Abstract
A universal increase in energy consumption and the dependency on fossil fuels have resulted in increasing severity of global warming, thus necessitating the search of new and environment-friendly energy sources. Hydrogen is as one of the energy sources that can resolve the abovementioned problems. Water splitting promotes ecofriendly hydrogen production without the formation of any greenhouse gas. The most common process for hydrogen production is electrolysis, wherein water molecules are separated into hydrogen and oxygen through electrochemical reactions. Solar-energy-induced chemical reactions, including photocatalysis and photoelectrochemistry, have gained considerable attention because of the simplicity of their procedures and use of solar radiation as the energy source. To improve performance of water splitting reactions, the use of catalysts has been widely investigated. For example, the novel-metal catalysts possessing extremely high catalytic properties for various reactions have been considered. However, due to the rarity and high costs of the novel-metal materials, the catalysts were considered unsuitable for universal use. Although other transition-metal-based materials have also been investigated, carbon-based materials, which are obtained from one of the most common elements on Earth, have potential as low-cost, nontoxic, high-performance catalysts for both photo and electrochemical reactions. Because abundancy, simplicity of synthesis routes, and excellent performance are the important factors for catalysts, easy optimization and many variations are possible in carbon-materials, making them more attractive. In particular, low-dimensional carbon materials, such as graphene and graphitic carbon nitride, exhibit excellent performance because of their unique electrical, mechanical, and catalytic properties. In this mini-review, we will discuss the performance of low-dimensional carbon-based materials for water splitting reactions.
1. Introduction
Increasing energy consumption rates and dependency on fossil fuels have resulted in wide-spread ecological contamination. The dependency on fossil fuels can be reduced by encouraging the use of hydrogen as an energy resource. Hydrogen has the potential to be an ideal energy carrier because it theoretically possesses high gravimetric energy density, can be produced easily through various methods, and, most significantly, produces zero CO2 emission rates. Hence, the use of hydrogen as an energy source has the potential to significantly lower current global pollution rates. In addition, water splitting systems that involve splitting of water into hydrogen and oxygen molecules can be considered as sustainable and reliable because they can produce high-purity hydrogen and oxygen without any destructive by-products. Moreover, these sustainable water-splitting systems can be powered by existing renewable energy sources such as solar, wind, hydro, and geothermal powers. Since electrochemical water splitting was first reported, it has been widely applied. Water electrolysis, an efficient and clean technology used for the generation of high-purity hydrogen, also shows excellent adaptability. Solar-powered water splitting systems are developed through the engagement of photocatalytic and photoelectrochemical systems that effectively convert incident solar energy into chemical bonds by using light-absorbing materials [1,2,3,4].
Hydrogen is regarded as a clean solar fuel because of its high free energy content, accessibility as a water resource, and production of water and oxygen as sole byproducts [5]. Additionally, hydrogen can be stored and transported easily. Currently, hydrogen is produced on a large scale by reforming hydrocarbons or through the gasification of fossil fuels, both of which produce a large amount of carbon dioxide (CO2) [6]. The production of hydrogen from water splitting is a feasible approach to obtain an environment-friendly energy source. Water splitting is composed of two half-reactions, namely, hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) [1,4,7], described by the following equations:
The methods of water splitting systems can be separated to electrochemistry, photoelectrochemisty, and photocatalysis, like shown in Figure 1. For the electrolysis of water which is shown in the left portion of Figure 1, the standard oxidization potential of the OER is defined as 1.23 V corresponding to a relative hydrogen electrode (RHE) and the reduction potential of HER is 0 V (vs. RHE) [6]. However, in the practical water splitting process, a larger applied potential is required because of disadvantageous factors such as activation energy, ion, and gas diffusion as well as factors related to the device. This additional potential is called overpotential (η) [5]. Many studies have been conducted with the aim of finding suitable electrocatalysts that could significantly decrease this overpotential and promote the reaction rate so as to enhance the total cell efficiency [2,3].
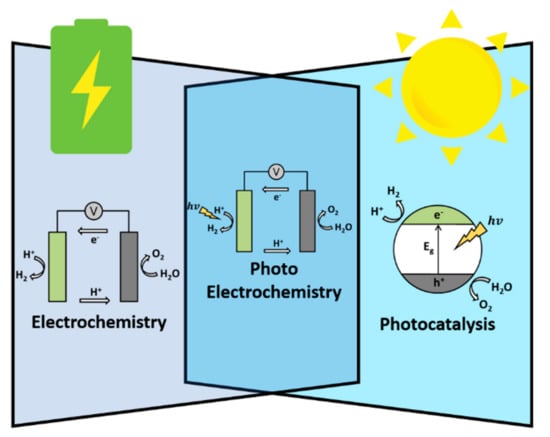
Figure 1.
Schematic illustration of the three water splitting methods.
Numerous semiconductor materials have exhibited the capability to split water under solar irradiation [8,9]. The simplest photocatalytic water splitting process, shown in right portion of Figure 1, is accomplished through the use of a single semiconductor loaded with co-catalysts as surface sites for redox reactions [10,11]. By absorbing the photon of solar irradiation, the electrons in the valence band (VB) of the semiconductor are excited to the conduction band (CB), generating electron-hole pairs (EHPs) [12]. The generated EHPs migrate to the surface sites before any catalytic reactions can occur if the potentials of the CB and VB fulfill the requirements for water reduction and water oxidation. According to the values of water oxidation and reduction potentials, a minimum bandgap energy of 1.23 eV or larger is required to split water [13]. However, in practice, a slightly larger bandgap is required due to overpotential-related losses. Photocatalytic water splitting systems are extremely simple and have lower operating costs; however, the limited efficiency of the conversion of sunlight into chemical energy due to poor charge separation and charge recombination, along with a lack of light absorption, remains a challenge to be resolved [5,14].
The PEC cell, the center portion of Figure 1, is another promising device for use in solar-driven water splitting reactions [5]. The main component of the PEC cell is the semiconductor photoelectrodes, which convert incident photons to EHPs. These electrons and holes are spatially separated from each other due to the presence of an electric field inside the semiconductor. The photogenerated electrons are swept toward the conducting back contact and are transported to the counter-electrode via an external wire [13]. Similarly, to EC, the photocathode reduces water into hydrogen, whereas photoanode oxidizes water into oxygen. The challenge remains to design a PEC device with both a highly active photoanode and photocathode [15]. Half-reaction PEC cells, composed of noble-metal-based counter electrode along with a photocathode/photoanode, are more practical as these counter electrodes are usually highly active for water splitting reactions [6,14,16].
Carbon-based materials have attracted considerable attention for use in a variety of chemical reactions, such as HER, OER, ORR owing to their low cost, high abundancy and highly stable catalytic properties [8,9,17,18]. Additionally, the low-dimensional structure of carbon-based materials, including graphene [19,20], graphene oxide (GO) [12,21], reduced graphene oxide (rGO) [22], graphitic carbon nitride (g-C3N4) [23,24], graphene quantum dots (GQDs) [25,26], and graphene quantum sheets (GQSs) [27,28], has prompted intensive interest in photocatalysis, PEC, and EC [29]. Two-dimensional (2D) layered materials, such as graphene and its family, can act as “the building blocks” for the catalysts with their ultrathin plane structure [20]. These materials are “built” and held together by van der Waals force, and, by controlling the interlayer distance, the catalytic reactions are significantly accelerated [11,30]. GQDs and GQSs are zero-dimensional carbon materials that have been attracting huge interest due to merits, including analogous structure framework and constituent units to graphene, increment of active sites, and quantum confinement effects [25]. The bandgap and the alignment of band edges of these low-dimensional materials can be easily controlled by their thickness, atomic arrangement, functionalization, or in combination with other materials. In this review, we summarize the low-dimensional carbon-based materials for the EC, PEC, and photocatalytic water splitting system [7,31].
2. Low Dimension Carbon-Based Materials
2.1. Graphene
Graphene is a monoatomic layer of an indefinite number of carbon atoms arranged in a 2D hexagonal lattice [9]. Graphene provides a non-bandgap semiconductor material with high mobility and conductivity. The hexagonal ring of sp2 hybridized carbon atoms is comprised of three strong in-plane sigma bonds and one pi bond perpendicular to the planes. The perpendicular pi(π) bond enables graphene to form graphite, which is composed of multiple layers of graphene through van der Waals forces between layers [29]. With its unique mechanical, optic, electric, and thermal properties [32], graphene has shown favorability in PEC reactions. With regard to thickness, graphene is approximately 100 times stronger than the strongest steel [33]. Nearly transparent graphene has a white-light absorption rate that is approximately 2.3%, with an ability to transmit 97.7% of incident light [32]. This optical property is well suited for use as a co-catalyst in photocatalysis and PEC systems as the incident light directed towards the photocatalyst and photoelectrode is largely unimpaired. Due to its 2-dimensional structure, graphene is the only form of carbon in which every atom is available for chemical reaction on both sides [34]. Its large exposed surface area of 2630 m2·g−1 [33,35] coupled with its 2D structure acts as an excellent platform for photoelectrochemical applications. High electronic mobility, up to 200,000 cm2·V−1·s−1 [33], enables charge transfers during redox reactions, especially for HER. Graphene is an excellent thermal conductor which is also well known for its remarkable mechanical stability and electrical conductivity [20,35,36,37].
Graphene has been applied to photocatalytic water splitting reactions as well as PEC systems due to its excellent conductive properties and large surface area. As a co-catalyst, N-doped monolayered graphene has been employed on Si photocathodes so as to investigate the catalytic activity of graphene and the effect of nitrogen doping in the PEC system. Sim et al. [38] have transferred monolayers of N-doped graphene to the surface of Si photocathodes following their growth on Cu foil. Compared to both the untreated Si photocathode and the photocathode with the undoped graphene layer, nitrogen doping showed an improvement in PEC performance with a significant positive shift in the onset potential without any changes in the saturated current density. Subsequently, the group investigated the layer dependence of graphene in HER catalysis. As shown in Figure 2a,b, a double-layer of graphene exhibits a greater anodic shift of the onset potential, thereby increasing the photon-to-current conversion efficiency. The analysis shows lower charge-transfer resistance and increments of the junction effect in the double-layer graphene/Si structure, compared to the structure with mono-/multi-layer graphene [39].
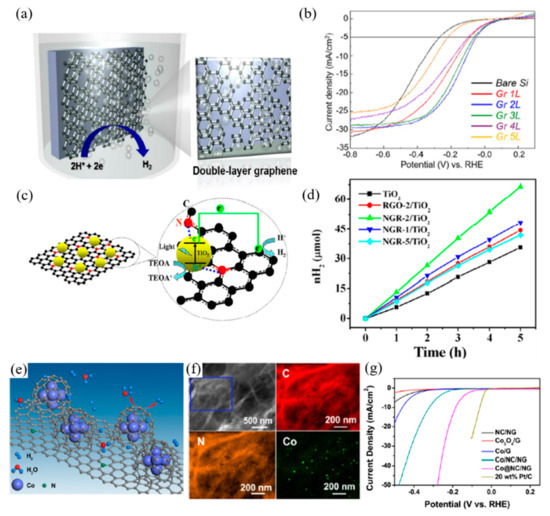
Figure 2.
Graphene applied in photoelectrochemical cells (a,b), as photocatalysts (c,d), and electrochemical cells. (e–g) for water splitting reactions. (a) Schematic of double-layer graphene on p-silicon photocathode, (b) Photocurrent density–potential (J–E) curves of multiple layers of graphene on lightly boron-doped planar p-Si [39], (c) Schematic illustration for the strong coupling between TiO2 and N atoms in N-doped graphene sheets (NGR), enhanced photoinduced charge transfer, and photocatalytic hydrogen generation, (d) The time course of hydrogen production from a 50 mL aqueous solution containing 10 vol% TEOA aqueous solution with different photocatalysts [42], (e) Schematic representation of the HER process at the surface Co/NGR, (f) EDS elemental maps of C, N, and Co for Co/NGR, (g) Polarization curves for HER in 0.5M H2SO4 at a glassy carbon electrode modified with indicated catalysts [44].
Owing to its outstanding electrical properties, graphene can effectively improve the charge separation and suppress the recombination of the excited carriers, generated by the photocatalysts or photoelectrodes. Lv et al. [40] investigated the synergetic effect of graphene with TiO2 and Cu for improved photocatalytic reaction. While the TiO2 generates electron-hole pairs under light irradiation, charge carriers are efficiently separated due to the high charge carrier transfer and mobility of graphene. PEC measurement also demonstrates an increment of photocurrent density with graphene as a co-catalyst, compared to ones without graphene. When coupled with graphene, enhancement of PEC performance of the photoelectrode materials with low diffusion lengths and low absorption rates, such as Fe2O3, was witnessed. The high conductive properties of graphene compensate for the loss of efficiency caused by low diffusion lengths. Yoon et al. [41] reported on graphene grown in an inverse opal structure and loaded with Fe2O3. The composition of Fe2O3 and inverse opal graphene reached a photocurrent density of 1.62 mA·cm−2 at 1.5 V vs. RHE, under AM 1.5 G, which is 1.38-fold higher than that of pristine Fe2O3 with 1.17 mA·cm−2. The inverse opal structure of the graphene can also play an important role in the charge transferring mechanism where it facilitates the fast transfer of electrons, generated by Fe2O3 loading.
Chemical doping of heteroatoms such as boron and nitrogen is one of the most practical approaches to modify the diverse properties of graphene. Doping can cause semiconducting behaviors in graphene with different valence electrons of the heteroatoms. Additionally, the catalytic activity of graphene towards photocatalytic reactions is enhanced due to the formation of active regions caused by asymmetric spin and charge distributions during doping. Doping with nitrogen has been considered as a means of producing an ideal photocatalyst. Nitrogen-doped graphene (NGR) has shown great potential in metal-free photocatalysts. Along with their unique semiconducting and catalytic properties, it can also be used as a supporting matrix of semiconductor-based photocatalysts in enhancing photocatalytic activity. Mou et al. [42] have synthesized NGR functionalized with TiO2 nanoparticles through a solvothermal treatment approach. Photocatalytic reaction and PEC performance were measured to investigate the role of the NGR. NGR/TiO2 composite showed a photocurrent density of 7.0 μA·cm−2, whereas the TiO2 was about 1.5 μA·cm−2. The photocurrent density of TiO2 composite with reduced graphene oxide (rGO), which was also investigated as a comparison, was approximately 4.5 μA·cm−2, which demonstrates that NGR has a higher charge transfer rate than rGO. This high charge transfer rate facilitates the efficient generated-charge separation and disables charge recombination for enhanced hydrogen evolution.
The electrochemical study of modified NGR with a transition metal was conducted for the overall water splitting reactions. Joy et al. [43] incorporated nickel on NGR nanoribbons, schematics shown in Figure 2e, to enhance the electrochemical OER. Through one-pot synthesis, nickel nanoparticles are randomly distributed on to the NGR. The nitrogen and nickel dopants open the active centers on graphene, thus improving electrocatalytic reactions. The Ni-NGR composite improved the catalytic reaction with a Tafel value of 62 mV·dec−1 and a highest current density of 120 mA·cm−2 at 1.75 V vs. RHE. Zhou et al. [44] wrapped cobalt nanoparticles with nitrogen-doped carbon on NGR for efficient hydrogen production. In the first step of synthesis, cobalt precursor and cyanamide were adsorbed onto a layer of graphene oxide, and, through thermal decomposition of cyanamide and reduction of Co, N-doped carbon is wrapped over Co nanoparticles. An onset potential of −49 mV vs. RHE was achieved by the Co/NGR with an overpotential of 10 mA cm−2 which is approximately ~200 mV with a Tafel value of 79.3 mV·dec−1.
2.2. Graphene Oxide (GO)/Reduced Graphene Oxide (rGO)
Zero bandgaps of graphene have proved challenging for their use as a photocatalyst. Efforts have been made to overcome this challenge by modifying and manipulating graphene so as to activate its surface for reactions. Graphene oxide is a derivative of graphene, tuned according to the degree of oxidation. With an extended wavelength of light absorption, graphene oxide can be used in photocatalysis for H2 production, CO2 reduction, and pollutant degradation [12,29]. Due to graphene’s zero band-gap, electron-hole pairs generated through illumination, instantaneously degenerate through recombination. Therefore, a band-gap opening is necessary in converting metallic graphene into a defective semiconductor graphene [45]. Oxidized graphene, containing oxygen functionalities, behaves as a p-type semiconductor due to lower electron mobility [35]. Oxygenated functional groups transform conductive sp2 carbons of graphene into nonconductive sp3 bonds Resulting in the creation of the band-gap opening an increase in the oxygen content stimulates the formation of sp3 carbon among the graphitic sp2 carbons, thereby enlarging the bandgap opening. The bandgap of graphene oxide has been determined to be between 2.4~4.3 eV [9]. With the increment of oxygen contents, the valance band’s maximum position gradually changes while the position of the conduction band minimum remains unchanged. Due to their tunable properties, graphene oxide sheets exhibit multiple functionalities along with remarkable performance in solar-driven water splitting reactions [21].
With its large specific surface area and excellent electrical properties, graphene oxide is regarded as being an ideal support for catalysts. Zhou et al. [46] have grown MoS2 on graphene oxide as a hierarchical framework electrocatalyst for HER. The MoS2/GO framework hydrogels were prepared with the use of a simple hydrothermal process for a binder-free synthesis. This 3D hierarchical framework of MoS2/GO showed exhibited a specific surface area and electrical conductivity due to the π–π interaction between 2D layer properties of both MoS2 and GO. With a ratio of 2:5, MoS2 and GO respectively, showed superior catalytic activity towards HER with an overpotential of 107 mV, which is comparatively higher than that of pure GO, as shown in Figure 3b. The Tafel slope of approximately 86.3 mV·dec−1 was achieved with a 2:5 ratio. Moreover, the reaction exhibited a high level of stability with little or no change in the cathodic current during 8 h of continuous operation. The overall EC performance of the composite was superior to previous MoS2 catalyst’s performance, suggesting that GO is a well-suited framework for electrocatalysts. Hu et al. [47] have also supported MoSx on GO with different degrees of oxidation through the use of facile wet chemical methods as an electrocatalyst for HER. Appling different weight ratios of graphite and KMnO4, GO, with various degrees of oxidation, was measured with Raman spectroscopy, as shown in Figure 3c. Peaks at ~1350, 1590, and 2700 cm−1 indicate the D, G, and 2D bands of graphene, respectively. The relative intensity ratio of ID/IG shows the degree of oxidation. MoSx/GO composite with 1:4 ratio of graphite and KMnO4, respectively, denoted as MoSx/GO2 with ID/IG ratio of 0.88, showed improved HER performance, as shown in Figure 3d. A composite with GO2 exhibited the best performance improvement with an overpotential of 180 mV and a Tafel value of 60.5 mV·dec−1. The MoSx/GO2 composite has the smallest charge transfer resistance value among all of the prepared samples, owing to the rapid electron transport between the active site and the electrode. It should be noted that by modifying the degree of oxidation in GO, catalytic performance can be enhanced.
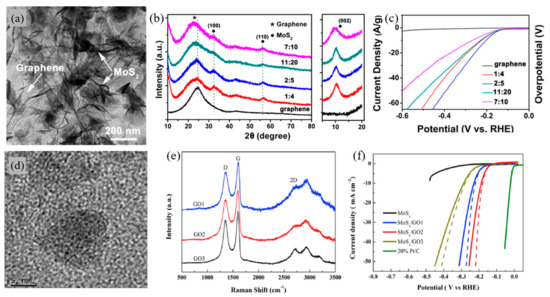
Figure 3.
Graphene oxide (GO) applied in electrochemical cells for water splitting reactions. (a) Representative TEM image of the MoS2/Graphene, (b) XRD patterns of the MoS2/graphene hierarchical frameworks at different MoS2 loadings and zoom-in of the low-angle region of the XRD profiles, (c) Polarization curves of MoS2/graphene 3D framework at different MoS2 loadings. The currents were normalized to the total mass of the catalysts [46]. (d) HR-TEM image of the MoSx/GO nanocomposite, (e) Raman spectra of different GO, (f) Linear sweep voltammogram (LSV) curves in 0.5M H2SO4 for different MoSx/GO electrocatalysts [47].
Reduced-Graphene Oxide (rGO) occurs when graphene oxide is reduced for the purposes of increasing the quantity of active sites available for photoelectrochemical reactions [48]. rGO is known to suppress charge recombination and enhance charge separation owing to its excellent electrical properties [22]. Additionally, oxygenated functional groups, like GO, have shown an increment of active sites for chemical reactions in water splitting. With a bandgap range of 1~1.69 eV, depending on the degree of reduction, rGO can form a heterojunction with other light-absorbing materials, thereby enhancing charge separation. With excellent conductive properties, rGO plays a crucial role in the PEC system, as well as EC systems. As mentioned above, the photoanode collects the hole for OER while the electrons are collected by the photocathode for the HER in the EC/PEC system. The improved charge separation with rGO suppresses the surface recombination of photogenerated charge carriers, thereby, enhancing the overall water splitting reactions [29].
Sim et al. [7] investigated the utilization of reduced graphene oxide as a cocatalyst with silicon nanowire to enhance the PEC response towards HER. SiNW has gained significant attention in the area of photon-assisted light-harvesting, owing to its narrow bandgap and its promising photovoltaic applicability. However, due to the high overpotential required for hydrogen generation, the use of the silicon electrode is highly challenging. rGO, with its exceptional PEC water splitting capability and fast-interfacial charge transfer, is a suitable co-catalyst. rGO displayed remarkable catalytic activity towards PEC HER on SiNW photocathodes, as shown in Figure 4b, with a solar-to-hydrogen (STH) efficiency rate 3.47 times higher than bare SiNW. The overpotential of rGO-SiNW measured 0.239 V vs. RHE at a current density of −10 mA·cm−2 and was calculated to 3.16% of half solar-to-hydrogen conversion efficiency whereas the bare SiNW was measured at 0.09 V overpotential and 0.91% half STH. The onset potential at −1 mA·cm−2 exhibited a positive shift from 0.179 V of SiNW to 0.326 V of rGO-SiNW, thereby proving an enhancement of PEC HER.
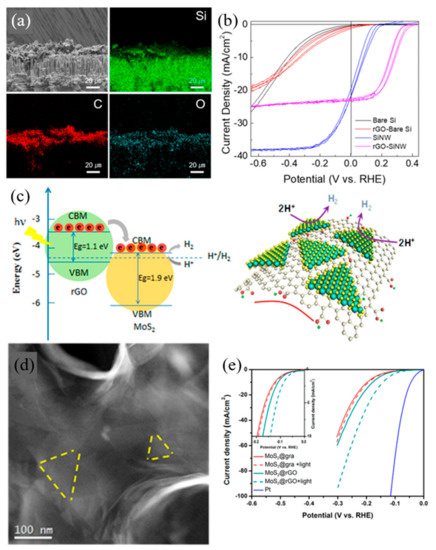
Figure 4.
Reduced graphene oxide (rGO) applied in photoelectrochemical cells (a,b), as photocatalysts (c), and electrochemical cells (d,e) for water splitting reactions. (a) Cross-Sectional SEM image of rGO on silicon nanowire (SiNW) photoelectrode and corresponding EDS elemental mapping describing the presence of Si, C and O, (b) Photoelectrochemical responses for bare Si, SiNW, rGO-bare Si and rGO-SiNW. Different lines of the same color represent repeated measurements at the same condition [7], (c) Schematic illustration of band alignment and mechanism of photo-excited charge transfer in MoS2/rGO (left) and Schematic image of MoS2/rGO, (d) HAADF-STEM image showing the morphology of MoS2/rGO, (e) Polarization curves with/without light irradiation [49].
Zhang et al. [49] have worked with MoS2 and rGO heterojunctions for photo-enhanced HER, as shown in Figure 4c,d. In their work, the nanoporous heterojunctions were comprised of 3D nanoporous rGO as the light absorber and a 2D monolayer of MoS2 as the electron transfer bridge and the electrocatalyst for PEC hydrogen production, respectively. High electric conductivity and high photoresponsivity of rGO showed a synergetic effect with MoS2, the HER electrocatalyst. Also, utilizing the tunablity and gaps of rGO, is advantageous in generating a built-in electric field so as to suppress electron-hole recombination and to promote the catalytic activity of MoS2. Compared to obvious photo-enhancement with rGO, MoS2 with graphene showed a minor light response, evidence that rGO plays a crucial role in the PEC system of HER catalysis. With graphene, overpotentials at a current density of −10 mA·cm−2 were 197 mV under dark and 193 mV under one-sun illumination resulting in 4 mV of photo-enhancement. MoS2/rGO resulted in 32 mV photo-enhancement with an overpotential of 173 mV under dark, and 141 mV under illumination at −10 mA·cm−2 current density, which is eight times the enhancement of MoS2/graphene, as shown in Figure 4e.
Inorganic semiconductor photoelectrodes composed of transition metal dichalcogenides (oxides, sulfides, and selenides) such as ZnS [50], ZnO [51], Fe2O3 [52,53], have been investigated with the rGO as a cocatalyst. Even with large bandgaps these semiconductors have fast-interfacial charge transferring properties of rGO thus enhancing performance of solar-driven water splitting reactions. Hou et al. [54] have investigated the role of rGO in PEC water splitting reactions. α-Fe2O3/rGO/BiV1−xMoxO4 Core-Shell heterojunction arrays were synthesized with and without rGO. A BiV1−xMoxO4 photocatalyst absorbed the visible light thereby generating the electron-hole pair. Assisted by rGO, these electrons are then transferred through ferrite to the cathode. Due to the conductive properties of rGO, the electrons are efficiently transferred so as to inhibit the recombination of photogenerated electron-hole pairs. This mechanism was also shown with other PEC systems.
Similarly, Ning et al. [55] have investigated rGO with TiO2/NiFe-layered double hydroxide (NiFe-LDH) composite for enhanced PEC performance. To begin with, TiO2, under illumination, generates the electron-hole pairs. Following this, owing to the band energy difference and chemical binding interactions between the TiO2/rGO/NiFe-LDH components, the rapid migration of electrons to the current collector occurred due to the superior electron mobility exhibited by rGO. Simultaneously, the holes are collected to NiFe-LDH along with the oxidation of Fe, which acts as the active site for water oxidation. With the support of rGO, the PEC was greatly enhanced as compared to TiO2/NiFe-LDH composite and TiO2 with rGO only. These works have shown the role of conductive rGO in PEC water splitting reactions. The pristine TiO2 nanoarrays displayed 0.92 mA·cm−2 photocurrent density at 0.6 V (vs. SCE) which is relatively low compared to 1.18 mA·cm−2 of TiO2/NiFe-LDH, 1.5 mA·cm−2 of TiO2/rGO, and 1.74 mA·cm−2 of the ternary TiO2/rGO/NiFe-LDH nanoarrays. An overall 1.89-fold enhancement of photocurrent density was observed. The increment of photocurrent density between TiO2/LDH and TiO2/rGO/LDH can be indicated to highlight the role of rGO in this ternary nanoarray structure. 1.74 mA·cm−2 photocurrent density of TiO2/rGO/LDH is 1.47-fold higher than 1.18 mA·cm−2 of TiO2/LDH. This can also be noticed through the comparison between the TiO2/rGO and bare TiO2. TiO2/rGO has exhibited 1.63 times greater photocurrent density than bare TiO2 nanoarrays. Similar results have been shown using other metal oxide semiconductors such as α-Fe2O3 and WO3 with rGO/NiFe-LDH decorations. The improved charge transport, arising from rGO, allows for better separation of the electron-hole pair to suppress surface recombination thereby enhancing PEC systems.
2.3. Graphitic Carbon Nitride
Graphitic carbon nitride (g-C3N4) is regarded as a good photocatalyst for solar fuel conversion and pollutant degradation due to its moderate bandgap of 2.7 eV, good chemical stability under light irradiation, low cost and nontoxicity [56,57]. The pyridinic and quaternary nitrogen atoms act as active sites for chemical reactions, making g-C3N4 a favorable catalyst in water splitting reactions [36]. Considerable work has been done to optimize its performance, such as doping, forming heterojunctions [58,59], metal nanoparticle decoration, and copolymerization. A g-C3N4 powder can be easily synthesized via inexpensive raw materials, such as urea, melamine, dicyandiamide, under high temperatures, further adding to its advantages as a catalyst in water splitting reactions [23,24]. Through techniques, such as spin coating, drop-casting, and the doctor blade method, g-C3N4 powder can be deposited onto solid substrates in PEC water splitting systems [60].
Peng et al. [61] adopted the doctor blade method for the synthesis of porous carbon nitride films on a fluorine-doped tin oxide (FTO) substrate, which showed a high of 13 μA·cm−2 at 1.23 V vs. RHE, which decreased to 60% of the initial photocurrent density after 2 h during long-term measurement. Mohamed et al. [62] synthesized g-C3N4 through thermal polymerization from urea. This was then deposited onto the FTO substrate using the spin coating method. The highest photocurrent density of approximately 21 μA·cm−2 was obtained in this study at 1.23 V vs. Ag/AgCl in 0.5M Na2SO4 electrolyte. Spray coating on to FTO glass, another conventional method, was carried out by Sima et al. [63] The group synthesized g-C3N4 by pyrolysis of urea. This was then dispersed in methanol to attain exfoliated nanosheets of g-C3N4 for spray coating. As deposition is time dependent, 3 μA·cm−2 of photocurrent density was obtained at 1.23 V vs. RHE when the FTO was sprayed for 270 s. As mentioned above, doctor blade methods and spin coating methods are simple and straightforward. However, the low levels of uniformity of g-C3N4 films obtained through these methods has limited the applications of g-C3N4 in PEC systems. As a result, other experiments into obtaining a uniform coating with even coverage over the substrate have been researched. For example, thermal deposition techniques have been found to produce films with higher uniformity and coverage on the substrates.
Bian et al. [64] have introduced a thermal vapor condensation method for the deposition of g-C3N4 on a large area of different substrates. Through heating precursors, such as melamine, dicyandiamide, to a temperature greater than 300 °C, precursor vapor is generated in the crucible. Condensation of the vapor can easily deposit g-C3N4 onto the surface of the substrate, which is positioned at the top of the crucible. The g-C3N4 was also grown on FTO glass as well as ITO glass, silica, and glass. Even though the morphologies on the glass substrate were less uniform, the deposition on FTO, ITO, and silica substrates was successfully grown uniformly. The group also reported that using dicyandiamide, thiourea, and urea could not achieve a g-C3N4 film with as much uniformity as using melamine as the precursor. The g-C3N4 film grown on FTO glass, processed at 600 °C showed the best photoresponsivity with an onset potential of 0.35 V vs. RHE. The photocurrent density of 0.12 mA·cm−2 at 1.55 V vs. RHE was also achieved with Na2S as the sacrificial reagent.
Lu et al. [65] reported a thermal vapor liquid-polymerization for the framework g-C3N4 film on the FTO glass substrate directly. Through the framework film, the PEC performance of g-C3N4 was improved due to its uniform surface, increased light-harvesting ability, optical performance, and reduced recombination of photogenerated EHP. It has been indicated that using the framework structure enhances the light-harvesting ability of g-C3N4 by increasing the reflections of light as well as enhancing the photogenerated charge separation due to the faster charge transfer rate. The photocurrent density of the framework g-C3N4 film, shown in Figure 5c, reached 89 μA·cm−2, which is twice the current density of 40 μA·cm−2 in dark conditions.
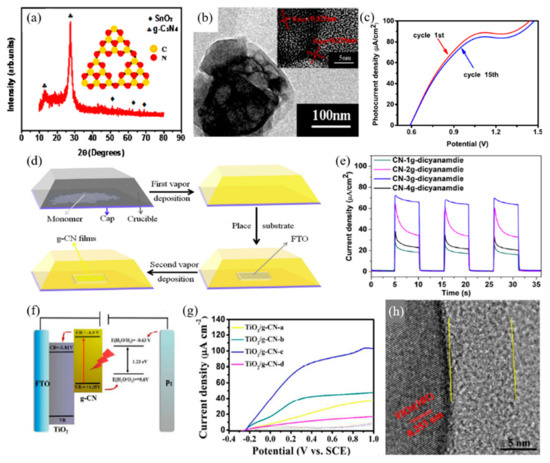
Figure 5.
Graphitic carbon nitride (g-C3N4) applied in photoelectrochemical cells for water splitting reactions. (a) XRD of the framework g-C3N4 film on FTO, inset shows the tri-s-triazine units of g-C3N4, (b) TEM image of the framework g-C3N4, inset shows the HRTEM image, (c) Linear sweep voltammograms (LSV) curve of the framework g-C3N4 film [65]. (d) Schematic diagram of the thermal vapor deposition (TVD) procedure for deposition of g-C3N4 films on FTO. (e) Transient photocurrent density under light illumination of the g-C3N4 films prepared using different amount of dicyanamide [23]. (f) Schematic diagram of PEC water oxidation mechanism on the TiO2/g-C3N4 arrays under visible light, (g) Linear sweep voltammograms (LSV) of TiO2/g-C3N4 Core-shell Arrays under illumination (λ > 420 nm) and dark states. (h) HRTEM image of TiO2/g-C3N4 composite [66].
Similarly, Lv et al. [23] reported a two-step thermal vapor deposition process of depositing g-C3N4 films. As shown in Figure 5d, the film was firstly deposited onto the inner wall of the crucible through the first TVD method, following this the g-C3N4 attached to the crucible was vaporized through the second process, allowing it to grow on the substrate. The deposition of the g-C3N4 film was determined by temperature, where the film failed to grow on the substrate below 450 °C and fully detached above 580 °C. The optimal deposition temperature was set selected at approximately 500 °C. As for the PEC properties, the quantity of precursor showed an important effect. In Figure 5e, the quantity of dicyanamide was controlled from 1 g to 4 g, with PEC performance improving as the quantity increases. However, the use of 4 g of the monomer exhibited a sharp decrease in photocurrent density where dicyanamide was used as the precursor. This two-step method exhibited the highest photocurrent density compared to melamine and cyanamide, with 63, 52 and 39 μA·cm−2, respectively. This two-step TVD deposition strategy can be employed in the synthesis of heterojunctions for the further enhancement of PEC performance.
The g-C3N4 electrodes, synthesized directly to the substrates through the thermal vapor deposition method, shows higher photocurrent density overall compared to one that has been deposited through conventional doctor blade or spin/spray coating methods. However, it should be noted that for mass production more straight forward methods are preferable as mass production through thermal vapor deposition methods is limited. Studies of g-C3N4 in forming heterojunctions with other photoelectrodes have been developed so as to enhance the efficiency of the PEC system, compensating for the loss of simplicity in the synthesis process [59]. The excellent conductive properties of g-C3N4, allowing for the formation of heterojunctions with other semiconductors, enhances charge separation and increases the water splitting reactions. Fan et al. [66] fabricated TiO2/g-C3N4 core-shell arrays to investigate the synergetic effects between TiO2 and g-C3N4 for the PEC water splitting system. Firstly, TiO2 arrays were grown on FTO glass through the hydrothermal process, then decorations of g-C3N4 layers were grown on top of TiO2 through a solvothermal process. The formation of heterojunctions between TiO2 and g-C3N4, shown in Figure 5f, allows for the efficient transfer and separation of photogenerated charge carriers for the enhancement of PEC systems. The growth of TiO2 and the quantity of g-C3N4 were studied as both can be a factor in the PEC system. With g-C3N4, the TiO2 arrays grown for 4 h showed the highest photocurrent density value of 43 μA·cm−2 at 0.6 V vs. SCE, which is 19.7 times higher than bare TiO2, and 11.7 times higher than pure g-C3N4. The quantity of g-C3N4 was controlled by the quantity of precursor used. As the amount of the precursor increases, the PEC performance tends to decrease due to the reverse recombination of photogenerated charge carriers within the overgrown g-C3N4. The addition of 1 mmol of cyanuric acid and 0.5 mmol of melamine in the deposition of g-C3N4 on top of TiO2, resulted in the highest PEC performance observed at 81 μA·cm−2, which is 36 times higher than that of pure TiO2.
A similar concept of heterojunctions of TiO2 and g-C3N4 has been raised by Liu et al. [58] through the anodizing of titanium foil to form TiO2 nanotube arrays (TNTAs) then decorating with g-C3N4. For comparison, amorphous TiO2, Ti foil without anodic oxidation treatment was also investigated. The photocurrent density of g-C3N4 decorated TNTAs (g-C3N4/TNTA) was about 0.86 mA·cm−2 at 1.23 V vs. RHE while pristine TNTAs only measured approximately 0.40 mA·cm−2 at 1.23 V. The photocurrent densities of g-C3N4decorated TiO2 and pristine TiO2 were determined to be 0.19 and 0.07 mA·cm−2, respectively. The lower current density of TiO2, compared to TNTAs, refers to the distinct photo-response of TNTAs. However, in both cases of g-C3N4/TNTAs and g-C3N4/TiO2, an increase in photocurrent density occurred with g-C3N4 decoration. The photocurrent density of g-C3N4/TNTAs was twice the density of pristine TNTA, while amorphous TiO2 increased to a 2.7-fold higher photocurrent density with the g-C3N4 decorations.
The high content of nitrogen in carbon material has facilitated high reactivity during the chemical reactions. g-C3N4 has also shown excellent performance in EC water splitting. Ma et al. [67] have conducted studies on a g-C3N4/Carbon Nanotube (CNT) three-dimensional (3D) composite for OER electrocatalysts. The 2D layer of g-C3N4 showed outstanding activity for OER originating from its high nitrogen contents, along with its unique porous structure with CNT. The composite showed an operating potential of 1.60 V vs. RHE to achieve the current density of 10 mA·cm−2 with a Tafel value of 83 mV·dec−1, which is a similar value to that of 1.59 V vs. RHE and 90 mV·dec−1 for IrO2-CNT, one of the noble-metal catalysts. The composite with 2D g-C3N4 showed improved OER performance compared to that with bulk g-C3N4, owing to an increase in surface area in the 2D structure of g-C3N4. Additionally, it can be noted that comparatively poor electrical conductivity is compensated through the smooth current transfer from the π–π interaction of the two carbon-based materials.
Sulfur-modified g-C3N4, reported by Kale et al. [3], has shown excellent catalytic properties due to minimization of the activation energy for the OER. Inspired by melamine nanogeodes (MNG), the group synthesized the g-C3N4 nanostructure from MNG powder, which was obtained through a simple hydrothermal process with melamine. Geodes are hollow, circular volcanic rocks formed in nature. The multiwalled closed cage structure of nano-sized geodes of melamine is mixed with a sulfur precursor which then undergoes a two-step heating process in order to obtain S-modified g-C3N4. The overpotential of 290 mV achieved with this structure is lower than that of the novel-metal as well as that of non-metal, metal/metal oxide catalysts.
2.4. Graphene Quantum Dots/Graphene Quantum Sheets
Graphene quantum dots (GQDs) are zero-dimensional (0D) derivatives of graphene with a diameter of up to ~20 nm [25]. Due to quantum confinement and edge effects, GQDs possess a distinct bandgap and increment of surface area [68]. They possess characteristics such as low-toxicity, chemical inertness, and biocompatibility, facilitating their application in many fields, especially those of photoelectronics and catalysis [69]. Additionally, GQD possesses a high degree of crystallinity relating to superior electron-transport capability and an enhanced carrier lifetime [70,71,72].
The electrocatalytic performance of nitrogen functionalized inter-connected GQDs (c-GQDs) was reported by Kundu et al. [73] GQDs were treated with hydrazine hydrate in acidic conditions so as to synthesize 3D c-GQDs. 0D GQDs are inter-connected through a form of hydrazine bonding, as shown in Figure 6a. The c-GQDs exhibited a smaller overpotential of 220 mV with 20% Pt/C was benchmarked at 50 mV, as shown in Figure 6b. The c-GQDs also exhibited excellent durability even after 1000 cycles. The results of synthesizing c-GQDs shows that it is a promising candidate for hydrogen production and opens new prospects for carbon-based catalysts. Lou et al. [74] reported on graphene quantum dots/gold (Au) hybrid nanoparticles as an electrocatalyst for HER, as shown in Figure 6c,d. Au nanoparticles are known for their high catalytic activity supporting metal oxide catalysts, as well as carbon-based catalysts, including GQDs. The hybrid nanoparticles showed enhanced electrochemical performance towards HER, compared to pure GQDs and pure Au nanoparticles. The Tafel value of both GQDs/Au and pure Au nanoparticle is approximately equal at 75–78 mV·dec−1. However, as shown in Figure 6e, the overpotential of 0.14 V was achieved with GQDs/Au hybrid nanoparticles for HER, whereas pure Au nanoparticles exhibit a higher overpotential of 0.27 V, indicating that hybrid nanoparticles exhibit significantly higher electrocatalytic activity than pure Au.
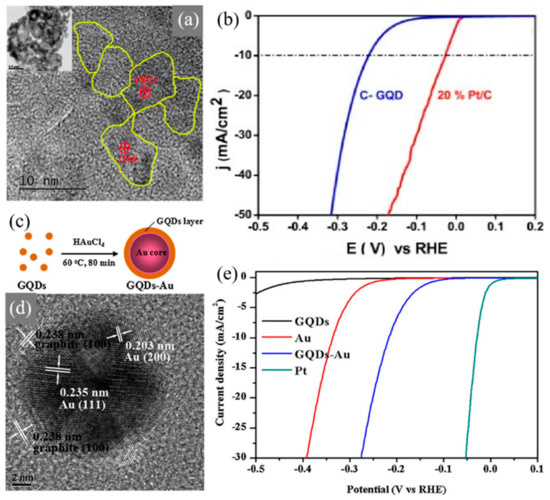
Figure 6.
Graphene quantum dots (GQDs) applied in electrochemical cells for water splitting reactions. (a) HRTEM image of inter-connected graphene quantum dots (c-GQDs), inset shows low magnification image of c-GQDs. (b) Polarization curve for 20% Pt/C and c-GQDs [73]. (c) Schematic illustration of the strategy for GQDs-Au preparation. (d) TEM image of single GQDs-Au. (e) Polarization curves obtained with several catalysts as indicated [74].
Atom-thick Graphene Quantum Sheets (GQSs) were introduced into the PEC system as a co-catalyst for the water splitting system [75]. Moon et al. [27] introduced nitrogen-doped graphene quantum sheets (N-GQSs) for solar-driven hydrogen evolution, as shown in Figure 7. N-GQSs can be synthesized through a solvent-free method, which is highly advantageous compared to the complicated wet-chemical reactions required for synthesis of GQDs. By applying nitrogen plasma to as-grown graphene on Cu, the sheet of graphene can be converted to N-GQSs. The resulting N-GQSs can be transferred as a film-like layer or easily dispersed in an organic solvent to be transferred onto a Si substrate as a PEC catalyst for HER. Sim et al. [28] applied the N-GQSs to both planar Si as well as a porous Si photoelectrode. By inducing N-GQSs, the onset potential was positively shifted by 0.29 V for planar Si, and 0.09 V for porous Si. With a potential of −5 mA·cm−2 similar values of 0.3 V and 0.11 V, planar Si and porous Si, respectively, were observed. The positive shift of the overall J–E curves induced by the N-GQSs indicates an enhancement of HER performance. The group further experimented with N-GQSs on Si nanowire (SiNW). Similarly, an enhanced HER performance can be observed with a positive shift of 0.09 V for the onset potential, in addition to a high ABPE of 2.29%, which is 2.5-fold higher than that of bare SiNW.
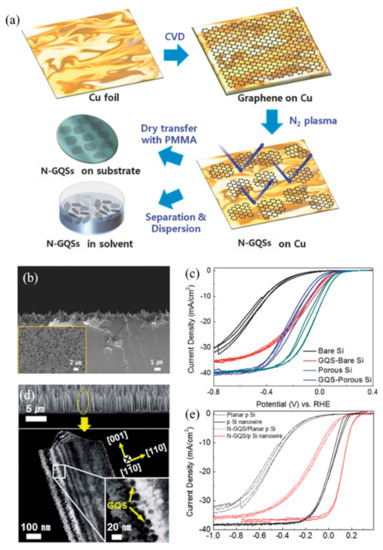
Figure 7.
Graphene quantum sheets (GQSs) applied in photoelectrochemical cells for water splitting reactions. (a) Schematic illustration of N-doped graphene quantum sheets (N-GQSs) fabrication processes. (b) Cross-section SEM image of porous Si, inset shows top-view SEM image of porous Si. (c) Cyclic Voltammetry (CV) of N-GQSs on bare Si and porous Si [27]. (d) Cross-section SEM image and dark-field TEM images of N-GQSs on p-type silicon nanowires (p-SiNWs). (e) Photocurrent density—potential (J–E) curves for lightly boron-doped planar p-Si electrode and silicon nanowire electrode deposited with N-GQSs [28].
We went through the low-dimensional carbon-based materials for the water splitting reactions. The materials are summarized below for electrochemical, photocatalysis, and photoelectrochemical system. The few key parameters measured for electrochemical systems are the Tafel slope and the overpotential to investigate the performance of the materials. Figure 8 shows a simple plot for the low-dimensional carbon-based materials in EC water splitting along with the detailed information and references in Table 1. As for the photocatalysis which is shown in Table 2, the H2 production rate and the light source are the important factor. Similar with photocatalysis, the intensity of the light source is a crucial factor in photoelectrochemical system. The onset potential measured when the current density reaches −1 mA·cm−2, and the overpotential, which is measured when the current density reaches −10 mA·cm−2, are the important factors in PEC hydrogen evolution along with the light intensity. However, in some cases where the materials show relatively low current density, the photocurrent density is measured to evaluate the performances. Likewise, the current density of the oxygen evolution performances is relatively low compared to HER, therefore the photocurrent density is measured along with the light intensity. The HER performances are shown in Table 3 while Table 4 shows the OER performances of the low-dimensional carbon-based materials.
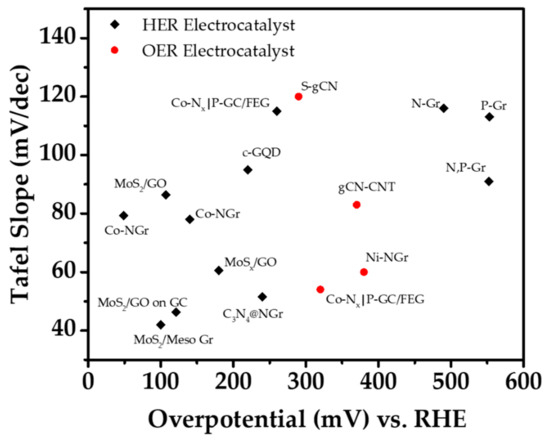
Figure 8.
Plot for carbon-based materials in electrochemical systems.

Table 1.
Carbon-based materials for electrochemical water splitting reactions.

Table 2.
Carbon-based photocatalysts for hydrogen production.

Table 3.
Carbon-based materials for photoelectrochemical HER.

Table 4.
Carbon-based materials for photoelectrochemical OER.
3. Conclusions
As the search for environment-friendly energy source progresses, hydrogen produced from water splitting reactions is proving to be a possible candidate. However, efficient energy conversion in water splitting reactions requires further optimization through the development of suitable catalysts. Novel-metal and numerous semiconductor-based materials are available for the enhancement of catalytic performance. However, low cost, non-toxic, abundant, and highly efficient catalysts are vital for their commercial use. Due to their unique electrical and catalytic properties, carbon materials exhibit outstanding performance as both catalyst and cocatalyst and are promising candidates for worldwide commercial use. Because of its layered 2D structure and π–conjugated planes, graphene exhibits excellent electrical properties, enhancing the separation of photogenerated EHPs and electron transfer for catalytic reactions. Furthermore, the transparent characteristic of graphene, makes it suitable as a cocatalyst also. The oxygenated functional groups of the GO and rGO act as active sites for the water splitting reaction with defects of the functional groups opening the bandgap thereby enabling the absorption of light energy. With the created bandgap, GO and rGO is also suitable for creating heterojunctions with other carbon materials as well as the semiconductor-based catalyst thereby improving charge separation and reducing recombination probability. The pyridinic and quaternary nitrogen atoms in g-C3N4 also act as reaction sites, similar to oxygenated functional groups in GO and rGO. The simple synthesis methods and high abundancy of these 2D materials are advantageous aspects of the multi-purpose catalyst for use in water splitting reactions. Furthermore, 0D GQDs and GQSs have also shown outstanding performance as catalysts. Due to the quantum confinement effects of these 0D materials, GQDs and GQSs exhibit increased surface area and distinct bandgaps, making them favorable catalysts.
In summary, low-dimensional carbon-based materials are being widely studied for the enhancement of photo and electrochemical water splitting reactions, as well as photocatalysis. These materials have been found to exhibit suitable characteristics for use as catalysts due to their exceptional performance and ecofriendly properties. Many derivatives of the carbon materials are available, and they have been co-opted for multiple uses. Numerous combinations of other catalysts, as well as other derivatives, are available due to the flexible optimization of the carbon materials. Low-dimensional carbon materials exhibit enormous potential as highly efficient, low cost and ecofriendly catalysts in water splitting reactions for the production of hydrogen as an energy source.
Author Contributions
Conceptualization, Y.L. and D.-K.L.; Investigation, Y.L.; Data curation, Y.L., D.-K.L. and S.M.K.; Writing—Original draft preparation, Y.L.; Writing—Review and editing, S.M.K. and D.-K.L.; Visualization, Y.L. and D.-K.L.; Supervision, W.P., S.Y.C. and U.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Basic Science Research Program through the National Research 309 Foundation of Korea funded by the Ministry of Science and ICR, grant number 2018R1A5A1025224 and the Human Resources Development program of the Korea Institute of Energy Technology Evaluation and Planning (KETEP) grant funded by the Korea government Ministry of Trade grant number 20194030202470. The work done by W.P. is supported by Sookmyung Women’s University, grant number 1-1903-2006.
Acknowledgments
The authors express sincere thanks the RFB project by Ministry of Trade, Industry and Energy.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
List of abbreviations mentioned in the main text.
| 0D | Zero-Dimensional |
| 2D | Two-Dimensional |
| 3D | Three-Dimensional |
| BVO | BiVO4 |
| CB | Conduction Band |
| c-GQD | Inter-Connected Graphene Quantum Dots |
| CN | Carbon Nitride |
| CNT | Carbon Nanotube |
| CV | Cyclic Voltammetry |
| EC | Electrochemistry/Electrochemical |
| EDS | Energy Dispersive X-ray Spectroscopy |
| EHP | Electron-Hole Pair |
| FTO | Fluorine-doped Tin Oxide |
| g-C3N4 | Graphitic Carbon Nitride |
| GO | Graphene Oxide |
| GO QD | Graphene Oxide Quantum Dot |
| GQD | Graphene Quantum Dot |
| GQS | Graphene Quantum Sheet |
| Gr | Graphene |
| HAADF-STEM | High-angle Annular Dark-field Scanning Transmission Electron Microscopy |
| HER | Hydrogen Evolution Reaction |
| HRTEM | High-resolution Transmission Electron Microscopy |
| ITO | Indium-doped Tin Oxide |
| LDH | Layered Double Hydroxide |
| LSV | Linear Sweep Voltammetry |
| MNG | Melamine Nanogeodes |
| N-GQD | N-doped Graphene Quantum Dot |
| N-GQS | N-doped Graphene Quantum Sheet |
| NGR | N-doped Graphene |
| NP | Nanoparticle |
| OER | Oxygen Evolution Reaction |
| PEC | Photoelectrochemisty / Photoelectrochemical |
| rGO | Reduced Graphene Oxide |
| SEM | Scanning Electron Microscopy |
| SiNW | Silicon Nanowire |
| TCN | Tubular Carbon Nitride |
| TEOA | Triethanolamine |
| TNTA | TiO2 Nanotube Arrays |
| TVD | Thermal Vapor Deposition |
| VB | Valance Band |
| XRD | X-ray Diffraction |
References
- Sim, Y.; John, J.; Surendran, S.; Moon, B.; Sim, U. Efficient photoelectrochemical water splitting reaction using electrodeposited Co3Se4 catalyst. Appl. Sci. 2019, 9, 16. [Google Scholar] [CrossRef]
- An, T.-Y.; Surendran, S.; Kim, H.; Choe, W.-S.; Kim, J.K.; Sim, U. A polydopamine-mediated biomimetic facile synthesis of molybdenum carbide-phosphide nanodots encapsulated in carbon shell for electrochemical hydrogen evolution reaction with long-term durability. Compos. Part B Eng. 2019, 175, 107071. [Google Scholar] [CrossRef]
- Kale, V.S.; Sim, U.; Yang, J.; Jin, K.; Chae, S.I.; Chang, W.J.; Sinha, A.K.; Ha, H.; Hwang, C.-C.; An, J.; et al. Sulfur-modified graphitic carbon nitride nanostructures as an efficient electrocatalyst for water oxidation. Small 2017, 13, 1603893. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Sim, U.; Kim, D.J.; Ahn, H.-Y.; An, J.; Ha, H.; Choi, K.S.; Jeon, C.; Lee, J.; Nam, K.T.; et al. Hierarchical carbon–silicon nanowire heterostructures for the hydrogen evolution reaction. Nanoscale 2018, 10, 13936–13941. [Google Scholar] [CrossRef]
- Qi, J.; Zhang, W.; Cao, R. Solar-to-hydrogen energy conversion based on water splitting. Adv. Energy Mater. 2018, 8, 1701620. [Google Scholar] [CrossRef]
- Sim, U.; Jin, K.; Oh, S.; Jeong, D.; Moon, J.; Oh, J.; Nam, K.T. Hydrogen production by electrolysis and photoelectrochemical system. In Handbook of Clean Energy Systems; Wiley: Hoboken, NJ, USA, 2015; pp. 1–42. [Google Scholar]
- Sim, Y.; John, J.; Moon, J.; Sim, U. Photo-assisted hydrogen evolution with reduced graphene oxide catalyst on silicon nanowire photocathode. Appl. Sci. 2018, 8, 2046. [Google Scholar] [CrossRef]
- Kumar, P.; Boukherroub, R.; Shankar, K. Sunlight-driven water-splitting using two-dimensional carbon based semiconductors. J. Mater. Chem. A 2018, 6, 12876–12931. [Google Scholar] [CrossRef]
- Albero, J.; Mateo, D.; García, H. Graphene-based materials as efficient photocatalysts for water splitting. Molecules 2019, 24, 906. [Google Scholar] [CrossRef]
- Gan, X.; Lei, D.; Wong, K.-Y. Two-dimensional layered nanomaterials for visible-light-driven photocatalytic water splitting. Mater. Today Energy 2018, 10, 352–367. [Google Scholar] [CrossRef]
- Su, T.; Shao, Q.; Qin, Z.; Guo, Z.; Wu, Z. Role of interfaces in two-dimensional photocatalyst for water splitting. ACS Catal. 2018, 8, 2253–2276. [Google Scholar] [CrossRef]
- Yeh, T.-F.; Syu, J.-M.; Cheng, C.; Chang, T.-H.; Teng, H. Graphite oxide as a photocatalyst for hydrogen production from water. Adv. Funct. Mater. 2010, 20, 2255–2262. [Google Scholar] [CrossRef]
- Jiang, C.; Moniz, S.J.A.; Wang, A.; Zhang, T.; Tang, J. Photoelectrochemical devices for solar water splitting—Materials and challenges. Chem. Soc. Rev. 2017, 46, 4645–4660. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.G.; Warren, E.L.; McKone, J.R.; Boettcher, S.W.; Mi, Q.; Santori, E.A.; Lewis, N.S. Solar water splitting cells. Chem. Rev. 2010, 110, 6446–6473. [Google Scholar] [CrossRef] [PubMed]
- Joy, J.; Mathew, J.; George, S.C. Nanomaterials for photoelectrochemical water splitting—Review. Int. J. Hydrogen Energy 2018, 43, 4804–4817. [Google Scholar] [CrossRef]
- Chen, Z.; Jaramillo, T.F.; Deutsch, T.G.; Kleiman-Shwarsctein, A.; Forman, A.J.; Gaillard, N.; Garland, R.; Takanabe, K.; Heske, C.; Sunkara, M.; et al. Accelerating materials development for photoelectrochemical hydrogen production: Standards for methods, definitions, and reporting protocols. J. Mater. Res. 2010, 25, 3–16. [Google Scholar] [CrossRef]
- Wang, X.; Vasileff, A.; Jiao, Y.; Zheng, Y.; Qiao, S.Z. Electronic and structural engineering of carbon-based metal-free electrocatalysts for water splitting. Adv. Mater. 2019, 31, 1803625. [Google Scholar] [CrossRef]
- Peng, G.; Albero, J.; Garcia, H.; Shalom, M. A water-splitting carbon nitride photoelectrochemical cell with efficient charge separation and remarkably low onset potential. Angew. Chem. 2018, 130, 16033–16037. [Google Scholar] [CrossRef]
- Singh, V.; Joung, D.; Zhai, L.; Das, S.; Khondaker, S.I.; Seal, S. Graphene based materials: Past, present and future. Prog. Mater. Sci. 2011, 56, 1178–1271. [Google Scholar] [CrossRef]
- Yazyev, O.V.; Chen, Y.P. Polycrystalline graphene and other two-dimensional materials. Nat. Nanotechnol. 2014, 9, 755–767. [Google Scholar] [CrossRef]
- Yeh, T.-F.; Cihlář, J.; Chang, C.-Y.; Cheng, C.; Teng, H. Roles of graphene oxide in photocatalytic water splitting. Mater. Today 2013, 16, 78–84. [Google Scholar] [CrossRef]
- Iwase, A.; Ng, Y.H.; Ishiguro, Y.; Kudo, A.; Amal, R. Reduced graphene oxide as a solid-state electron mediator in z-scheme photocatalytic water splitting under visible light. J. Am. Chem. Soc. 2011, 133, 11054–11057. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Cao, M.; Shi, W.; Wang, M.; Shen, Y. A new strategy of preparing uniform graphitic carbon nitride films for photoelectrochemical application. Carbon 2017, 117, 343–350. [Google Scholar] [CrossRef]
- Volokh, M.; Peng, G.; Barrio, J.; Shalom, M. Carbon nitride materials for water splitting photoelectrochemical cells. Angew. Chem. Int. Ed. 2019, 58, 6138–6151. [Google Scholar] [CrossRef]
- Yan, Y.; Chen, J.; Li, N.; Tian, J.; Li, K.; Jiang, J.; Liu, J.; Tian, Q.; Chen, P. Systematic bandgap engineering of graphene quantum dots and applications for photocatalytic water splitting and CO2 reduction. ACS Nano 2018, 12, 3523–3532. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Hou, F.; Zhang, H.; Jiang, B.; Hu, S.; Wu, B.; Wang, Y.; Fu, H. Graphene quantum-dot-modified hexagonal tubular carbon nitride for visible-light photocatalytic hydrogen evolution. Chem. Cat. Chem. 2018, 10, 1330–1335. [Google Scholar] [CrossRef]
- Moon, J.; An, J.; Sim, U.; Cho, S.-P.; Kang, J.H.; Chung, C.; Seo, J.-H.; Lee, J.; Nam, K.T.; Hong, B.H. One-step synthesis of n-doped graphene quantum sheets from monolayer graphene by nitrogen plasma. Adv. Mater. 2014, 26, 3501–3505. [Google Scholar] [CrossRef]
- Sim, U.; Moon, J.; An, J.; Kang, J.H.; Jerng, S.E.; Moon, J.; Cho, S.-P.; Hong, B.H.; Nam, K.T. N-doped graphene quantum sheets on silicon nanowire photocathodes for hydrogen production. Energy Environ. Sci. 2015, 8, 1329–1338. [Google Scholar] [CrossRef]
- Xie, G.; Zhang, K.; Guo, B.; Liu, Q.; Fang, L.; Gong, J.R. Graphene-based materials for hydrogen generation from light-driven water splitting. Adv. Mater. 2013, 25, 3820–3839. [Google Scholar] [CrossRef]
- Hasani, A.; Tekalgne, M.; Van Le, Q.; Jang, H.W.; Kim, S.Y. Two-dimensional materials as catalysts for solar fuels: Hydrogen evolution reaction and CO2 reduction. J. Mater. Chem. A 2019, 7, 430–454. [Google Scholar] [CrossRef]
- Sa, R.; Ma, Z.; Li, Q.; Wu, K. Interfacial electronic structure and charge transfer of hybrid graphene quantum dot and graphitic carbon nitride nanocomposites: Insights into high efficiency for photocatalytic solar water splitting. Phys. Chem. Chem. Phys. 2016, 18, 1050–1058. [Google Scholar]
- Apell, S.P.; Hanson, G.W.; Hägglund, C. High Optical Absorption in Grapheme. 2012. Available online: https://arxiv.org/abs/1201.3071 (accessed on 24 December 2019).
- Bunch, J.S. Mechanical and Electrical Properties of Graphene Sheets; Cornell University Ithaca: Ithaca, NY, USA, 2008. [Google Scholar]
- Li, M.; Zhang, L.; Xu, Q.; Niu, J.; Xia, Z. N-doped graphene as catalysts for oxygen reduction and oxygen evolution reactions: Theoretical considerations. J. Catal. 2014, 314, 66–72. [Google Scholar] [CrossRef]
- Xu, X.; Liu, C.; Sun, Z.; Cao, T.; Zhang, Z.; Wang, E.; Liu, Z.; Liu, K. Interfacial engineering in graphene bandgap. Chem. Soc. Rev. 2018, 47, 3059–3099. [Google Scholar] [CrossRef] [PubMed]
- Raziq, F.; Qu, Y.; Zhang, X.; Humayun, M.; Wu, J.; Zada, A.; Yu, H.-T.; Sun, X.; Jing, L. Enhanced cocatalyst-free visible-light activities for photocatalytic fuel production of g-C3N4 by trapping holes and transferring electrons. J. Phys. Chem. C 2015, 120, 98–107. [Google Scholar] [CrossRef]
- Hou, Y.; Qiu, M.; Zhang, T.; Ma, J.; Liu, S.; Zhuang, X.; Yuan, C.; Feng, X. Efficient electrochemical and photoelectrochemical water splitting by a 3D nanostructured carbon supported on flexible exfoliated graphene foil. Adv. Mater. 2017, 29, 1604480. [Google Scholar] [CrossRef] [PubMed]
- Sim, U.; Moon, J.; Lee, J.; An, J.; Ahn, H.-Y.; Kim, D.J.; Jo, I.; Jeon, C.; Han, S.; Hong, B.H.; et al. Double-layer graphene outperforming monolayer as catalyst on silicon photocathode for hydrogen production. ACS Appl. Mater. Interfaces 2017, 9, 3570–3580. [Google Scholar] [CrossRef]
- Mou, Z.; Wu, Y.; Sun, J.; Yang, P.; Du, Y.; Lu, C. TiO2 Nanoparticles-functionalized n-doped graphene with superior interfacial contact and enhanced charge separation for photocatalytic hydrogen generation. ACS Appl. Mater. Interfaces 2014, 6, 13798–13806. [Google Scholar] [CrossRef]
- Zhou, W.; Zhou, J.; Zhou, Y.; Lu, J.; Zhou, K.; Yang, L.; Tang, Z.; Li, L.; Chen, S. N-doped carbon-wrapped cobalt nanoparticles on n-doped graphene nanosheets for high-efficiency hydrogen production. Chem. Mater. 2015, 27, 2026–2032. [Google Scholar] [CrossRef]
- Sim, U.; Yang, T.-Y.; Moon, J.; An, J.; Hwang, J.; Seo, J.-H.; Lee, J.; Kim, K.Y.; Lee, J.; Han, S.; et al. N-doped monolayer graphene catalyst on silicon photocathode for hydrogen production. Energy Environ. Sci. 2013, 6, 3658. [Google Scholar] [CrossRef]
- Lv, X.-J.; Zhou, S.-X.; Zhang, C.; Chang, H.-X.; Chen, Y.; Fu, W.-F. Synergetic effect of Cu and graphene as cocatalyst on TiO2 for enhanced photocatalytic hydrogen evolution from solar water splitting. J. Mater. Chem. 2012, 22, 18542. [Google Scholar] [CrossRef]
- Yoon, K.-Y.; Lee, J.-S.; Kim, K.; Bak, C.H.; Kim, S.-I.; Kim, J.-B.; Jang, J.-H. Hematite-based photoelectrochemical water splitting supported by inverse opal structures of graphene. ACS Appl. Mater. Interfaces 2014, 6, 22634–22639. [Google Scholar] [CrossRef]
- Joy, J.; Sekar, A.; Vijayaraghavan, S.; Kumar, P.; Pillai, V.K.; Alwarappan, S. Nickel-incorporated, nitrogen-doped graphene nanoribbons as efficient electrocatalysts for oxygen evolution reaction. J. Electrochem. Soc. 2018, 165, H141–H146. [Google Scholar] [CrossRef]
- Compton, O.C.; Nguyen, S.T. Graphene oxide, highly reduced graphene oxide, and graphene: Versatile building blocks for carbon-based materials. Small 2010, 6, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhou, K.; Hou, D.; Liu, X.; Li, G.; Sang, Y.; Liu, H.; Li, L.; Chen, S. Three-dimensional hierarchical frameworks based on MoS2 nanosheets self-assembled on graphene oxide for efficient electrocatalytic hydrogen evolution. ACS Appl. Mater. Interfaces 2014, 6, 21534–21540. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.-H.; Shang, X.; Han, G.-Q.; Dong, B.; Liu, Y.-R.; Li, X.; Chai, Y.-M.; Liu, Y.-Q.; Liu, C.-G. MoSx supported graphene oxides with different degree of oxidation as efficient electrocatalysts for hydrogen evolution. Carbon 2016, 100, 236–242. [Google Scholar] [CrossRef]
- Sehrawat, P.; Islam, S.; Mishra, P.; Ahmad, S. Reduced graphene oxide (rGO) based wideband optical sensor and the role of Temperature, Defect States and Quantum Efficiency. Sci. Rep. 2018, 8, 3537. [Google Scholar]
- Zhang, Y.; Du, J.; Wang, Z.; Luo, M.; Tian, Y.; Fujita, T.; Xue, Q.; Chen, M. Three-dimensional nanoporous heterojunction of monolayer MoS2@rGO for photoenhanced hydrogen evolution reaction. ACS Appl. Energy Mater. 2018, 1, 2183–2191. [Google Scholar] [CrossRef]
- Mahvelati-Shamsabadi, T.; Goharshadi, E.K. ZnS nanospheres/reduced graphene oxide photoanode for highly efficient solar water oxidation. Sol. Energy 2018, 161, 226–234. [Google Scholar] [CrossRef]
- Ghorbani, M.; Abdizadeh, H.; Taheri, M.; Golobostanfard, M.R. Enhanced photoelectrochemical water splitting in hierarchical porous ZnO/Reduced graphene oxide nanocomposite synthesized by sol-gel method. Int. J. Hydrogen Energy 2018, 43, 7754–7763. [Google Scholar] [CrossRef]
- Nasiri, M.; Sangpour, P.; Yousefzadeh, S.; Bagheri, M. Elevated temperature annealed α-Fe2O3/reduced graphene oxide nanocomposite photoanode for photoelectrochemical water oxidation. J. Environ. Chem. Eng. 2019, 7, 102999. [Google Scholar] [CrossRef]
- Tamirat, A.G.; Su, W.-N.; Dubale, A.A.; Pan, C.-J.; Chen, H.-M.; Ayele, D.W.; Lee, J.-F.; Hwang, B.-J. Efficient photoelectrochemical water splitting using three dimensional urchin-like hematite nanostructure modified with reduced graphene oxide. J. Power Sources 2015, 287, 119–128. [Google Scholar] [CrossRef]
- Hou, Y.; Zuo, F.; Dagg, A.; Feng, P. Visible light-driven α-Fe2O3 nanorod/graphene/BiV1–xMoxO4 core/shell heterojunction array for efficient photoelectrochemical water splitting. Nano Lett. 2012, 12, 6464–6473. [Google Scholar] [CrossRef] [PubMed]
- Ning, F.; Shao, M.; Xu, S.; Fu, Y.; Zhang, R.; Wei, M.; Evans, D.G.; Duan, X. TiO2/graphene/NiFe-layered double hydroxide nanorod array photoanodes for efficient photoelectrochemical water splitting. Energy Environ. Sci. 2016, 9, 2633–2643. [Google Scholar] [CrossRef]
- Thomas, A.; Fischer, A.; Goettmann, F.; Antonietti, M.; Müller, J.-O.; Schlögl, R.; Carlsson, J.M. Graphitic carbon nitride materials: Variation of structure and morphology and their use as metal-free catalysts. J. Mater. Chem. 2008, 18, 4893. [Google Scholar] [CrossRef]
- Wang, Z.; Jin, B.; Zou, G.; Zhang, K.; Hu, X.; Park, J.H. Rationally designed copper-modified polymeric carbon nitride as a photocathode for solar water splitting. ChemSusChem 2019, 12, 866–872. [Google Scholar] [CrossRef]
- Liu, C.; Wang, F.; Zhang, J.; Wang, K.; Qiu, Y.; Liang, Q.; Chen, Z. Efficient photoelectrochemical water splitting by g-C3N4/TiO2 nanotube array heterostructures. Nano Micro Lett. 2018, 10, 37. [Google Scholar] [CrossRef]
- Fan, X.; Wang, T.; Gao, B.; Xie, X.; Zhang, S.; Meng, X.; Gong, H.; Guo, Y.; Huang, X.; He, J. Layered double hydroxides decorated graphic carbon nitride film as efficient photoanodes for photoelectrochemical water splitting. Catal. Today 2019, 335, 423–428. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Chen, Z.P.; Moehwald, H.; Fiechter, S.; van de Krol, R.; Wen, L.; Jiang, L.; Antonietti, M. Microcontact-printing-assisted access of graphitic carbon nitride films with favorable textures toward photoelectrochemical application. Adv. Mater. 2015, 27, 712–718. [Google Scholar] [CrossRef]
- Peng, G.; Xing, L.; Barrio, J.; Volokh, M.; Shalom, M. Frontispiz: A general synthesis of porous carbon nitride films with tunable surface area and photophysical properties. Angew. Chem. 2018, 130, 1186–1192. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Safaei, J.; Ismail, A.F.; Jailani, M.F.A.M.; Khalid, M.N.; Noh, M.F.M.; Aadenan, A.; Nasir, S.N.S.; Sagu, J.S.; Teridi, M.A.M. The influences of post-annealing temperatures on fabrication graphitic carbon nitride, (g-C3N4) thin film. Appl. Surf. Sci. 2019, 489, 92–100. [Google Scholar] [CrossRef]
- Sima, M.; Vasile, E.; Sima, A.; Preda, N.; Logofatu, C. Graphitic carbon nitride based photoanodes prepared by spray coating method. Int. J. Hydrogen Energy 2019, 44, 24430–24440. [Google Scholar] [CrossRef]
- Bian, J.; Li, Q.; Huang, C.; Li, J.; Guo, Y.; Zaw, M.; Zhang, R.-Q. Thermal vapor condensation of uniform graphitic carbon nitride films with remarkable photocurrent density for photoelectrochemical applications. Nano Energy 2015, 15, 353–361. [Google Scholar] [CrossRef]
- Lu, X.; Liu, Z.; Li, J.; Zhang, J.; Guo, Z. Novel framework g-C3N4 film as efficient photoanode for photoelectrochemical water splitting. Appl. Catal. B Environ. 2017, 209, 657–662. [Google Scholar] [CrossRef]
- Fan, X.; Wang, T.; Gao, B.; Gong, H.; Xue, H.; Guo, H.; Song, L.; Xia, W.; Huang, X.; He, J. Preparation of the TiO2/graphic carbon nitride core–shell array as a photoanode for efficient photoelectrochemical water splitting. Langmuir 2016, 32, 13322–13332. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.Y.; Dai, S.; Jaroniec, M.; Qiao, S.Z. Graphitic carbon nitride nanosheet–carbon nanotube three-dimensional porous composites as high-performance oxygen evolution electrocatalysts. Angew. Chem. 2014, 126, 7409–7413. [Google Scholar] [CrossRef]
- Yeh, T.-F.; Teng, C.-Y.; Chen, S.-J.; Teng, H. Nitrogen-doped graphene oxide quantum dots as photocatalysts for overall water-splitting under visible light illumination. Adv. Mater. 2014, 26, 3297–3303. [Google Scholar] [CrossRef]
- Guo, C.X.; Dong, Y.; Bin Yang, H.; Li, C.M. Graphene quantum dots as a green sensitizer to functionalize ZnO nanowire arrays on F-Doped SnO2 glass for enhanced photoelectrochemical water splitting. Adv. Energy Mater. 2013, 3, 997–1003. [Google Scholar] [CrossRef]
- Bian, S.; Zhou, C.; Li, P.; Xi, F.; Liu, J.; Dong, X. Graphene quantum dots decorated titania nanosheets heterojunction: Efficient charge separation and enhanced visible-light photocatalytic performance. ChemCatChem 2017, 9, 3349–3357. [Google Scholar] [CrossRef]
- Zeng, Z.; Li, Y.-B.; Chen, S.; Chen, P.; Xiao, F.-X. Insight into the charge transport correlation in Au x clusters and graphene quantum dots deposited on TiO2 nanotubes for photoelectrochemical oxygen evolution. J. Mater. Chem. A 2018, 6, 11154–11162. [Google Scholar] [CrossRef]
- Chan, D.K.L.; Cheung, P.L.; Yu, J.C. A visible-light-driven composite photocatalyst of TiO2 nanotube arrays and graphene quantum dots. Beilstein J. Nanotechnol. 2014, 5, 689–695. [Google Scholar] [CrossRef]
- Kundu, S.; Malik, B.; Pattanayak, D.K.; Pitchai, R.; Pillai, V.K. Unraveling the hydrogen evolution reaction active sites in n-functionalized interconnected graphene quantum dots. ChemistrySelect 2017, 2, 4511–4515. [Google Scholar] [CrossRef]
- Luo, P.; Jiang, L.; Zhang, W.; Guan, X. Graphene quantum dots/Au hybrid nanoparticles as electrocatalyst for hydrogen evolution reaction. Chem. Phys. Lett. 2015, 641, 29–32. [Google Scholar] [CrossRef]
- Yang, K.D.; Ha, Y.; Sim, U.; An, J.; Lee, C.W.; Jin, K.; Kim, Y.; Park, J.; Hong, J.S.; Lee, J.H.; et al. Graphene quantum sheet catalyzed silicon photocathode for selective CO2 conversion to CO. Adv. Funct. Mater. 2016, 26, 233–242. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiao, Y.; Li, L.H.; Xing, T.; Chen, Y.; Jaroniec, M.; Qiao, S.Z. Toward design of synergistically active carbon-based catalysts for electrocatalytic hydrogen evolution. ACS Nano 2014, 8, 5290–5296. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Jiao, Y.; Zhu, Y.; Li, L.H.; Han, Y.; Chen, Y.; Du, A.; Jaroniec, M.; Qiao, S.Z. Hydrogen evolution by a metal-free electrocatalyst. Nat. Commun. 2014, 5, 3783. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.; Zhu, J.; Bian, X.; Zhu, L.; Scanlon, M.D.; Girault, H.; Liu, B. MoS 2 formed on mesoporous graphene as a highly active catalyst for hydrogen evolution. Adv. Funct. Mater. 2013, 23, 5326–5333. [Google Scholar] [CrossRef]
- Zhang, F.-J.; Kong, C.; Li, X.; Sun, X.-Y.; Xie, W.-J.; Oh, W.-C. Synthesis and characterization of MoS2/Graphene-TiO2 ternary photocatalysts for high-efficiency hydrogen production under visible light. J. Korean Ceram. Soc. 2019, 56, 284–290. [Google Scholar] [CrossRef]
- Li, Z.; Ma, Y.; Hu, X.; Liu, E.; Fan, J. Enhanced photocatalytic H2 production over dual-cocatalyst-modified g-C3N4 heterojunctions. Chin. J. Catal. 2019, 40, 434–445. [Google Scholar] [CrossRef]
- Wu, Y.; Yan, M.; Gao, J.; Lv, P.; Liu, X.; Li, C.; Yan, Y. Fabrication of nitrogen-doped graphene quantum dots-Cu2O catalysts for enhanced photocatalytic hydrogen evolution. Nano 2018, 13, 1850099. [Google Scholar] [CrossRef]
- Huang, Z.; Zhong, P.; Wang, C.; Zhang, X.; Zhang, C. Silicon nanowires/reduced graphene oxide composites for enhanced photoelectrochemical properties. ACS Appl. Mater. Interfaces 2013, 5, 1961–1966. [Google Scholar] [CrossRef]
- Dubale, A.A.; Su, W.-N.; Tamirat, A.G.; Pan, C.-J.; Aragaw, B.A.; Chen, H.-M.; Chen, C.-H.; Hwang, B.-J. The synergetic effect of graphene on Cu2O nanowire arrays as a highly efficient hydrogen evolution photocathode in water splitting. J. Mater. Chem. A 2014, 2, 18383–18397. [Google Scholar] [CrossRef]
- Shah, A.K.; Sahu, T.K.; Banik, A.; Gogoi, D.; Peela, N.R.; Qureshi, M. Reduced graphene oxide modified CuBi2O4 as an efficient and noble metal free photocathode for superior photoelectrochemical hydrogen production. Sustain. Energy Fuels 2019, 3, 1554–1561. [Google Scholar] [CrossRef]
- Momeni, M.M.; Ghayeb, Y.; Menati, M. Fabrication, characterization and photoelectrochemical properties of cuprous oxide-reduced graphene oxide photocatalysts for hydrogen generation. J. Mater. Sci. Mater. Electron. 2018, 29, 4136–4146. [Google Scholar] [CrossRef]
- Kang, S.; Jang, J.; Pawar, R.C.; Ahn, S.; Lee, C.S. Direct coating of a g-C3N4 layer onto one-dimensional TiO2 nanocluster/nanorod films for photoactive applications. Dalton Trans. 2018, 47, 7237–7244. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, R.; Li, H.; Wei, X.; Feng, J.; Liu, K.; Dang, Y.; Zhou, A. Efficient and stable photoelectrochemical seawater splitting with TiO2@g-C3N4 nanorod arrays decorated by Co-Pi. J. Phys. Chem. C 2015, 119, 20283–20292. [Google Scholar] [CrossRef]
- Alam, K.M.; Kumar, P.; Kar, P.; Thakur, U.K.; Zeng, S.; Cui, K.; Shankar, K. Enhanced charge separation in g-C3N4–BiOI heterostructures for visible light driven photoelectrochemical water splitting. Nanoscale Adv. 2019, 1, 1460–1471. [Google Scholar] [CrossRef]
- Peng, G.; Volokh, M.; Tzadikov, J.; Sun, J.; Shalom, M. Carbon nitride/reduced graphene oxide film with enhanced electron diffusion length: An efficient photo-electrochemical cell for hydrogen generation. Adv. Energy Mater. 2018, 8, 1800566. [Google Scholar] [CrossRef]
- Soltani, T.; Tayyebi, A.; Lee, B.-K. Efficient promotion of charge separation with reduced graphene oxide (rGO) in BiVO4/rGO photoanode for greatly enhanced photoelectrochemical water splitting. Sol. Energy Mater. Sol. Cells 2018, 185, 325–332. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Hur, S.H.; Kim, E.J.; Rajagopalan, B.; Babu, K.F.; Senthilkumar, V.; Chung, J.S.; Choi, W.M.; Kim, Y.S. Highly-ordered maghemite/reduced graphene oxide nanocomposites for high-performance photoelectrochemical water splitting. RSC Adv. 2015, 5, 29159–29166. [Google Scholar] [CrossRef]
- Majumder, T.; Debnath, K.; Dhar, S.; Hmar, J.J.L.; Mondal, S.P. Nitrogen-doped graphene quantum dot-decorated ZnO nanorods for improved electrochemical solar energy conversion. Energy Technol. 2016, 4, 950–958. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).