Synthesis of Polypyrrole/Reduced Graphene Oxide Hybrids via Hydrothermal Treatment for Energy Storage Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Graphene Oxide
2.2. Preparation of Polypyrrole
2.3. Hydrothermal Self-Assembly of PPy/GO-HT Hybrids
2.4. Characterization Methods
2.5. Electrochemical Measurements
3. Results and Discussion
3.1. Porous Texture of the Hybrid Materials and Their Precursors
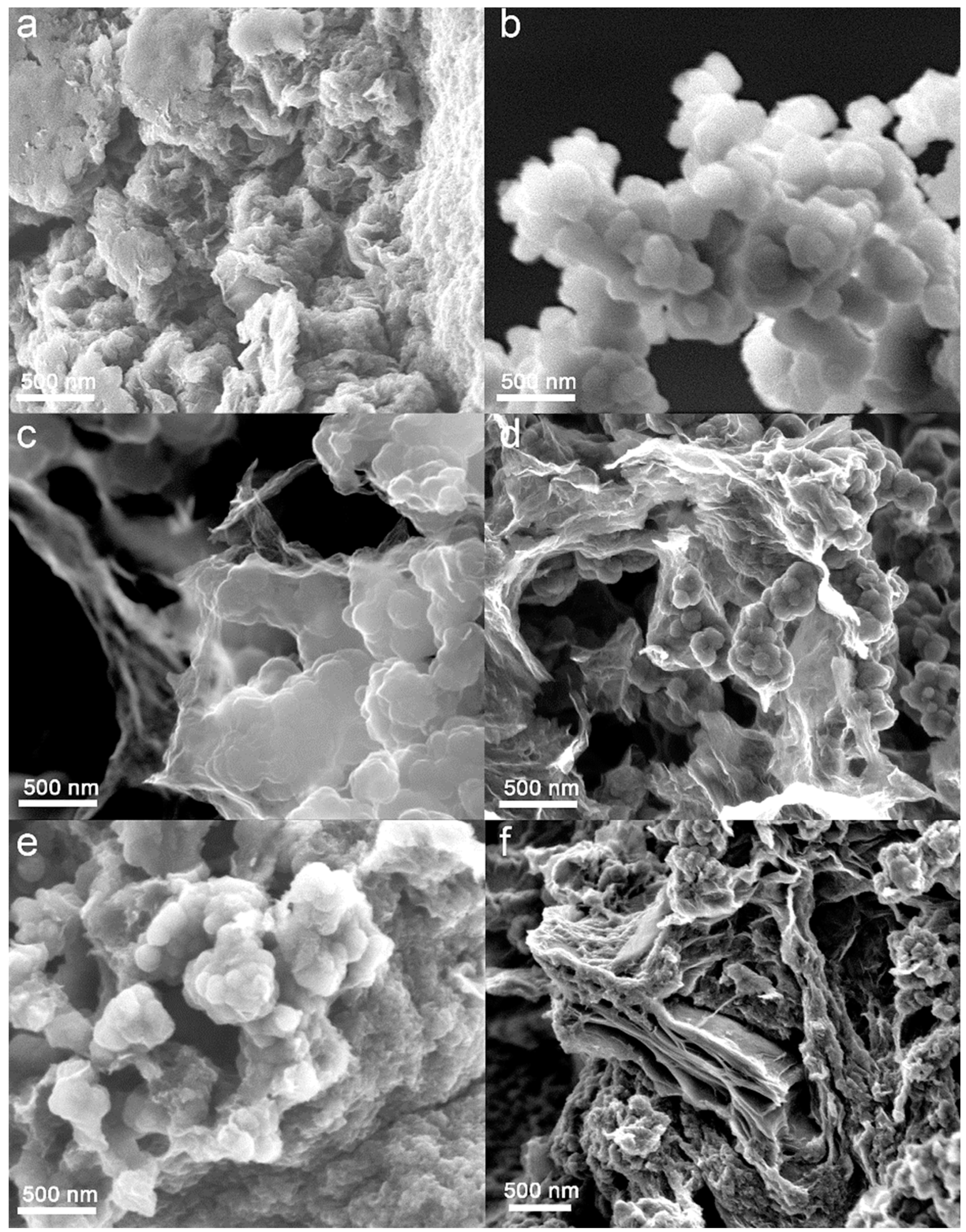
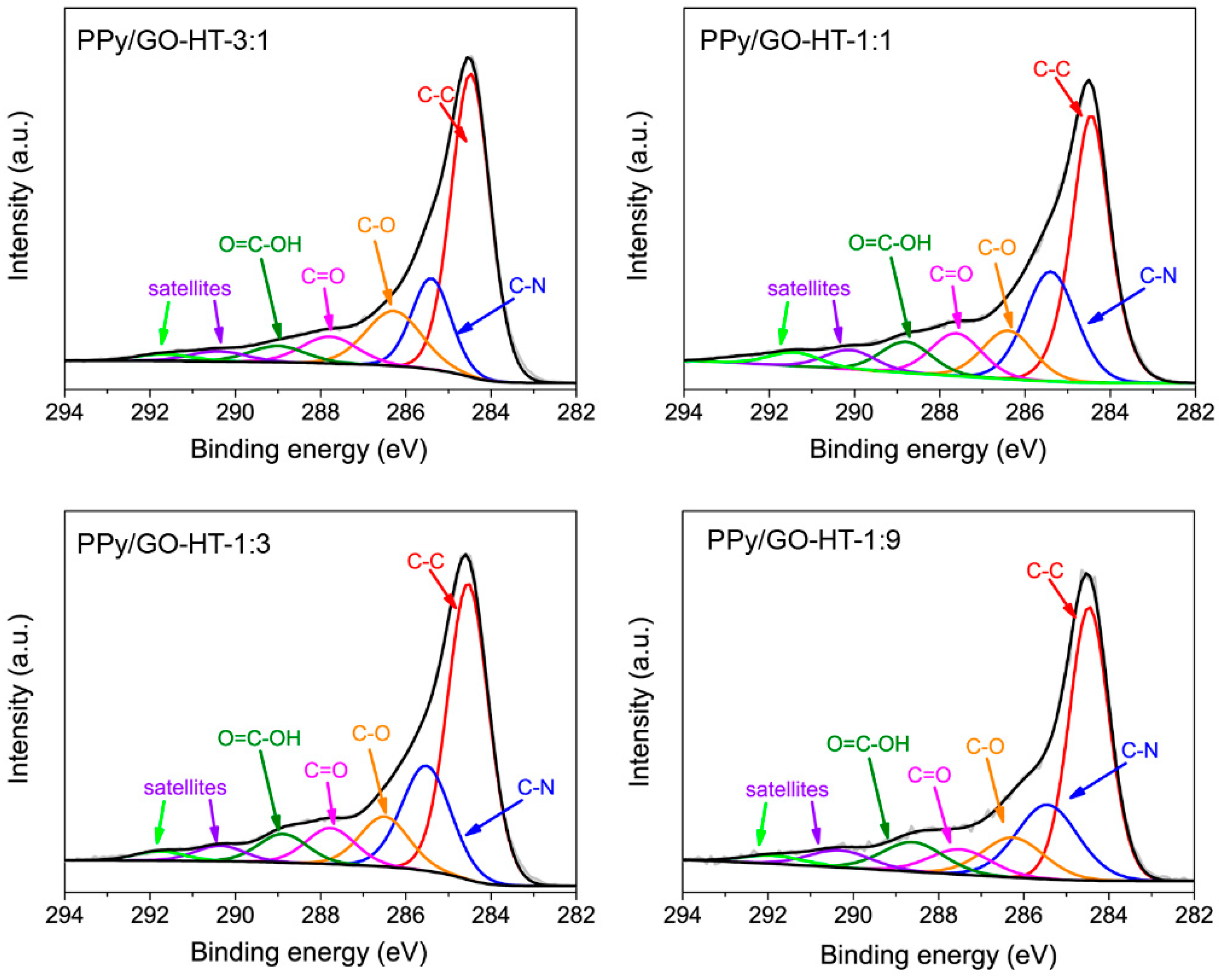
3.2. Morphology and Chemical Structure of the Binary Composites and Their Precursors
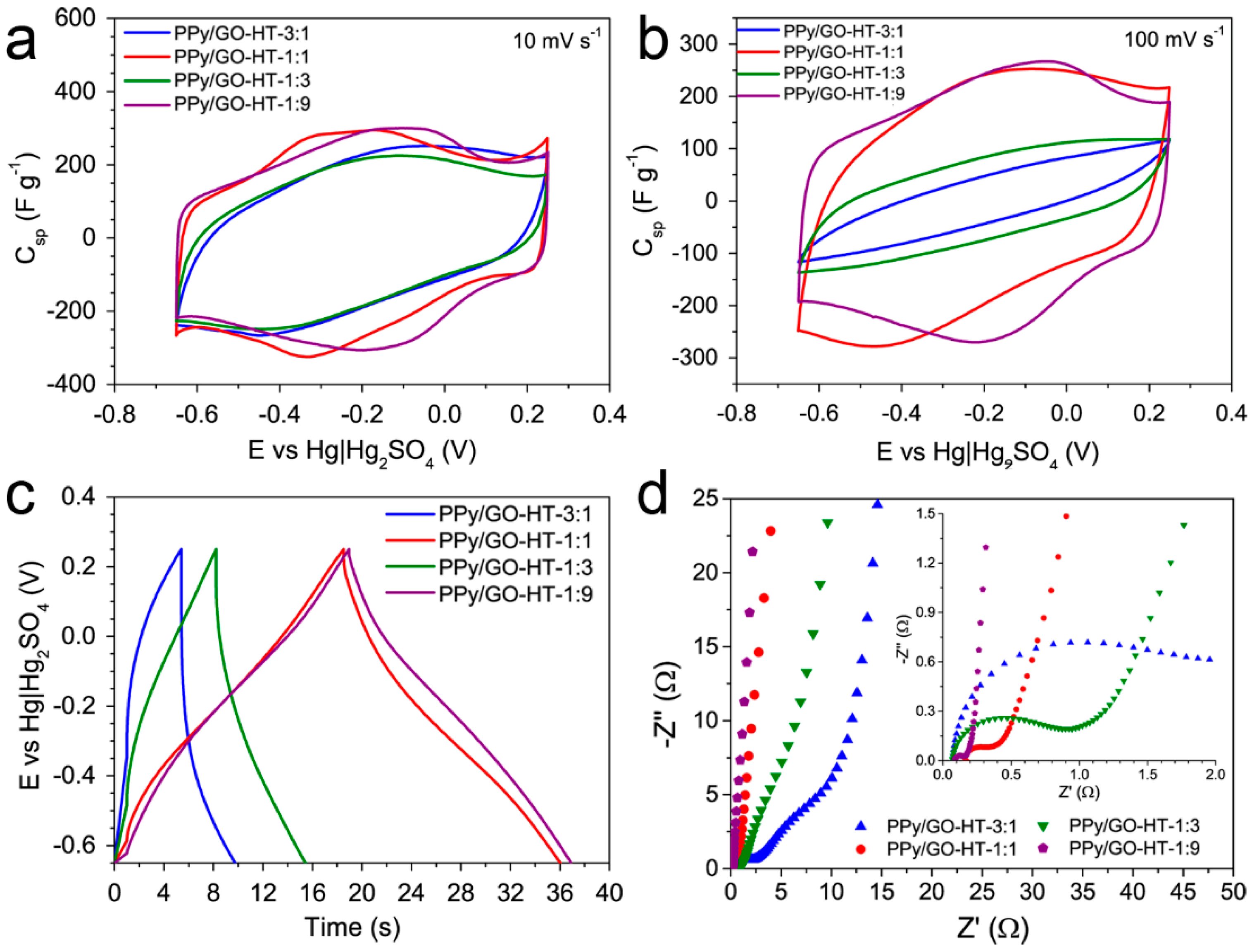
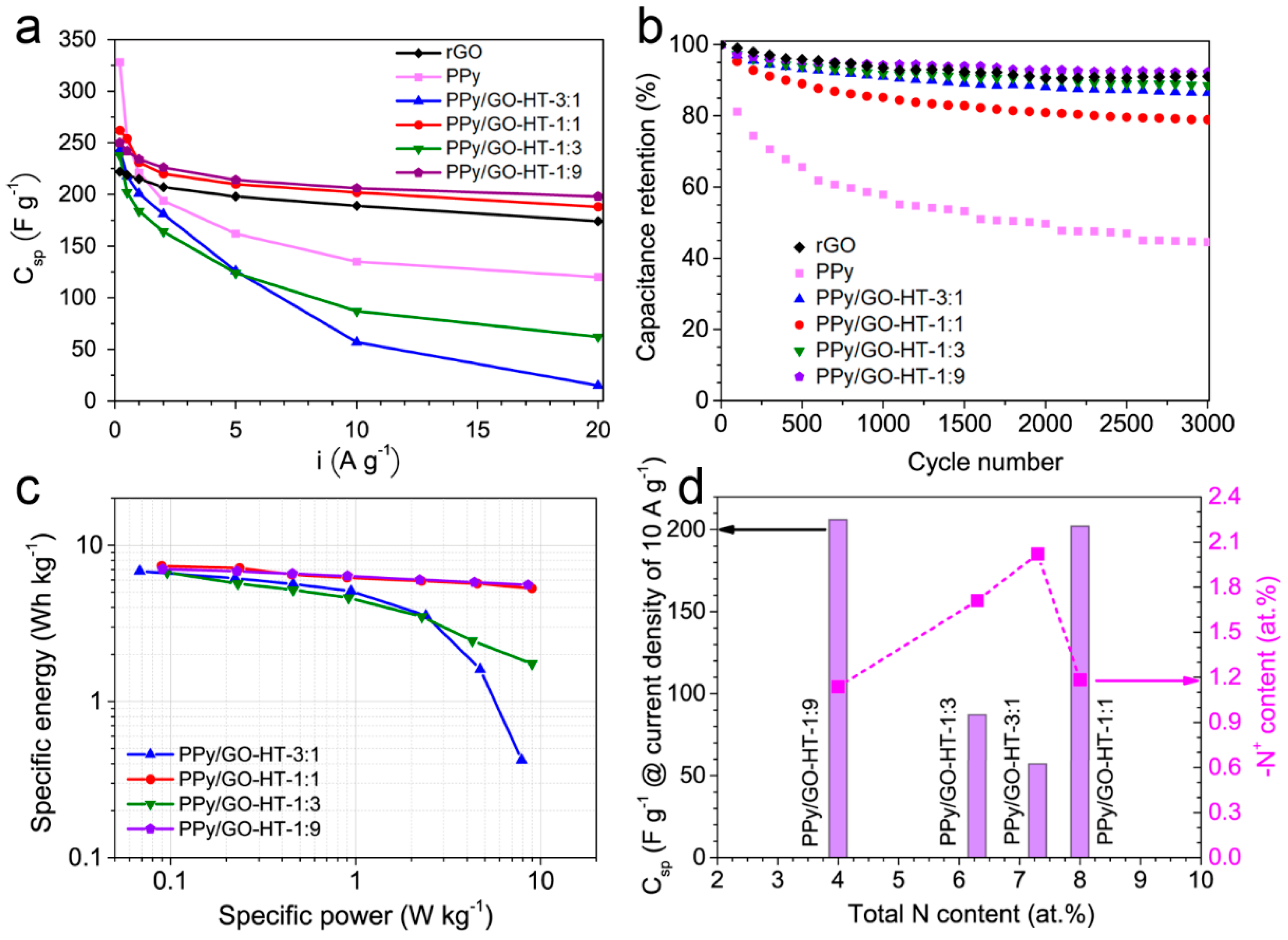
3.3. Electrochemical Performance of the PPy/GO-HT Hybrids
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eftekhari, A. The mechanism of ultrafast supercapacitors. J. Mater. Chem. A 2018, 6, 2866–2876. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, Y.; Cheng, L.; Zheng, L.; Li, Y.; Yuan, W.; Yuan, X. Graphene-based architectures for electrochemical capacitors. Energy Storage Mater. 2016, 5, 8–32. [Google Scholar] [CrossRef]
- Yu, G.; Xie, X.; Pan, L.; Bao, Z.; Cui, Y. Hybrid nanostructured materials for high-performance electrochemical capacitors. Nano Energy 2013, 2, 213–234. [Google Scholar] [CrossRef]
- Moyseowicz, A.; Śliwak, A.; Gryglewicz, G. Influence of structural and textural parameters of carbon nanofibers on their capacitive behavior. J. Mater. Sci. 2015, 51, 3431–3439. [Google Scholar] [CrossRef]
- Candelaria, S.L.; Shao, Y.; Zhou, W.; Li, X.; Xiao, J.; Zhang, J.-G.; Wang, Y.; Liu, J.; Li, J.; Cao, G. Nanostructured carbon for energy storage and conversion. Nano Energy 2012, 1, 195–220. [Google Scholar] [CrossRef]
- Śliwak, A.; Grzyb, B.; Díez, N.; Gryglewicz, G. Nitrogen-doped reduced graphene oxide as electrode material for high rate supercapacitors. Appl. Surf. Sci. 2017, 399, 265–271. [Google Scholar] [CrossRef]
- Augustyn, V.; Simon, P.; Dunn, B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 2014, 7, 1597–1614. [Google Scholar] [CrossRef]
- Moyseowicz, A. Scalable one-pot synthesis of bismuth sulfide nanorods as an electrode active material for energy storage applications. J. Solid State Electrochem. 2019, 23, 1191–1199. [Google Scholar] [CrossRef]
- Śliwak, A.; Moyseowicz, A.; Gryglewicz, G. Hydrothermal-assisted synthesis of an iron nitride-carbon composite as a novel electrode material for supercapacitors. J. Mater. Chem. A 2017, 5, 5680–5684. [Google Scholar] [CrossRef]
- Abdelhamid, M.E.; O’Mullane, A.P.; Snook, G.A. Storing energy in plastics: A review on conducting polymers & their role in electrochemical energy storage. RSC Adv. 2015, 5, 11611–11626. [Google Scholar]
- Zhan, C.; Yu, G.; Lu, Y.; Wang, L.; Wujcik, E.; Wei, S. Conductive polymer nanocomposites: A critical review of modern advanced devices. J. Mater. Chem. C 2017, 5, 1569–1585. [Google Scholar] [CrossRef]
- Eftekhari, A.; Mohamedi, M. Tailoring pseudocapacitive materials from a mechanistic perspective. Mater. Today Energy 2017, 6, 211–229. [Google Scholar] [CrossRef]
- Janáky, C.; Visy, C. Conducting polymer-based hybrid assemblies for electrochemical sensing: A materials science perspective. Anal. Bioanal. Chem. 2013, 405, 3489–3511. [Google Scholar] [CrossRef] [PubMed]
- Dubal, D.P.; Ayyad, O.; Ruiz, V.; Gómez-Romero, P. Hybrid energy storage: The merging of battery and supercapacitor chemistries. Chem. Soc. Rev. 2015, 44, 1777–1790. [Google Scholar] [CrossRef] [PubMed]
- Moyseowicz, A.; Śliwak, A.; Miniach, E.; Gryglewicz, G. Polypyrrole/iron oxide/reduced graphene oxide ternary composite as a binderless electrode material with high cyclic stability for supercapacitors. Compos. Part B Eng. 2017, 109, 23–29. [Google Scholar] [CrossRef]
- Moyseowicz, A.; González, Z.; Menéndez, R.; Gryglewicz, G. Three-dimensional poly(aniline-co-pyrrole)/thermally reduced graphene oxide composite as a binder-free electrode for high-performance supercapacitors. Compos. Part B Eng. 2018, 145, 232–239. [Google Scholar] [CrossRef]
- Yin, J.; Chang, R.; Shui, Y.; Zhao, X. Preparation and enhanced electro-responsive characteristic of reduced graphene oxide/polypyrrole composite sheet suspensions. Soft Matter 2013, 9, 7468–7478. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, L.; Hu, N.; Yang, Z.; Wei, H.; Zhang, Y. Reduced graphene oxide/polypyrrole nanotube papers for flexible all-solid-state supercapacitors with excellent rate capability and high energy density. J. Power Sources 2016, 302, 39–45. [Google Scholar] [CrossRef]
- Barakzehi, M.; Montazer, M.; Sharif, F.; Norby, T.; Chatzitakis, A. A textile-based wearable supercapacitor using reduced graphene oxide/polypyrrole composite. Electrochim. Acta 2019, 305, 187–196. [Google Scholar] [CrossRef]
- Fan, L.-Q.; Liu, G.-J.; Wu, J.-H.; Liu, L.; Lin, J.-M.; Wei, Y.-L. Asymmetric supercapacitor based on graphene oxide/polypyrrole composite and activated carbon electrodes. Electrochim. Acta 2014, 137, 26–33. [Google Scholar] [CrossRef]
- Zhu, J.; Xu, Y.; Wang, J.; Wang, J.; Bai, Y.; Du, X. Morphology controllable nano-sheet polypyrrole–graphene composites for high-rate supercapacitor. Phys. Chem. Chem. Phys. 2015, 17, 19885–19894. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Yang, J.J.; Li, S.S.; Wang, Z.; Xiao, T.Y.; Qian, Y.H.; Yu, S.H. Hydrothermal synthesis of macroscopic nitrogen-doped graphene hydrogels for ultrafast supercapacitor. Nano Energy 2013, 2, 249–256. [Google Scholar] [CrossRef]
- Moyseowicz, A.; Gryglewicz, G. Hydrothermal-assisted synthesis of a porous polyaniline/reduced graphene oxide composite as a high-performance electrode material for supercapacitors. Compos. Part B Eng. 2019, 159, 4–12. [Google Scholar] [CrossRef]
- Wang, R.; Han, M.; Zhao, Q.; Ren, Z.; Guo, X.; Xu, C.; Hu, N.; Lu, L. Hydrothermal synthesis of nanostructured graphene/polyaniline composites as high-capacitance electrode materials for supercapacitors. Sci. Rep. 2017, 7, 44562. [Google Scholar] [CrossRef] [PubMed]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Díez, N.; Śliwak, A.; Gryglewicz, S.; Grzyb, B.; Gryglewicz, G. Enhanced reduction of graphene oxide by high-pressure hydrothermal treatment. RSC Adv. 2015, 5, 81831–81837. [Google Scholar] [CrossRef]
- Alothman, Z.A. A Review: Fundamental aspects of silicate mesoporous materials. Materials 2012, 5, 2874–2902. [Google Scholar] [CrossRef]
- Li, J.; Östling, M. Prevention of graphene restacking for performance boost of supercapacitors—A review. Crystals 2013, 3, 163–190. [Google Scholar] [CrossRef]
- Shen, J.; Hu, Y.; Li, C.; Qin, C.; Ye, M. Synthesis of amphiphilic graphene nanoplatelets. Small 2009, 5, 82–85. [Google Scholar] [CrossRef]
- Moussa, M.; El-Kady, M.F.; Abdel-Azeim, S.; Kaner, R.B.; Majewski, P.; Ma, J. Compact, flexible conducting polymer/graphene nanocomposites for supercapacitors of high volumetric energy density. Compos. Sci. Technol. 2018, 160, 50–59. [Google Scholar] [CrossRef]
- Devi, M.; Kumar, A. In-situ reduced graphene oxide nanosheets–polypyrrole nanotubes nanocomposites for supercapacitor applications. Synth. Met. 2016, 222, 318–329. [Google Scholar] [CrossRef]
- Desimoni, E.; Brunetti, B. X-Ray photoelectron spectroscopic characterization of chemically modified electrodes used as chemical sensors and biosensors: A review. Chemosensors 2015, 3, 70–117. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, Q.; Wang, J.; Huang, X.; Bai, H. A self-assembly route to porous polyaniline/reduced graphene oxide composite materials with molecular-level uniformity for high-performance supercapacitors. Energy Environ. Sci. 2018, 11, 1280–1286. [Google Scholar] [CrossRef]
- Rajagopalan, R.; Iroh, J.O. Characterization of polyaniline–polypyrrole composite coatings on low carbon steel: A XPS and infrared spectroscopy study. Appl. Surf. Sci. 2003, 218, 58–69. [Google Scholar] [CrossRef]
- Zhang, R.; Li, L.; Chen, L.; Zhang, G.; Shi, K. N-doped carbon nanotubes synthesized in high yield and decorated with CeO2 and SnO2 nanoparticles. J. Alloys Compd. 2011, 509, 8620–8624. [Google Scholar] [CrossRef]
- Xu, L.Q.; Liu, Y.L.; Neoh, K.-G.; Kang, E.-T.; Fu, G.D. Reduction of graphene oxide by aniline with its concomitant oxidative polymerization. Macromol. Rapid Commun. 2011, 32, 684–688. [Google Scholar] [CrossRef]
- Śliwak, A.; Grzyb, B.; Ćwikła, J.; Gryglewicz, G. Influence of wet oxidation of herringbone carbon nanofibers on the pseudocapacitance effect. Carbon N. Y. 2013, 64, 324–333. [Google Scholar] [CrossRef]
- Iurchenkova, A.A.; Fedorovskaya, E.O.; Asanov, I.P.; Arkhipov, V.E.; Popov, K.M.; Baskakova, K.I.; Okotrub, A.V. MWCNT buckypaper/polypyrrole nanocomposites for supercapasitor application. Electrochim. Acta 2020, 335, 135700. [Google Scholar] [CrossRef]
- Pruna, A.I.; Rosas-Laverde, N.M.; Busquets Mataix, D. Effect of deposition parameters on electrochemical properties of polypyrrole-graphene oxide films. Materials 2020, 13, 624. [Google Scholar] [CrossRef]
- Huang, Y.; Tao, J.; Meng, W.; Zhu, M.; Huang, Y.; Fu, Y.; Gao, Y.; Zhi, C. Super-high rate stretchable polypyrrole-based supercapacitors with excellent cycling stability. Nano Energy 2015, 11, 518–525. [Google Scholar] [CrossRef]
- Liu, T.; Ding, J.; Su, Z.; Wei, G. Porous two-dimensional materials for energy applications: Innovations and challenges. Mater. Today Energy 2017, 6, 79–95. [Google Scholar] [CrossRef]
- Malik, R.; Lata, S.; Malik, R.S. Study of supercapacitive pursuance of polypyrrole/sulphonated poly (ether ether ketone)/multi walled carbon nanotubes composites for energy storage. J. Energy Storage 2020, 27, 101162. [Google Scholar] [CrossRef]
- Qu, Y.; Lu, C.; Su, Y.; Cui, D.; He, Y.; Zhang, C.; Cai, M.; Zhang, F.; Feng, X.; Zhuang, X. Hierarchical-graphene-coupled polyaniline aerogels for electrochemical energy storage. Carbon 2018, 127, 77–84. [Google Scholar] [CrossRef]
- Yang, J.; Weng, W.; Liang, Y.; Zhang, Y.; Yang, L.; Luo, X.; Liu, Q.; Zhu, M. Heterogeneous graphene/polypyrrole multilayered microtube with enhanced capacitance. Electrochim. Acta 2019, 304, 378–385. [Google Scholar] [CrossRef]
- Azman, N.H.N.; Mamat Mat Nazir, M.S.; Ngee, L.H.; Sulaiman, Y. Graphene-based ternary composites for supercapacitors. Int. J. Energy Res. 2018, 42, 2104–2116. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, X.; Chen, Y.; Yu, P.; Wang, C.; Ma, Y. Enhanced capacitance and rate capability of graphene/polypyrrole composite as electrode material for supercapacitors. J. Power Sources 2011, 196, 5990–5996. [Google Scholar] [CrossRef]
- Lota, K.; Lota, G.; Sierczynska, A.; Acznik, I. Carbon/polypyrrole composites for electrochemical capacitors. Synth. Met. 2015, 203, 44–48. [Google Scholar] [CrossRef]
- Stoller, M.D.; Ruoff, R.S. Best practice methods for determining an electrode material’s performance for ultracapacitors. Energy Environ. Sci. 2010, 3, 1294–1301. [Google Scholar] [CrossRef]
- Pope, M.A.; Korkut, S.; Punckt, C.; Aksay, I.A. Supercapacitor electrodes produced through evaporative consolidation of graphene oxide-water-ionic liquid gels. J. Electrochem. Soc. 2013, 160, A1653–A1660. [Google Scholar] [CrossRef]
- Yassine, M.; Fabris, D. Performance of commercially available supercapacitors. Energies 2017, 10, 1340. [Google Scholar] [CrossRef]
- Venkatesh, S.; Vishista, K. Identification of the best chemical equivalent ratio to produce emeraldine salt exhibiting better pseudo capacitance. Electrochim. Acta 2018, 263, 76–84. [Google Scholar] [CrossRef]
- Tabačiarová, J.; Mičušík, M.; Fedorko, P.; Omastová, M. Study of polypyrrole aging by XPS, FTIR and conductivity measurements. Polym. Degrad. Stab. 2015, 120, 392–401. [Google Scholar] [CrossRef]


| Material | SBET | Vtotal | Vmes | Vmes/Vtotal |
|---|---|---|---|---|
| (m2 g−1) | (cm3 g−1) | (cm3 g−1) | ||
| rGO | 51 | 0.031 | 0.010 | 0.32 |
| PPy | 20 | 0.024 | 0.022 | 0.76 |
| PPy/GO-HT-3:1 | 82 | 0.095 | 0.064 | 0.67 |
| PPy/GO-HT-1:1 | 199 | 0.168 | 0.091 | 0.54 |
| PPy/GO-HT-1:3 | 122 | 0.105 | 0.060 | 0.57 |
| PPy/GO-HT-1:9 | 25 | 0.025 | 0.016 | 0.64 |
| Material | C | N | O | –N= | –NH– | Doping Level N+/N |
|---|---|---|---|---|---|---|
| at.% | % | |||||
| rGO | 82.9 | – | 17.1 | – | – | – |
| PPy | 75 | 12.2 | 12.8 | 3 | 70 | 27 |
| PPy/GO-HT-3:1 | 81.5 | 7.3 | 11.2 | 9 | 55 | 33 |
| PPy/GO-HT-1:1 | 78.2 | 8 | 13.8 | 8 | 63 | 22 |
| PPy/GO-HT-1:3 | 80.3 | 6.3 | 13.4 | 8 | 55 | 31 |
| PPy/GO-HT-1:9 | 82 | 4.1 | 13.9 | 12 | 57 | 28 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moyseowicz, A.; Pająk, K.; Gajewska, K.; Gryglewicz, G. Synthesis of Polypyrrole/Reduced Graphene Oxide Hybrids via Hydrothermal Treatment for Energy Storage Applications. Materials 2020, 13, 2273. https://doi.org/10.3390/ma13102273
Moyseowicz A, Pająk K, Gajewska K, Gryglewicz G. Synthesis of Polypyrrole/Reduced Graphene Oxide Hybrids via Hydrothermal Treatment for Energy Storage Applications. Materials. 2020; 13(10):2273. https://doi.org/10.3390/ma13102273
Chicago/Turabian StyleMoyseowicz, Adam, Krzysztof Pająk, Katarzyna Gajewska, and Grażyna Gryglewicz. 2020. "Synthesis of Polypyrrole/Reduced Graphene Oxide Hybrids via Hydrothermal Treatment for Energy Storage Applications" Materials 13, no. 10: 2273. https://doi.org/10.3390/ma13102273
APA StyleMoyseowicz, A., Pająk, K., Gajewska, K., & Gryglewicz, G. (2020). Synthesis of Polypyrrole/Reduced Graphene Oxide Hybrids via Hydrothermal Treatment for Energy Storage Applications. Materials, 13(10), 2273. https://doi.org/10.3390/ma13102273






