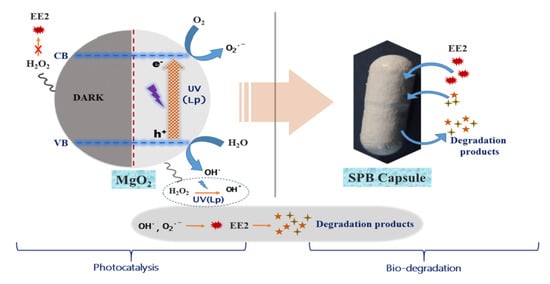
LP-UV-Nano MgO2 Pretreated Catalysis Followed by Small Bioreactor Platform Capsules Treatment for Superior Kinetic Degradation Performance of 17α-Ethynylestradiol
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Characterization
2.2. Analytical Procedures
2.3. Photocatalysis Experiments
2.4. Estimation of H2O2 by Potassium Permanganate (KMnO4) Titration
2.5. Colorimetric Estimation of H2O2 Using Strips
2.6. Estimation of ·OH Radicals
2.7. Photocatalytic Degradation of EE2
2.8. Encapsulation of R. zopfii in Membrane-Based Capsules
2.9. Activation of SBP Capsules
2.10. Biodegradation of EE2 by SBP Capsules
2.11. Photocatalytic Degradation of EE2 by MgO2 Followed by Biodegradation
2.12. EE2 Adsorption to the SBP Capsule Membrane
3. Results
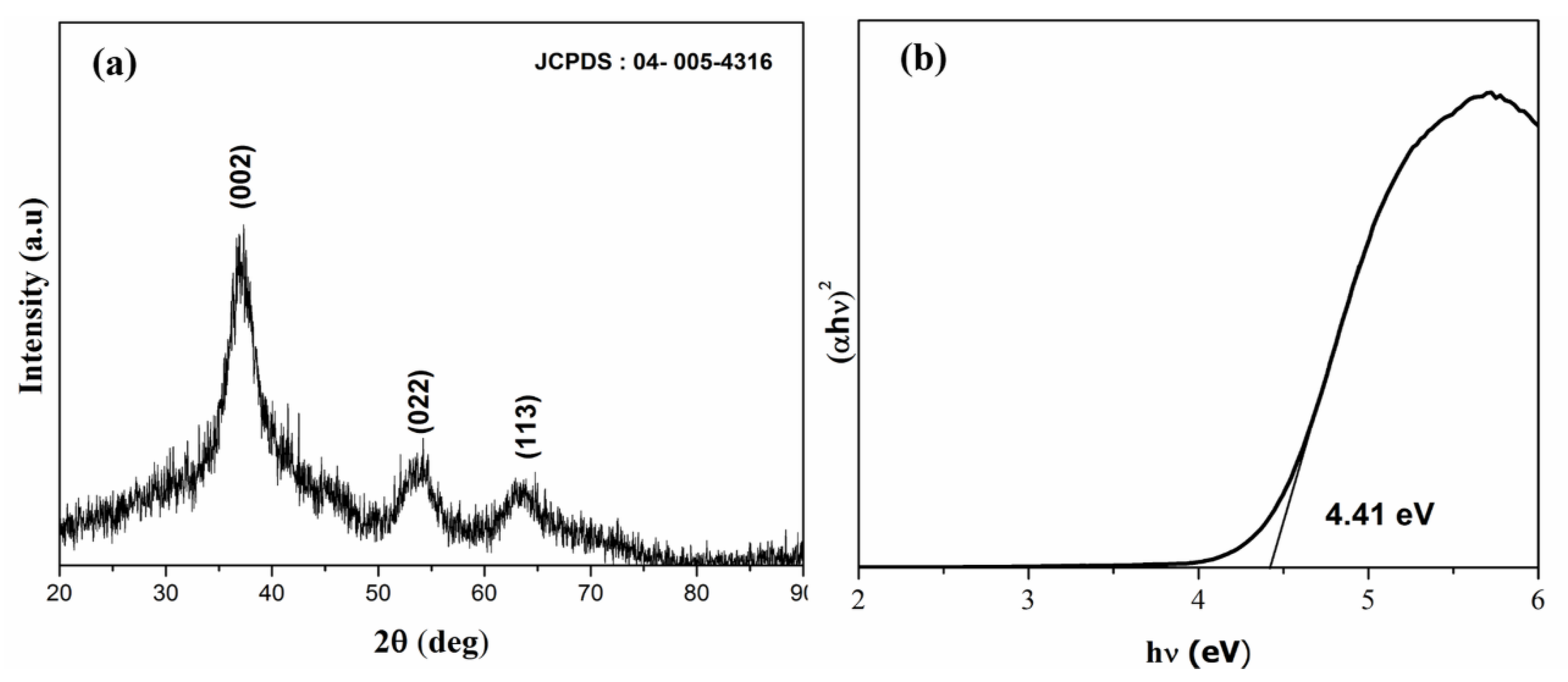
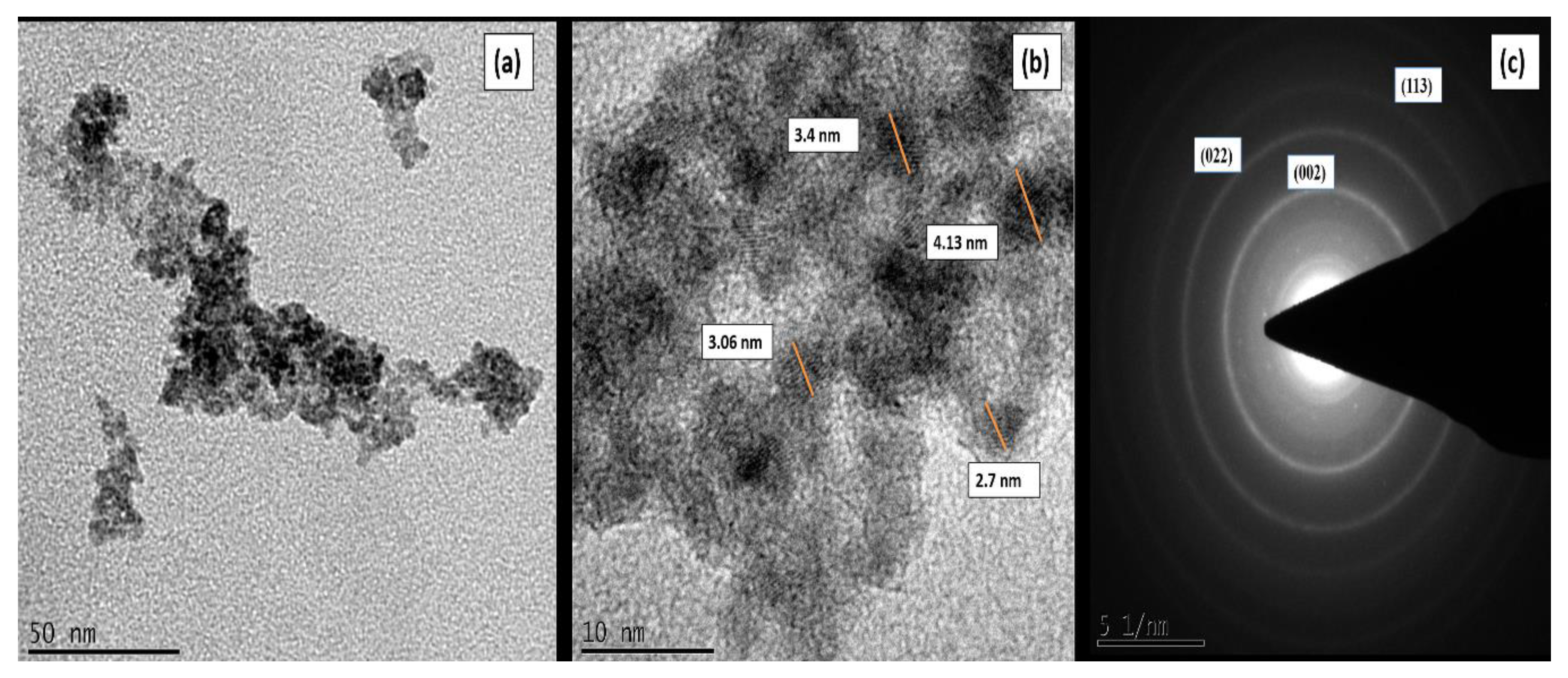
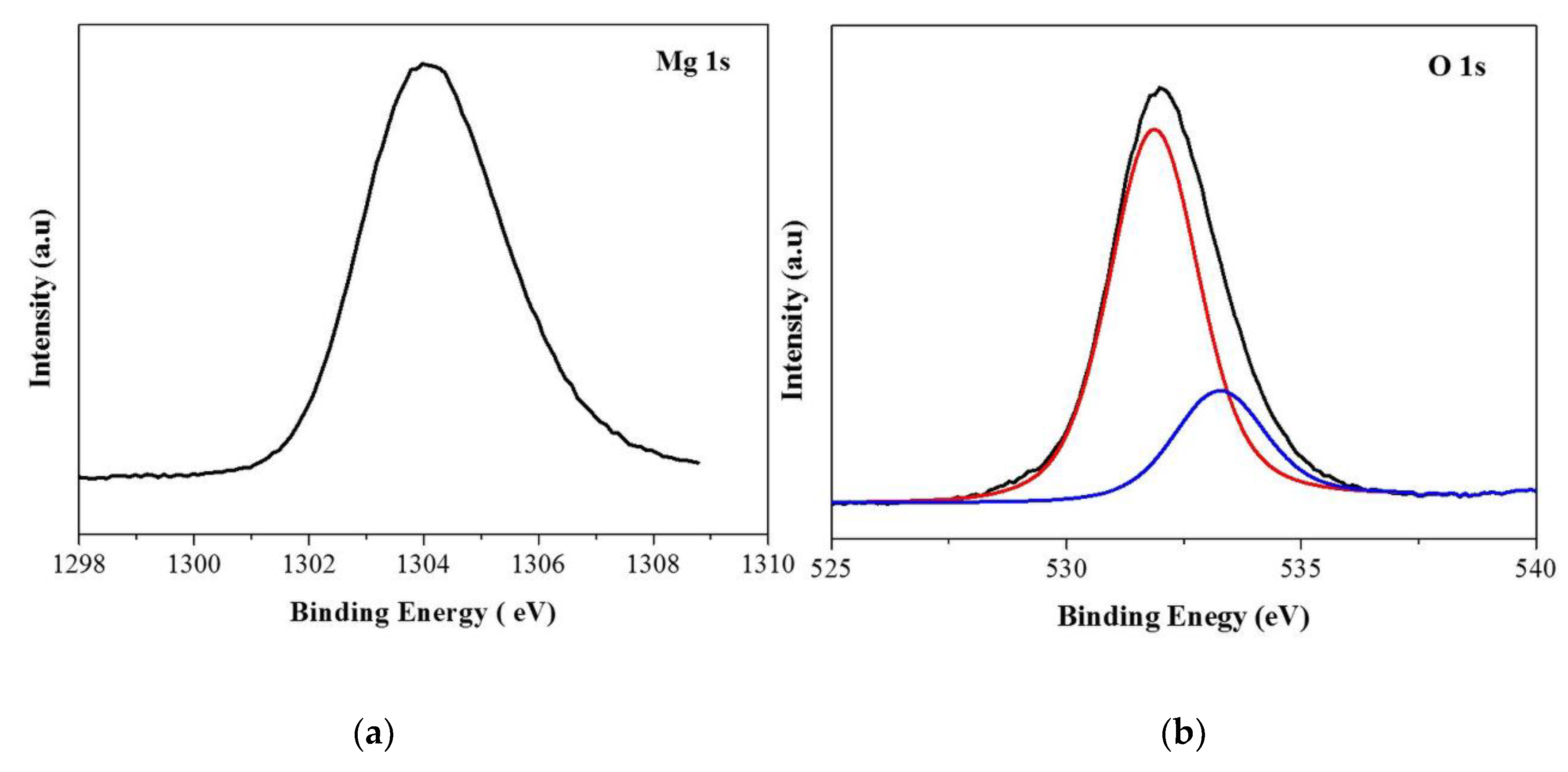
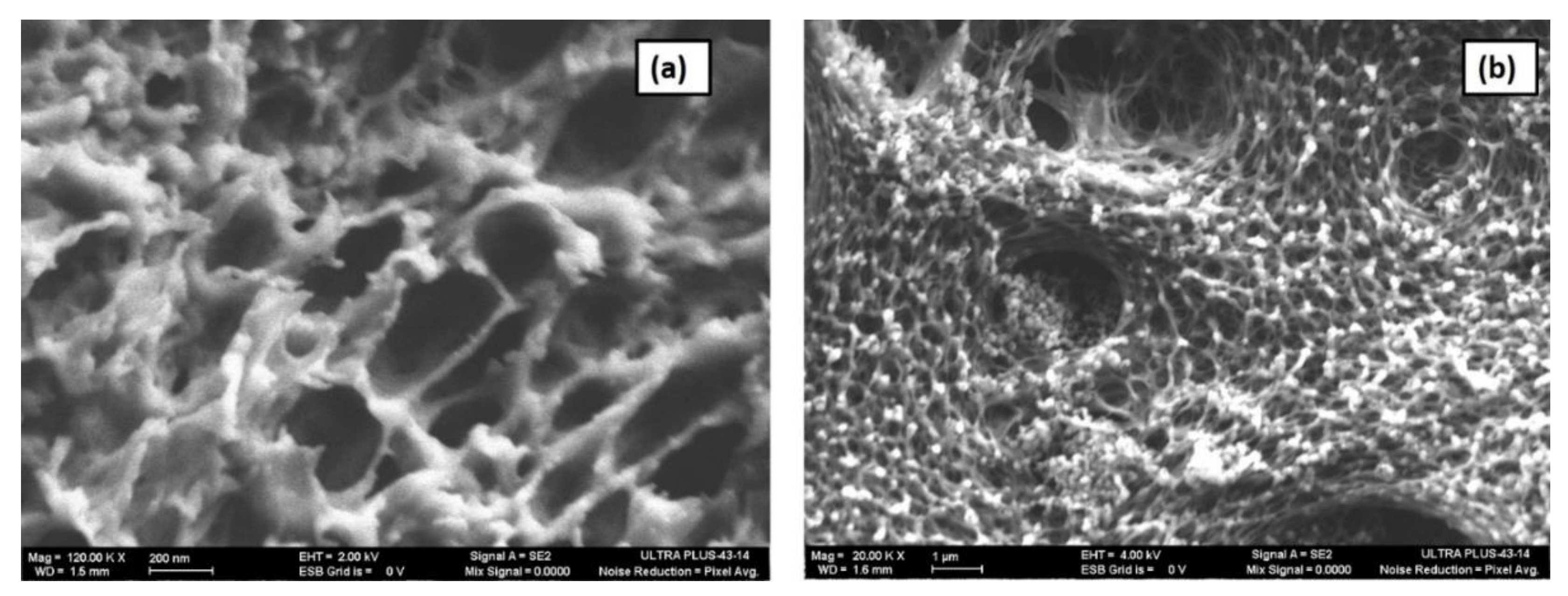
3.1. Material Characterization
3.2. ROS from Aqueous Suspensions of MgO2
3.3. Photocatalytic Degradation of EE2
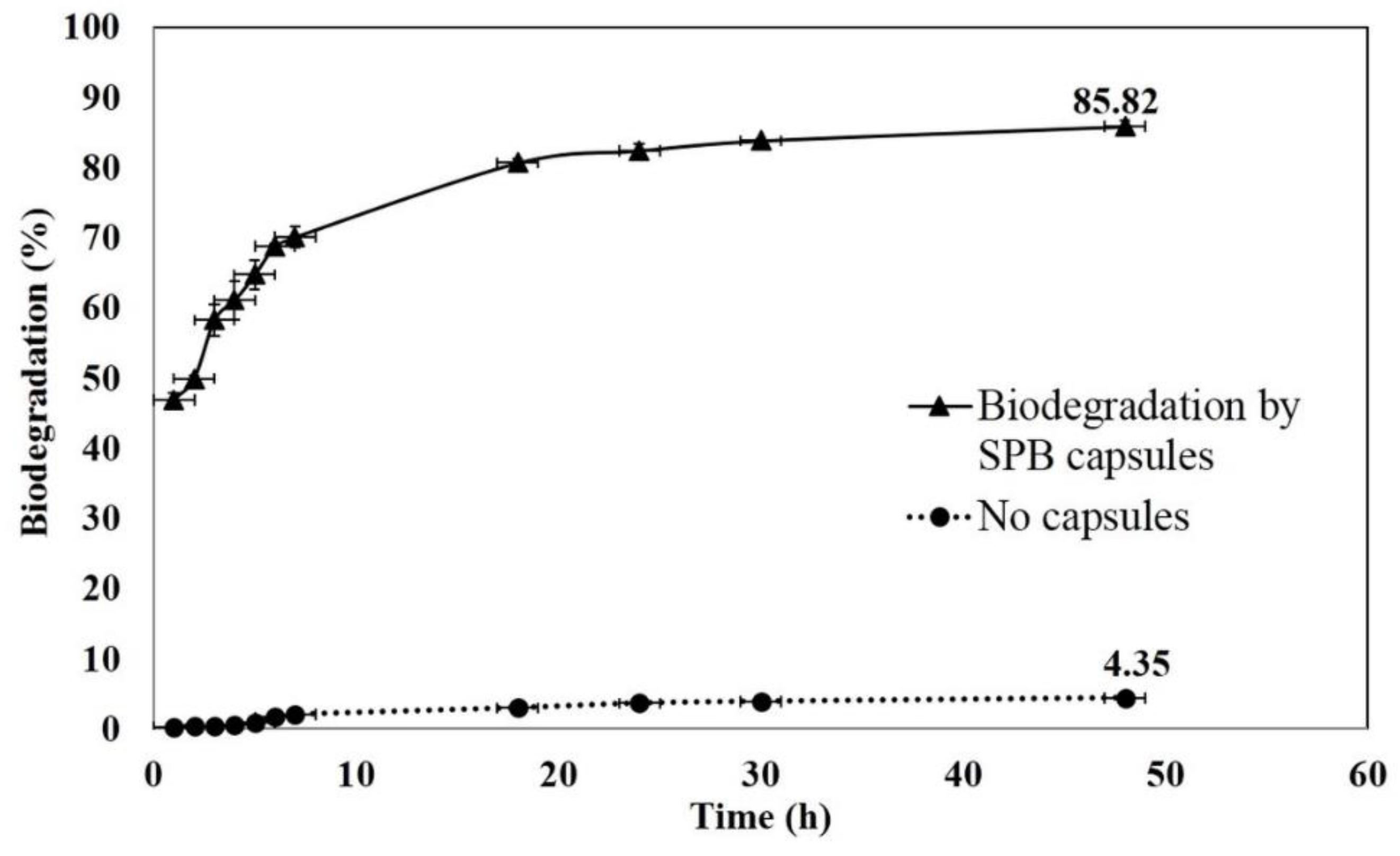
3.4. Biodegradation of EE2 by SBP Capsules
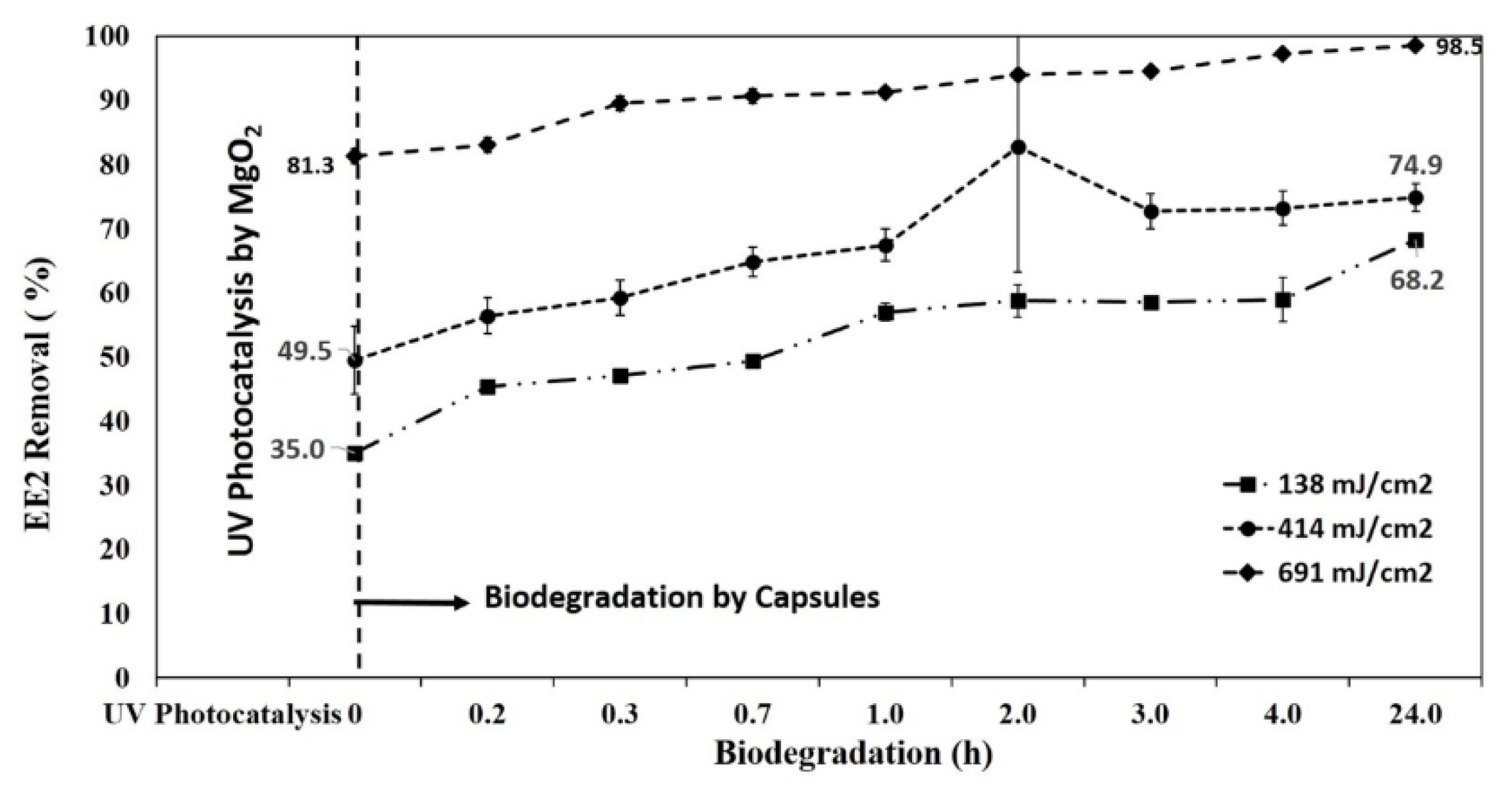
3.5. Photocatalytic Degradation of EE2 by MgO2/LP-UV Followed by Biodegradation by SBP Capsules
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Adeel, M.; Song, X.; Wang, Y.; Francis, D.; Yang, Y. Environmental impact of estrogens on human, animal and plant life: A critical review. Environ. Int. 2017, 99, 107–119. [Google Scholar] [CrossRef]
- Sarkar, S.; Ali, S.; Rehmann, L.; Nakhla, G.; Ray, M.B. Degradation of estrone in water and wastewater by various advanced oxidation processes. J. Hazard. Mater. 2014, 278, 16–24. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, P.; Wu, F.; Deng, N.; Liu, J.; Fang, T. Degradation of 17α-ethinylestradiol in aqueous solution by ozonation. J. Hazard. Mater. 2006, 133, 291–298. [Google Scholar] [CrossRef]
- Rosenfeldt, E.J.; Linden, K.G. Degradation of endocrine disrupting chemicals bisphenol A, ethinyl estradiol, and estradiol during UV photolysis and advanced oxidation processes. Environ. Sci. Technol. 2004, 38, 5476–5483. [Google Scholar] [CrossRef]
- Frontistis, Z.; Xekoukoulotakis, N.P.; Hapeshi, E.; Venieri, D.; Fatta-Kassinos, D.; Mantzavinos, D. Fast degradation of estrogen hormones in environmental matrices by photo-Fenton oxidation under simulated solar radiation. Chem. Eng. J. 2011, 178, 175–182. [Google Scholar] [CrossRef]
- Shappell, N.W.; Vrabel, M.A.; Madsen, P.J.; Harrington, G.; Billey, L.O.; Hakk, H.; Larsen, G.L.; Beach, E.S.; Horwitz, C.P.; Ro, K.; et al. Destruction of estrogens using Fe-TAML/peroxide catalysis. Environ. Sci. Technol. 2008, 42, 1296–1300. [Google Scholar] [CrossRef]
- Krishnan, S.; Rawindran, H.; Sinnathambi, C.M.; Lim, J.W. Comparison of various advanced oxidation processes used in remediation of industrial wastewater laden with recalcitrant pollutants. IOP Conf. Ser. Mater. Sci. Eng. 2017, 206, 012089. [Google Scholar] [CrossRef]
- Wolanov, Y.; Prikhodchenko, P.V.; Medvedev, A.G.; Pedahzur, R.; Lev, O. Zinc dioxide nanoparticulates: A hydrogen peroxide source at moderate pH. Environ. Sci. Technol. 2013, 47, 8769–8774. [Google Scholar] [CrossRef]
- Menashe, O.; Kurzbaum, E. Small-bioreactor platform technology as a municipal wastewater additive treatment. Water Sci. Technol. 2014, 69, 504–510. [Google Scholar] [CrossRef]
- Azaizeh, H.; Kurzbaum, E.; Said, O.; Jaradat, H.; Menashe, O. The potential of autochthonous microbial culture encapsulation in a confined environment for phenol biodegradation. Environ. Sci. Pollut. Res. 2015, 22, 15179–15187. [Google Scholar] [CrossRef]
- Kurzbaum, E.; Raizner, Y.; Cohen, O.; Suckeveriene, R.Y.; Kulikov, A.; Hakimi, B.; Iasur Kruh, L.; Armon, R.; Farber, Y.; Menashe, O. Encapsulated pseudomonas putida for phenol biodegradation: Use of a structural membrane for construction of a well-organized confined particle. Water Res. 2017, 121, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Bar Oz, Y.; Mamane, H.; Menashe, O.; Cohen-Yaniv, V.; Kumar, R.; Iasur Kruh, L.; Kurzbaum, E. Treatment of olive mill wastewater using ozonation followed by an encapsulated acclimated biomass. J. Environ. Chem. Eng. 2018, 6, 5014–5023. [Google Scholar] [CrossRef]
- Larcher, S.; Yargeau, V. Biodegradation of 17α-ethinylestradiol by heterotrophic bacteria. Environ. Pollut. 2013, 173, 17–22. [Google Scholar] [CrossRef]
- Haiyan, R.; Shulan, J.; ud din Ahmad, N.; Dao, W.; Chengwu, C. Degradation characteristics and metabolic pathway of 17α-ethynylestradiol by Sphingobacterium sp. JCR5. Chemosphere 2007, 66, 340–346. [Google Scholar] [CrossRef]
- Yoshimoto, T.; Nagai, F.; Fujimoto, J.; Watanabe, K.; Mizukoshi, H.; Makino, T.; Kimura, K.; Saino, H.; Sawada, H.; Omura, H. Degradation of estrogens by Rhodococcus zopfii and Rhodococcus equi isolates from activated sludge in wastewater treatment plants. Appl. Environ. Microbiol. 2004, 70, 5283–5289. [Google Scholar] [CrossRef]
- Taylor-Edmonds, L.; Lichi, T.; Rotstein-Mayer, A.; Mamane, H. The impact of dose, irradiance and growth conditions on Aspergillus niger (renamed A. brasiliensis) spores low-pressure (LP) UV inactivation. J. Environ. Sci. Heal. Part A Toxic/Hazardous Subst. Environ. Eng. 2015, 50, 341–347. [Google Scholar] [CrossRef]
- Cho, M.; Chung, H.; Choi, W.; Yoon, J. Linear correlation between inactivation of E. coli and OH radical concentration in TiO2 photocatalytic disinfection. Water Res. 2004, 38, 1069–1077. [Google Scholar] [CrossRef]
- Menashe, O. Microorganism comprising particles and uses of same. Patent WO/2010/122545, 28 October 2010. [Google Scholar]
- Lakshmi Prasanna, V.; Vijayaraghavan, R. Simultaneous fenton-photocatalytic reactions through a new single catalyst (nano ZnO2 /Fe2+) for dye degradation. J. Phys. Chem. C 2017, 121, 18557–18563. [Google Scholar] [CrossRef]
- Zhou, Y.; Fang, X.; Wang, T.; Hu, Y.; Lu, J. Chelating agents enhanced CaO2 oxidation of bisphenol a catalyzed by Fe3+ and reuse of ferric sludge as a source of catalyst. Chem. Eng. J. 2017, 313, 638–645. [Google Scholar] [CrossRef]
- Wu, D.; Bai, Y.; Wang, W.; Xia, H.; Tan, F.; Zhang, S.; Su, B.; Wang, X.; Qiao, X.; Wong, P.K. Highly pure MgO2 nanoparticles as robust solid oxidant for enhanced Fenton-like degradation of organic contaminants. J. Hazard. Mater. 2019, 374, 319–328. [Google Scholar] [CrossRef]
- Zhang, Z.; Feng, Y.; Liu, Y.; Sun, Q.; Gao, P.; Ren, N. Kinetic degradation model and estrogenicity changes of EE2 (17α-ethinylestradiol) in aqueous solution by UV and UV/H2O2 technology. J. Hazard. Mater. 2010, 181, 1127–1133. [Google Scholar] [CrossRef]
- Zhang, A.; Li, Y. Removal of phenolic endocrine disrupting compounds from waste activated sludge using UV, H2O2, and UV/H2O2 oxidation processes: Effects of reaction conditions and sludge matrix. Sci. Total Environ. 2014, 493, 307–323. [Google Scholar] [CrossRef]
- Duan, L.; Wang, H.; Sun, Y.; Xie, X. Biodegradation of phenol from wastewater by microorganism immobilized in bentonite and carboxymethyl cellulose gel. Chem. Eng. Commun. 2016, 203, 948–956. [Google Scholar] [CrossRef]
- González, G.; Herrera, G.; García, M.T.; Peña, M. Biodegradation of phenolic industrial wastewater in a fluidized bed bioreactor with immobilized cells of Pseudomonas putida. Bioresour. Technol. 2001, 80, 137–142. [Google Scholar] [CrossRef]
- Mohd-Towel, R.; Amir, A.; Abdul-Talib, S. Physical Characterization of Rhodococcus zopfii DSM 44108 bacteria isolated from municipal sludge. Appl. Mech. Mater. 2015, 773, 1307–1311. [Google Scholar] [CrossRef]
- Yu, C.-P.; Roh, H.; Chu, K.-H. 17β-estradiol-degrading bacteria isolated from activated sludge. Environ. Sci. Technol. 2007, 41, 486–492. [Google Scholar] [CrossRef]
- O’Grady, D.; Evangelista, S.; Yargeau, V. Removal of Aqueous 17α-ethinylestradiol by Rhodococcus species. Environ. Eng. Sci. 2009, 26, 1393–1400. [Google Scholar] [CrossRef]
- Oturan, M.A.; Aaron, J. Advanced oxidation processes in water/wastewater treatment: Principles and applications. A review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2577–2641. [Google Scholar] [CrossRef]
- Li, G.; Park, S.; Kang, D.-W.; Krajmalnik-Brown, R.; Rittmann, B.E. 2,4,5-trichlorophenol degradation using a novel TiO2 -coated biofilm carrier: Roles of adsorption, photocatalysis, and biodegradation. Environ. Sci. Technol. 2011, 45, 8359–8367. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Y.; Xiao, G.; Tang, Z.; Wang, M.; Liu, F.; Zhu, X. Simultaneous photocatalytic and microbial degradation of dye-containing wastewater by a novel g-C3N4-P25/photosynthetic bacteria composite. PLoS ONE 2017, 12, e0172747. [Google Scholar] [CrossRef] [PubMed]


| Time (min) | Concentration of H2O2 (ppm) |
|---|---|
| 0 | 0.2–5 |
| 5 | 0.2–5 |
| 25 | 0.4–10 |
| 35 | 20–30 |
| 60 | 20–30 |
| % Biodegradation of EE2 at the Specified Time (h) | ||||
|---|---|---|---|---|
| Dose (mJ/cm2) | 0 | 1 | 3 | 4 |
| 0 | 0 | 47 | 58 | 61 |
| 138 | 35 | 57 | 58 | 59 |
| 414 | 50 | 67 | 72 | 73 |
| 691 | 81 | 91 | 94 | 97 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaddadi, L.P.; Avisar, D.; Vadivel, V.K.; Menashe, O.; Kurzbaum, E.; Cohen-Yaniv, V.; Mamane, H. LP-UV-Nano MgO2 Pretreated Catalysis Followed by Small Bioreactor Platform Capsules Treatment for Superior Kinetic Degradation Performance of 17α-Ethynylestradiol. Materials 2020, 13, 83. https://doi.org/10.3390/ma13010083
Vaddadi LP, Avisar D, Vadivel VK, Menashe O, Kurzbaum E, Cohen-Yaniv V, Mamane H. LP-UV-Nano MgO2 Pretreated Catalysis Followed by Small Bioreactor Platform Capsules Treatment for Superior Kinetic Degradation Performance of 17α-Ethynylestradiol. Materials. 2020; 13(1):83. https://doi.org/10.3390/ma13010083
Chicago/Turabian StyleVaddadi, Lakshmi Prasanna, Dror Avisar, Vinod Kumar Vadivel, Ofir Menashe, Eyal Kurzbaum, Vered Cohen-Yaniv, and Hadas Mamane. 2020. "LP-UV-Nano MgO2 Pretreated Catalysis Followed by Small Bioreactor Platform Capsules Treatment for Superior Kinetic Degradation Performance of 17α-Ethynylestradiol" Materials 13, no. 1: 83. https://doi.org/10.3390/ma13010083
APA StyleVaddadi, L. P., Avisar, D., Vadivel, V. K., Menashe, O., Kurzbaum, E., Cohen-Yaniv, V., & Mamane, H. (2020). LP-UV-Nano MgO2 Pretreated Catalysis Followed by Small Bioreactor Platform Capsules Treatment for Superior Kinetic Degradation Performance of 17α-Ethynylestradiol. Materials, 13(1), 83. https://doi.org/10.3390/ma13010083