Using In Situ Polymerization to Increase Puncture Resistance and Induce Reversible Formability in Silk Membranes
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Surface Modification of Silk Fibroin Films
2.3. Atomic Force Microscopy
2.4. Water Contact Angle
2.5. Needle Penetration Tests
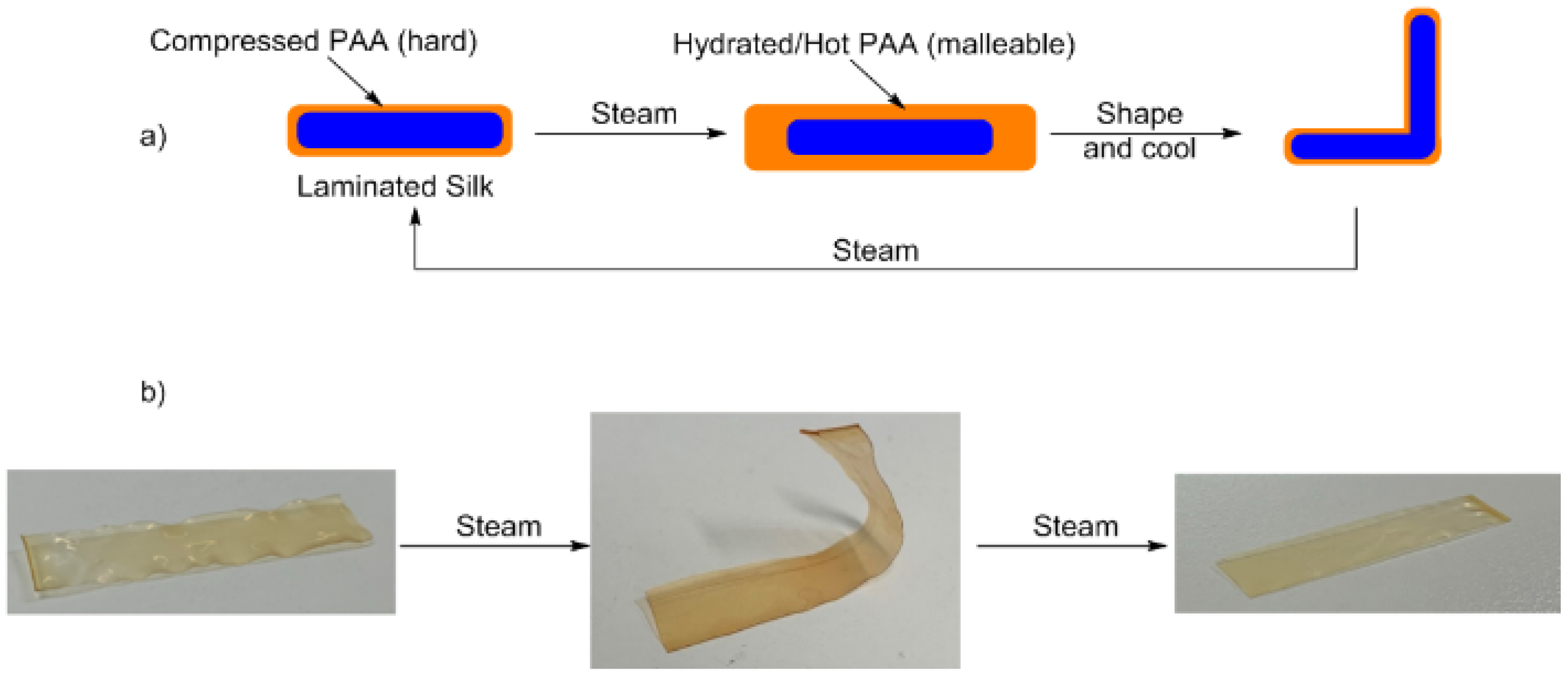
2.6. Membrane Malleability Procedure
2.7. Statistical Analysis
3. Results and Discussion
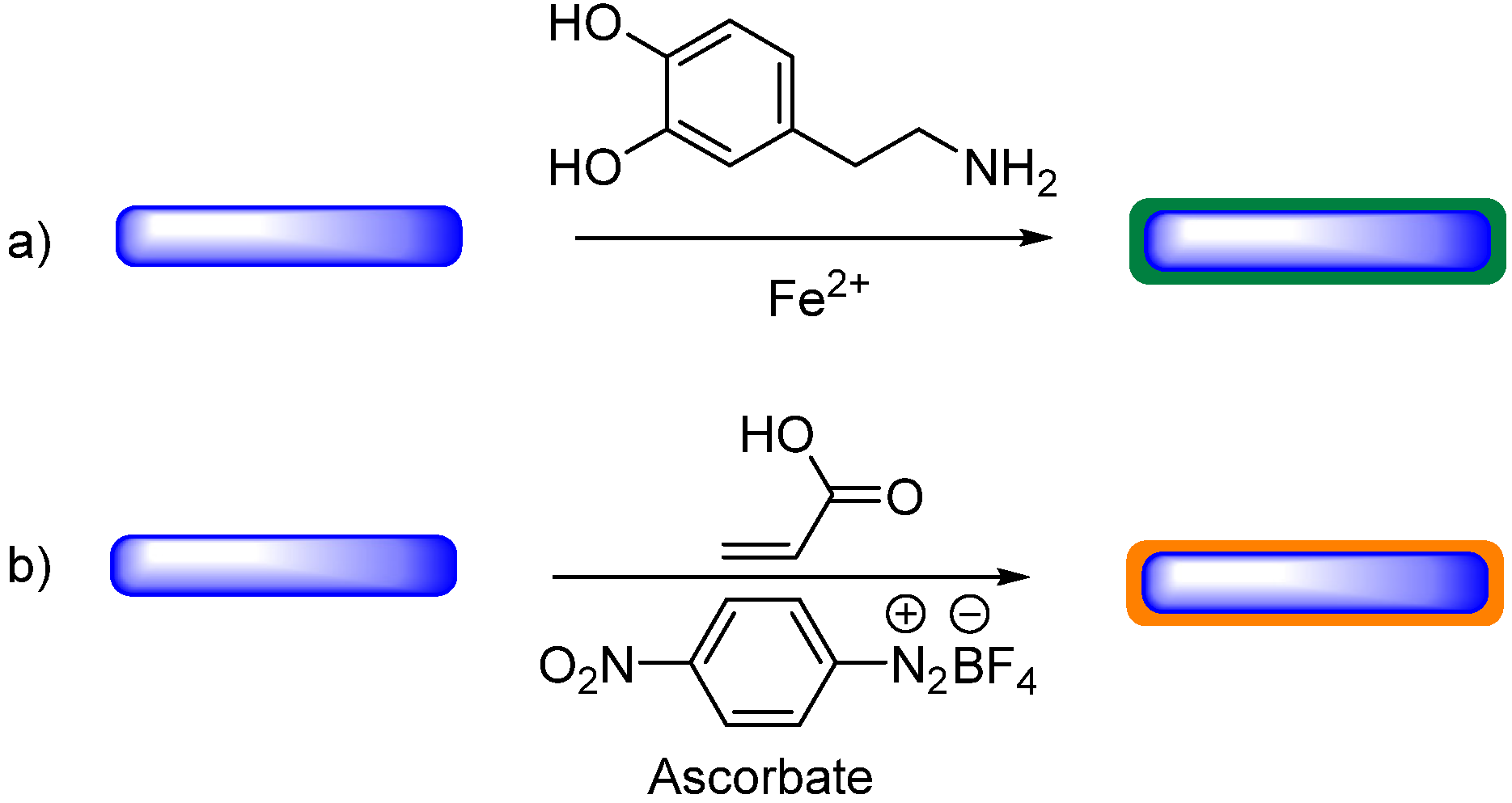
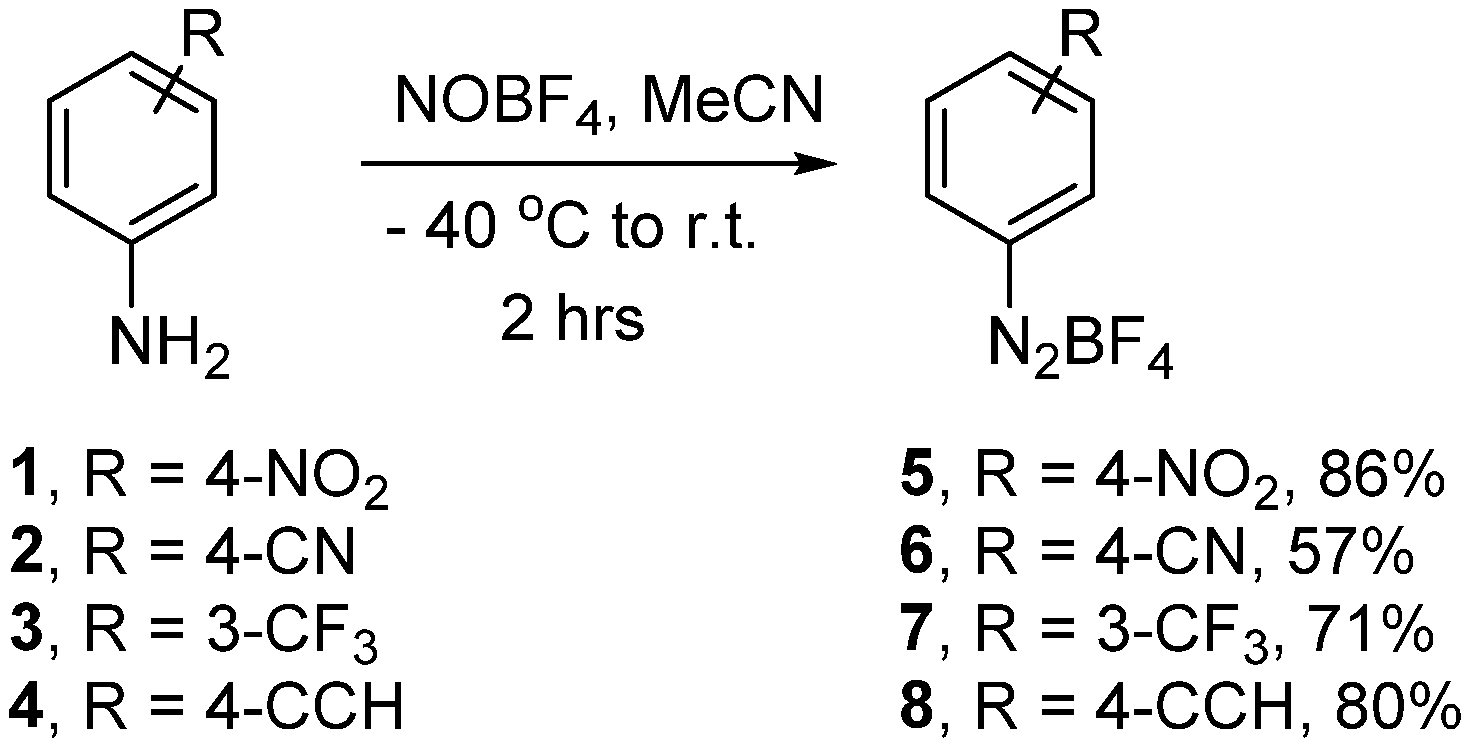
3.1. Optimisation Surface Modification Procedure
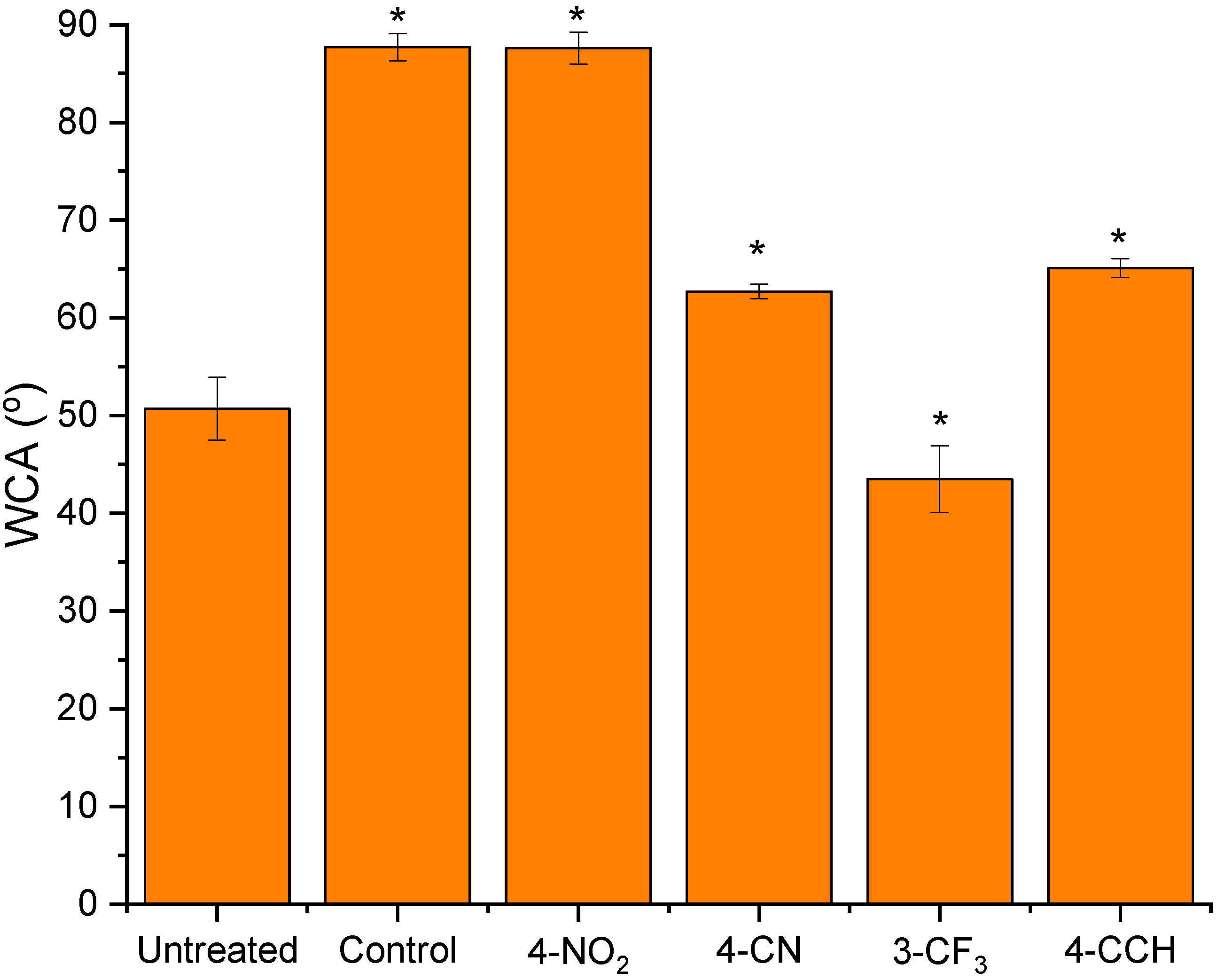
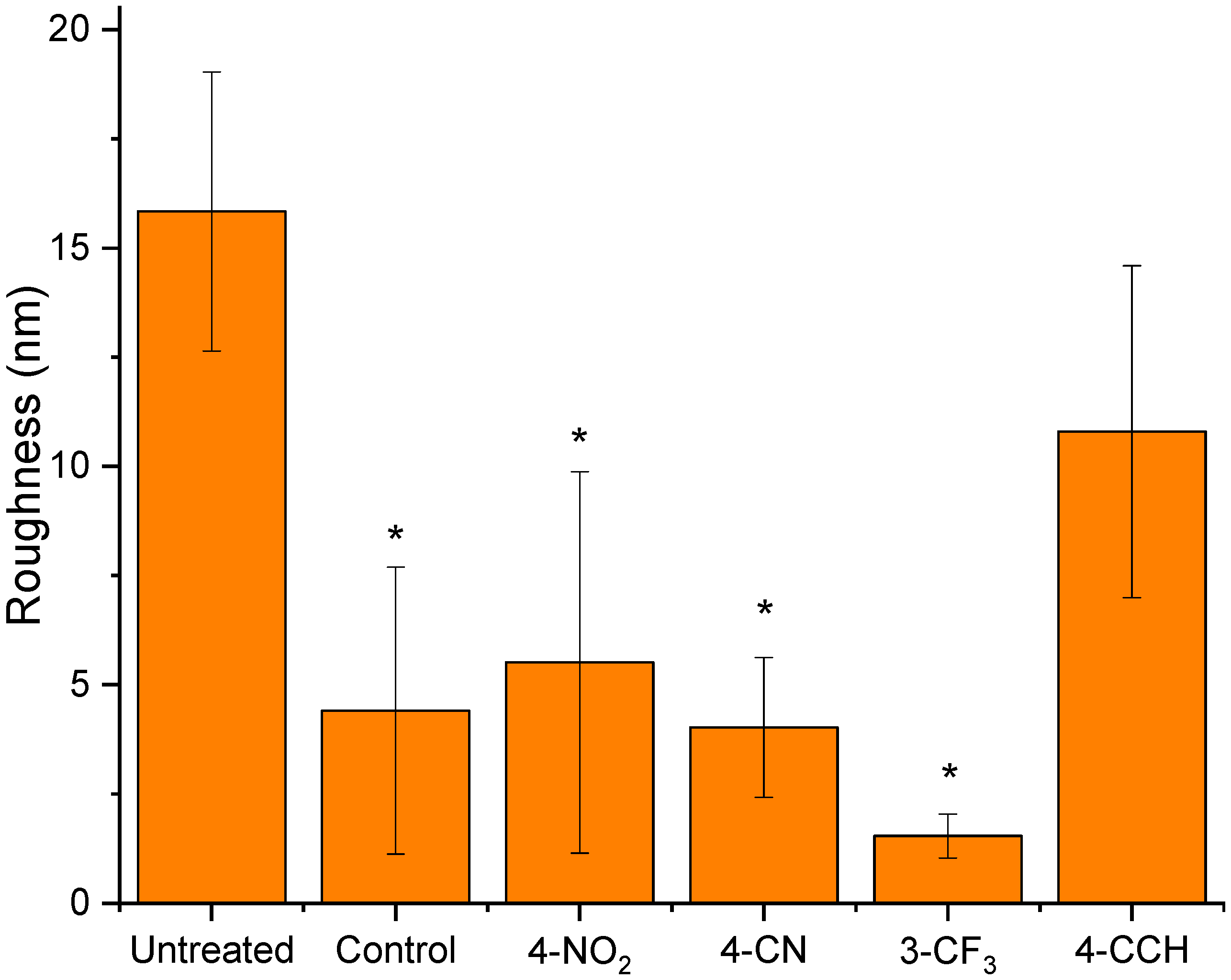
3.2. Physical Characterisation of the Treated Membranes
3.3. Physical Characterisation of Treated Silk Membranes
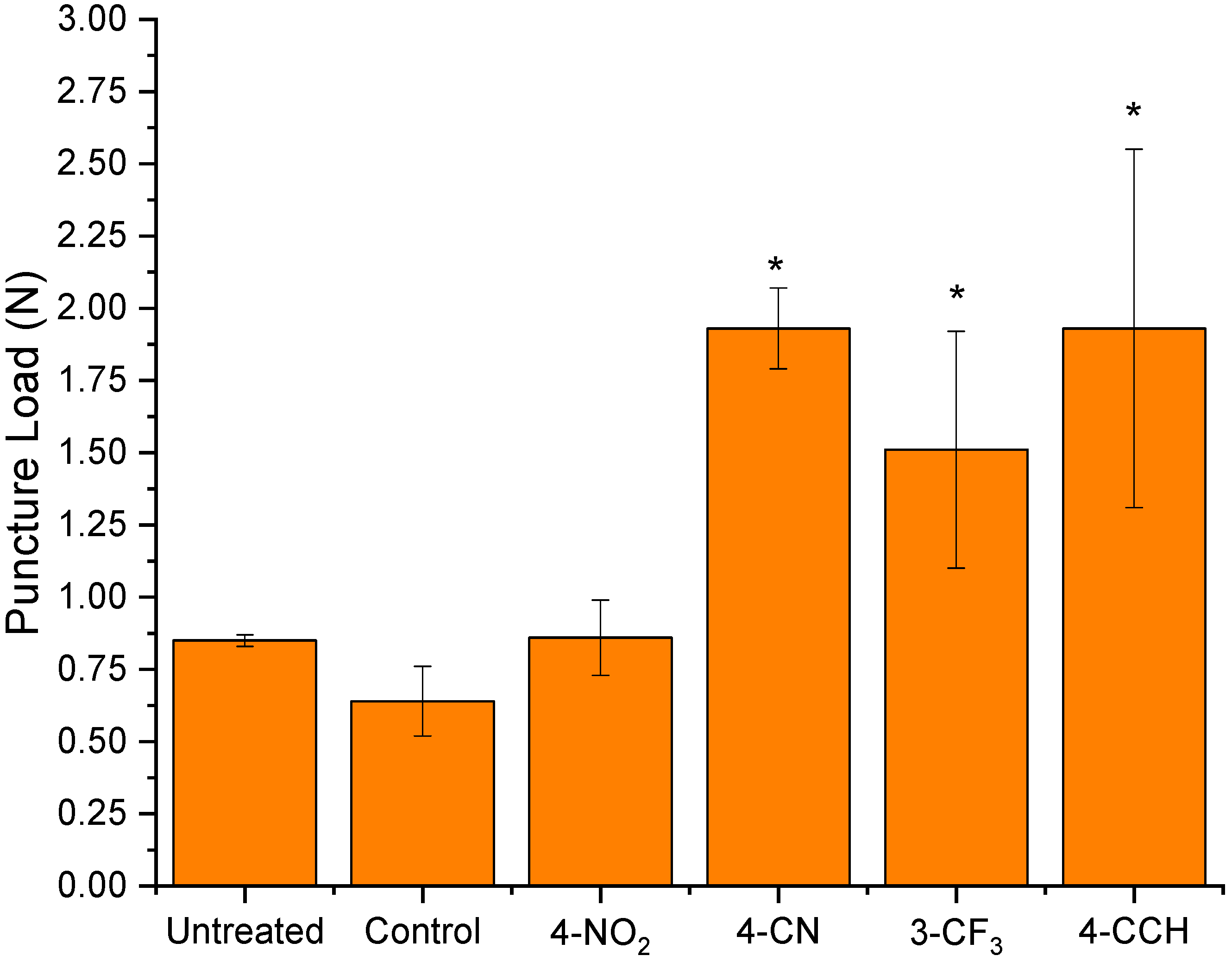
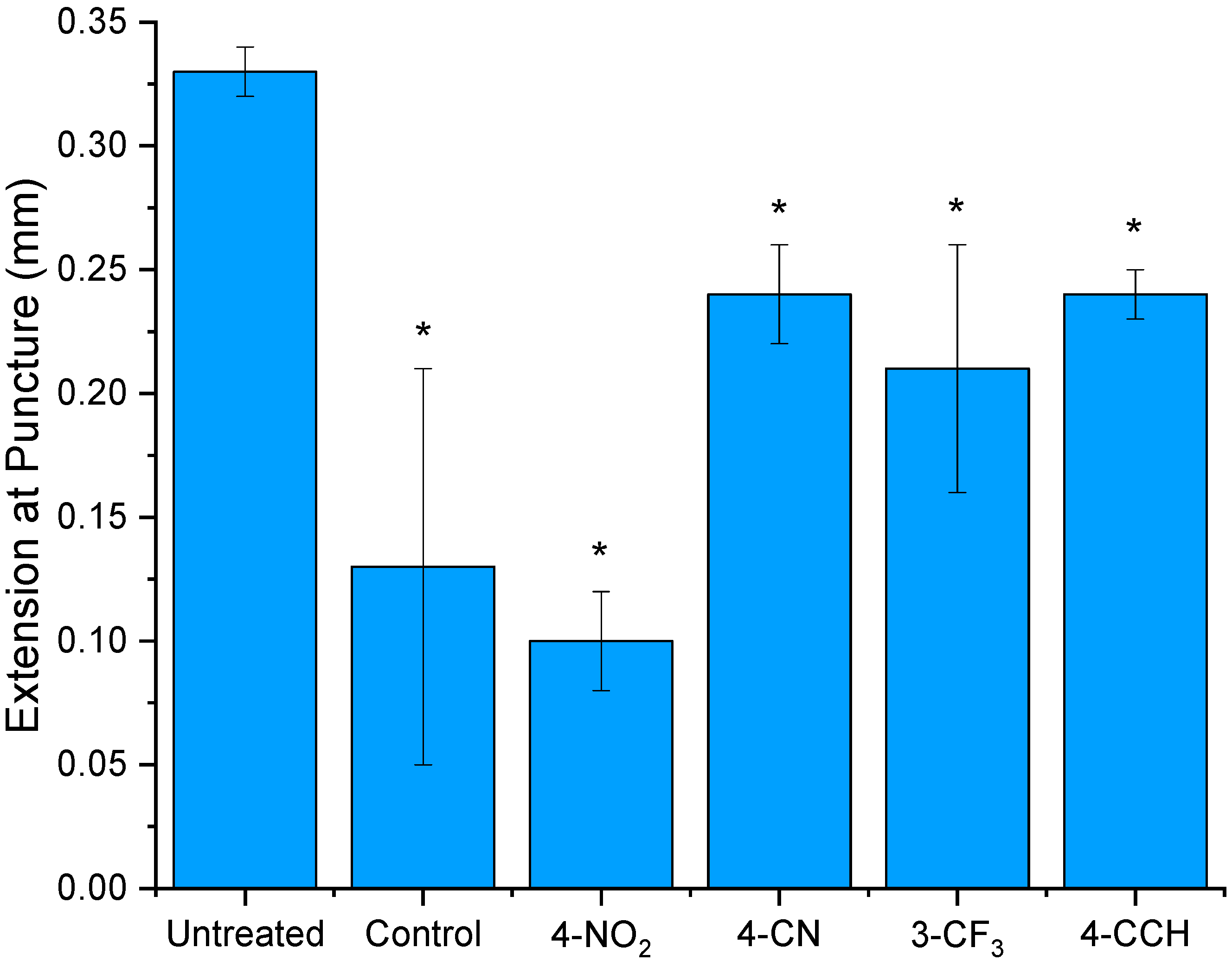
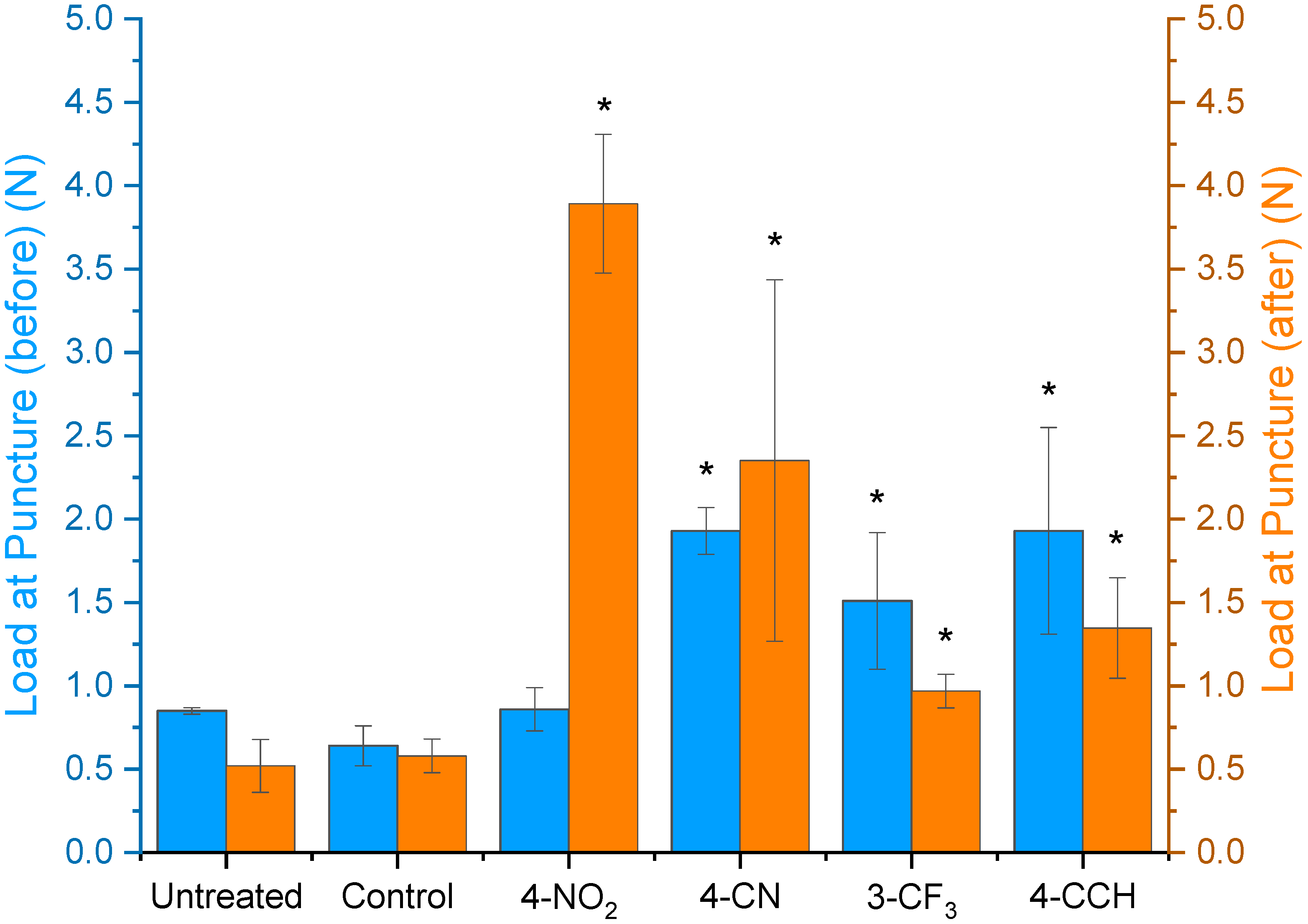
Needle Puncture Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cao, Y.; Liu, F.; Chen, Y.; Yu, T.; Lou, D.; Guo, Y.; Li, P.; Wang, Z.; Ran, H. Drug release from core-shell PVA/silk fibroin nanoparticles fabricated by one-step electrospraying. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Jin, H.-J.; Park, J.; Valluzzi, R.; Cebe, P.; Kaplan, D.L. Biomaterial Films of Bombyx Mori Silk Fibroin with Poly(ethylene oxide). Biomacromolecules 2004, 5, 711–717. [Google Scholar] [CrossRef]
- Hu, X.; Kaplan, D.L.; Cebe, P. Determining Beta-Sheet Crystallinity in Fibrous Proteins by Thermal Analysis and Infrared Spectroscopy. Macromolecules 2006, 39, 6161–6170. [Google Scholar] [CrossRef]
- Vepari, C.P.; Kaplan, D.L. Covalently immobilized enzyme gradients within three-dimensional porous scaffolds. Biotechnol. Bioeng. 2006, 93, 1130–1137. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kim, H.J.; Vunjak-Novakovic, G.; Kaplan, D.L. Stem cell-based tissue engineering with silk biomaterials. Biomaterials 2006, 27, 6064–6082. [Google Scholar] [CrossRef] [PubMed]
- Landry, M.J.; Applegate, M.B.; Bushuyev, O.S.; Omenetto, F.G.; Kaplan, D.L.; Cronin-Golomb, M.; Barrett, C.J. Photo-induced structural modification of silk gels containing azobenzene side groups. Soft Matter 2017, 13, 2903–2906. [Google Scholar] [CrossRef]
- Allardyce, B.J.; Rajkhowa, R.; Dilley, R.J.; Redmond, S.L.; Atlas, M.D.; Wang, X. Glycerol-plasticised silk membranes made using formic acid are ductile, transparent and degradation-resistant. Mater. Sci. Eng. C 2017, 80, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.G.; Scheibel, T.R. Composite materials based on silk proteins. Prog. Polym. Sci. 2010, 35, 1093–1115. [Google Scholar] [CrossRef]
- Wojcieszak, M.; Percot, A.; Colomban, P. Regenerated silk matrix composite materials reinforced by silk fibres: Relationship between processing and mechanical properties. J. Compos. Mater. 2018, 52, 2301–2311. [Google Scholar] [CrossRef]
- Shah, D.U.; Porter, D.; Vollrath, F. Can silk become an effective reinforcing fibre? A property comparison with flax and glass reinforced composites. Compos. Sci. Technol. 2014, 101, 173–183. [Google Scholar] [CrossRef]
- Saba, N.; Jawaid, M.; Alothman, O.Y.; Paridah, M.; Hassan, A. Recent advances in epoxy resin, natural fiber-reinforced epoxy composites and their applications. J. Reinf. Plas. Compos. 2016, 35, 447–470. [Google Scholar] [CrossRef]
- Pereira, R.F.P.; Silva, M.M.; de Zea Bermudez, V. Bombyx mori Silk Fibers: An Outstanding Family of Materials. Macromol. Mater. Eng. 2015, 300, 1171–1198. [Google Scholar] [CrossRef]
- Ravindra Rama, S.; Rai, S.K. Performance analysis of waste silk fabric-reinforced vinyl ester resin laminates. J. Compos. Mater. 2011, 45, 2475–2480. [Google Scholar] [CrossRef]
- Murphy, A.R.; John, P.S.; Kaplan, D.L. Modification of silk fibroin using diazonium coupling chemistry and the effects on hMSC proliferation and differentiation. Biomaterials 2008, 29, 2829–2838. [Google Scholar] [CrossRef] [PubMed]
- Romero, I.S.; Schurr, M.L.; Lally, J.V.; Kotlik, M.Z.; Murphy, A.R. Enhancing the Interface in Silk–Polypyrrole Composites through Chemical Modification of Silk Fibroin. ACS Appl. Mater. Interfaces 2013, 5, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.E.; Moreau, J.E.; Berman, A.M.; McSherry, H.J.; Coburn, J.M.; Schmidt, D.F.; Kaplan, D.L. Shape Memory Silk Protein Sponges for Minimally Invasive Tissue Regeneration. Adv. Healthc. Mater. 2017, 6, 1600762. [Google Scholar] [CrossRef]
- Raynal, L.; Allardyce, B.J.; Wang, X.; Dilley, R.J.; Rajkhowa, R.; Henderson, L.C. Facile and versatile solid state surface modification of silk fibroin membranes using click chemistry. J. Mater. Chem. B 2018, 6, 8037–8042. [Google Scholar] [CrossRef]
- Das, S.; Pati, D.; Tiwari, N.; Nisal, A.; Sen Gupta, S. Synthesis of silk fibroin-glycopolypeptide conjugates and their recognition with lectin. Biomacromolecules 2012, 13, 3695–3702. [Google Scholar] [CrossRef]
- Love, C.J.; Serban, B.A.; Katashima, T.; Numata, K.; Serban, M.A. Mechanistic Insights into Silk Fibroin’s Adhesive Properties via Chemical Functionalization of Serine Side Chains. ACS Biomater. Sci. Eng. 2019, 5, 5960–5967. [Google Scholar] [CrossRef]
- Beggs, K.M.; Servinis, L.; Gengenbach, T.R.; Huson, M.G.; Fox, B.L.; Henderson, L.C. A systematic study of carbon fibre surface grafting via in situ diazonium generation for improved interfacial shear strength in epoxy matrix composites. Compos. Sci. Technol. 2015, 118, 31–38. [Google Scholar] [CrossRef]
- Huang, L.; Li, C.; Yuan, W.; Shi, G. Strong composite films with layered structures prepared by casting silk fibroin–graphene oxide hydrogels. Nanoscale 2013, 5, 3780–3786. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.Z.; Wu, X.L.; Luo, M.Q. Silk Fibroin Sol-Gel Transitions in Different Solutions. Adv. Mater. Res. 2011, 175–176, 153–157. [Google Scholar] [CrossRef]
- Yang, S.H.; Kang, S.M.; Lee, K.B.; Chung, T.D.; Lee, H.; Choi, I.S. Mussel-inspired encapsulation and functionalization of individual yeast cells. J. Am. Chem. Soc. 2011, 133, 2795–2797. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.M.; You, I.; Cho, W.K.; Shon, H.K.; Lee, T.G.; Choi, I.S.; Karp, J.M.; Lee, H. One-Step Modification of Superhydrophobic Surfaces by a Mussel-Inspired Polymer Coating. Angew. Chem. Int. Ed. 2010, 49, 9401–9404. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Choi, I.S.; Nam, Y. A biofunctionalization scheme for neural interfaces using polydopamine polymer. Biomaterials 2011, 32, 6374–6380. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.; Ku, S.H.; Lee, H.; Park, C.B. Mussel-Inspired Polydopamine Coating as a Universal Route to Hydroxyapatite Crystallization. Adv. Funct. Mater. 2010, 20, 2132–2139. [Google Scholar] [CrossRef]
- Ryou, M.H.; Lee, Y.M.; Park, J.K.; Choi, J.W. Mussel-inspired polydopamine-treated polyethylene separators for high-power li-ion batteries. Adv. Mater. 2011, 23, 3066–3070. [Google Scholar] [CrossRef]
- Zhou, Q.; Wu, W.; Zhou, S.; Xing, T.; Sun, G.; Chen, G. Polydopamine-induced growth of mineralized γ-FeOOH nanorods for construction of silk fabric with excellent superhydrophobicity, flame retardancy and UV resistance. Chem. Eng. J. 2020, 382, 122988. [Google Scholar] [CrossRef]
- Deniau, G.; Azoulay, L.; Bougerolles, L.; Palacin, S. Surface Electroinitiated Emulsion Polymerization: Grafted Organic Coatings from Aqueous Solutions. Chem. Mater. 2006, 18, 5421–5428. [Google Scholar] [CrossRef]
- Eyckens, D.J.; Arnold, C.L.; Randall, J.D.; Stojcevski, F.; Hendlmeier, A.; Stanfield, M.K.; Pinson, J.; Gengenbach, T.R.; Alexander, R.; Soulsby, L.C.; et al. Fiber with Butterfly Wings: Creating Colored Carbon Fibers with Increased Strength, Adhesion, and Reversible Malleability. ACS Appl. Mater. Interfaces 2019, 11, 41617–41625. [Google Scholar] [CrossRef]
- Mévellec, V.; Roussel, S.; Tessier, L.; Chancolon, J.; Mayne-L’Hermite, M.; Deniau, G.; Viel, P.; Palacin, S. Grafting Polymers on Surfaces: A New Powerful and Versatile Diazonium Salt-Based One-Step Process in Aqueous Media. Chem. Mater. 2007, 19, 6323–6330. [Google Scholar] [CrossRef]
- Tessier, L.; Deniau, G.; Charleux, B.; Palacin, S. Surface Electroinitiated Emulsion Polymerization (SEEP): A Mechanistic Approach. Chem. Mater. 2009, 21, 4261–4274. [Google Scholar] [CrossRef]
- Jennings, J.; He, G.; Howdle, S.M.; Zetterlund, P.B. Block copolymer synthesis by controlled/living radical polymerisation in heterogeneous systems. Chem. Soc. Rev. 2016, 45, 5055–5084. [Google Scholar] [CrossRef]
- Arnold, C.L.; Eyckens, D.J.; Servinis, L.; Nave, M.D.; Yin, H.; Marceau, R.K.W.; Pinson, J.; Demir, B.; Walsh, T.R.; Henderson, L.C. Simultaneously increasing the hydrophobicity and interfacial adhesion of carbon fibres: A simple pathway to install passive functionality into composites. J. Mater. Chem. A 2019, 7, 13483–13494. [Google Scholar] [CrossRef]
- van der Walt, J.P.; Hopsu-Havu, V.K. A colour reaction for the differentiation of ascomycetous and hemibasidiomycetous yeasts. Antonie Van Leeuwenhoek 1976, 42, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Allardyce, B.J.; Rajkhowa, R.; Kalita, S.; Dilley, R.J.; Wang, X.; Liu, X. Silk particles, microfibres and nanofibres: A comparative study of their functions in 3D printing hydrogel scaffolds. Mater. Sci. Eng. C 2019, 103, 109784. [Google Scholar] [CrossRef] [PubMed]
- Polyacrylic Acid (130,000 Da). 2020. Available online: https://www.sigmaaldrich.com/australia.html (accessed on 10 April 2020).


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emonson, N.S.; Eyckens, D.J.; Allardyce, B.J.; Hendlmeier, A.; Stanfield, M.K.; Soulsby, L.C.; Stojcevski, F.; Henderson, L.C. Using In Situ Polymerization to Increase Puncture Resistance and Induce Reversible Formability in Silk Membranes. Materials 2020, 13, 2252. https://doi.org/10.3390/ma13102252
Emonson NS, Eyckens DJ, Allardyce BJ, Hendlmeier A, Stanfield MK, Soulsby LC, Stojcevski F, Henderson LC. Using In Situ Polymerization to Increase Puncture Resistance and Induce Reversible Formability in Silk Membranes. Materials. 2020; 13(10):2252. https://doi.org/10.3390/ma13102252
Chicago/Turabian StyleEmonson, Nicholas S., Daniel J. Eyckens, Benjamin J. Allardyce, Andreas Hendlmeier, Melissa K. Stanfield, Lachlan C. Soulsby, Filip Stojcevski, and Luke C. Henderson. 2020. "Using In Situ Polymerization to Increase Puncture Resistance and Induce Reversible Formability in Silk Membranes" Materials 13, no. 10: 2252. https://doi.org/10.3390/ma13102252
APA StyleEmonson, N. S., Eyckens, D. J., Allardyce, B. J., Hendlmeier, A., Stanfield, M. K., Soulsby, L. C., Stojcevski, F., & Henderson, L. C. (2020). Using In Situ Polymerization to Increase Puncture Resistance and Induce Reversible Formability in Silk Membranes. Materials, 13(10), 2252. https://doi.org/10.3390/ma13102252






