Water Decontamination with Magnetic Particles by Adsorption and Chemical Degradation. Influence of the Manufacturing Parameters
Abstract
1. Introduction
2. Materials and Methods
2.1. Magnetic Particle Manufacturing
2.2. Sorption Experiments
2.3. Fenton Experiments
3. Results
3.1. Magnetic Particle Manufacturing
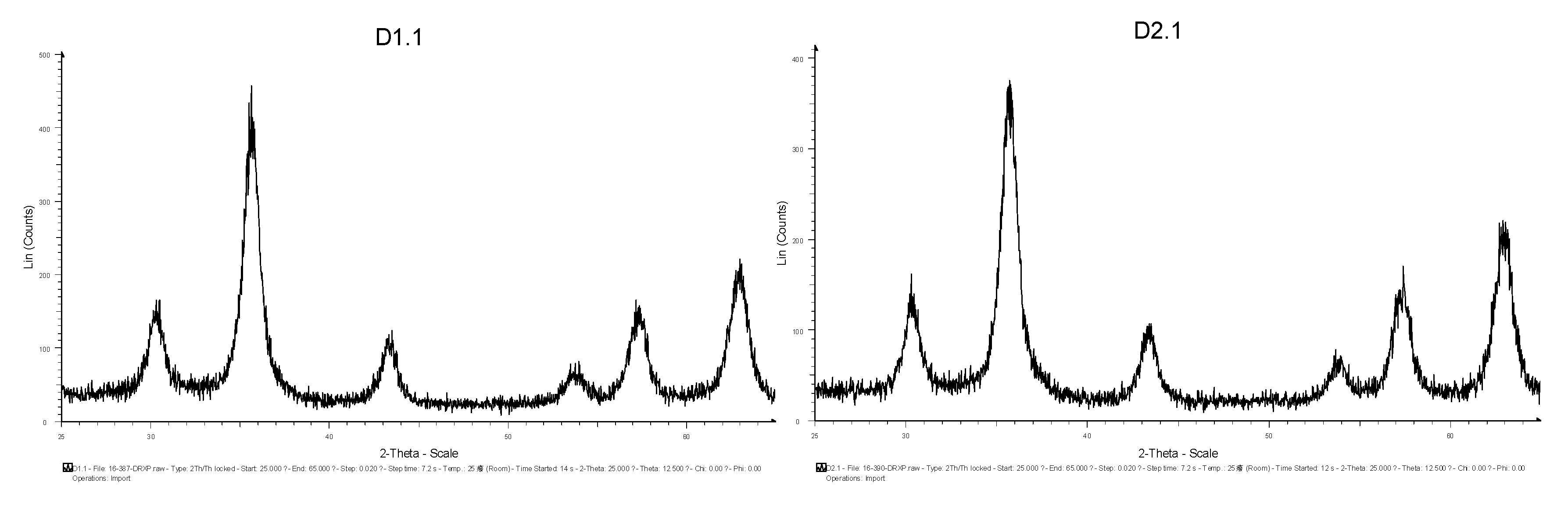
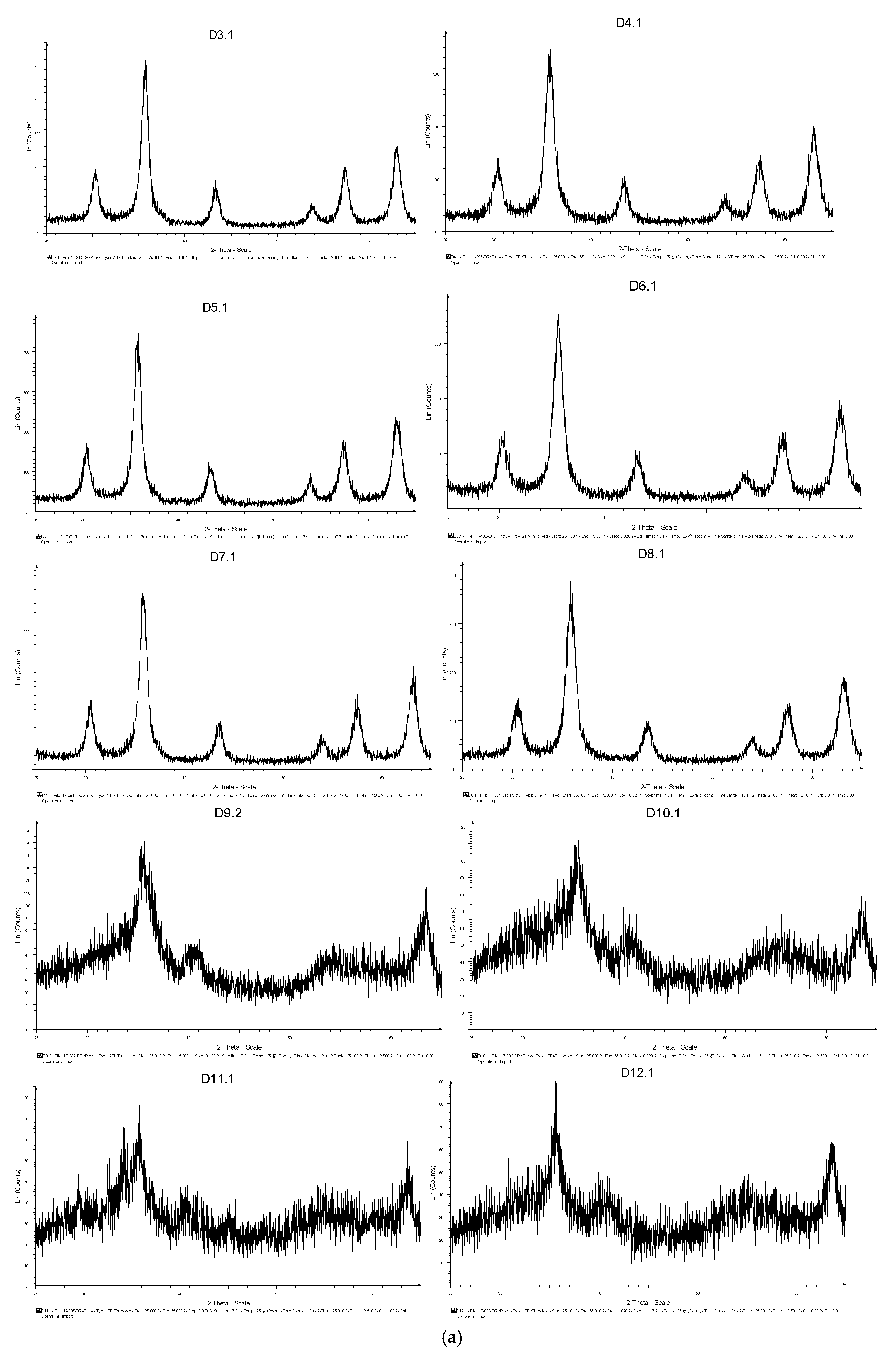
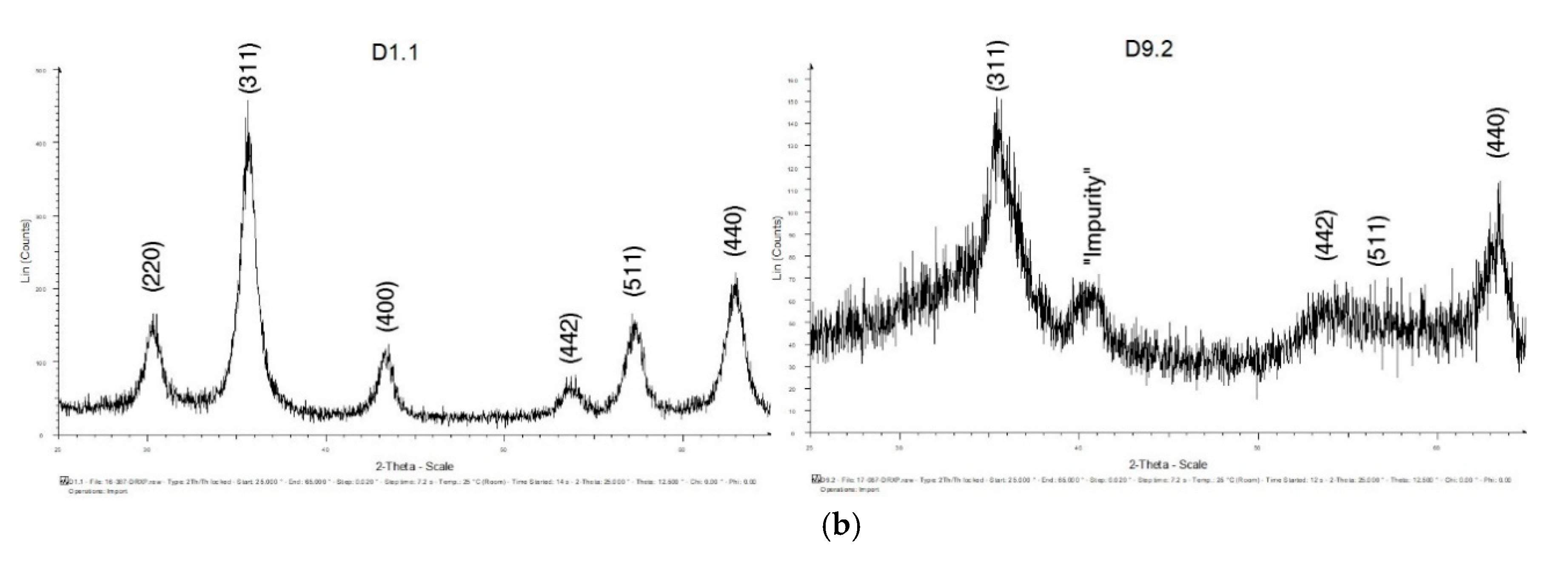
3.1.1. XRD Analysis
3.1.2. Magnetic Susceptibility Determination
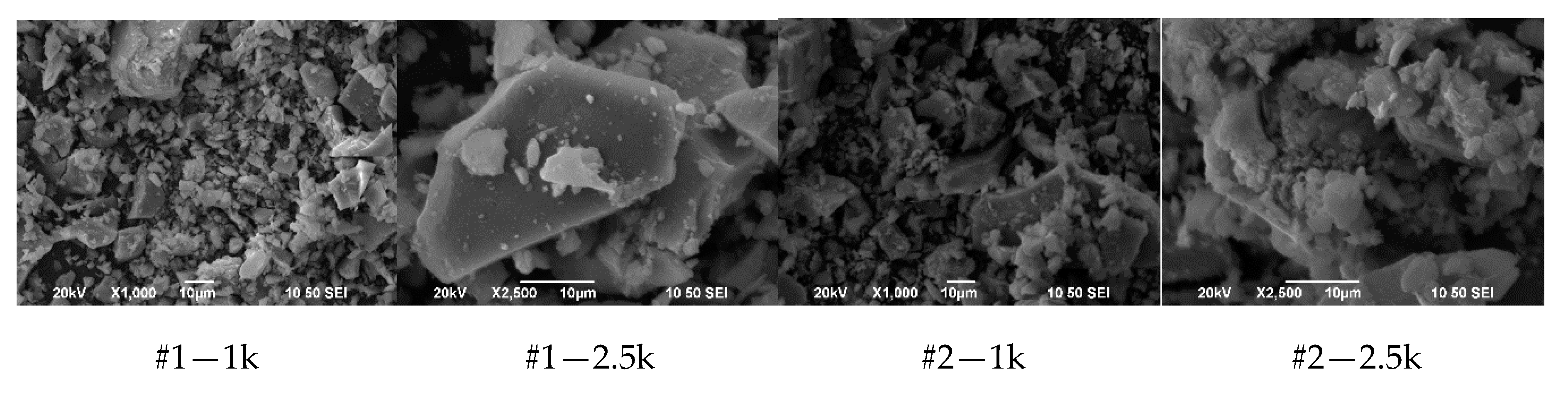
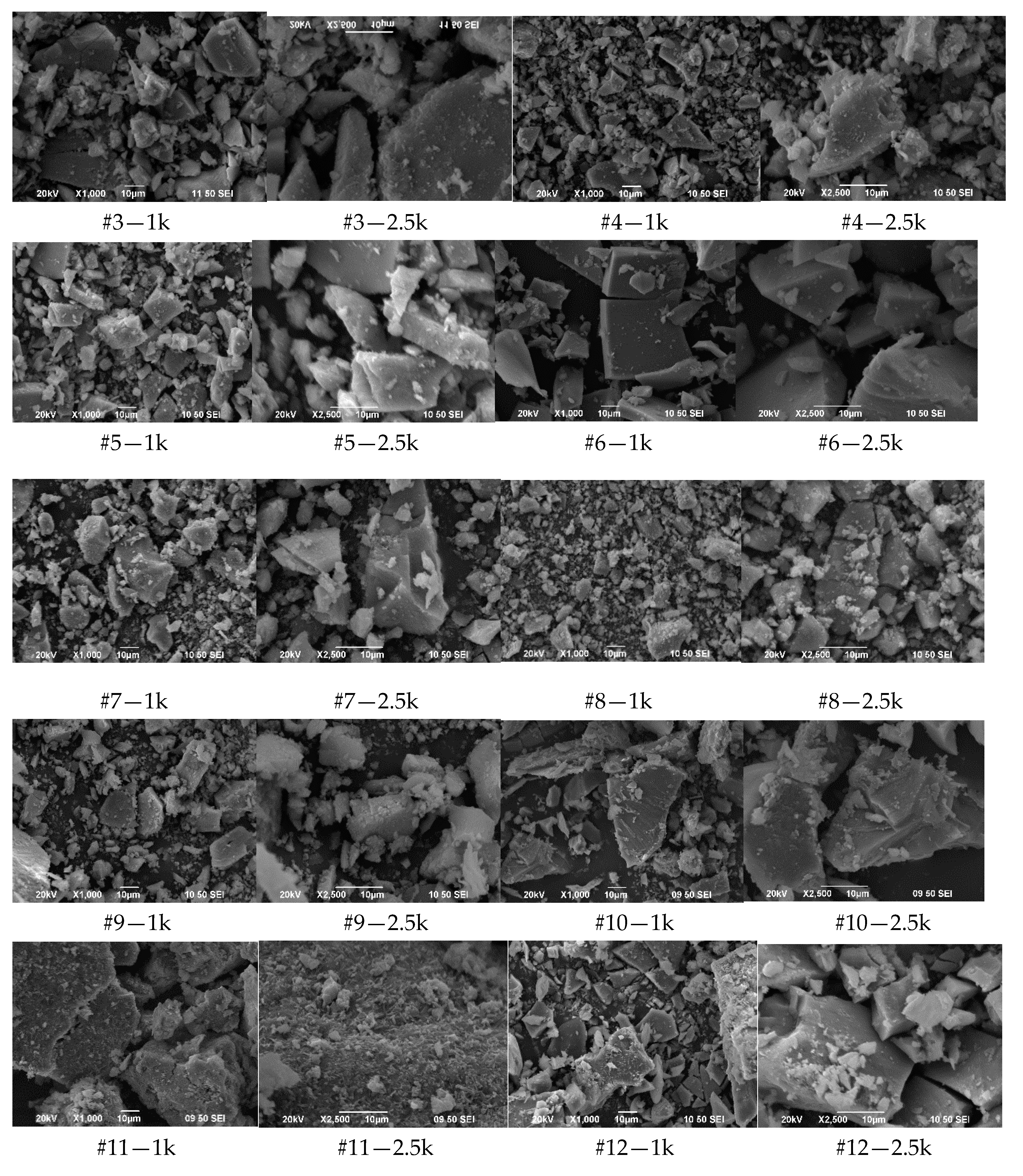
3.1.3. SEM Images
3.1.4. Discussion of Results
- Factor 1: Alkaline base
- Factor 2: Drying Temperature
- Factor 3: Initial concentration of Fe2SO4∙7H2O
- Factor 4: Surfactant
- (a)
- Without surfactant/Tween 80: experiments 1, 2 and 3;
- (b)
- Without surfactant/Citric acid: experiments 1, 4 and 6;
- (c)
- Tween 80/Citric acid: experiments 3 and 6;
- (d)
- Without surfactant/Tween 80 (drying at 90 °C): experiments 5 and 7
- Optimized values for production
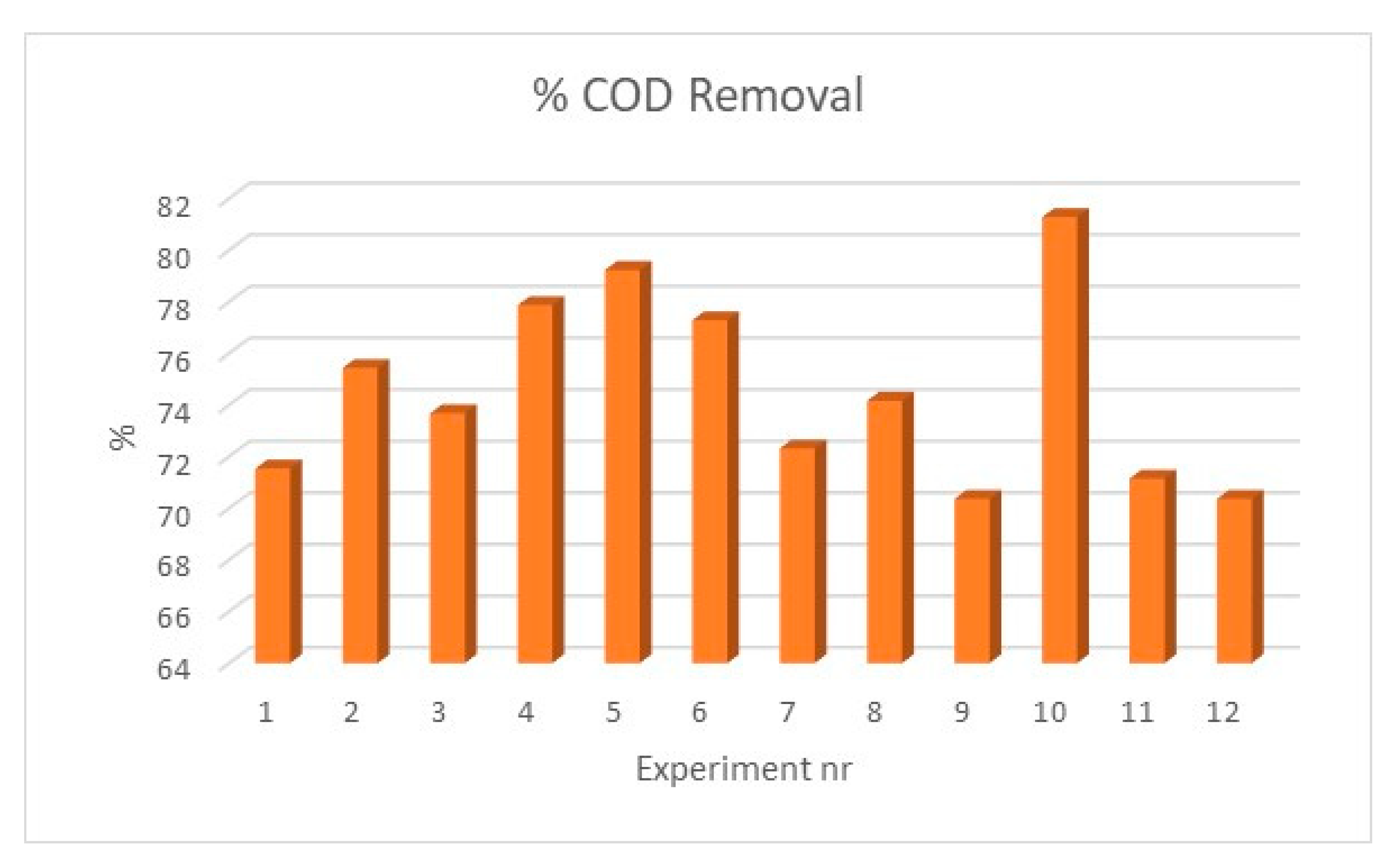
3.2. Sorption Experiments
Discussion of Results
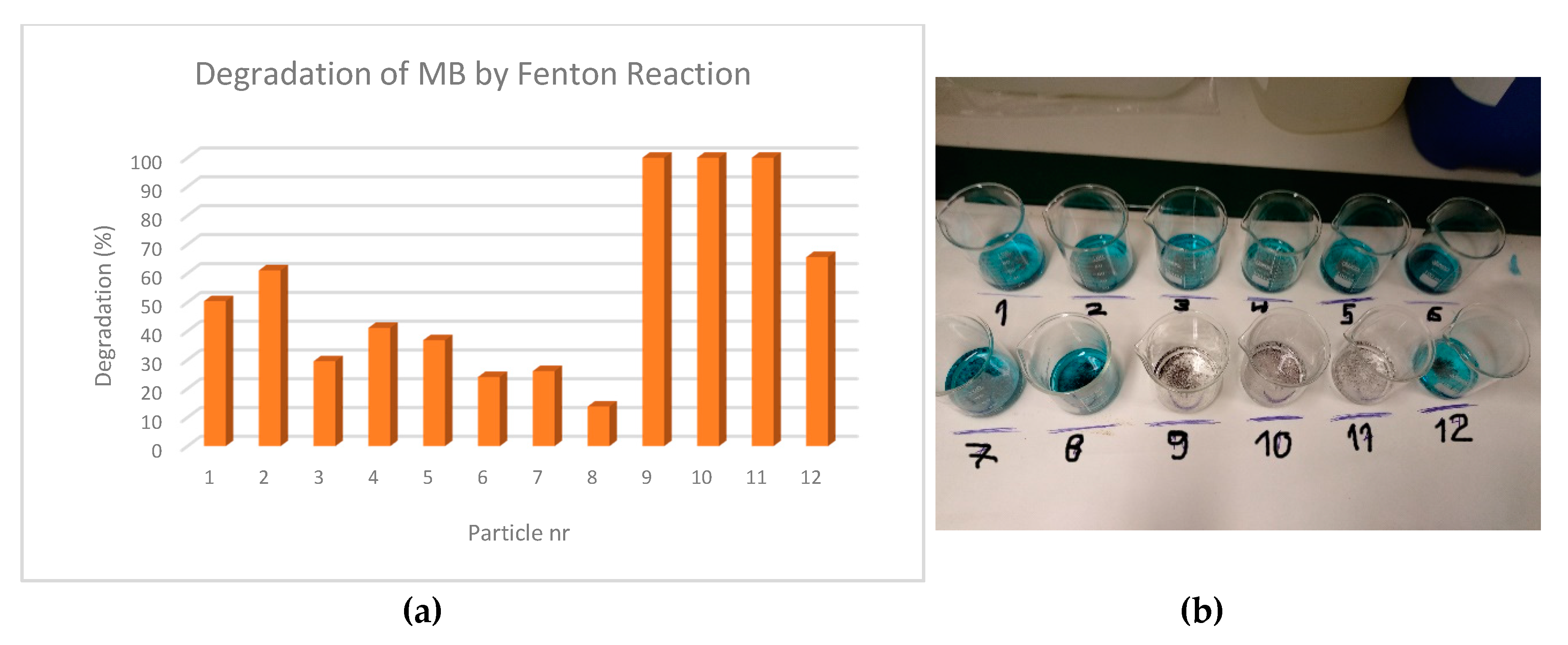
3.3. Fenton Experiments
Discussion of Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ning, G.; Xiaowen, D.; Yuting, C.; Futao, H.; Tianhua, L.; Qianli, J. An Ultrasensitive Electrochemical Immunosensor for HIV p24 Based on Fe3O4@SiO2 Nanomagnetic Probes and Nanogold Colloid-Labeled Enzyme–Antibody Copolymer as Signal Tag. Materials 2013, 6, 1255–1269. [Google Scholar]
- Akbarzadeh, A.; Samiei, M.; Davaran, S. Magnetic nanoparticles: Preparation, physical properties, and applications in biomedicine. Nanoscale Res. Lett. 2012, 7, 144. [Google Scholar] [CrossRef]
- Fouriki, A.; Dobson, J. Nanomagnetic Gene Transfection for Non-Viral Gene Delivery in NIH 3T3 Mouse Embryonic Fibroblasts. Materials 2013, 6, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Moraes, S.S.; Tavallaie, R.; Sandiford, L.; Tilley, R.D.; Gooding, J.J. Gold coated magnetic nanoparticles: From preparation to surface modification for analytical and biomedical applications. Chem. Commun. 2016, 52, 7528–7540. [Google Scholar] [CrossRef] [PubMed]
- Dabaghi, M.; Hilger, I. Magnetic Nanoparticles Behavior in Biological Solutions; The Impact of Clustering Tendency on Sedimentation Velocity and Cell Uptake. Materials 2020, 13, 1644. [Google Scholar] [CrossRef]
- Farag, R.K.; Labena, A.; Fakhry, S.H.; Safwat, G.; Diab, A.; Atta, A.M. Antimicrobial Activity of Hybrids Terpolymers Based on Magnetite Hydrogel Nanocomposites. Materials 2019, 12, 3604. [Google Scholar] [CrossRef]
- Kuhar, L.; Sanderson, R.; Koch, K. Magnetic Nanoparticles: Properties and Potential Applications. Pure and Applied Chemistry. Pure Appl. Chem. 2006, 78, 1793–1801. [Google Scholar]
- Marsooli, M.A.; Fasihi-Ramandi, M.; Adib, K.; Pourmasoud, S.; Ahmadi, F.; Ganjali, M.R.; Nasab, A.S.; Nasrabadi, M.R.; Plonska-Brzezinska, M.E. Preparation and Characterization of Magnetic Fe3O4/CdWO4 and Fe3O4/CdWO4/PrVO4 Nanoparticles and Investigation of Their Photocatalytic and Anticancer Properties on PANC1 Cells. Materials 2019, 12, 3274. [Google Scholar] [CrossRef]
- Paun, I.A.; Calin, B.S.; Mustaciosu, C.C.; Mihailescu, M.; Moldovan, A.; Crisan, O.; Leca, A.; Luculescu, C.R. 3D Superparamagnetic Scaffolds for Bone Mineralization under Static Magnetic Field Stimulation. Materials 2019, 12, 2834. [Google Scholar] [CrossRef]
- Wang, S.; Niu, R.; Yang, Y.; Zhou, X.; Wang, Y. Aptamer-functionalized chitosan magnetic nanoparticles as a novel adsorbent for selective extraction of ochratoxin A. Int. J. Biol. Macromol. 2020, 15315, 583–590. [Google Scholar] [CrossRef]
- Gu, T.; Wang, J.; Xia, H.; Wang, S.; Yu, X. Direct Electrochemistry and Electrocatalysis of Horseradish Peroxidase Immobilized in a DNA/Chitosan-Fe3O4 Magnetic Nanoparticle Bio-Complex Film. Materials 2014, 7, 1069–1083. [Google Scholar] [CrossRef] [PubMed]
- Tanasa, E.; Zaharia, C.; Hudita, A.; Radu, I.-C.; Galateanu, B. Impact of the magnetic field on 3T3-E1 preosteoblasts inside SMART silk fibroin-based scaffolds decorated with magnetic nanoparticles. Mater. Sci. Eng.: C 2020, 110, 110714. [Google Scholar] [CrossRef] [PubMed]
- Majidi, S.; Sehrig, F.; Mussa-Farkhani, S.; Soleymani-Goloujeh, M.; Akbarzadeh, A. Current methods for synthesis of magnetic nanoparticles. Artif. Cells Nanomed. Biotech. 2014, 44, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Staniland, S.S.; Rawlings, A.; Bramble, J.; Tolosa, J.; Wilson, O.; García-Martínez, J.C.; Binns, C. Chapter 3—Novel Methods for the Synthesis of Magnetic Nanoparticles. In Frontiers of Nanoscience; Chris, B., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 6, pp. 85–128. [Google Scholar]
- Augusto, P.A.; Castelo-Grande, T.; Vargas, D.; Pascual, A.; Hernández, L.; Estevez, A.M.; Barbosa, D. Upscale Design, Process Development and Economic Analysis of Industrial Plants for Nanomagnetic Particle Production for Environmental and Biomedical Use. Materials 2020, in press. [Google Scholar]
- Castelo-Grande, T.; Augusto, P.A.; Monteiro, P.; Barbosa, D. Design and Application of a Membrane Bioreactor Reactor Unit to Upgrade and Enhance the Required Performance of an Installed Wastewater Treatment Plant. Asian-Pac. J. Chem. Eng. 2010, 5, 73–82. [Google Scholar] [CrossRef]
- Castelo-Grande, T.; Augusto, P.A.; Monteiro, P.; Barbosa, D. Reduction of Solids in Leachate Streams by Membrane Bioreactors: Mass Transfer Topics and Fouling Problems. Defect Diffus. Forum 2008, 273–276, 770–775. [Google Scholar]
- Augusto, P.A.; Castelo-Grande, T.; Augusto, P. Magnetic classification in Health Sciences and in Chemical Engineering. Chem. Eng. J. 2005, 111, 85–90. [Google Scholar] [CrossRef]
- El-Dib, F.I.; Mohamed, D.E.; El-Shamy, O.A.A.; Mishrif, M.R. Study the adsorption properties of magnetite nanoparticles in the presence of different synthesized surfactants for heavy metal ions removal. Egypt. J. Pet. 2020, 29, 1–7. [Google Scholar] [CrossRef]
- Elkady, M.; Hassan, H.S.; Hashim, A. Immobilization of Magnetic Nanoparticles onto Amine-Modified Nano-Silica Gel for Copper Ions Remediation. Materials 2016, 9, 460. [Google Scholar] [CrossRef] [PubMed]
- Vamvakidis, K.; Kostitsi, T.K.; Makridis, A.; Dendrinou-Samara, C. Diverse Surface Chemistry of Cobalt Ferrite Nanoparticles to Optimize Copper (II) Removal from Aqueous Media. Materials 2020, 13, 1537. [Google Scholar] [CrossRef]
- Gutierrez, A.M.; Dziubla, T.D.; Hilt, J.Z. Recent advances on iron oxide magnetic nanoparticles as sorbents of organic pollutants in water and wastewater treatment. Rev. Environ. Health 2017, 32, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Elessawy, N.A.; Gouda, M.H.; Ali, S.M.; Salerno, M.; Mohy-Eldin, M.S. Effective Elimination of Contaminant Antibiotics Using High-Surface-Area Magnetic-Functionalized Graphene Nanocomposites Developed from Plastic Waste. Materials 2020, 13, 1517. [Google Scholar] [CrossRef] [PubMed]
- Augusto, P.A.; Castelo-Grande, T.; Merchan, L.; Estevez, A.M.; Quintero, X.; Barbosa, D. Landfill leachate treatment by sorption in magnetic particles: Preliminary study. Sci. Total Environ. 2019, 648, 638–668. [Google Scholar] [CrossRef]
- Estevez, A.M.; Rodriguez, J.M.; Alvaro, A.; Augusto, P.A.; Jiménez, O.; Castelo-Grande, T.; Barbosa, D. Preparation, Characterization, and Testing of Magnetic Carriers for Arsenic Removal From Water. IEEE Trans. Magn. 2008, 44, 1–8. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Chen, D.; Qin, Q.; Wu, Y.; Huang, F.; Li, W. Preparation of FeOOH/Cu with High Catalytic Activity for Degradation of Organic Dyes. Materials 2019, 12, 338. [Google Scholar] [CrossRef]
- Xu, L.; Wang, J. Fenton-like degradation of 2, 4-dichlorophenol using Fe3O4 magnetic nanoparticles. Appl. Catal. B: Environ. 2012, 123–124, 117–126. [Google Scholar] [CrossRef]
- Calza, P.; Di Sarro, J.; Magnacca, G.; Prevot, A.B.; Laurenti, E. Use of Low-Cost Magnetic Materials Containing Waste Derivatives for the (Photo)-Fenton Removal of Organic Pollutants. Materials 2019, 12, 3942. [Google Scholar] [CrossRef]
- Luo, W.; Zhu, L.; Wang, N.; Tang, H.; Cao, M.; She, Y. Efficient Removal of Organic Pollutants with Magnetic Nanoscaled BiFeO3 as a Reusable Heterogeneous Fenton-Like Catalyst. Environ. Sci. Tech. 2010, 44, 1786–1791. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Shi, W.; Bao, J.; Yang, X. A Fenton-Like Nanocatalyst Based on Easily Separated Magnetic Nanorings for Oxidation and Degradation of Dye Pollutant. Materials 2020, 13, 332. [Google Scholar] [CrossRef]
- Hongping, H.E.; Zhong, Y.; Liang, X.; Wei, T.A.N.; Jianxi, Z.H.U.; Wang, C.Y. Natural Magnetite: An efficient catalyst for the degradation of organic contaminant. Sci. Rep. 2015, 5, 1–10. [Google Scholar]
- Liu, Q.; Hu, S.; Yang, Z.; Zhang, X.; Ge, J. Green Synthesis of Composite Graphene Aerogels with Robust Magnetism for Effective Water Remediation. Materials 2019, 12, 4106. [Google Scholar] [CrossRef] [PubMed]
- Masudi, A.; Harimisa, G.E.; Ghafar, N.A.; Jusoh, N.W.C. Magnetite-based catalysts for wastewater treatment. Environ. Sci. Pollut. Res. 2020, 27, 4664–4682. [Google Scholar] [CrossRef] [PubMed]
- Aono, H.; Hirazawa, H.; Naohara, T.; Maehara, T.; Kikkawa, H.; Watanabe, Y. Synthesis of fine magnetite powder using reverse coprecipitation method and its heating properties by applying AC magnetic field. Mater. Res. Bull. 2005, 40, 1126–1135. [Google Scholar] [CrossRef]
- Chomoucka, J.; Drbohlavova, J.; Huska, D.; Adam, V.; Kizek, R.; Hubalek, J. Magnetic nanoparticles and targeted drug delivering. Pharmacol. Res. 2010, 62, 144–149. [Google Scholar] [CrossRef]
- Kazemzadeh, H.; Ataie, A.; Rashchi, F. In situ synthesis of silica-coated magnetic nanoparticles by reverse coprecipitation method. J. Supercond. Nov. Magn. 2012, 25, 2803–2808. [Google Scholar] [CrossRef]
- Mahmed, N.; Heczko, O.; Lancok, A.; Hannula, S.-P. The magnetic and oxidation behavior of bare and silica-coated iron oxide nanoparticles synthesized by reverse co-precipitation of ferrous ion (Fe2+) in ambient atmosphere. J. Magn. Magn. Mater. 2014, 353, 15–22. [Google Scholar] [CrossRef]
- Mahmed, N.; Heczko, O.; Söderberg, O.; Hannula, S.-P. Room Temperature Synthesis of Magnetite (Fe3-O4) Nanoparticles by a Simple Reverse Co-Precipitation Method. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2011; Volume 18. [Google Scholar]
- Castelo-Grande, T.; Augusto, P.A.; Estevéz, A.M.; Barbosa, D.; Vargas, D.; González, M.; Diego, M.; Martin, E.; Rodriguez, B.; Sanchez, A. Nanomagnetic Particles Synthesis: Comparision of Methods and Applications. In Proceedings of the 20th International Conference on Magnetism, Barcelona, Spain, 5–10 July 2015; pp. 345–346. [Google Scholar]
- Augusto, P.A.; Castelo-Grande, T.; Hernandez, L.; Estevez, A.M.; Barbosa, D. Regeneration and Re-use of Spent Magnetite Particles after Heterogeneous Fenton Process. Chem. Eng. J. (submitted).
- Augusto, P.A.; Castelo-Grande, T.; Estevez, A.M.; Barbosa, D. Application of magnetism and nanomagnetism in water, leachates and soils remediation. In Proceedings of the 5th International Congress on Water, Waste and Energy Management, Paris, France, 22–24 July 2019; pp. 45–52. [Google Scholar]

| Experiment n. | Alkaline Base | Surfactant | FeSO4 (M) | Drying T (°C) |
|---|---|---|---|---|
| 1 | NH4OH | – | 0.2 | 50 |
| 2 | NH4OH | Tween 80 a | 0.2 | 50 |
| 3 | NH4OH | Tween 80 b | 0.2 | 50 |
| 4 | NH4OH | Citric acid a | 0.2 | 50 |
| 5 | NH4OH | – | 0.2 | 90 |
| 6 | NH4OH | Citric acid b | 0.2 | 50 |
| 7 | NH4OH | Tween 80 a | 0.2 | 90 |
| 8 | NH4OH | – | 0.4 | 50 |
| 9 | NaOH | – | 0.2 | 50 |
| 10 | NaOH | – | 0.2 | 50 |
| 11 | NaOH | Tween 80 b | 0.2 | 50 |
| 12 | NaOH | Citric acid b | 0.2 | 50 |
| Experiment nr. | Crystallites Size (Å) | χm (×105) (SI Units) |
|---|---|---|
| 1 | 92.9 | 7507.7 |
| 2 | 92.4 | 10,485.2 |
| 3 | 98.3 | 10,643.4 |
| 4 | 85.7 | 8367.7 |
| 5 | 95.5 | 11,752.8 |
| 6 | 88.6 | 10,225.3 |
| 7 | 111.3 | 11,346.3 |
| 8 | 96.3 | 9060.1 |
| 9 | 45.3 | 116.0 |
| 10 | 87.0 | 63.8 |
| 11 | 90.7 | 123.6 |
| 12 | 71.0 | 141.8 |
| Particles nr. | COD (mgO2/L) | Removal (%) |
|---|---|---|
| Initial | 1026 | – |
| 1 | 292 | 71.5 |
| 2 | 252 | 75.4 |
| 3 | 270 | 73.7 |
| 4 | 227 | 77.9 |
| 5 | 213 | 79,2 |
| 6 | 233 | 77.3 |
| 7 | 284 | 72.3 |
| 8 | 265 | 74.2 |
| 9 | 304 | 70.4 |
| 10 | 192 | 81.3 |
| 11 | 296 | 71.2 |
| 12 | 304 | 70.4 |
| Particles nr. | Relative Absorbance | % Degradation |
|---|---|---|
| 1 | 0.50 | 50.4 |
| 2 | 0.39 | 61.0 |
| 3 | 0.70 | 29.5 |
| 4 | 0.59 | 41.1 |
| 5 | 0.63 | 36.8 |
| 6 | 0.76 | 24.0 |
| 7 | 0.74 | 26.1 |
| 8 | 0.86 | 13.8 |
| 9 | 0.00 | 100.0 |
| 10 | 0.00 | 100.0 |
| 11 | 0.00 | 100.0 |
| 12 | 0.34 | 65.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Augusto, P.A.; Castelo-Grande, T.; Vargas, D.; Hernández, L.; Merchán, L.; Estevez, A.M.; Gómez, J.; Compaña, J.M.; Barbosa, D. Water Decontamination with Magnetic Particles by Adsorption and Chemical Degradation. Influence of the Manufacturing Parameters. Materials 2020, 13, 2219. https://doi.org/10.3390/ma13102219
Augusto PA, Castelo-Grande T, Vargas D, Hernández L, Merchán L, Estevez AM, Gómez J, Compaña JM, Barbosa D. Water Decontamination with Magnetic Particles by Adsorption and Chemical Degradation. Influence of the Manufacturing Parameters. Materials. 2020; 13(10):2219. https://doi.org/10.3390/ma13102219
Chicago/Turabian StyleAugusto, Paulo A, Teresa Castelo-Grande, Diana Vargas, Lorenzo Hernández, Leticia Merchán, Angel M Estevez, Juan Gómez, José M Compaña, and Domingos Barbosa. 2020. "Water Decontamination with Magnetic Particles by Adsorption and Chemical Degradation. Influence of the Manufacturing Parameters" Materials 13, no. 10: 2219. https://doi.org/10.3390/ma13102219
APA StyleAugusto, P. A., Castelo-Grande, T., Vargas, D., Hernández, L., Merchán, L., Estevez, A. M., Gómez, J., Compaña, J. M., & Barbosa, D. (2020). Water Decontamination with Magnetic Particles by Adsorption and Chemical Degradation. Influence of the Manufacturing Parameters. Materials, 13(10), 2219. https://doi.org/10.3390/ma13102219





