Photoelectrochemical Water Splitting Reaction System Based on Metal-Organic Halide Perovskites
Abstract
1. Introduction
2. Properties of Materials and Mechanisms of the PEC Cell
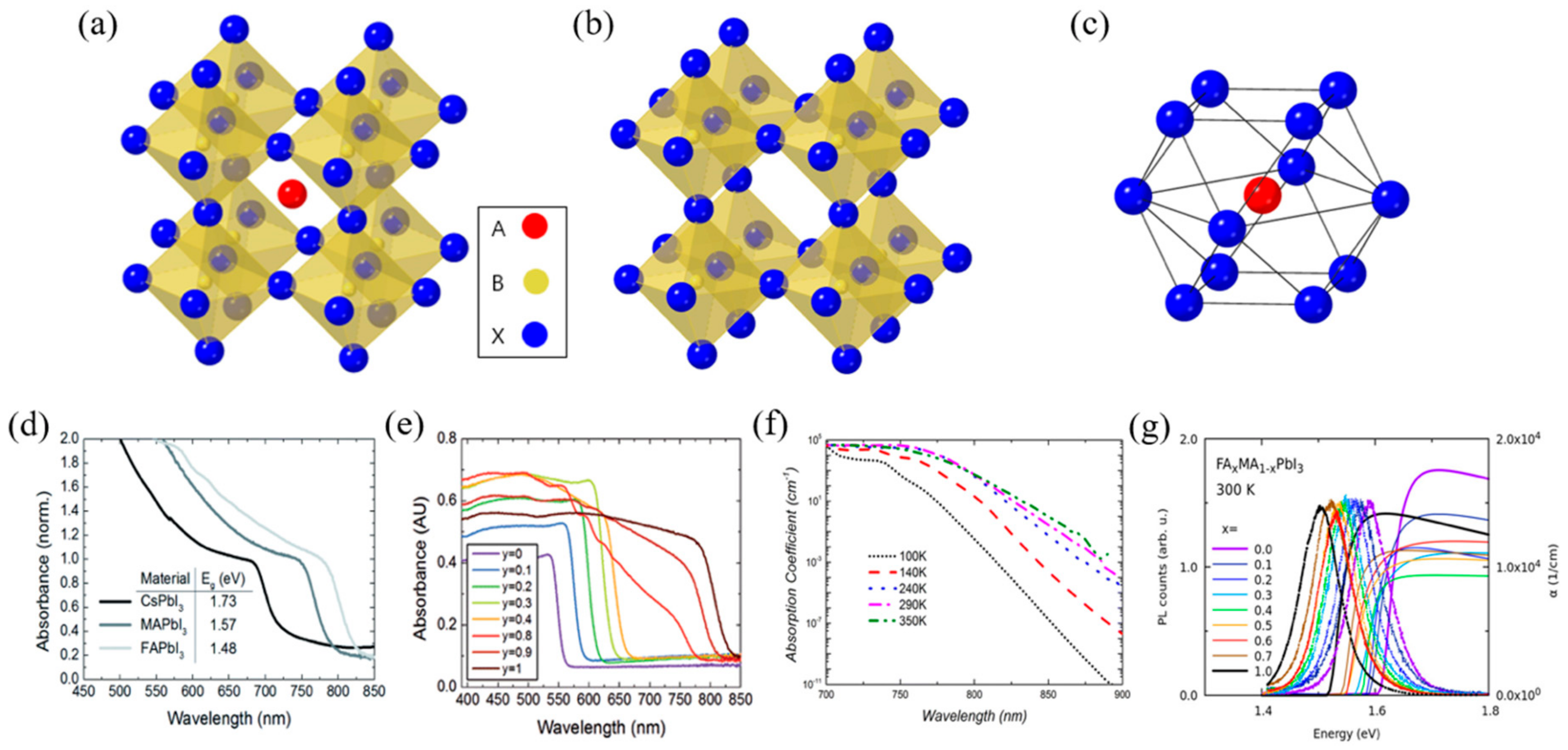
2.1. Intrinsic Properties of Perovskite Materials
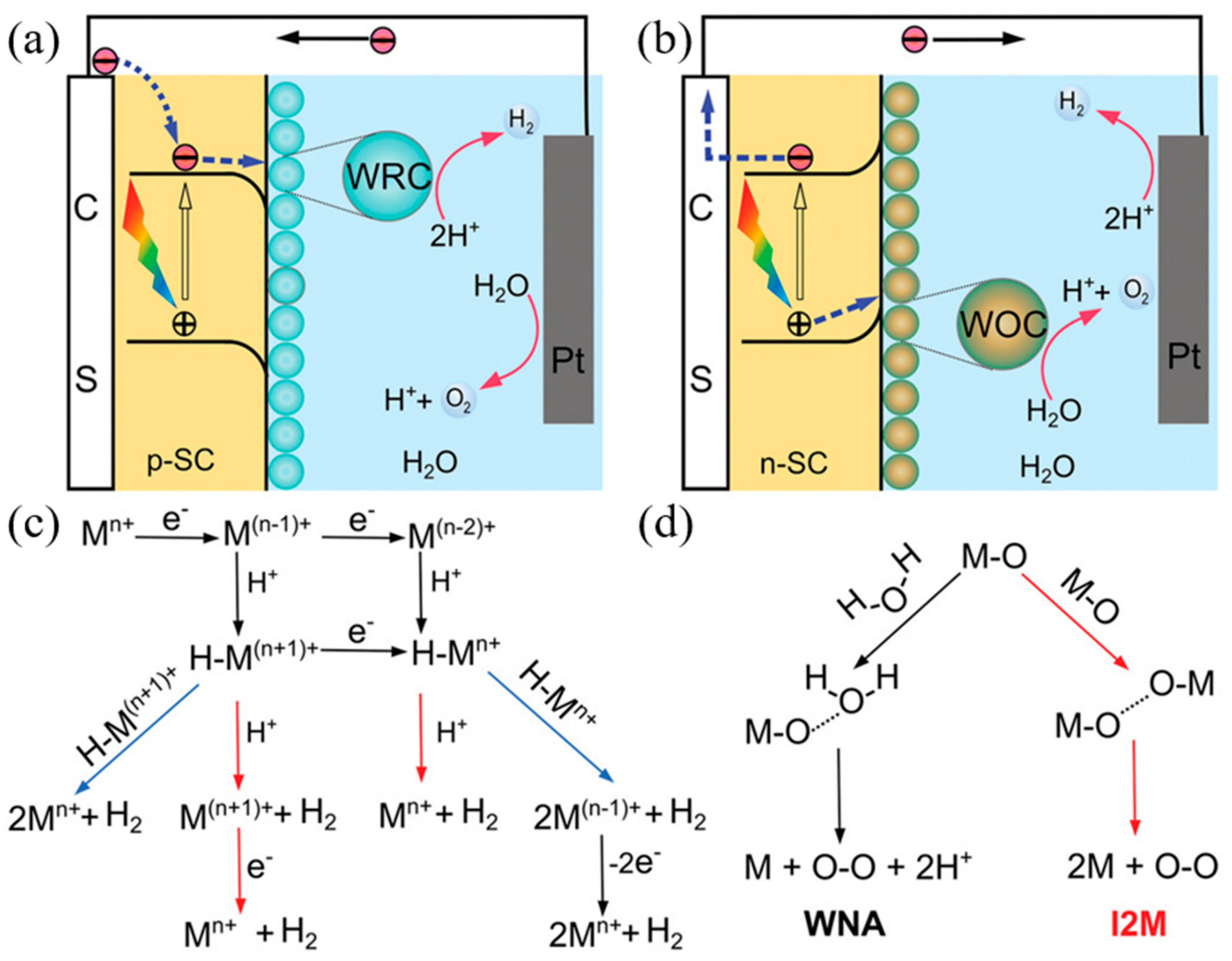
2.2. Principle of Photoelectrochemical Water Splitting Reaction
2.3. Mechanism of Perovskite PEC Cells
3. Recent Progress on Perovskite-based Photoelectrochemical Cells
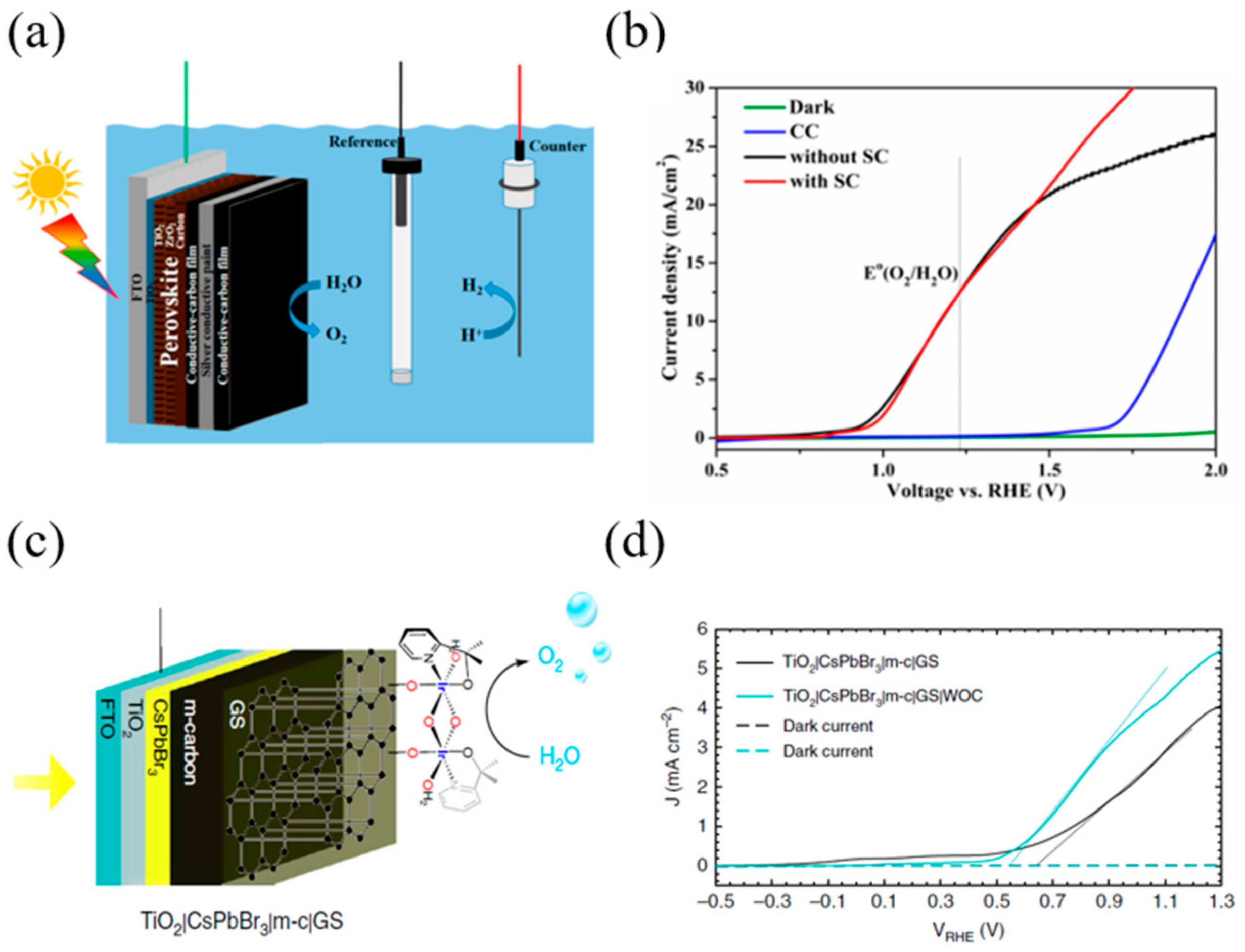
3.1. n–i–p Configuration Perovskite-Based PEC Cell for Oxygen Evolution
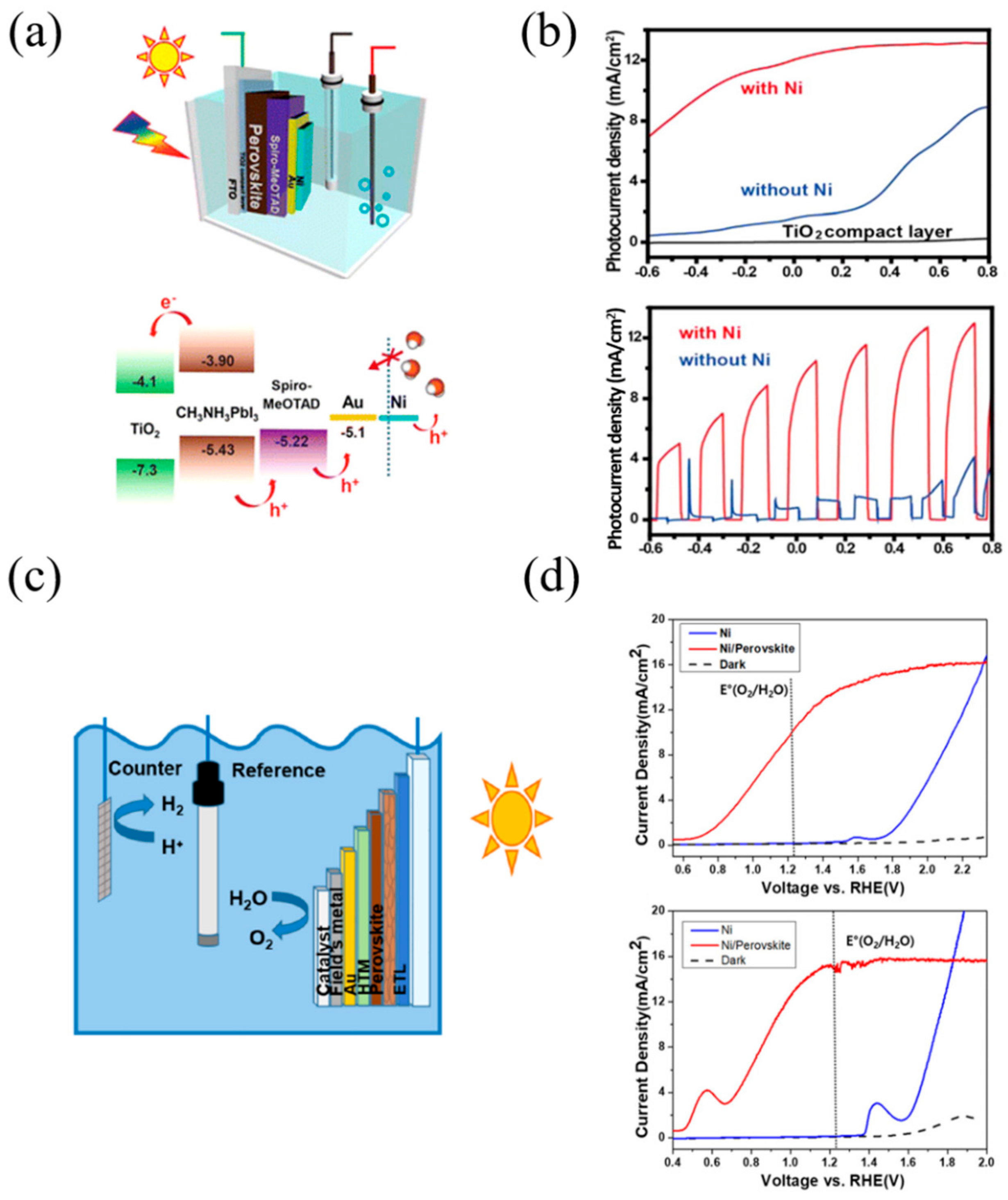
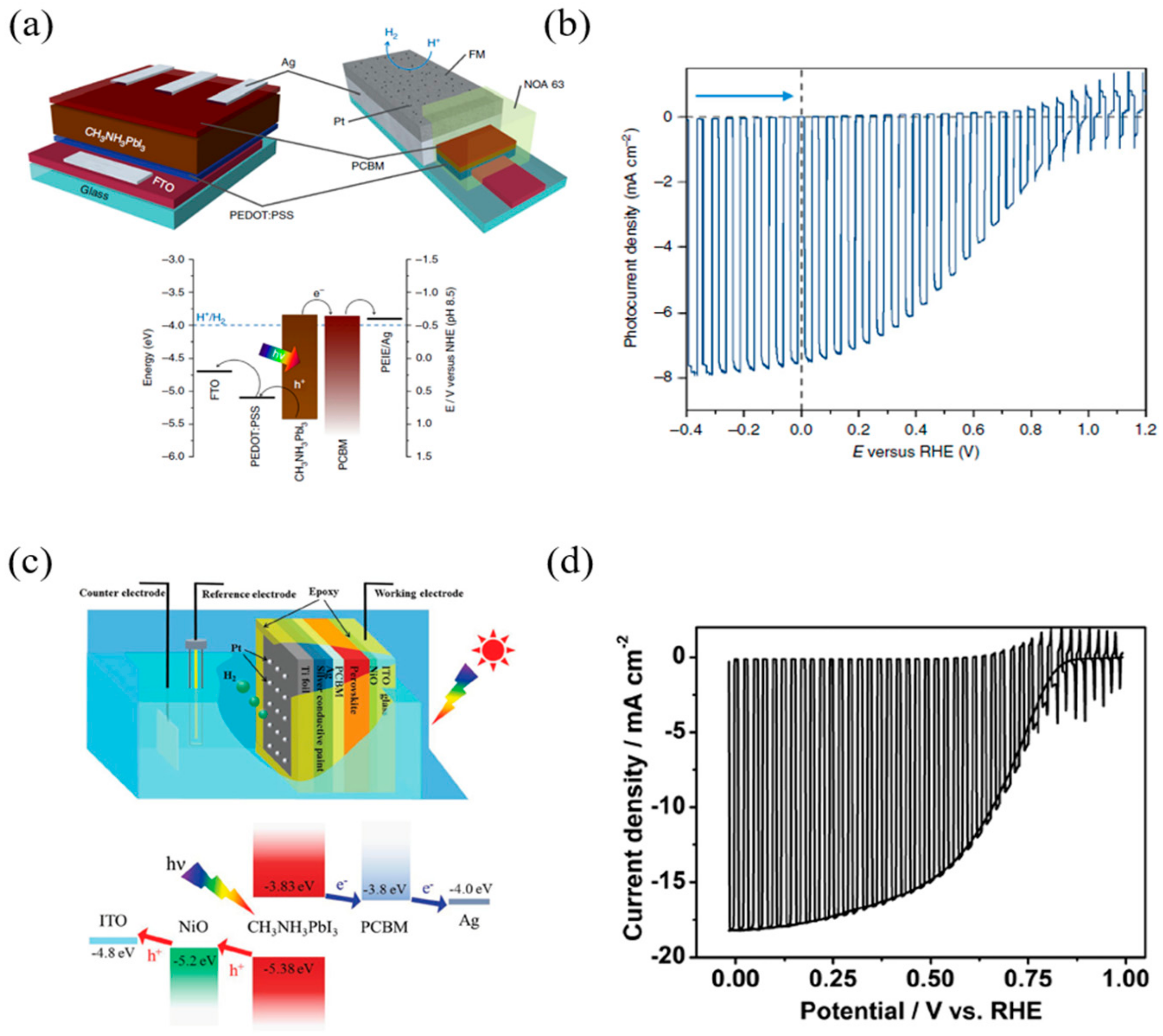
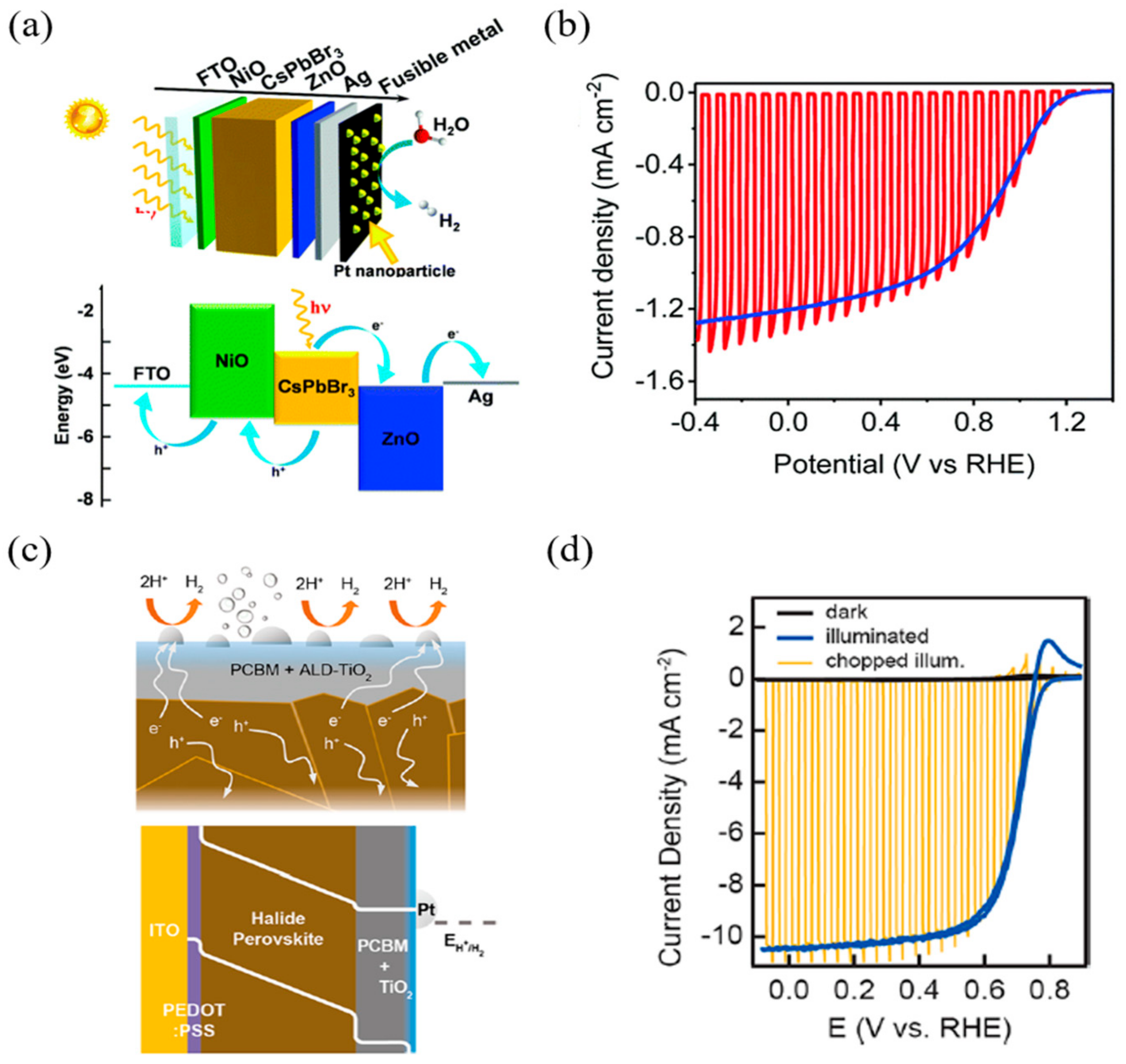
3.2. p–i–n Configuration Perovskite-Based PEC Cell for Hydrogen Evolution
4. Stability Issues of Perovskite PEC Cells
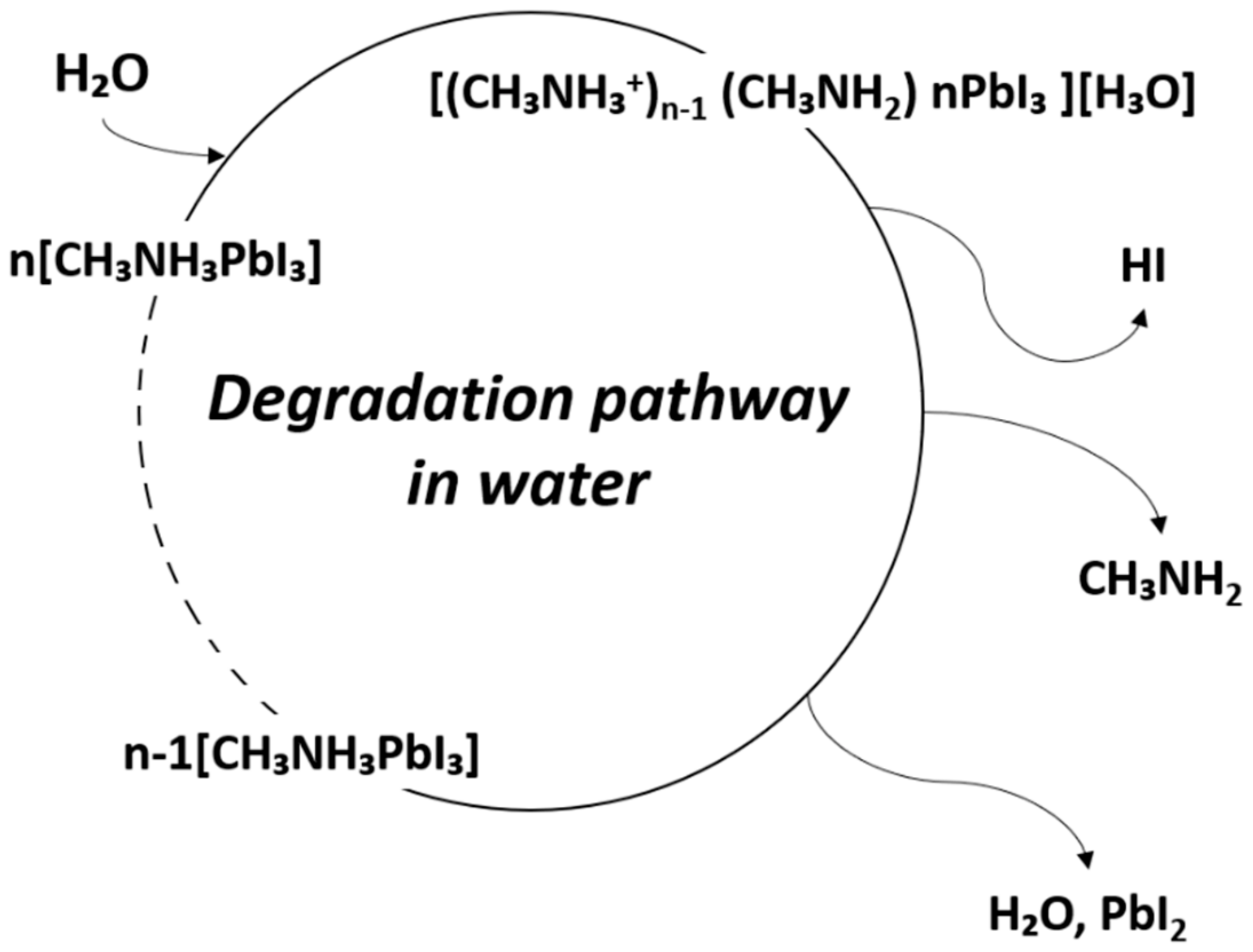
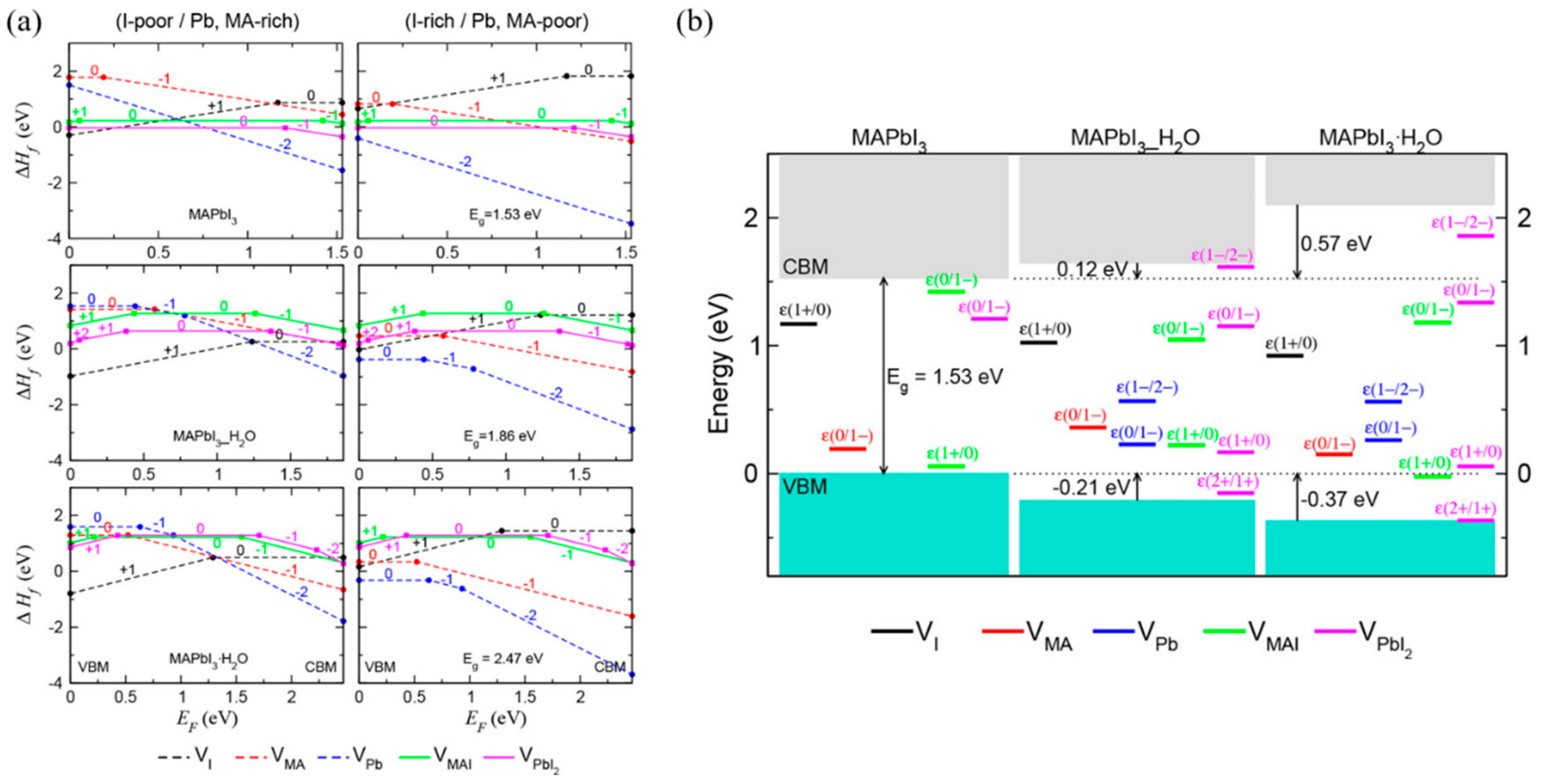
4.1. Degradation of Perovskite Material by Moisture
4.2. Passivation Strategies for Improving Stability
4.3. Enhanced Stability through the Introduction of Inorganic Perovskites
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- UNFCCC. The Paris Agreement. In Proceedings of the Paris Climate Change Conference, COP 21, Paris, France, 29 January 2016. [Google Scholar]
- Sim, U.; Moon, J.; An, J.; Kang, J.H.; Jerng, S.E.; Moon, J.; Cho, S.-P.; Hong, B.H.; Nam, K.T. N-Doped Graphene Quantum Sheets on Silicon Nanowire Photocathodes for Hydrogen Production. Energy Environ. Sci. 2015, 8, 1329–1338. [Google Scholar] [CrossRef]
- Khaselev, O.; Turner, J.A. A Monolithic Photovoltaic-Photoelectrochemical Device for Hydrogen Production Via Water Splitting. Science 1998, 280, 425–427. [Google Scholar] [CrossRef] [PubMed]
- Sim, Y.; John, J.; Surendran, S.; Moon, B.; Sim, U. Efficient Photoelectrochemical Water Splitting Reaction using Electrodeposited Co3Se4 Catalyst. Appl. Sci. 2019, 9, 16. [Google Scholar] [CrossRef]
- An, T.-Y.; Surendran, S.; Kim, H.; Choe, W.-S.; Kim, J.K.; Sim, U. A Polydopamine-Mediated Biomimetic Facile Synthesis of Molybdenum Carbide-Phosphide Nanodots Encapsulated in Carbon Shell for Electrochemical Hydrogen Evolution Reaction with Long-Term Durability. Compos. Part B Eng. 2019, 175, 107071. [Google Scholar] [CrossRef]
- Marsen, B.; Cole, B.; Miller, E.L. Photoelectrolysis of Water Using Thin Copper Gallium Diselenide Electrodes. Sol. Energy Mater. Sol. Cells 2008, 92, 1054–1058. [Google Scholar] [CrossRef]
- Goodey, A.P.; Eichfeld, S.M.; Lew, K.-K.; Redwing, J.M.; Mallouk, T.E. Silicon Nanowire Array Photoelectrochemical Cells. J. Am. Chem. Soc. 2007, 129, 12344–12345. [Google Scholar] [CrossRef]
- Sun, J.; Zhong, D.K.; Gamelin, D.R. Composite Photoanodes for Photoelectrochemical Solar Water Splitting. Energy Environ. Sci. 2010, 3, 1252–1261. [Google Scholar] [CrossRef]
- Weng, B.; Grice, C.R.; Ge, J.; Poudel, T.; Deng, X.; Yan, Y. Barium Bismuth Niobate Double Perovskite/Tungsten Oxide Nanosheet Photoanode for High-Performance Photoelectrochemical Water Splitting. Adv. Energy Mater. 2018, 8, 1701655. [Google Scholar] [CrossRef]
- Galkowski, K.; Mitioglu, A.; Miyata, A.; Plochocka, P.; Portugall, O.; Eperon, G.E.; Wang, J.T.-W.; Stergiopoulos, T.; Stranks, S.D.; Snaith, H.J. Determination of the Exciton Binding Energy and Effective Masses for Methylammonium and Formamidinium Lead Tri-Halide Perovskite Semiconductors. Energy Environ. Sci. 2016, 9, 962–970. [Google Scholar] [CrossRef]
- Yin, W.J.; Shi, T.; Yan, Y. Unique Properties of Halide Perovskites as Possible Origins of the Superior Solar Cell Performance. Adv. Mater. 2014, 26, 4653–4658. [Google Scholar] [CrossRef]
- Mitzi, D.B.; Feild, C.A.; Schlesinger, Z.; Laibowitz, R.B. Transport, Optical, and Magnetic Properties of the Conducting Halide Perovskite CH3NH3SnI3. J. Solid State Chem. 1995, 114, 159–163. [Google Scholar] [CrossRef]
- Gharibzadeh, S.; Nejand, B.A.; Moshaii, A.; Mohammadian, N.; Alizadeh, A.H.; Mohammadpour, R.; Ahmadi, V.; Alizadeh, A. Two-Step Physical Deposition of a Compact CuI Hole-Transport Layer and the Formation of an Interfacial Species in Perovskite Solar Cells. Chem. Sus. Chem. 2016, 9, 1929–1937. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Ma, J.; Xie, F.; Li, L.; Chen, J.; Fan, J.; Zhao, N. Organic Cation-Dependent Degradation Mechanism of Organotin Halide Perovskites. Adv. Funct. Mater. 2016, 26, 3417–3423. [Google Scholar] [CrossRef]
- Han, Y.; Meyer, S.; Dkhissi, Y.; Weber, K.; Pringle, J.M.; Bach, U.; Spiccia, L.; Cheng, Y.-B. Degradation Observations of Encapsulated Planar CH 3 NH 3 PbI 3 Perovskite Solar Cells at High Temperatures and Humidity. J. Mater. Chem. A 2015, 3, 8139–8147. [Google Scholar] [CrossRef]
- Sabba, D.; Kumar, M.H.; Wong, L.H.; Barber, J.; Grätzel, M.; Mathews, N. Perovskite–Hematite Tandem Cells for Efficient Overall Solar Driven Water Splitting. Nano Lett. 2015, 15, 3833–3839. [Google Scholar]
- Chen, Y.-S.; Manser, J.S.; Kamat, P.V. All Solution-Processed Lead Halide Perovskite-BiVO4 Tandem Assembly for Photolytic Solar Fuels Production. J. Am. Chem. Soc. 2015, 137, 974–981. [Google Scholar] [CrossRef]
- Qiu, Y.; Liu, W.; Chen, W.; Zhou, G.; Hsu, P.-C.; Zhang, R.; Liang, Z.; Fan, S.; Zhang, Y.; Cui, Y. Efficient Solar-Driven Water Splitting by Nanocone BiVO4-Perovskite Tandem Cells. Sci. Adv. 2016, 2, e1501764. [Google Scholar] [CrossRef]
- Milić, J.V.; Im, J.-H.; Kubicki, D.J.; Ummadisingu, A.; Seo, J.-Y.; Li, Y.; Ruiz-Preciado, M.A.; Dar, M.I.; Zakeeruddin, S.M.; Emsley, L.; et al. Supramolecular Engineering for Formamidinium-Based Layered 2D Perovskite Solar Cells: Structural Complexity and Dynamics Revealed by Solid-State NMR Spectroscopy. Adv. Eng. Mater. 2019, 9, 1900284. [Google Scholar] [CrossRef]
- Tejuca, L.G.; Fierro, J.L.G. Properties and Applications of Perovskite-Type Oxides; CRC Press: Boca Raton, FL, USA, 1992. [Google Scholar]
- Pena, M.A.; Fierro, J.L.G. Chemical Structures and Performance of Perovskite Oxides. Chem. Rev. 2001, 101, 1981–2018. [Google Scholar] [CrossRef]
- Tan, Z.-K.; Moghaddam, R.S.; Lai, M.L.; Docampo, P.; Higler, R.; Deschler, F.; Price, M.; Sadhanala, A.; Pazos, L.M.; Credgington, D. Bright Light-Emitting Diodes Based on Organometal Halide Perovskite. Nat. Nanotechnol. 2014, 9, 687. [Google Scholar] [CrossRef]
- Sutherland, B.R.; Sargent, E.H. Perovskite Photonic Sources. Nat. Photonics 2016, 10, 295. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, X.; Liang, L.; Bao, J.; Li, S.; Yang, W.; Xie, Y. High-Performance Flexible Broadband Photodetector Based on Organolead Halide Perovskite. Adv. Funct. Mater. 2014, 24, 7373–7380. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, S.; Jin, S.; Li, H.; Zhai, T. Crystal Organometal Halide Perovskites with Promising Optoelectronic Applications. J. Mater. Chem. C 2016, 4, 11–27. [Google Scholar] [CrossRef]
- Li, Z.; Yang, M.; Park, J.-S.; Wei, S.-H.; Berry, J.J.; Zhu, K. Stabilizing Perovskite Structures by Tuning Tolerance factor: Formation of Formamidinium and Cesium Lead Iodide Solid-State Alloys. Chem. Mater. 2015, 28, 284–292. [Google Scholar] [CrossRef]
- Tanaka, K.; Takahashi, T.; Ban, T.; Kondo, T.; Uchida, K.; Miura, N. Comparative Study on the Excitons in Lead-Halide-Based Perovskite-Type Crystals CH3NH3PbBr3 CH3NH3PbI3. Solid State Commun. 2003, 127, 619–623. [Google Scholar] [CrossRef]
- Wu, K.; Bera, A.; Ma, C.; Du, Y.; Yang, Y.; Li, L.; Wu, T. Temperature-Dependent Excitonic Photoluminescence of Hybrid Organometal Halide Perovskite Films. Phys. Chem. Chem. Phys. 2014, 16, 22476–22481. [Google Scholar] [CrossRef]
- Chung, I.; Song, J.-H.; Im, J.; Androulakis, J.; Malliakas, C.D.; Li, H.; Freeman, A.J.; Kenney, J.T.; Kanatzidis, M.G. CsSnI3: Semiconductor or Metal? High Electrical Conductivity and Strong Near-Infrared Photoluminescence from a Single Material. High Hole Mobility and Phase-Transitions. J. Am. Chem. Soc. 2012, 134, 8579–8587. [Google Scholar] [CrossRef]
- Eperon, G.E.; Stranks, S.D.; Menelaou, C.; Johnston, M.B.; Herz, L.M.; Snaith, H.J. Formamidinium Lead Trihalide: A Broadly Tunable Perovskite for Efficient Planar Heterojunction Solar Cells. Energy Environ. Sci. 2014, 7, 982–988. [Google Scholar] [CrossRef]
- Zhang, F.; Zhong, H.; Chen, C.; Wu, X.-G.; Hu, X.; Huang, H.; Han, J.; Zou, B.; Dong, Y. Brightly Luminescent and Color-Tunable Colloidal CH3NH3PbX3 (X = Br, I, Cl) Quantum Dots: Potential Alternatives for Display Technology. ACS Nano 2015, 9, 4533–4542. [Google Scholar] [CrossRef]
- Barugkin, C.; Cong, J.; Duong, T.; Rahman, S.; Nguyen, H.T.; Macdonald, D.; White, T.P.; Catchpole, K.R. Ultralow Absorption Coefficient and Temperature Dependence of Radiative Recombination of CH3NH3PbI3 Perovskite from Photoluminescence. J. Phys. Chem. Lett. 2015, 6, 767–772. [Google Scholar] [CrossRef]
- Alonso, M.I.; Charles, B.; Francisco-López, A.; Garriga, M.; Weller, M.T.; Goñi, A.R. Spectroscopic Ellipsometry Study of FA x MA1−x PbI3 Hybrid Perovskite Single Crystals. J. Vac. Sci. Technol. B Nanotechnol. Microelectron. Mater. Process. Meas. Phenom. 2019, 37, 062901. [Google Scholar]
- Green, M.A.; Ho-Baillie, A.; Snaith, H.J. The Emergence of Perovskite Solar Cells. Nat. Photonics 2014, 8, 506. [Google Scholar] [CrossRef]
- Chilvery, A.; Das, S.; Guggilla, P.; Brantley, C.; Sunda-Meya, A. A Perspective on the Recent Progress in Solution-Processed Methods for Highly Efficient Perovskite Solar Cells. Sci. Technol. Adv. Mater. 2016, 17, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Mitzi, D.B. Synthesis, Structure, and Properties of Organic-Inorganic Perovskites and Related Materials. Prog. Inorg. Chem. 1999, 48, 1–121. [Google Scholar]
- Kieslich, G.; Sun, S.; Cheetham, A.K. An Extended Tolerance Factor Approach for Organic–Inorganic Perovskites. Chem. Sci. 2015, 6, 3430–3433. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.; Glover, E.N.K.; Bronstein, H.; Scanlon, D.O.; Palgrave, R.G. On the Application of the Tolerance Factor to Inorganic and Hybrid Halide Perovskites: A Revised System. Chem. Sci. 2016, 7, 4548–4556. [Google Scholar] [CrossRef] [PubMed]
- Reaney, I.M.; Colla, E.L.; Setter, N. Dielectric and Structural Characteristics of Ba-And Sr-Based Complex Perovskites as a Function of Tolerance Factor. Jpn. J. Appl. Phys. 1994, 33, 3984. [Google Scholar] [CrossRef]
- Koh, T.M.; Fu, K.; Fang, Y.; Chen, S.; Sum, T.C.; Mathews, N.; Mhaisalkar, S.G.; Boix, P.P.; Baikie, T. Formamidinium-Containing Metal-Halide: An Alternative Material for Near-IR Absorption Perovskite Solar Cells. J. Phys. Chem. C 2013, 118, 16458–16462. [Google Scholar] [CrossRef]
- Stoumpos, C.C.; Malliakas, C.D.; Kanatzidis, M.G. Semiconducting Tin and Lead Iodide Perovskites with Organic Cations: Phase Transitions, High Mobilities, and Near-Infrared Photoluminescent Properties. Inorg. Chem. 2013, 52, 9019–9038. [Google Scholar] [CrossRef]
- Ball, J.M.; Lee, M.M.; Hey, A.; Snaith, H.J. Low-Temperature Processed Meso-Superstructured to Thin-Film Perovskite Solar Cells. Energy Environ. Sci. 2013, 6, 1739–1743. [Google Scholar] [CrossRef]
- Kitazawa, N.; Watanabe, Y.; Nakamura, Y. Optical Properties of CH 3 NH 3 PbX 3 (X = halogen) and Their Mixed-Halide Crystals. J. Mater. Sci. 2002, 37, 3585–3587. [Google Scholar] [CrossRef]
- Hu, F.; Zhang, H.; Sun, C.; Yin, C.; Lv, B.; Zhang, C.; Yu, W.W.; Wang, X.; Zhang, Y.; Xiao, M. Superior Optical Properties of Perovskite Nanocrystals as Single Photon Emitters. ACS Nano 2015, 9, 12410–12416. [Google Scholar] [CrossRef] [PubMed]
- D’innocenzo, V.; Grancini, G.; Alcocer, M.J.P.; Kandada, A.R.S.; Stranks, S.D.; Lee, M.M.; Lanzani, G.; Snaith, H.J.; Petrozza, A. Excitons Versus Free Charges in Organo-Lead Tri-Halide Perovskites. Nat. Commun. 2014, 5, 3586. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-C.; Wu, J.-R.; Tseng, Z.-L.; Chen, C.-C.; Chang, S.; Huang, J.-K.; Lee, K.-L.; Cheng, H.-M. Annealing Effect on (FAPbI3) 1−x (MAPbBr3) x Perovskite Films in Inverted-Type Perovskite Solar Cells. Materials 2016, 9, 747. [Google Scholar] [CrossRef]
- D’Innocenzo, V.; Srimath Kandada, A.R.; De Bastiani, M.; Gandini, M.; Petrozza, A. Tuning the Light Emission Properties by Band Gap Engineering in Hybrid Lead Halide Perovskite. J. Am. Chem. Soc. 2014, 136, 17730–17733. [Google Scholar] [CrossRef]
- Dong, Q.; Fang, Y.; Shao, Y.; Mulligan, P.; Qiu, J.; Cao, L.; Huang, J. Electron-Hole Diffusion Lengths> 175 μm in Solution-Grown CH3NH3PbI3 Single Crystals. Science 2015, 347, 967–970. [Google Scholar] [CrossRef]
- Han, Q.; Bae, S.H.; Sun, P.; Hsieh, Y.T.; Yang, Y.; Rim, Y.S.; Zhao, H.; Chen, Q.; Shi, W.; Li, G. Single Crystal Formamidinium Lead Iodide (FAPbI3): Insight into the Structural, Optical, and Electrical Properties. Adv. Mater. 2016, 28, 2253–2258. [Google Scholar] [CrossRef]
- Shi, D.; Adinolfi, V.; Comin, R.; Yuan, M.; Alarousu, E.; Buin, A.; Chen, Y.; Hoogland, S.; Rothenberger, A.; Katsiev, K. Low Trap-State Density and Long Carrier Diffusion in Organolead Trihalide Perovskite Single Crystals. Science 2015, 347, 519–522. [Google Scholar] [CrossRef]
- Milot, R.L.; Eperon, G.E.; Snaith, H.J.; Johnston, M.B.; Herz, L.M. Temperature-Dependent Charge-Carrier Dynamics in CH3NH3PbI3 Perovskite Thin Films. Adv. Funct. Mater. 2015, 25, 6218–6227. [Google Scholar] [CrossRef]
- Oga, H.; Saeki, A.; Ogomi, Y.; Hayase, S.; Seki, S. Improved Understanding of the Electronic and Energetic Landscapes of Perovskite Solar Cells: High Local Charge Carrier Mobility, Reduced Recombination, and Extremely Shallow Traps. J. Am. Chem. Soc. 2014, 136, 13818–13825. [Google Scholar] [CrossRef]
- Wehrenfennig, C.; Eperon, G.E.; Johnston, M.B.; Snaith, H.J.; Herz, L.M. High Charge Carrier Mobilities and Lifetimes in Organolead Trihalide Perovskites. Adv. Mater. 2014, 26, 1584–1589. [Google Scholar] [CrossRef] [PubMed]
- Ponseca, C.S., Jr.; Savenije, T.J.; Abdellah, M.; Zheng, K.; Yartsev, A.; Pascher, T.R.; Harlang, T.; Chabera, P.; Pullerits, T.; Stepanov, A. Organometal Halide Perovskite Solar Cell Materials Rationalized: Ultrafast Charge Generation, High and Microsecond-Long Balanced Mobilities, and Slow Recombination. J. Am. Chem. Soc. 2014, 136, 5189–5192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, M.J.; Pang, S.P.; Huang, C.S.; Zhou, Z.M.; Wang, D.; Wang, N.; Cui, G.L. Carrier Transport in CH3NH3PbI3 Films with Different Thickness for Perovskite Solar Cells. Adv. Mater. Interfaces 2016, 3, 1600327. [Google Scholar] [CrossRef]
- Kim, H.-S.; Lee, J.-W.; Yantara, N.; Boix, P.P.; Kulkarni, S.A.; Mhaisalkar, S.; Grätzel, M.; Park, N.-G. High Efficiency Solid-State Sensitized Solar Cell-Based on Submicrometer Rutile TiO2 Nanorod and CH3NH3PbI3 Perovskite Sensitizer. Nano Lett. 2013, 13, 2412–2417. [Google Scholar] [CrossRef]
- Correa-Baena, J.P.; Anaya, M.; Lozano, G.; Tress, W.; Domanski, K.; Saliba, M.; Matsui, T.; Jacobsson, T.J.; Calvo, M.E.; Abate, A. Unbroken Perovskite: Interplay of Morphology, Electro-Optical Properties, and Ionic Movement. Adv. Mater. 2016, 28, 5031–5037. [Google Scholar] [CrossRef]
- He, Z.; Zhong, C.; Huang, X.; Wong, W.Y.; Wu, H.; Chen, L.; Su, S.; Cao, Y. Simultaneous Enhancement of Open-Circuit Voltage, Short-Circuit Current Density, and Fill Factor in Polymer Solar Cells. Adv. Mater. 2011, 23, 4636–4643. [Google Scholar] [CrossRef]
- Sim, U.; Jeong, H.-Y.; Yang, T.-Y.; Nam, K.T. Nanostructural Dependence of Hydrogen Production in Silicon Photocathodes. J. Mater. Chem. A 2013, 1, 5414–5422. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, H.; Liu, P.; Shen, Y.; Cheng, C.; Hirata, A.; Fujita, T.; Tang, Z.; Chen, M. Versatile Nanoporous Bimetallic Phosphides Towards Electrochemical Water Splitting. Energy Environ. Sci. 2016, 9, 2257–2261. [Google Scholar] [CrossRef]
- Niu, F.; Wang, D.; Li, F.; Liu, Y.; Shen, S.; Meyer, T.J. Hybrid Photoelectrochemical Water Splitting Systems: From Interface Design to System Assembly. Adv. Energy Mater. 2019. [Google Scholar] [CrossRef]
- Berardi, S.; Drouet, S.; Francas, L.; Gimbert-Suriñach, C.; Guttentag, M.; Richmond, C.; Stoll, T.; Llobet, A. Molecular Artificial Photosynthesis. Chem. Soc. Rev. 2014, 43, 7501–7519. [Google Scholar] [CrossRef]
- Savini, A.; Belanzoni, P.; Bellachioma, G.; Zuccaccia, C.; Zuccaccia, D.; Macchioni, A. Activity and Degradation Pathways of Pentamethyl-Cyclopentadienyl-Iridium Catalysts for Water Oxidation. Green Chem. 2011, 13, 3360–3374. [Google Scholar] [CrossRef]
- Boix, P.P.; Nonomura, K.; Mathews, N.; Mhaisalkar, S.G. Current Progress and Future Perspectives for Organic/Inorganic Perovskite Solar Cells. Mater. Today 2014, 17, 16–23. [Google Scholar] [CrossRef]
- Sum, T.C.; Mathews, N. Advancements in Perovskite Solar Cells: Photophysics behind the Photovoltaics. Energy Environ. Sci. 2014, 7, 2518–2534. [Google Scholar] [CrossRef]
- Liu, R.; Zheng, Z.; Spurgeon, J.; Yang, X. Enhanced Photoelectrochemical Water-Splitting Performance of Semiconductors by Surface Passivation Layers. Energy Environ. Sci. 2014, 7, 2504–2517. [Google Scholar] [CrossRef]
- Leijtens, T.; Eperon, G.E.; Pathak, S.; Abate, A.; Lee, M.M.; Snaith, H.J. Overcoming Ultraviolet Light Instability of Sensitized TiO2 with Meso-Superstructured Organometal Tri-Halide Perovskite Solar Cells. Nat. Commun. 2013, 4, 2885. [Google Scholar] [CrossRef]
- Boschloo, G.; Fitzmaurice, D. Spectroelectrochemical Investigation of Surface states in Nanostructured TiO2 Electrodes. J. Phys. Chem. B 1999, 103, 2228–2231. [Google Scholar] [CrossRef]
- Choi, J.; Song, S.; Hörantner, M.T.; Snaith, H.J.; Park, T. Well-Defined Nanostructured, Single-Crystalline TiO2 Electron Transport Layer for Efficient Planar Perovskite Solar Cells. ACS Nano 2016, 10, 6029–6036. [Google Scholar] [CrossRef]
- Sun, Y.; Seo, J.H.; Takacs, C.J.; Seifter, J.; Heeger, A.J. Inverted Polymer Solar Cells Integrated with a Low-Temperature-Annealed Sol-gel-Derived ZnO Film as an Electron Transport Layer. Adv. Mater. 2011, 23, 1679–1683. [Google Scholar] [CrossRef]
- Shao, S.; Zheng, K.; Pullerits, T.; Zhang, F. Enhanced Performance of Inverted Polymer Solar Cells by Using Poly (Ethylene Oxide)-Modified ZnO as an Electron Transport layer. ACS Appl. Mater. Interfaces 2013, 5, 380–385. [Google Scholar] [CrossRef]
- Xing, G.; Mathews, N.; Sun, S.; Lim, S.S.; Lam, Y.M.; Grätzel, M.; Mhaisalkar, S.; Sum, T.C. Long-Range Balanced Electron-and Hole-Transport Lengths in Organic-Inorganic CH3NH3PbI3. Science 2013, 342, 344–347. [Google Scholar] [CrossRef]
- Kakavelakis, G.; Maksudov, T.; Konios, D.; Paradisanos, I.; Kioseoglou, G.; Stratakis, E.; Kymakis, E. Efficient and Highly Air Stable Planar Inverted Perovskite Solar Cells with Reduced Graphene Oxide Doped PCBM Electron Transporting Layer. Adv. Energy Mater. 2017, 7, 1602120. [Google Scholar] [CrossRef]
- Bai, Y.; Yu, H.; Zhu, Z.; Jiang, K.; Zhang, T.; Zhao, N.; Yang, S.; Yan, H. High Performance Inverted Structure Perovskite Solar Cells Based on a PCBM: Polystyrene Blend Electron Transport layer. J. Mater. Chem. A 2015, 3, 9098–9102. [Google Scholar] [CrossRef]
- Fabregat-Santiago, F.; Bisquert, J.; Cevey, L.; Chen, P.; Wang, M.; Zakeeruddin, S.M.; Grätzel, M. Electron Transport and Recombination in Solid-State Dye Solar Cell with Spiro-OMeTAD as Hole Conductor. J. Am. Chem. Soc. 2008, 131, 558–562. [Google Scholar] [CrossRef]
- Snaith, H.J.; Grätzel, M. Electron and Hole Transport through Mesoporous TiO2 Infiltrated with Spiro-MeOTAD. Adv. Mater. 2007, 19, 3643–3647. [Google Scholar] [CrossRef]
- Luo, H.; Lin, X.; Hou, X.; Pan, L.; Huang, S.; Chen, X. Efficient and Air-Stable Planar Perovskite Solar Cells Formed on Graphene-Oxide-Modified PEDOT: PSS Hole Transport Layer. Nano-Micro Lett. 2017, 9, 39. [Google Scholar] [CrossRef]
- Yan, W.; Li, Y.; Li, Y.; Ye, S.; Liu, Z.; Wang, S.; Bian, Z.; Huang, C. Stable High-Performance Hybrid Perovskite Solar Cells with Ultrathin Polythiophene as Hole-Transporting Layer. Nano Res. 2015, 8, 2474–2480. [Google Scholar] [CrossRef]
- Da, P.; Cha, M.; Sun, L.; Wu, Y.; Wang, Z.-S.; Zheng, G. High-Performance Perovskite Photoanode Enabled by Ni Passivation and Catalysis. Nano Lett. 2015, 15, 3452–3457. [Google Scholar] [CrossRef]
- Nam, S.; Mai, C.T.K.; Oh, I. Ultrastable Photoelectrodes for Solar Water Splitting Based on Organic Metal Halide Perovskite Fabricated by Lift-Off Process. ACS Appl. Mater. Interfaces 2018, 10, 14659–14664. [Google Scholar] [CrossRef]
- Tao, R.; Sun, Z.; Li, F.; Fang, W.; Xu, L. Achieving Organic Metal Halide Perovskite into a Conventional Photoelectrode: Outstanding Stability in Aqueous Solution and High-Efficient Photoelectrochemical Water Splitting. ACS Appl. Energy Mater. 2019, 2, 1969–1976. [Google Scholar] [CrossRef]
- Poli, I.; Hintermair, U.; Regue, M.; Kumar, S.; Sackville, E.V.; Baker, J.; Watson, T.M.; Eslava, S.; Cameron, P.J. Graphite-Protected CsPbBr3 Perovskite Photoanodes Functionalised with Water Oxidation Catalyst for Oxygen Evolution in Water. Nat. Commun. 2019, 10, 2097. [Google Scholar] [CrossRef]
- Crespo-Quesada, M.; Pazos-Outón, L.M.; Warnan, J.; Kuehnel, M.F.; Friend, R.H.; Reisner, E. Metal-Encapsulated Organolead Halide Perovskite Photocathode for Solar-Driven Hydrogen Evolution in Water. Nat. Commun. 2016, 7, 12555. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, Z.; Yu, W.; Wang, H.; Ma, W.; Zong, X.; Li, C. A Sandwich-Like Organolead Halide Perovskite Photocathode for Efficient and Durable Photoelectrochemical Hydrogen Evolution in Water. Adv. Energy Mater. 2018, 8, 1800795. [Google Scholar] [CrossRef]
- Gao, L.-F.; Luo, W.-J.; Yao, Y.-F.; Zou, Z.-G. An All-Inorganic Lead Halide Perovskite-Based Photocathode for Stable Water Reduction. Chem. Commun. 2018, 54, 11459–11462. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.S.; Pellin, M.J.; Martinson, A.B.F. Acid-Compatible Halide Perovskite Photocathodes Utilizing Atomic Layer Deposited TiO2 for Solar-Driven Hydrogen Evolution. ACS Energy Lett. 2019, 4, 293–298. [Google Scholar] [CrossRef]
- Hoang, M.T.; Pham, N.D.; Han, J.H.; Gardner, J.M.; Oh, I. Integrated Photoelectrolysis of Water Implemented on Organic Metal Halide Perovskite Photoelectrode. ACS Appl. Mater. Interfaces 2016, 8, 11904–11909. [Google Scholar] [CrossRef]
- Ahmad, S.; Sadhanala, A.; Hoye, R.L.Z.; Andrei, V.; Modarres, M.H.; Zhao, B.; Rongé, J.; Friend, R.H.; De Volder, M. Triple Cation based Perovskite Photocathodes with AZO Protective Layer for Hydrogen Production Applications. ACS Appl. Mater. Interfaces 2019, 11. [Google Scholar] [CrossRef]
- Suarez, B.; Gonzalez-Pedro, V.; Ripolles, T.S.; Sanchez, R.S.; Otero, L.; Mora-Sero, I. Recombination Study of Combined Halides (Cl, Br, I) Perovskite Solar Cells. J. Phys. Chem. Lett. 2014, 5, 1628–1635. [Google Scholar] [CrossRef]
- Christians, J.A.; Miranda Herrera, P.A.; Kamat, P.V. Transformation of the Excited State and Photovoltaic Efficiency of CH3NH3PbI3 Perovskite upon Controlled Exposure to Humidified Air. J. Am. Chem. Soc. 2015, 137, 1530–1538. [Google Scholar] [CrossRef]
- Leijtens, T.; Eperon, G.E.; Noel, N.K.; Habisreutinger, S.N.; Petrozza, A.; Snaith, H.J. Stability of Metal Halide Perovskite Solar Cells. Adv. Energy Mater. 2015, 5, 1500963. [Google Scholar] [CrossRef]
- Kye, Y.-H.; Yu, C.-J.; Jong, U.-G.; Chen, Y.; Walsh, A. Critical Role of Water in Defect Aggregation and Chemical Degradation of Perovskite Solar Cells. J. Phys. Chem. Lett. 2018, 9, 2196–2201. [Google Scholar] [CrossRef]
- Park, S.; Chang, W.J.; Lee, C.W.; Park, S.; Ahn, H.-Y.; Nam, K.T. Photocatalytic Hydrogen Generation from Hydriodic Acid Using Methylammonium Lead Iodide in Dynamic Equilibrium with Aqueous Solution. Nat. Energy 2017, 2, 16185. [Google Scholar] [CrossRef]
- Guarnera, S.; Abate, A.; Zhang, W.; Foster, J.M.; Richardson, G.; Petrozza, A.; Snaith, H.J. Improving the Long-Term Stability of Perovskite Solar Cells with a Porous Al2O3 Buffer Layer. J. Phys. Chem. Lett. 2015, 6, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Fang, X.; Lv, M.; Lin, B.; Zhang, S.; Ding, J.; Yuan, N. Improvement of the Humidity Stability of Organic-Inorganic Perovskite Solar Cells Using Ultrathin Al2O3 Layers Prepared by Atomic Layer Deposition. J. Mater. Chem. A 2015, 3, 5360–5367. [Google Scholar] [CrossRef]
- Liu, J.; Wu, Y.; Qin, C.; Yang, X.; Yasuda, T.; Islam, A.; Zhang, K.; Peng, W.; Chen, W.; Han, L. A Dopant-Free Hole-Transporting Material for Efficient and Stable Perovskite Solar Cells. Energy Environ. Sci. 2014, 7, 2963–2967. [Google Scholar] [CrossRef]
- Mei, A.; Li, X.; Liu, L.; Ku, Z.; Liu, T.; Rong, Y.; Xu, M.; Hu, M.; Chen, J.; Yang, Y. A Hole-Conductor–Free, Fully Printable Mesoscopic Perovskite Solar Cell with High Stability. Science 2014, 345, 295–298. [Google Scholar] [CrossRef]
- Dang, T.C.; Le, H.C.; Pham, D.L.; Nguyen, S.H.; Nguyen, T.T.O.; Nguyen, T.T.; Dai Nguyen, T. Synthesis of Perovskite Cs2SnI6 Film Via the Solution Processed Approach: First Study on the Photoelectrochemical Water Splitting Application. J. Alloys Compd. 2019, 805, 847–851. [Google Scholar] [CrossRef]
- Weng, B.; Xiao, Z.; Meng, W.; Grice, C.R.; Poudel, T.; Deng, X.; Yan, Y. Bandgap Engineering of Barium Bismuth Niobate Double Perovskite for Photoelectrochemical Water Oxidation. Adv. Energy Mater. 2017, 7, 1602260. [Google Scholar] [CrossRef]
- Bin, A.R.; Yusoff, M.; Jang, J. Highly Efficient Photoelectrochemical Water Splitting by a Hybrid Tandem Perovskite Solar Cell. Chem. Commun. 2016, 52, 5824–5827. [Google Scholar] [CrossRef]

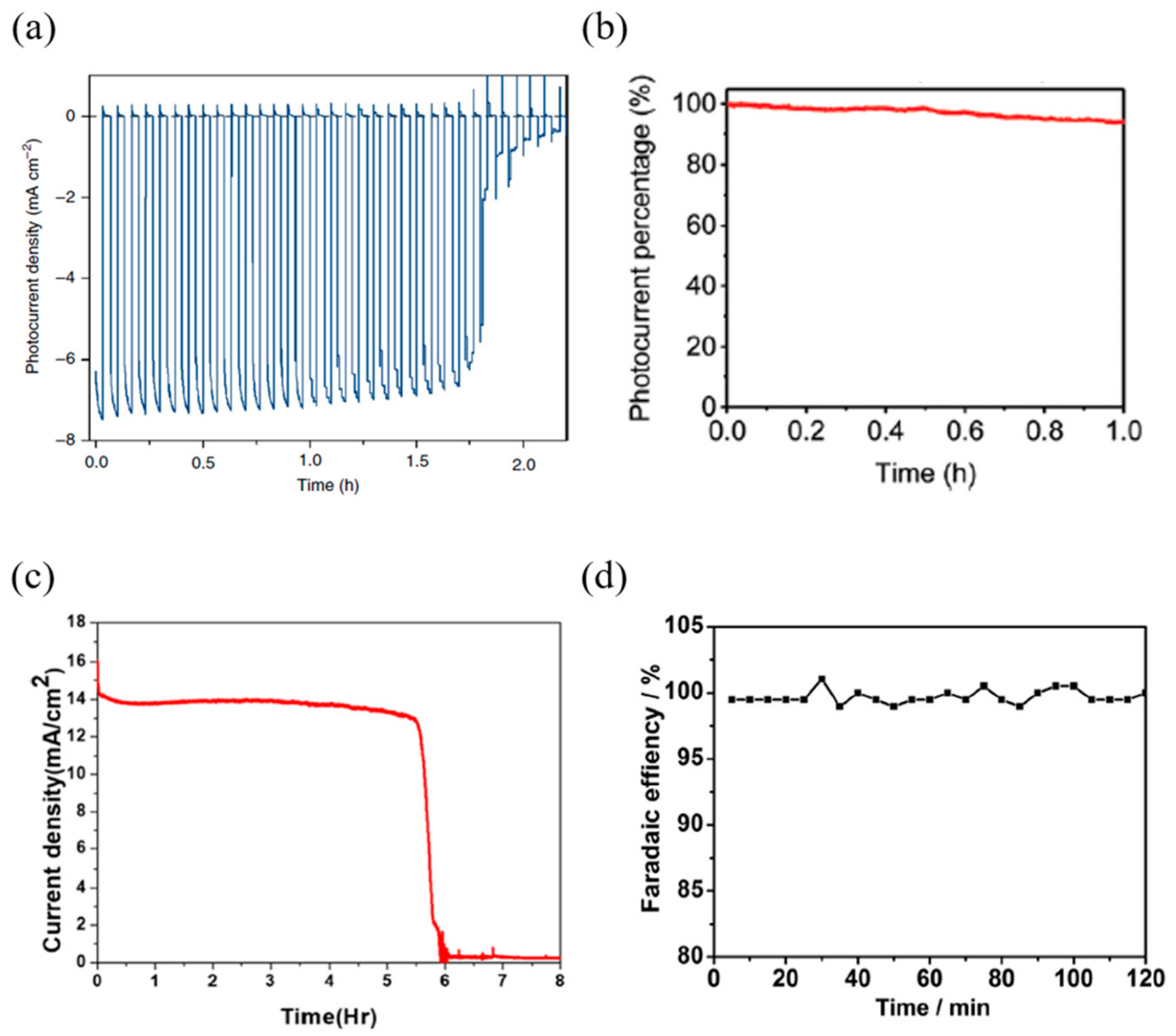
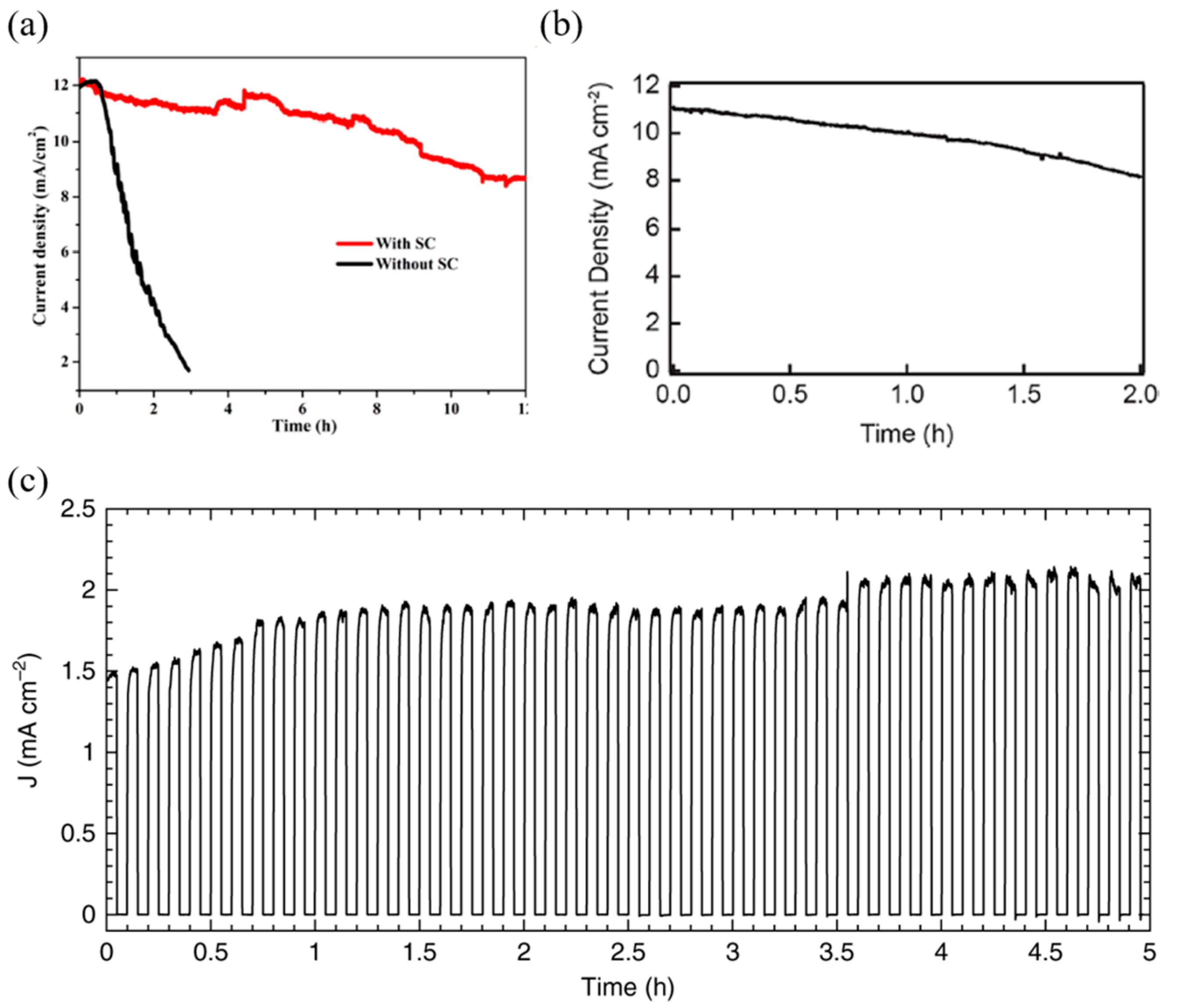
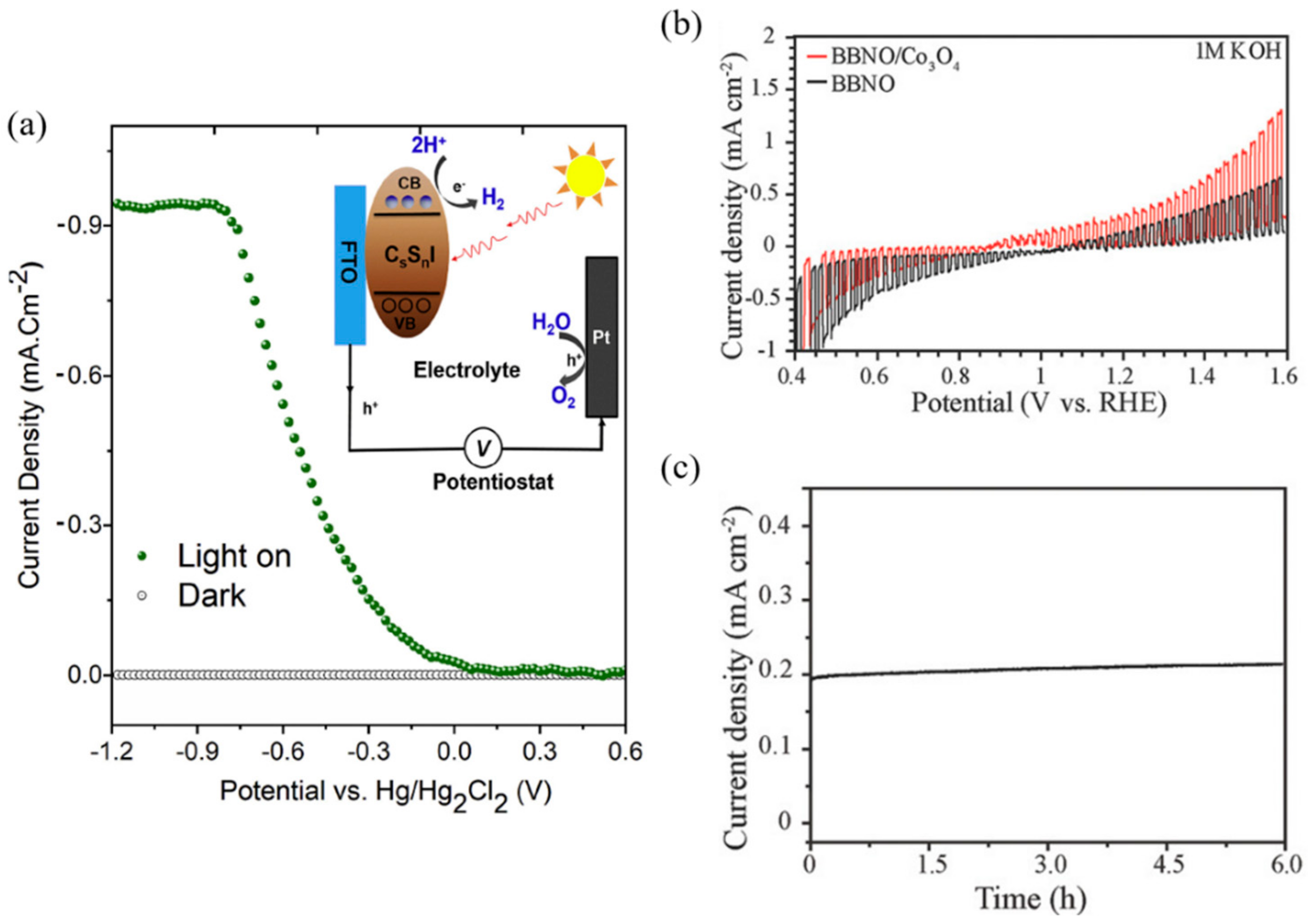
| Device Configuration | Passivation Method | Jsc | Voc | FF | PCE | Over Potential | Onset Potential | Stability | Photocurrent | Faradaic Efficiency | Ref | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n–i–p type | FTO/TiO2/MAPbI3/Spiro-OMeTAD/Au/Ni | Ni sputtering | 19 mA/cm2 | 0.95 V | 0.59 | 10% | −0.45 VAg/AgCl * | N/A | 100 s—50% | 10 mA/cm2 (at 0 VAg/AgCl) | N/A | [79] |
| FTO/TiO2/MAPbI3/Spiro-OMeTAD/Au/Field’s metal/Ni | Field’s metal encapsulation | 21 mA/cm2 | 0.98 V | 0.78 | 15.60% | −0.489 VRHE | 0.75 VRHE * | 5.5 h—50% | 14 mA/cm2.(at 1.23 VRHE) | N/A | [80] | |
| FTO/TiO2/(5-AVA)x(MA)1−xPbI3/CC/SC/CC | CC/SC paste doctor blade | N/A | N/A | N/A | N/A | 0.03 VRHE * | 0.8 VRHE * | 12 h—70% | 12.4 mA/cm2 (at 1.23 VRHE) | 82% | [81] | |
| FTO/TiO2/CsPbBr3/m-carbon/GS/WOC | m-carbon/GS doctor blade | 5.05 mA/cm2 | 1.4 V | 0.749 | 5.30% | N/A | 0.6 VRHE * | 30 h—50% | 2.5 mA/cm2 (at 1.23 VRHE) | N/A | [82] | |
| FTO/TiO2/MAPbI3/spiro-OMeTAD/Ni-Au/CNT-polymer composite | CNT-polymer composite | 18.3 mA/cm2 | 1.06 V | 0.74 | 14.4% | 0.85 VRHE * | 0.3 VRHE * | 10 h * | 9.2 mA/cm2 (at 1.23 VRHE) | N/A | [87] | |
| p–i–n type | FTO/PEDOT:PSS/MAPbI3/PCBM/Ag/Field’s metal/Pt | Field’s metal encapsulation | 15 mA/cm2 | 1 V | 0.54 | 8% | −0.2 VRHE * | 0.7 VRHE * | 1.8 h—50% | −9.8 mA/cm2 (at 0 VRHE) | 95.10% | [31] |
| ITO/NiO/MAPbI3/PCBM/Ag/Silver paint/Ti foil/Pt | Ti foil deposition | 20.38 mA/cm2 | 1.09 V | 0.729 | 16.10% | 0.69 VRHE * | 0.95 VRHE | 12 h | −18 mA/cm2 (at 0 VRHE) | 100%* | [84] | |
| FTO/NiO/CsPbBr3/ZnO/Ag/Field’s metal/Pt | Field’s metal encapsulation | 2.5 mA/cm2 | 1.37 V | 0.558 | 1.91% | N/A | 1.16 VRHE | 1 h—94% | −1.2 mA/cm2 (at 0 VRHE) | 90% | [85] | |
| ITO/PEDOT:PSS/Cs0.05(MA0.18FA0.83) 0.95Pb(I0.83Br0.17)3/PCBM/TiO2/Pt | TiO2 Atomic Layer Deposition | 21.4 mA/cm2 | 0.73 V | 0.655 | 9.63% | −0.05 VRHE | 0.68 VRHE | 2 h—75% | −10.5 mA/cm2 (at 0 VRHE) | N/A | [86] | |
| ITO/NiOx/CsFAMAPbI3/PCBM/AZO/FM/Pt | Field’s metal encapsulation | 23.15 mA/cm2 | 0.97 V | 0.814 | 18.20% | 0.3 VRHE * | 0.7 VRHE * | 18 h * | −14.3 mA/cm2 (at 0 VRHE) | 85% * | [88] | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.; Lee, D.-K.; Kim, S.M.; Park, W.; Sim, U. Photoelectrochemical Water Splitting Reaction System Based on Metal-Organic Halide Perovskites. Materials 2020, 13, 210. https://doi.org/10.3390/ma13010210
Kim D, Lee D-K, Kim SM, Park W, Sim U. Photoelectrochemical Water Splitting Reaction System Based on Metal-Organic Halide Perovskites. Materials. 2020; 13(1):210. https://doi.org/10.3390/ma13010210
Chicago/Turabian StyleKim, Dohun, Dong-Kyu Lee, Seong Min Kim, Woosung Park, and Uk Sim. 2020. "Photoelectrochemical Water Splitting Reaction System Based on Metal-Organic Halide Perovskites" Materials 13, no. 1: 210. https://doi.org/10.3390/ma13010210
APA StyleKim, D., Lee, D.-K., Kim, S. M., Park, W., & Sim, U. (2020). Photoelectrochemical Water Splitting Reaction System Based on Metal-Organic Halide Perovskites. Materials, 13(1), 210. https://doi.org/10.3390/ma13010210






