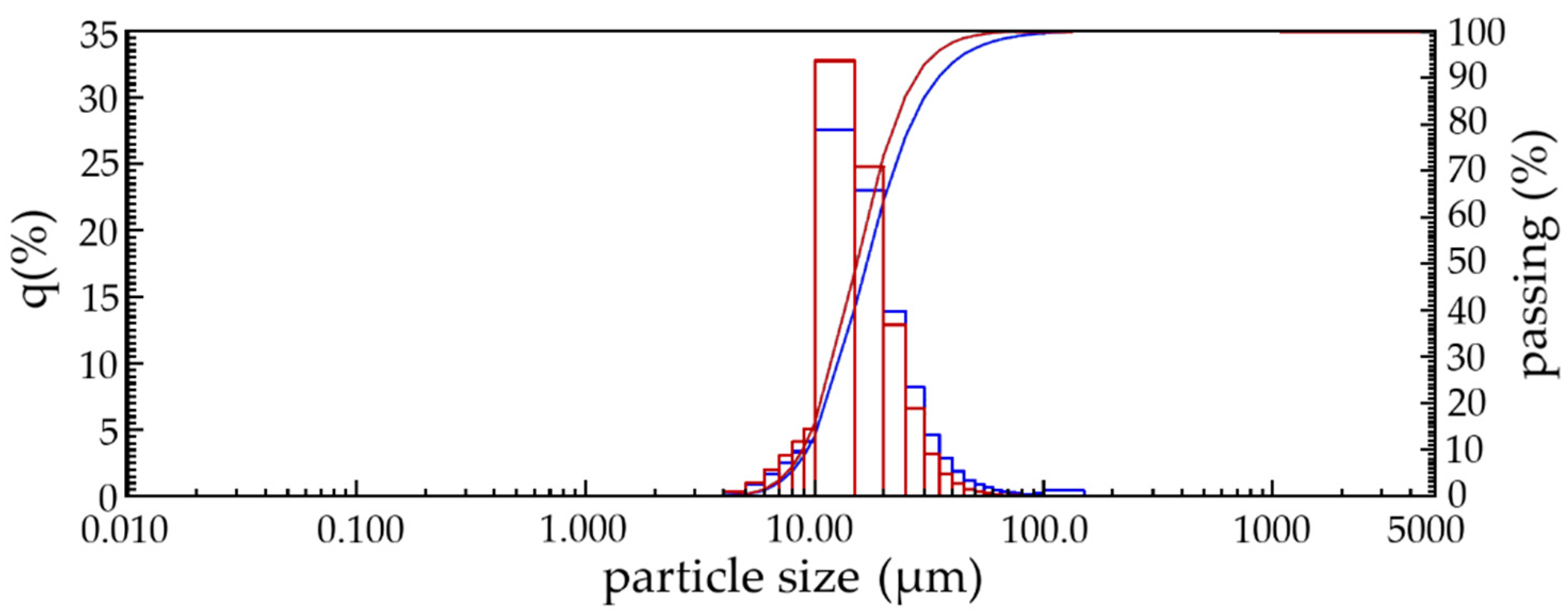
Determination of the size of lime dust particles in the quick and slake state (
Figure 1) by laser diffraction showed that its particle size composition is 8 to 30 microns, and after quenching the resulting material contains mainly particles with sizes from 6 to 20 µm. The average geometric particle size of lime dust before and after quenching is 16.7 and 12.9 µm, respectively. Considering such a small size of particles of lime dust, it can be argued that the destructive effect of calcinated particles on the properties of products made on its basis will be minimal.
As finely dispersed limestone in the work used by-products of crushing and sorting of limestone Balaklava deposit (Crimea) fraction to 5 mm, formed during the crushing and sorting of the original rock to the working fraction sent to the mine kiln for cooking. The main properties and content of the main rock-forming chemical compound calcium carbonate limestone are presented in
Table 2.
Limestone from the Balaklava deposit belongs to nummulite partially recrystallized (metamorphosed) limestone and is a sedimentary dense and sufficiently strong rock. The thermogram of limestone (
Figure 2) has one endothermic effect, corresponding to the decomposition of calcium carbonate. The endothermic effect has two peaks, which may indicate that the studied calcium carbonate is mainly represented by calcite and possibly contains minor impurities of dolomite [
23,
24].
2.2. Methods
Liquid high-pressure carbon dioxide in cylinders was used as a source of carbon dioxide in laboratory studies.
Research studies were carried out on the cylinder samples made by a method of semi-dry pressing of the molding mixtures, consisting of thoroughly mixed in a speed mixer of lime dust and finely dispersed limestone, passed through a sieve with the size of mesh 1.25 mm. The water content of the molding mixtures was 10% wt. These values are justified by the results presented in [
21,
22,
25]. The compositions of the molding mixtures are determined by the experimental conditions and are presented below. The 30 mm diameter testing cylinder samples were formed by pressing the raw mix in metal molds on a hydraulic press, with a simulation of double-sided counter-pressing. The diameter/height ratio was maintained within 1.
The forced carbonation of testing samples was carried out in the carbonation unit with automatic control and maintenance of the required concentration of CO
2 in it [
26]. The unit was additionally equipped with a buffer tank to prepare and maintain a constant concentration of CO
2 gas and air mixtures.
Research studies of complex influence of the composition of a raw mix, factors of reception, and also modes of the accelerated carbonation of pre-production of testing samples were spent by means of statistical methods of mathematical designing of the experiment [
27,
28]. Central composite rotatable design (CCRD) was adopted. A central composite rotatable design of the experiment—design points are located on three spheres: central points (zero-radius sphere), cube points and "star points". Rotatable designs allow getting the coefficients of models that predict the output value of the object with the same accuracy in all directions at the same distance from the center of the design. The obtained experimental data were processed using the STATISTICA 12 software package (StatSoft, Inc., Tulsa, USA). As a result of processing the testing data, the experimental-statistical (ES) models of each investigated parameter are formed, representing the equations of the second-order of the next kind:
where b
0, b
i, b
ij, b
ii is correlation coefficients determined as a result of mathematical and statistical processing of experimental data; z
i, z
j is the value of variable formulation and technological factors.
The significance of ES model correlation coefficients was determined with the help of the Student’s criterion; the second-order equation checked the adequacy of the object description by the Fisher’s F-criterion.
Two four-factor experiments differing only in the method of forced carbonation—dynamic (cyclic) and static—were implemented.
The principle of the applied dynamic method of carbonation implied accelerated carbonation of the lime binder within several cycles. One carbonation cycle included several consecutive operations: vacuumization of the sample chamber, holding under conditions of rarefaction, carbon dioxide supply to the carbonation chamber, holding the samples in CO2 environment of a certain concentration. The rarefaction in the chamber was 5000 Pa, the prepared in advance to the required concentration gas and air mixture of CO2 was fed into the chamber from a buffer tank. The gas, seeping through the pores of the product, returns to the buffer tank, where it is brought to the desired concentration by feeding the required amount of CO2. The duration of one cycle was 180 s.
The statistical method of carbonation was based on storing testing samples in carbon dioxide gas and air environment, without creating the resolution in the carbonation chamber. Duration of carbonation of testing samples corresponded to the duration of carbonation under the dynamic method.
The choice of factors in the experiment was made, taking into account the conclusions obtained in the analysis of the structure formation and properties of artificial stone based on lime carbonate hardening [
21,
22,
25]. Taking into account the influence of many factors influencing the lime carbonation process and the formation of material properties on its basis in this experiment varied: the concentration of CO
2 (z
1), the forming pressure of pressing the testing cylinder samples (z
2), the content of lime dust in the lime-lime composition equivalent to Ca(OH)
2 (z
3) and, directly, the modes of forced carbonation, the number of cycles and time of exposure to CO
2 on the testing samples (z
4). The designing conditions for the experiment are presented in
Table 3.
Main parameters of testing samples were compressive strength (Rc), average density (ρo), water resistance (KS), water absorption by mass (Wm) and carbonated layer thickness (δ).
Compressive strength, average density and water absorption by weight were determined using standard methods for building materials. The resistance of testing samples, i.e. the ability of the material to maintain its operating properties under prolonged exposure to water, was evaluated by the so-called softening factor, K
S, which is the ratio of compressive strength of the sample in a water-saturated state, R
w to the compressive strength of the material in a dry state, R
d by the formula:
Water-resistant materials are those with KS above 0.8.
The thickness of the carbonated layer, δ, was determined by spraying, on the surface, a 1% alcohol solution of phenolphthalein to the testing sample.
Testing samples were dried to zero humidity in a drying cabinet at 80 °C, before testing.
3. Results
Experimental data on the properties of testing samples carbonated by dynamic (1) and static (2) methods are presented in
Table 4.
The correlation coefficients of ES models, obtained as a result of statistical processing of testing data, are presented in
Table 5.
The estimation of the influence of separate technological factors shows that the importance of their influence on the formation of properties of materials based on lime carbonation hardening depending on the chosen method of production of artificial carbonation is different. So, if the dynamic method of carbonation (model 1), the most influencing factors can be called the factors for obtaining the testing samples, i.e. pressure pressing (z2) and lime dust content (z3), then in the static method (model 2), along with the main factor z2, the factors responsible directly for the process of carbonation curing, such as the concentration of CO2 (z1) and carbonation time (z4), become more decisive.
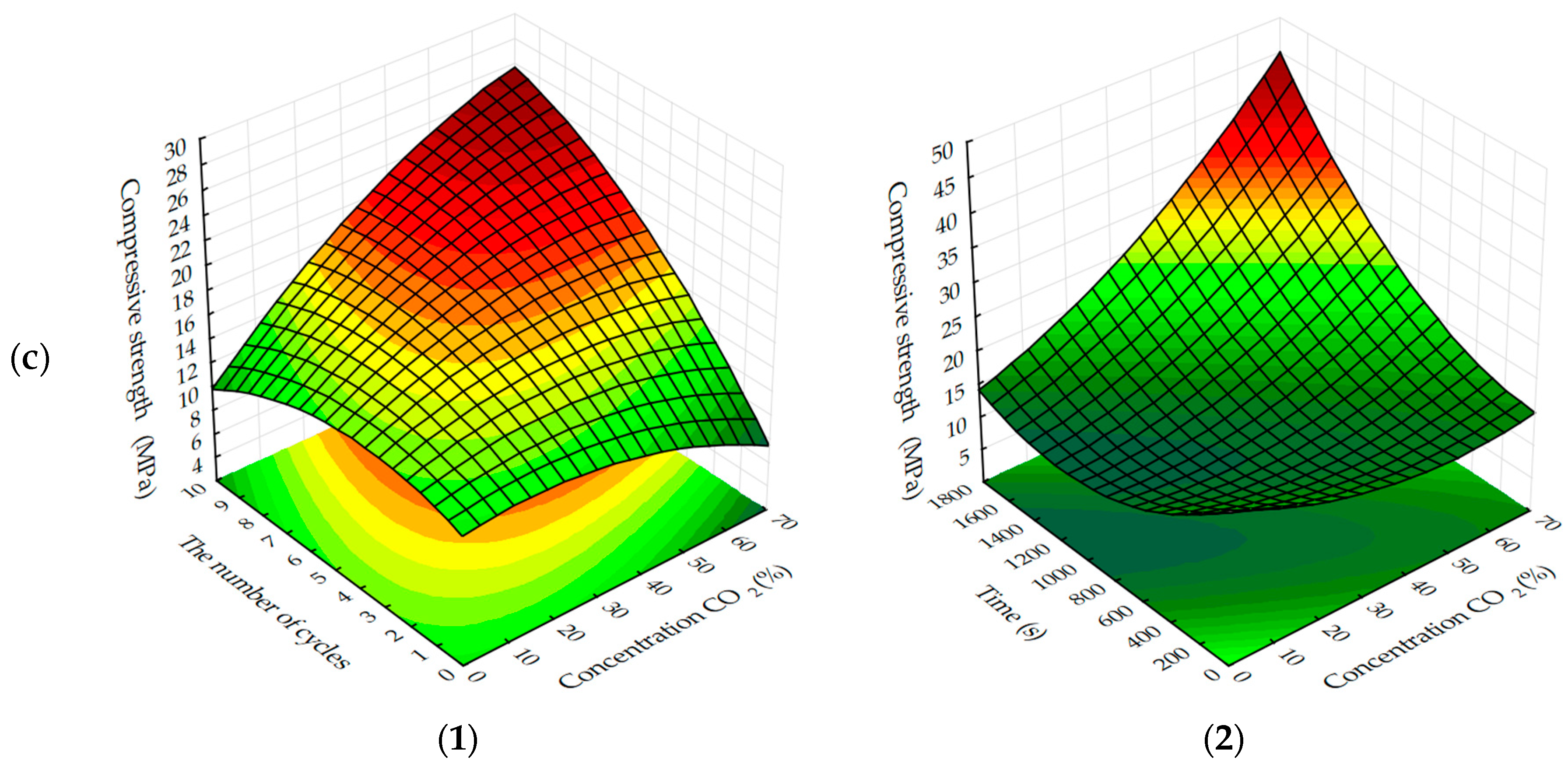
Analyzing the experimentally calculated results of the formation of compressive strength of testing samples depending on the investigated factors (
Figure 3 and
Figure 4), it is necessary to note the different character of response surfaces. Thus, in the dynamic method of the carbonation of the cylinder, samples of the response surface have a dome-shaped, convex-like parabola, which has the area of points reaching the maximum values of Rс, and in the static method of carbonation of the response surface, on the contrary, somewhat concave, reminiscent of a graph of the degree function, by which the compressive strength increases with the increase of variable factors. Such characteristic differences in the response surfaces indicate that the carbonation process is more intensive in a dynamic method than in a static method, however, the estimation of absolute values of the index Rс shows that in a static method, the compression strength of carbonated cylinder sample s with prolonged maintenance in an environment with a high concentration of CO
2 is significantly higher and tends to further increase.
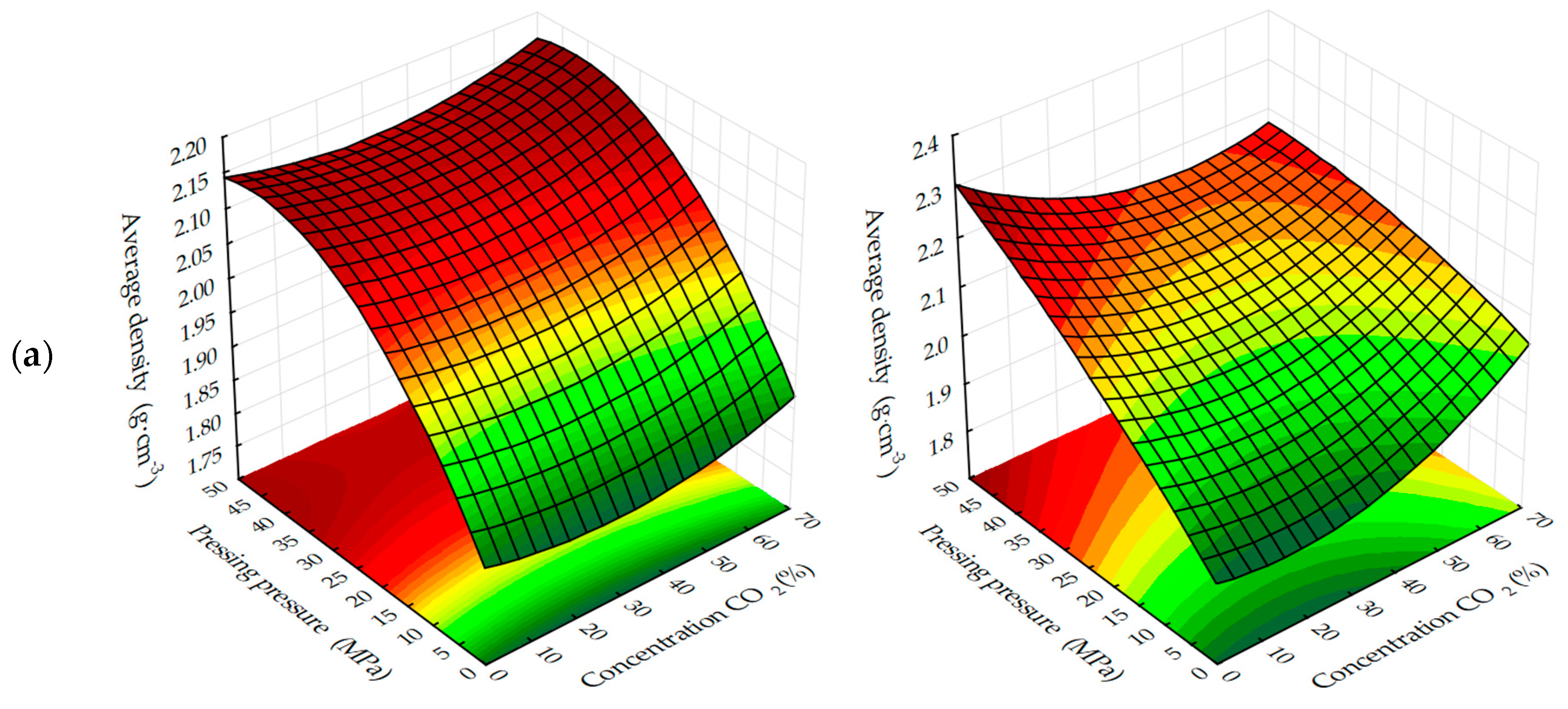
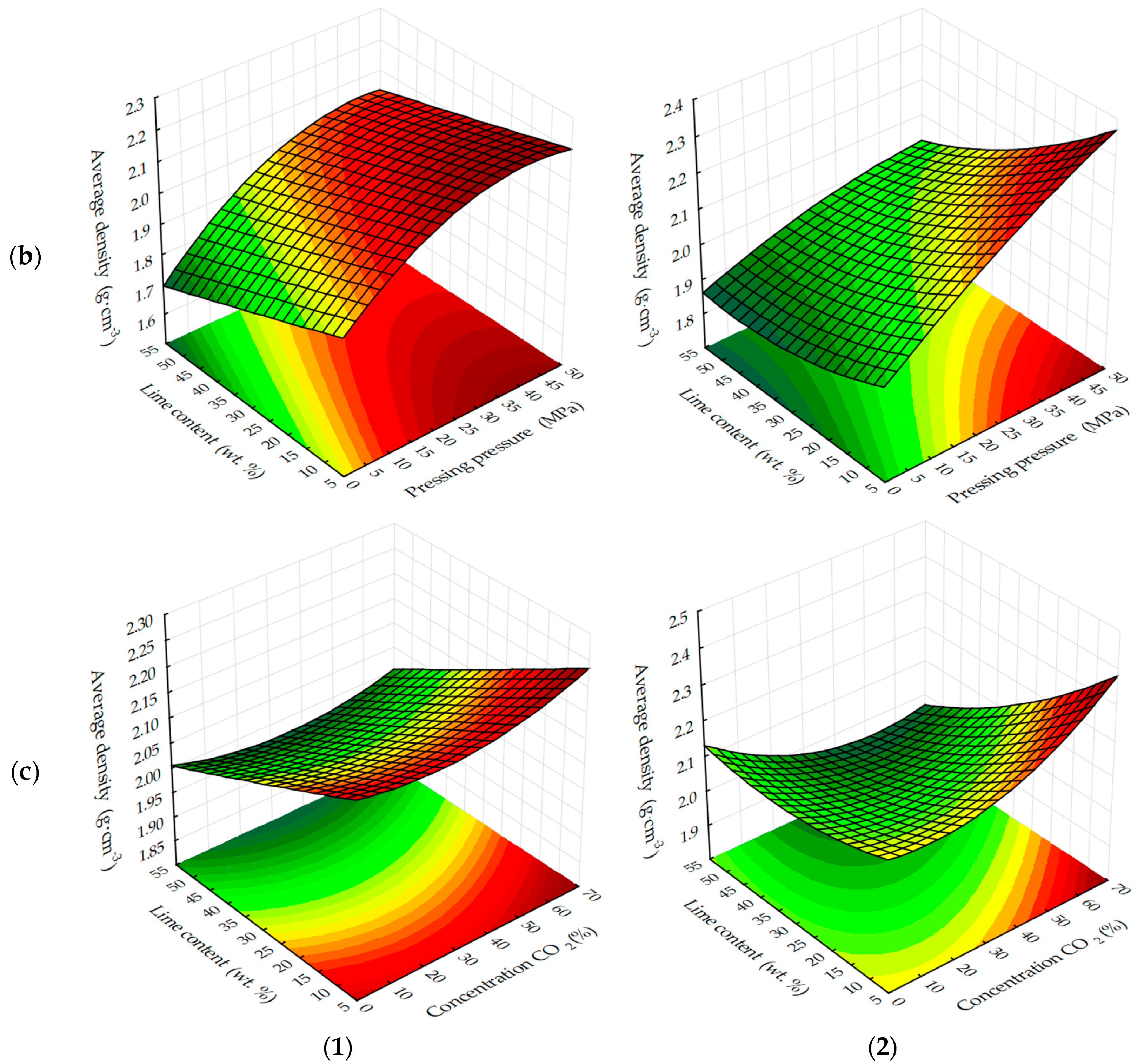
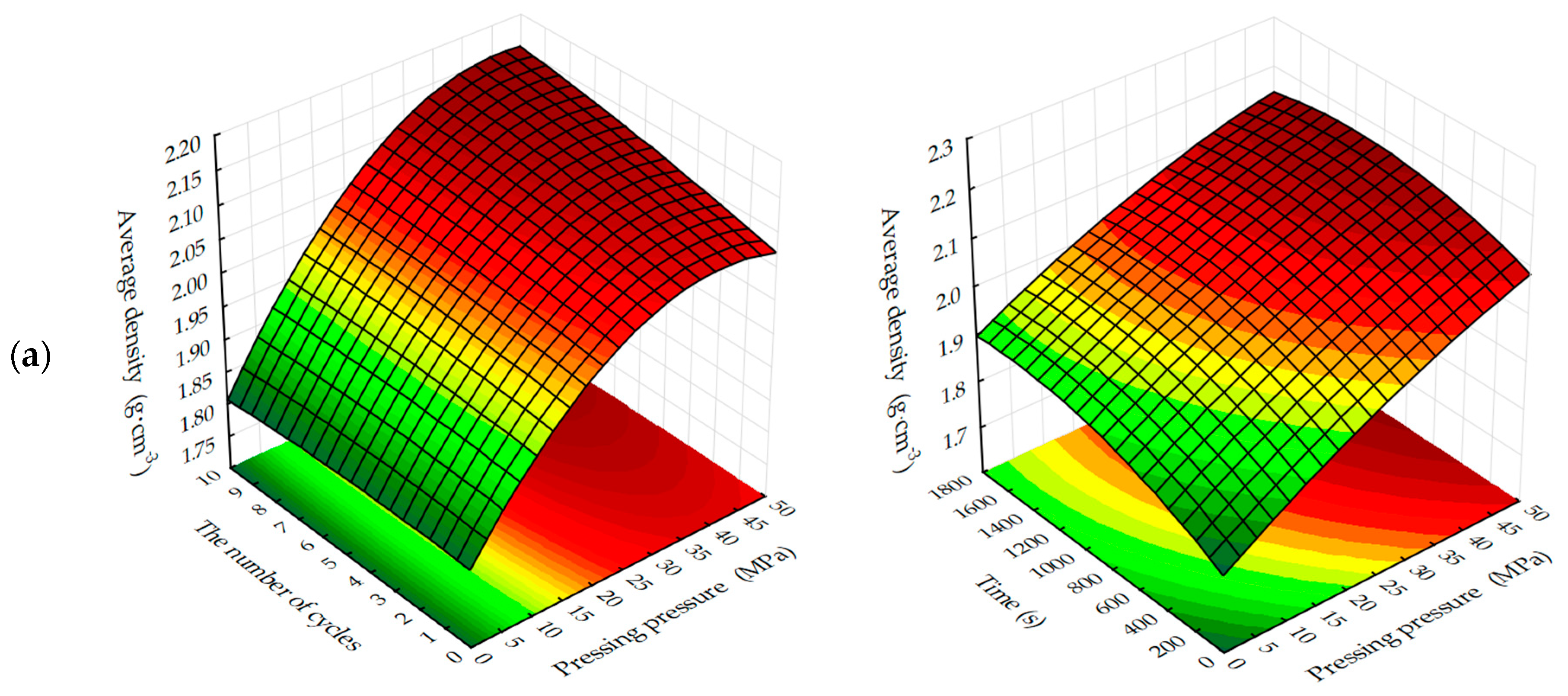
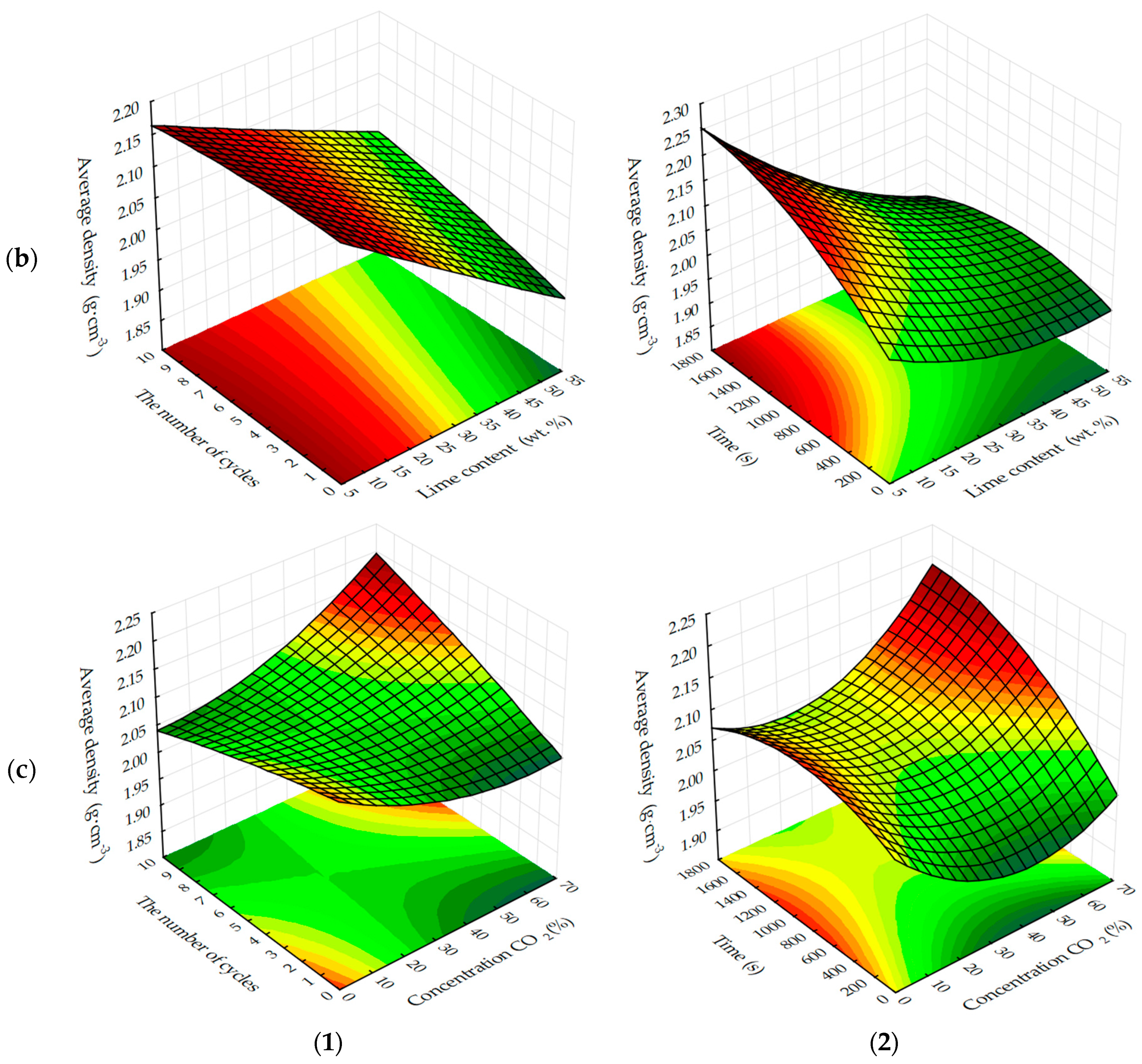
The response surfaces of changes in the average density of carbonated samples (
Figure 5 and
Figure 6) show that, regardless of the methods of carbonation, the index
ρo of testing samples increases with a decrease in the amount of lime dust and an increase in the forming pressure of pressing, due to the formation of a more dense structure of the material at the stage of production of cylinder samples and, practically, does not change with the variation of CO
2 concentration. Average density, as well as the compressive strength of testing samples, increases more intensively with the dynamic method of carbonation, but the absolute values of the index ρ
o, with an increase in the duration of exposure of samples in the carbon dioxide gas and air environment, are higher for the samples, the carbonation of which was carried out in a static mode.
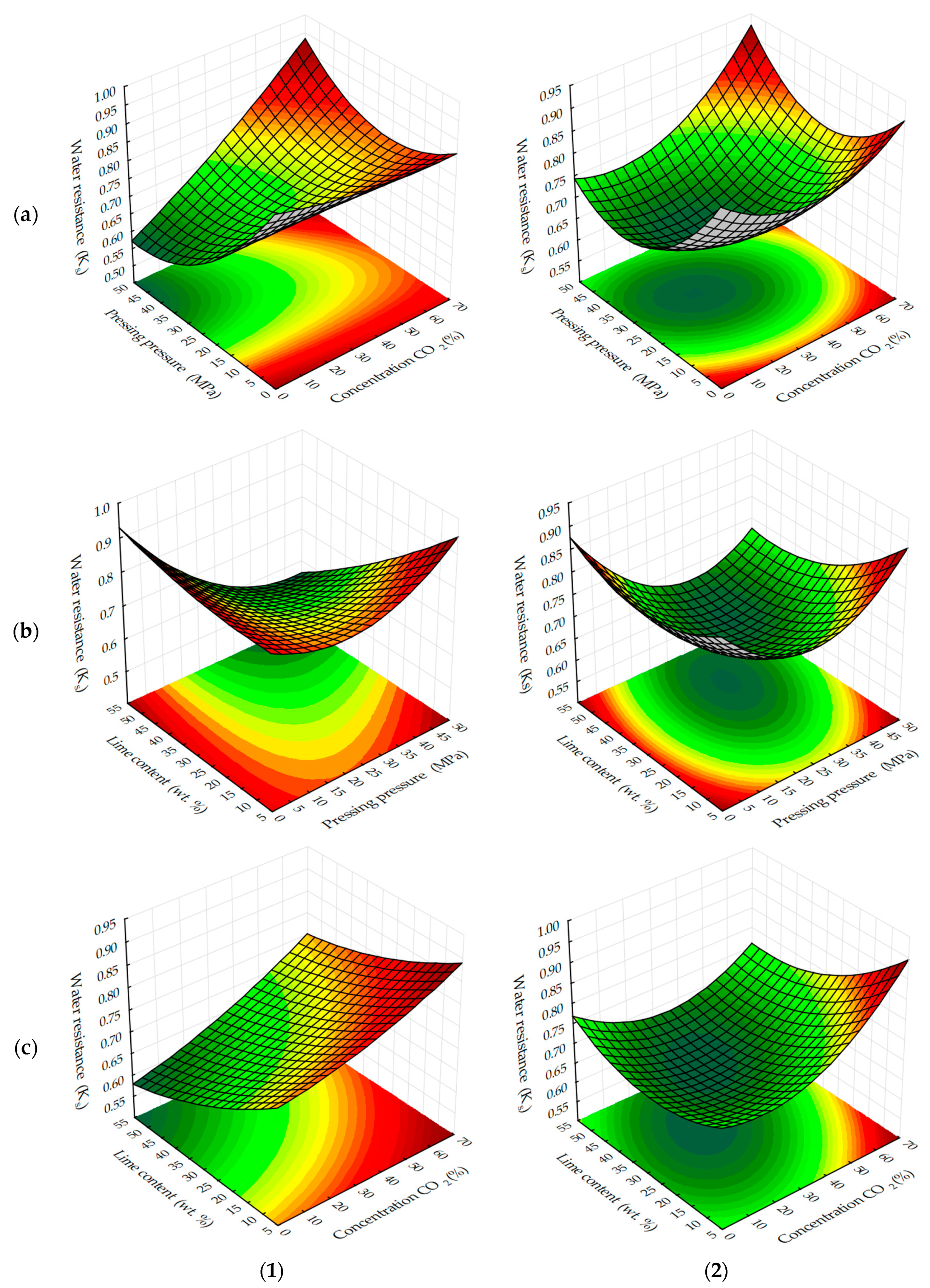
The areas in the graphs where the testing samples reach values at which they can be considered water-resistant, i.e. K
S ≥ 0.8, are not significant (
Figure 7 and
Figure 8), regardless of carbonation methods.
Water-resistance is mainly provided by carbonated cylinder samples obtained at forming pressures up to 10 MPa, as well as samples containing a minimum amount of lime dust (Ca(OH)2), in the composition of forming mixtures. Most likely, water resistance depends on the degree of carbonation contained in them Ca(OH)2, which under the above conditions and under accepted time factors, is characterized by maximum KS values.
In view of the above, it is also logical that as the duration of carbonation (
Figure 8) increases, so does the water resistance of the testing samples. Moreover, a more stable increase of this index is observed in samples obtained in the static mode of forced carbonation. With the dynamic carbonization method, K
S values corresponding to water-resistant materials are achieved earlier. For example, at the minimum content of lime dust (see
Figure 8c), a material with K
S ≥ 0.8 can be obtained on the fifth cycle, and with a static method of carbonation, the same index is achieved, approximately 1400, with the holding in an environment of CO
2, which corresponds to eight cycles, with a dynamic method of forced carbonation. Based on the results obtained, it can be argued that the water-resistance of carbonated materials depends on the degree of carbonation of the lime component, which in turn, depends on the initial structure density and carbonation time of samples based on lime dust and finely dispersed limestone.
Experimental data on changes in water absorption by weight of testing cylinder samples, based on lime dust and finely dispersed limestone (
Figure 9 and
Figure 10) show that the minimum achievable values of this parameter, depending on the varying factors in the experiment are in the range from 9% wt. to 10% wt. In contrast to the water resistance, the maximum values of which are achieved with the least effort to form testing samples; the minimum values of water absorption of testing samples, on the contrary, are achieved with the maximum effort of pressing testing samples.
Wm also decreases with a decrease in the content of lime dust in the studied samples, i.e., the most dispersed raw material component, which creates small open pores in the samples, contributing to water migration and water saturation. The change of modes of forced carbonation of testing samples does not change the general logical change of water absorption of samples, but it should be noted that in a static mode under equivalent conditions of variation of factors, Wm index is 1%–1.5% less than the mass.
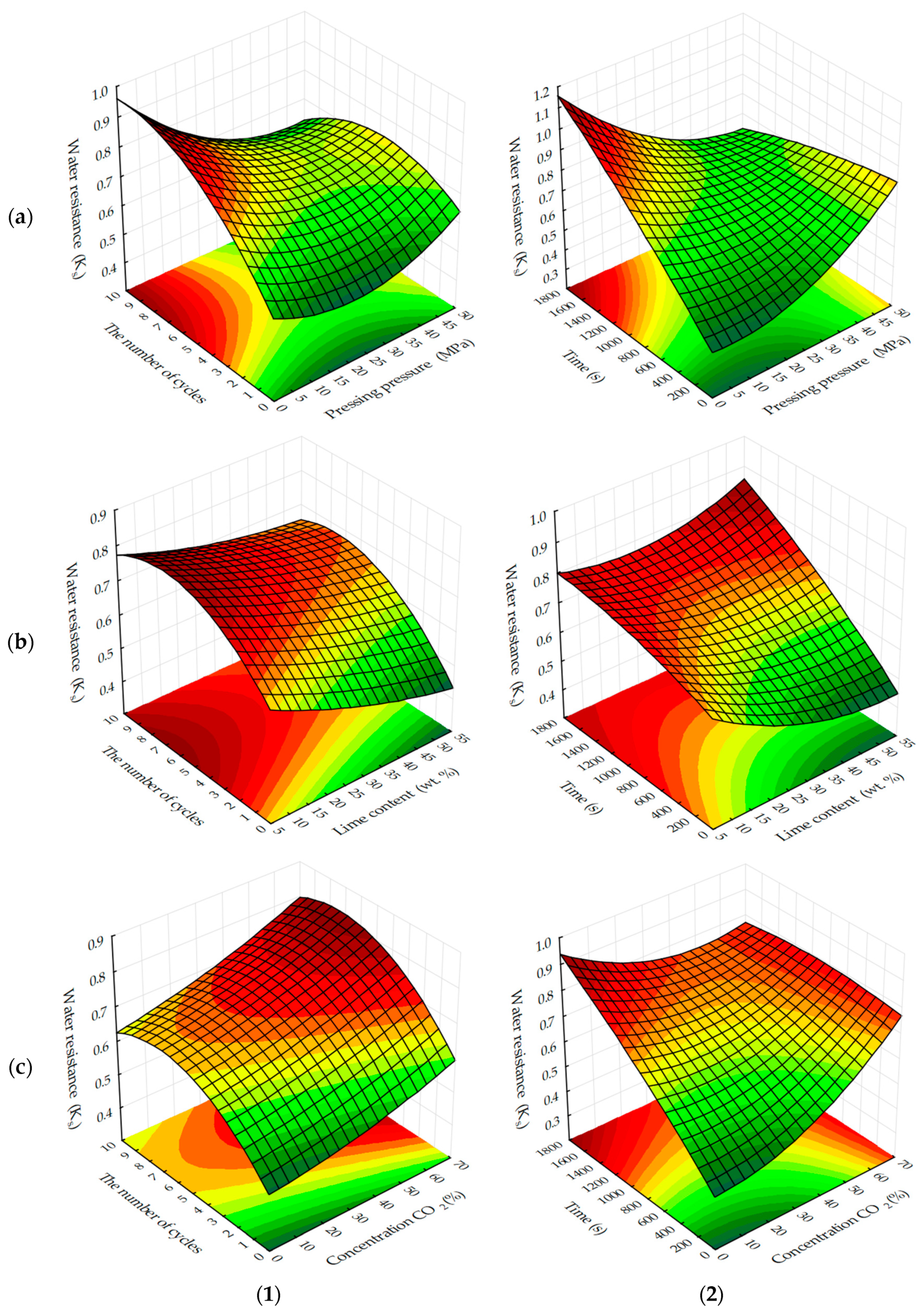
The analysis of response surfaces of the carbonated layer formation of testing samples (
Figure 11 and
Figure 12) shows that their character of change is very similar to that of water resistance change response surfaces (see
Figure 7 and
Figure 8). This indicates that the water-resistance of materials hardening due to forced carbonation determines the thickness of the formed carbonated layer, the size of which can indirectly be judged on the degree of carbonation of the lime component in the testing samples (
Figure 13). The size of the formed carbonated layer depends on the factors that determine the initial structure of the studied lime-liming samples, the content of lime dust and forming pressure pressing. Thus, with a reduction in lime dust content and pressing forming pressure; i.e., when the least dense initial structure of the material is created, the sample is carbonated almost completely (
Figure 13a).
In addition to the lime dust content and pressing forming pressure, the formation of the carbonated layer is largely dependent on the time of forced carbonate processing of sample cylinders and the concentration of CO
2 in the gas and air mixture fed into the carbonation chamber. As the duration of carbonizing treatment increases and the amount of carbon dioxide in the gas and air mixture increases, the carbonated layer constantly increases (see
Figure 12). Thus, absolute values of thickness of the carbonated layer formed in testing samples at a dynamic and static method of their forced carbonation are practically identical, and the main technological factor forming a layer is a time of forced carbonation. Slightly increased δ values observed in testing samples obtained in a static carbonation mode can be attributed to a longer contact time of the samples with CO
2, due to their continuous maintenance in the CO
2 gas and air environment, whereas in a dynamic method, due to the cyclic feeding of the gas and air mixture into the carbonation chamber, the total contact time of the samples with CO
2 was not much, but still less.
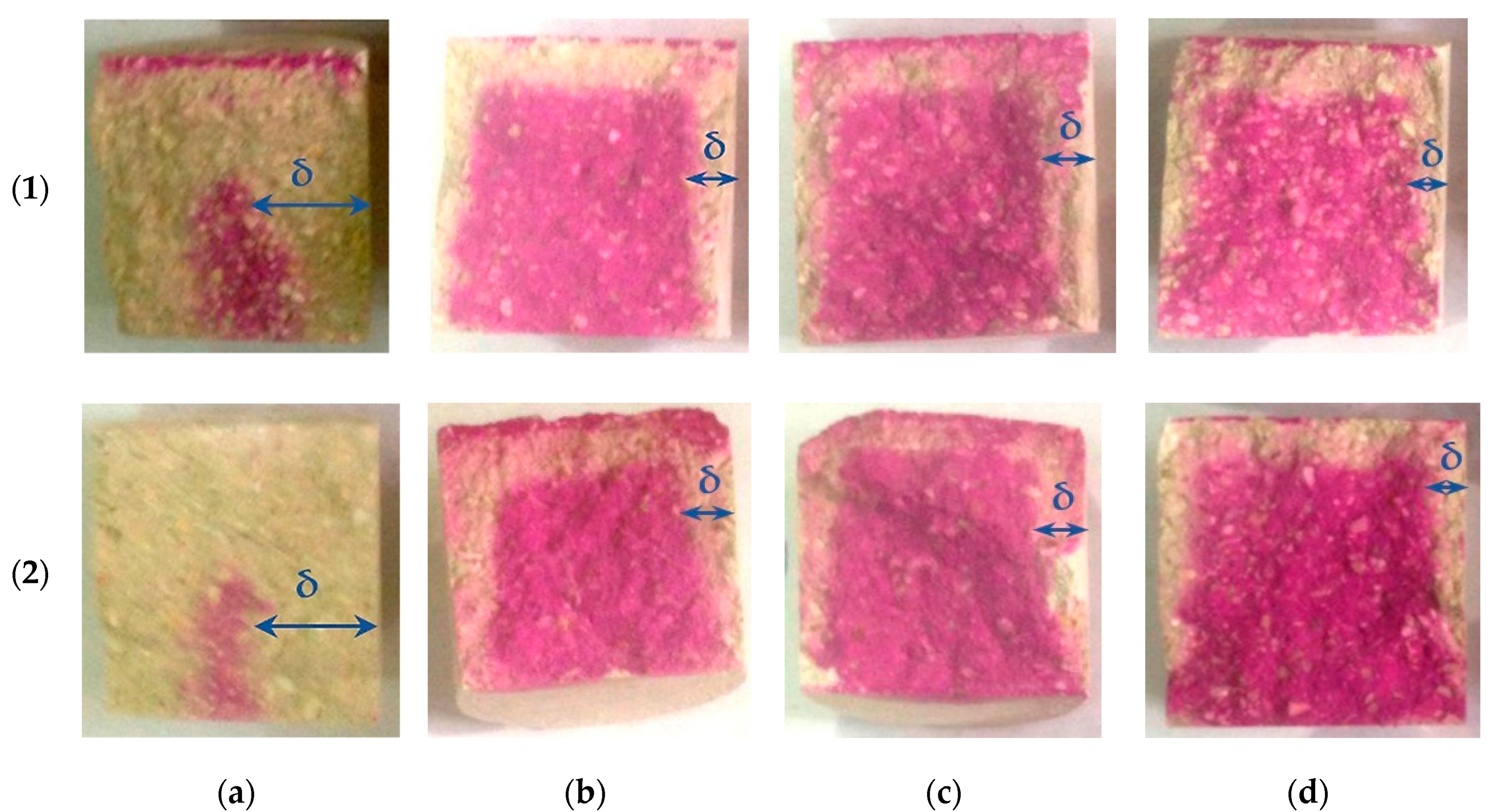
Photos in
Figure 13 show the carbonated layer formed in the testing samples as a result of their forced carbonation at different modes.
The carbonated layer was determined on the split cylinder samples by spraying the phenolphthalein alcohol solution to the sample. The visual examination of phenolphthalein-colored sample shows that the formation of carbonate stone, which makes up the carbonated layer, starts from the sample surface, gradually moving inward with the diffusion limitation of carbon dioxide, and the thickness of the layer is not the same in the surface area of the samples. There is practically no carbonated layer in the lower part of the testing samples. This can be explained by the dependence of the Ca(OH)
2 carbonation process from moisture. It is known [
22,
25,
29,
30,
31] that at a high humidity of the system chemical reaction of Ca(OH)
2, carbonation stops. Under the conditions of the experiment, water released as a result of intensive carbonation of the testing samples accumulated in the lower part and prevented the carbonation reaction in this scope of the samples. In this regard, one of the necessary conditions for the production of forced carbonate hardening of products is to ensure an intensive removal of moisture in the carbonation chambers, both from samples and from the chamber itself, for example, "airing" the chamber by a flow of gas and air mixture, with a constant concentration of CO
2.
A visual analysis of the sample also allowed one to determine that at close values of δ depth of CO2 penetration differs depending on the modes of forced carbonation of the cylinder samples. Thus, in samples carbonated by the dynamic method, the inner non-carbonated part after spraying phenolphthalein alcohol solution on it has a pale color in comparison with samples carbonated by the static method. This may indicate a deeper penetration of the carbon dioxide in forced carbonation in a dynamic (cyclic) mode. It should be noted that despite clear boundaries, the carbonated layer does not peel off the uncarbonated part of the sample, representing a solid heterogeneous conglomerate.
4. Discussion
As can be seen from the presented experimental calculation results of the study, it is unambiguously difficult to interpret them. It makes sense to consider the process of formation of the basic properties of carbonate hardening materials, depending on the intensity of their forced carbonation more complex, combining the maximum number of factors.
The graphical processing of experimental and calculated data of models of the formation of compressive strength of investigated lime-liming systems on the basis of lime dust, depending on the simultaneous influence of varying factors at various modes of carbonation is presented in
Figure 14,
Figure 15,
Figure 16,
Figure 17 and
Figure 18.
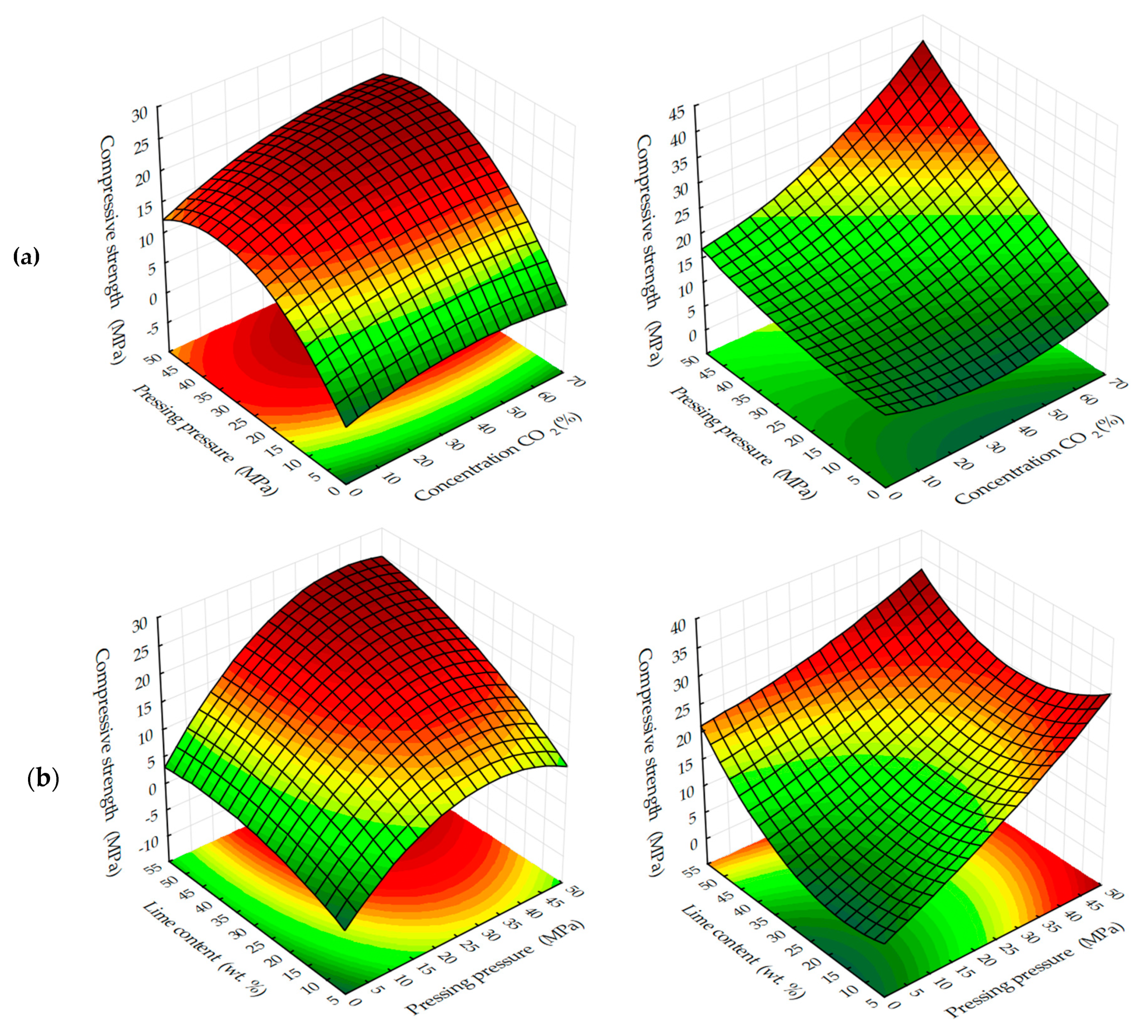
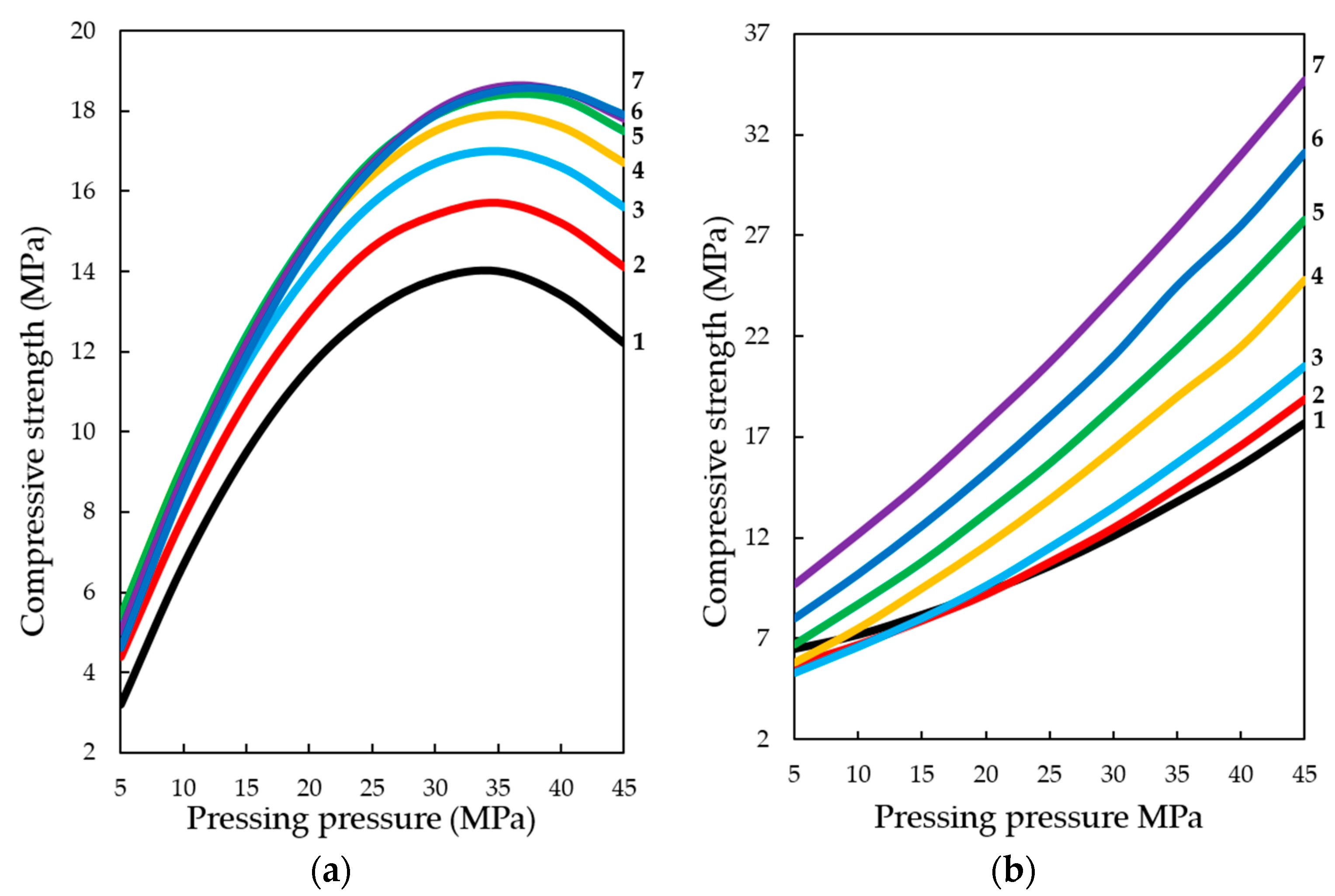
With the dynamic method of forced carbonate hardening (see
Figure 14a), the compressive strength of testing samples increases with an increase in CO
2 concentration in the gas and air mixture up to 50%; a further increase in CO
2 concentration up to 65% practically does not affect the change of R
c. A certain pattern of the dependence of R
c testing samples on the forming pressure of the pressing is traced. It can be assumed that with a further increase in the concentration of CO
2, the compressive strength will be reduced due to the developing corrosion and dissolution of newly formed CaCO
3 crystals, in accordance with the study of Cizer et al. [
29] According to the experimental data, the compressive strength increases with the increase in molding pressure up to 33–38 MPa, after which, with the increase in molding force up to 45 MPa, R
c values decrease, which, is explained by a denser structure of the material obtained at such high pressing pressures of the raw mix. Consequently, longer exposure times are required in environments with higher CO
2 concentrations. Thus, this fact of reduction of R
c proves that carbonation component, which forms the future structure of artificial stone from calcite crystals, actively participates in the formation of strength indices of pressed materials, the hardening of which is organized by carbonate type [
32,
33,
34].
With the static method of treatment of compositions with carbon dioxide (see
Figure 14b), the reverse picture is observed. The dynamics of the strength set of testing samples is lower than in the dynamic method, however, the absolute values of the strength values under similar varying conditions are higher, and the dynamics of the strength set itself tends to further increase, both with increasing forming pressure of pressing samples and with increasing concentration of CO
2 in the gas and air mixture fed into the carbonation chamber. This phenomenon shows that the acceleration of the lime carbonation reaction by actively seeping CO
2 through the samples, simulated in the experiment as a dynamic mode of carbonation, firstly, leads to a sharp formation of a large amount of water in the system, which clogs and closes the pores in the material, and complicates the diffusion of CO
2 in the pores of the material, and secondly, it can create conditions for the emergence in the system of aggressive carbon dioxide and the beginning of the dissolution and corrosion of calcite crystals of the newly formed carbonate structure [
23,
29,
35,
36,
37].
Estimating the obtained ES samples of forced carbonate hardening of samples on the basis of lime dust and limestone, it can be concluded that the most appropriate way to carry out the accelerated carbonation of materials based on lime, may be a combined method of artificial carbonation, which consists in creating a pre-discharge in the carbonation chamber, the subsequent supply of gas and air mixture of CO2 and further conducting the process in a static mode, maintaining a given concentration of CO2.
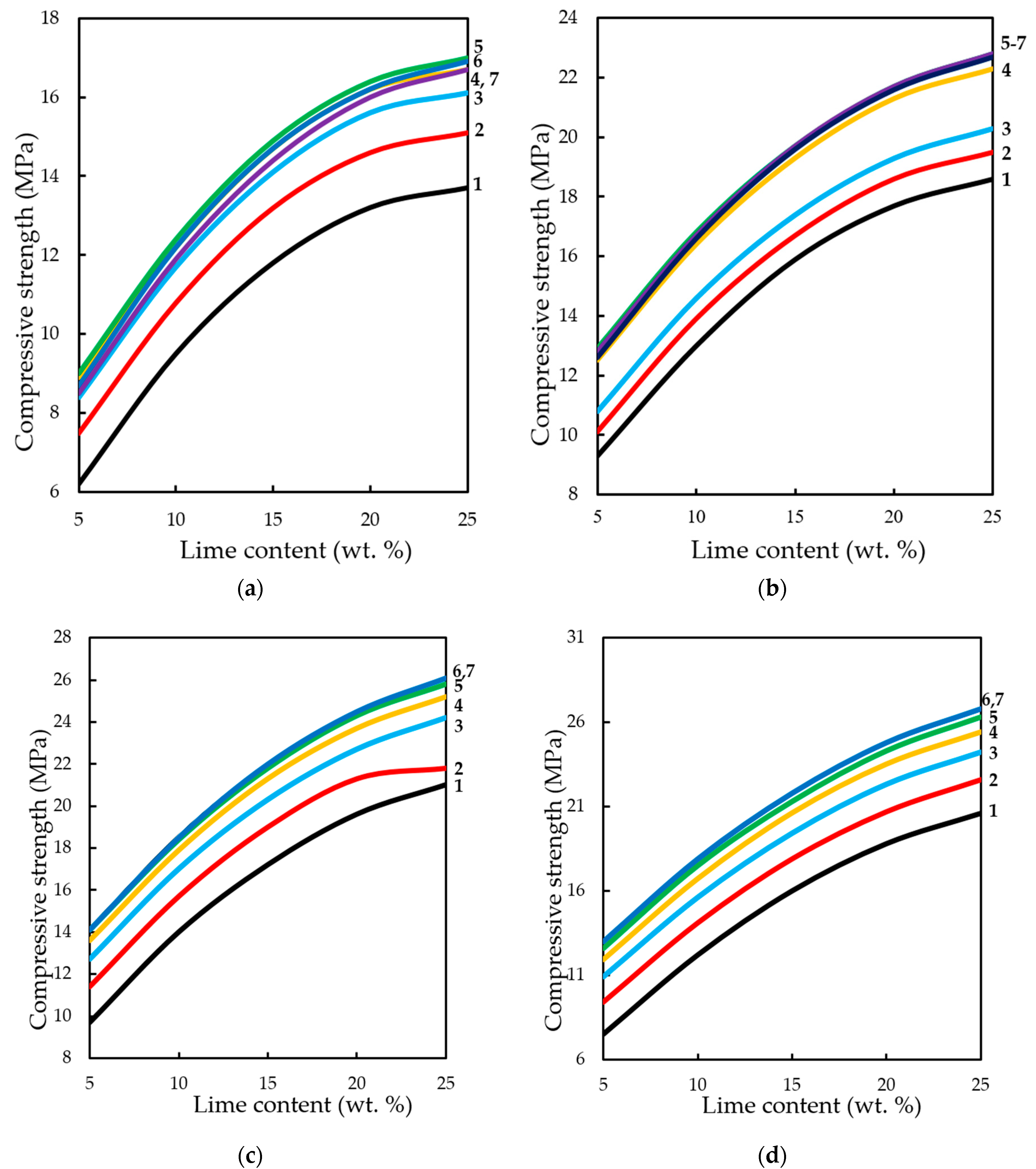
The analysis of changes in compression strength depending on the content of lime binder, forming the pressure of pressing and concentration of CO
2 in the gas and air mixture at different methods of carbonation, is presented in
Figure 15 and
Figure 16.
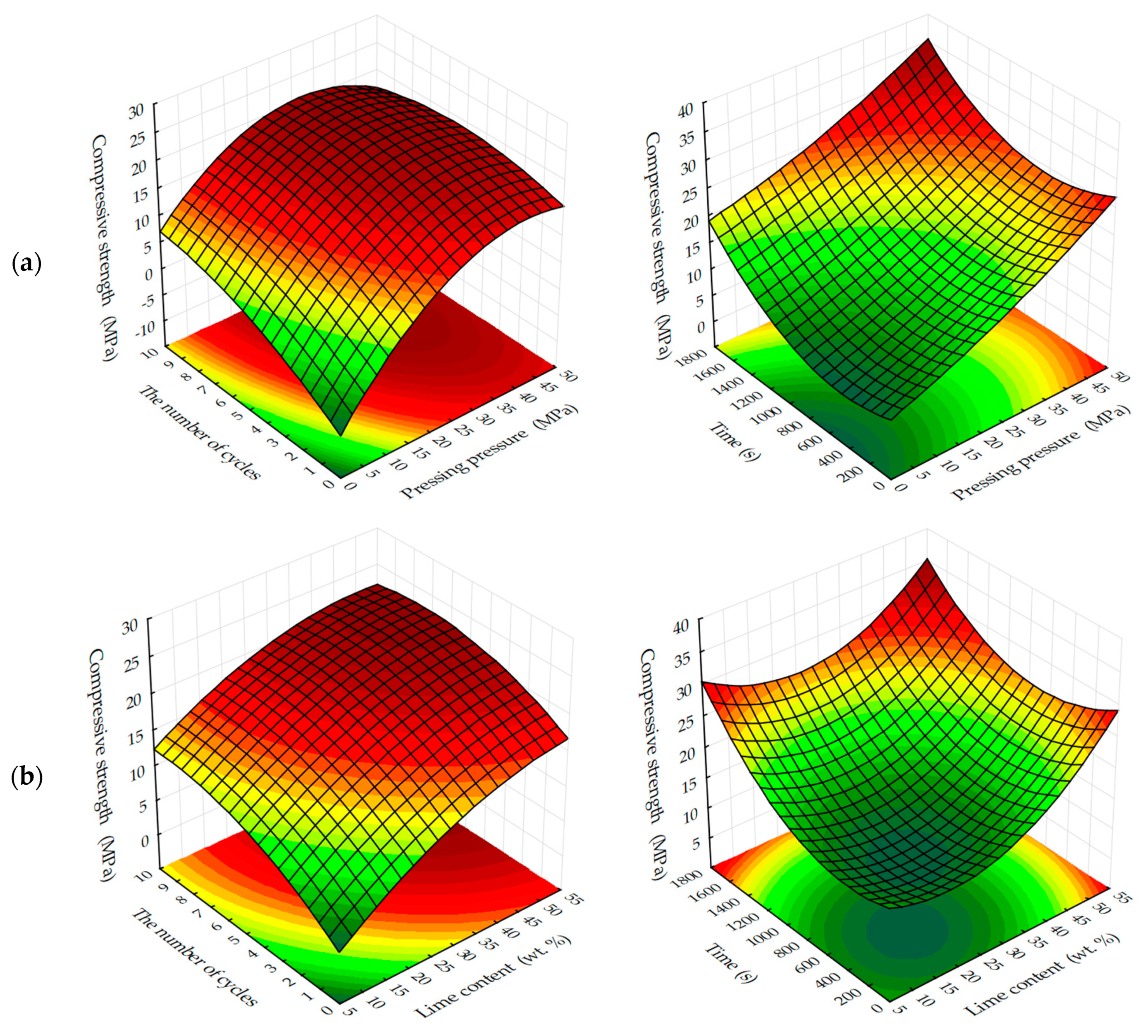
The dynamic method of carbonation (
Figure 15) shows that the compressive strength of testing samples increases with increasing binder content, as other factors change. However, as already noted, an increase in CO
2 concentration of more than 40%–50% of the compressive strength practically does not change, and with the growth of molding pressure of pressing increases the limit concentrations of CO
2, at which R
c practically does not change. Thus, for testing samples manufactured with a pressing force of 10 and 20 MPa, the maximum concentration of CO
2 is 40%, and for samples pressed at 30 and 40 MPa—60%.
In the conditions of static carbonation (
Figure 16) in testing samples with increasing content of Ca(OH)
2 up to 15% wt. in their composition, there is some decrease of compressive strength, with increasing content of lime in the composition—the strength increases. It seems that this could be an error in program calculation. The strength at the content of calcium hydroxide in the moulding mixtures within 5% wt.–15% wt. changes insignificantly, and with an increase in the content of Ca(OH)
2 in compositions over 15% wt., the strength increases markedly. It should be noted that the compressive strength of samples carbonated by the static method at increased pressing pressures (hyper-pressing) over 30 MPa is 25%–35% higher than that of similar samples obtained by the dynamic carbonation method.
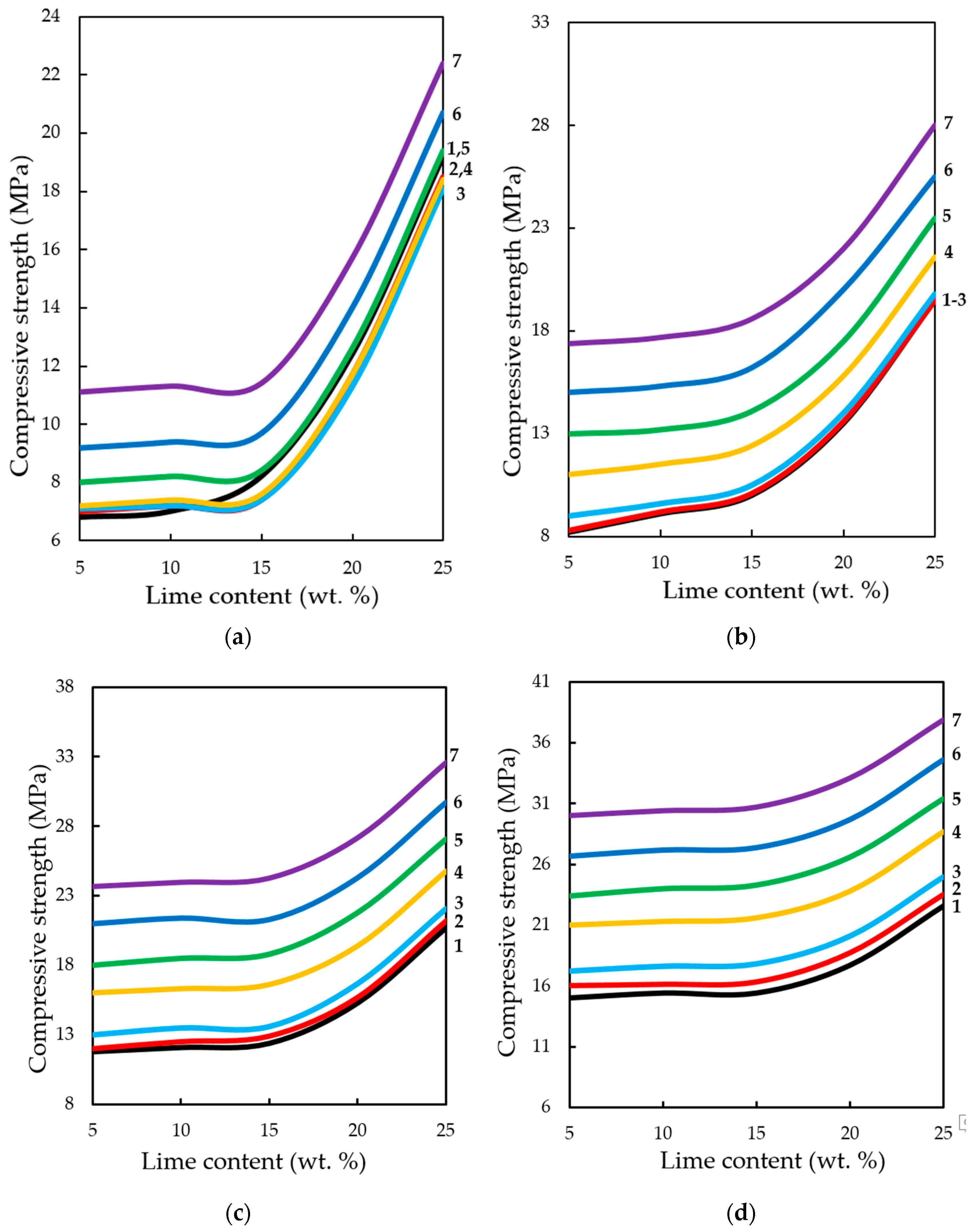
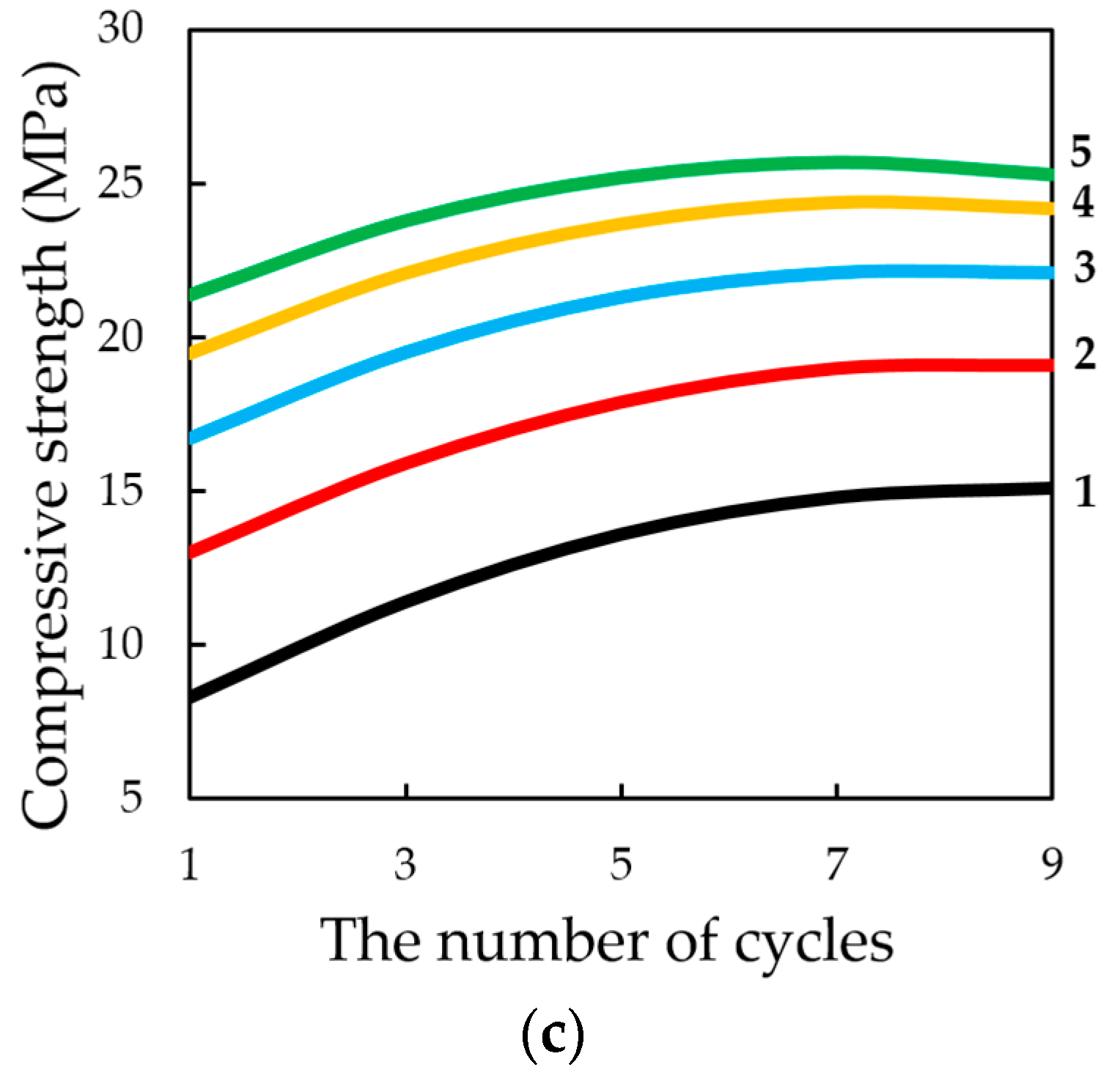
The study of the influence of the number of cycles in the dynamic method of carbonation on the change in compression strength of testing samples, presented in
Figure 17, shows that, in general, the strength increases with an increase in the number of cycles of carbonation, as well as with an increase in the number of lime binder in the compositions. However, it should be noted that the nature of growth of R
c is such that, with an increase in the number of cycles (more than 5) and molding pressure, the pressing growth of compressive strength is somewhat slowed down, due to difficult access from CO
2 particles to Ca(OH)
2 in the deep layers of the sample, due to the blocking pores of the material water released during the carbonation reaction Ca(OH)
2.
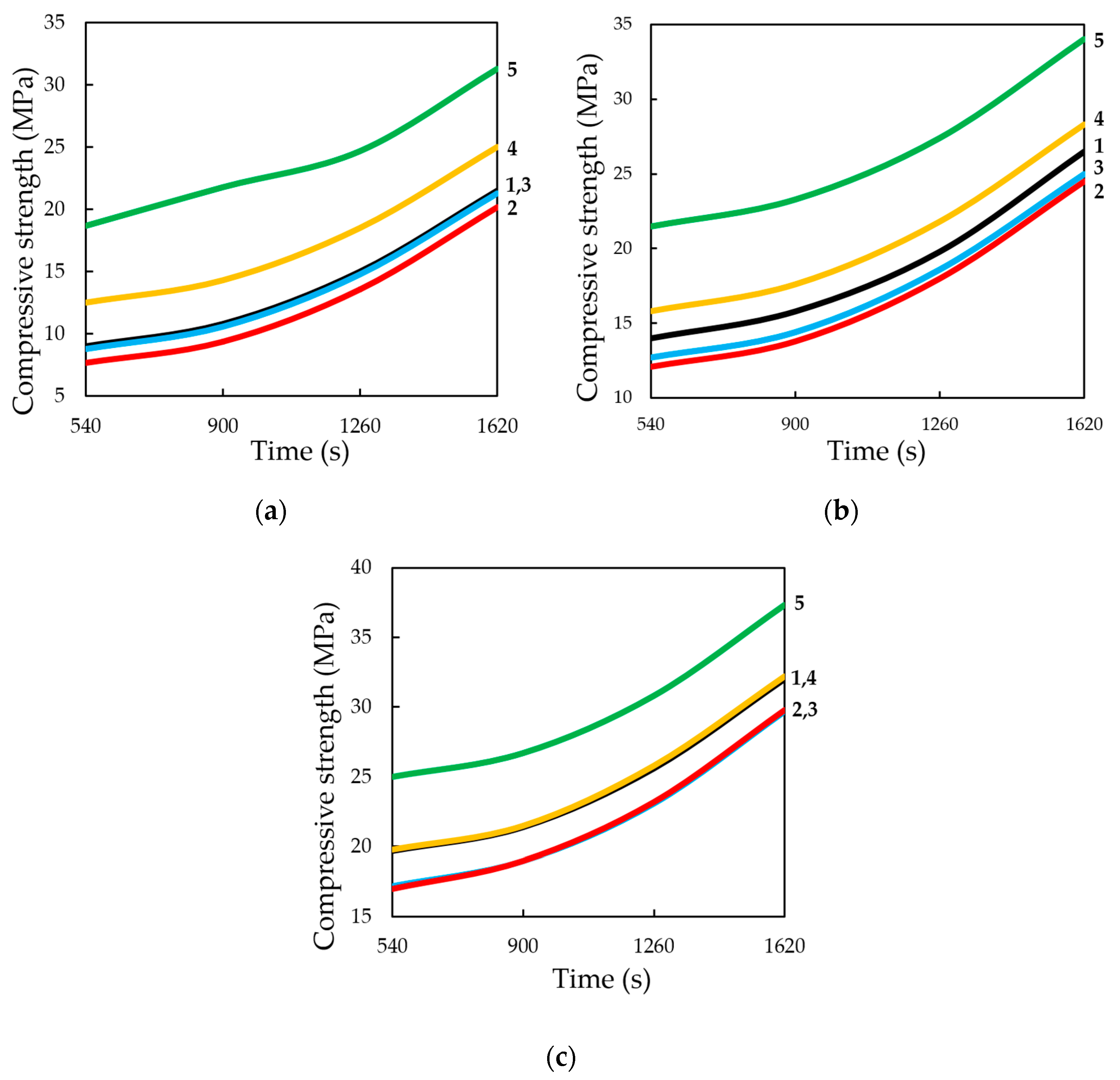
Compressive strength of samples at the static method of carbonation (
Figure 18) in contrast to the dynamic method, on the contrary, with increasing time of their treatment with carbon dioxide environment, increases to 1620 s, with a lime content of 25% wt. and the forming pressure of pressing 35 MPa is 37.3 MPa, which exceeds the compressive strength of samples obtained under similar conditions, the carbonation of which proceeded in the dynamic conditions of CO
2 supply more than 45%.
The formation of water-resistance of materials based on lime carbonation curing, as can be seen from the results obtained, depends on the formation of the carbonated layer, the thickening of which is facilitated by increasing the concentration and processing of samples in environments with a high concentration of CO
2. Increased factors such as molding pressure and lime content in samples, on the contrary, reduce the thickness of the carbonated layer, mainly due to the formation of a more dense initial structure of the material, which prevents the diffusion of CO
2 [
32,
38,
39,
40].
Water absorption by weight of carbonated samples determines the forming pressure of the pressing, with an increase in which the water absorption index decreases. Increasing the concentration of CO2 and the number of carbonation cycles also reduces the water absorption of samples and increasing the content of Ca(OH)2 in the raw mix increases the Wm indicator of carbonated material, impairing its hydrophysical properties.
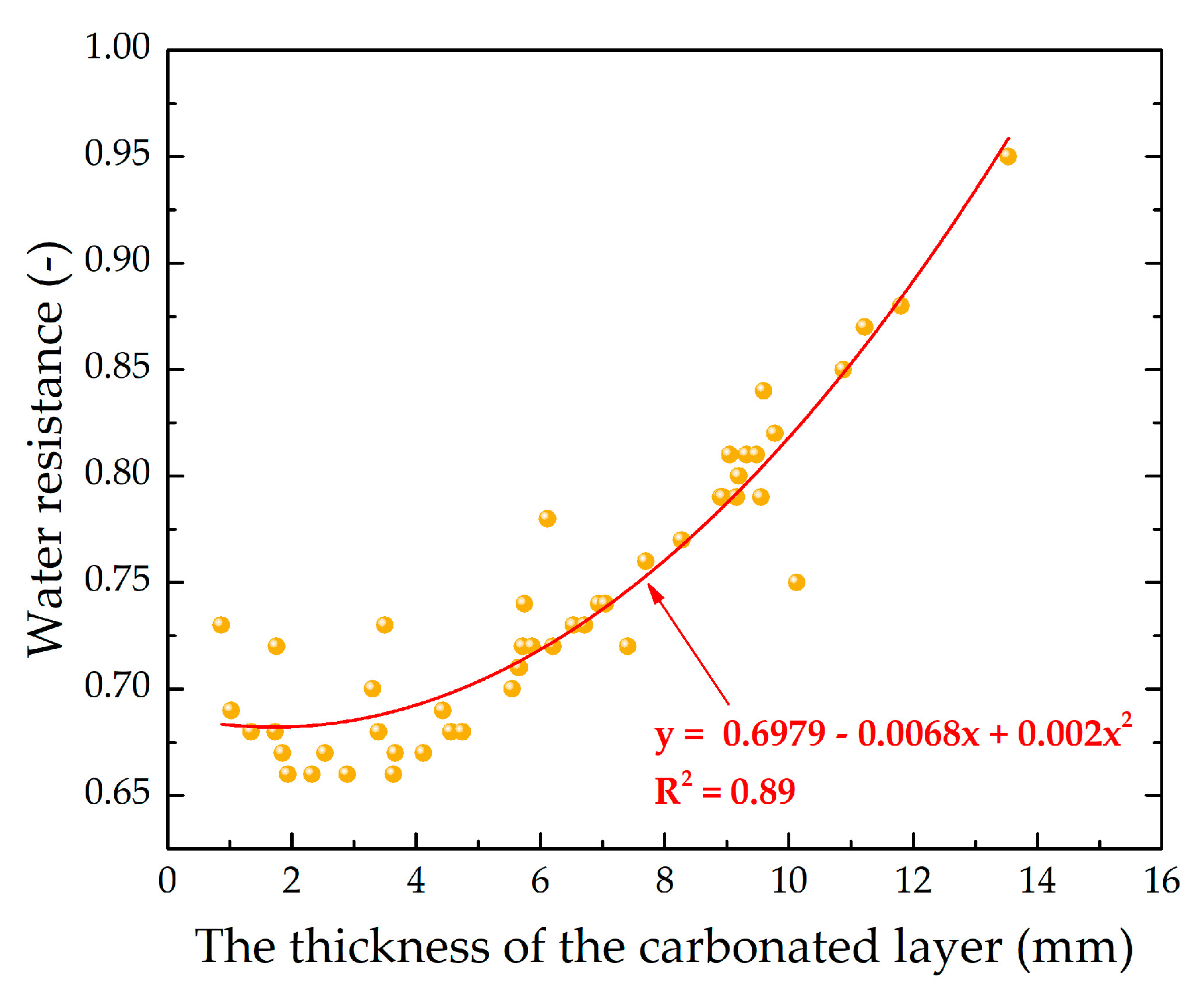
As was found during the analysis of the research results, the water-resistance of the testing samples depends on the degree of the carbonation of the lime binder, which can be indirectly characterized by the carbonated layer of the samples.
Figure 19 shows the graphical dependence of the water-resistance K
s on the thickness of the carbonated layer, δ, from which it is seen that the material acquires the properties of water resistance (K
s ≥ 0.8) at δ ≥ 9 mm.
In their work, Ergenç et al. [
35] showed that the carbonation chamber did not influence the compressive strength of the mortars, but worsened the flexural strength results. Mo et al. [
36] subjected cement pastes to CO
2 under pressure. Their research showed that this resulted in a rapid increase in compressive strength, as well as in microstructure compaction. Ahmad et al. [
37] checked the mechanical properties, precisely the compressive strength of the concrete, which was subjected to accelerated carbonation curing. Their research showed that when the concrete was exposed to a pressure of 414 kPa for 10 h, this resulted in an increase in compressive strength. This value increased by 114% compared to the reference sample. In study [
41], Moropoulou et al. concluded that mortar with addition of lime powder after 15 months of hardening showed a 236% increase of compressive strength. This is three times the strength increase compared to the reference sample. Chen et al. [
42] have observed that carbon curing significantly improves the early strength of cement mortars. However, this positive effect weakens with time. The compressive strength after 3 and 7 days retained initially an increased and then a reduced tendency as the curing time of carbonation was extended. The 28 day strength is slightly dependent on the duration of the carbonation. Based on their research, the authors determined that carbonation curing in combination with a suitable pre-curing can increase the initial compressive strength of the cement mortar. The impact on long term strength is nevertheless limited. Mo et al. [
43] in their work carbonated with CO
2 steel slag paste. The gas was at a pressure of 0.1 MPa. They observed that the creation of CaCO
3 in pastes reduced the amount of pores and thus improved their compressive strength. Studies carried out by Liu et al. [
44] indicated that for steel slag-cement binding materials, the best curing parameters were curing condition at 60 °C and carbonation lasting 7 h. Li et al. [
45] tested tricalcium silicate (C
3S) paste, which is the leading phase in Portland cement. The samples were subjected to accelerated curing with carbon dioxide (4-bar pressure). The results showed that after 2 h of curing, the paste reached a compressive strength of 27.5 MPa and after 24 h up to 62.9 MPa, where the samples subjected to hydration at the same time reached 5.2 MPa and 8.6 MPa, respectively. Cement blended with ground volcanic ash subjected to carbon curing was investigated by Seo et al. [
46] They observed a significant increase in compressive strength values. This did not include samples in which cement was replaced in 40% by ground volcanic ash. These samples were considered to be completely carbonated.
In this paper, the thickness of the carbonated layer, δ, was determined by spraying a 1% alcohol solution of phenolphthalein on the cleaved surface of the test sample (see
Figure 13). This method allowed only a qualitative analysis of the progress of the chemical reaction of carbonation. In further studies, it is advisable to study the depth of the carbonation layer in the samples, by changing the pH in the direction of the reaction front. In this case, the transition point pH < 9 should be taken as the carbonation depth. As indicated in the study [
47], at pH < 9, the brightness of the raspberry color begins to decrease until it disappears. To study the degree of carbonation of the material, it also makes sense to use Thermogravimetric analysis (TGA) in accordance with the work [
48].