Physical Hydrogels of Oxidized Polysaccharides and Poly(Vinyl Alcohol) for Wound Dressing Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
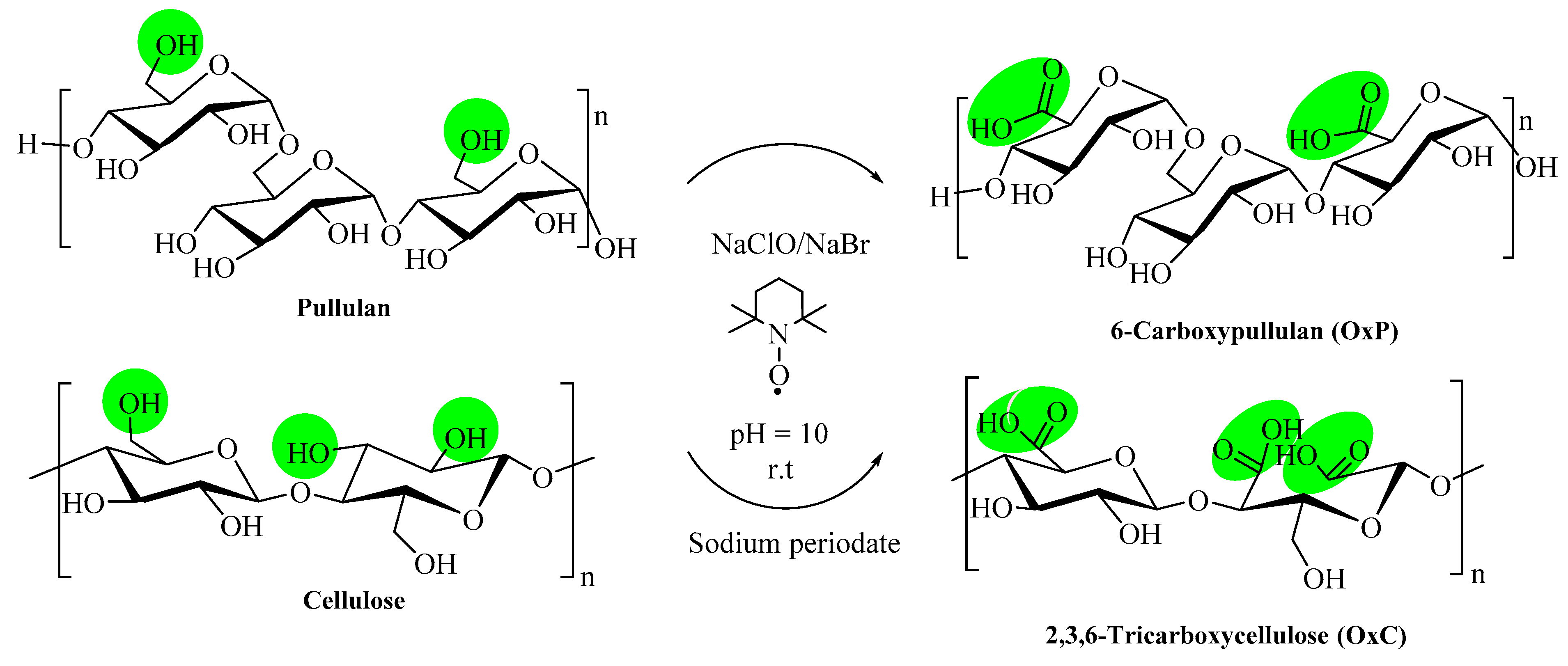
2.2. Preparation of Oxidized Polysaccharides
2.3. Preparation of the Composite Hydrogels
2.4. Fourier Transform Infrared Spectroscopy
2.5. Nuclear Magnetic Resonance Spectroscopy
2.6. Environmental Scanning Electron Microscopy
2.7. Swelling Measurements
2.8. Rheological Measurements
2.9. Cytotoxicity Tests
2.10. Loading of Hydrogels With L-Arginine
2.11. In Vitro Drug Release Studies
2.12. Analysis of In Vitro Drug Release Kinetics
3. Results and Discussions
3.1. Synthesis and Characterization of Oxidized Polysaccharide Samples
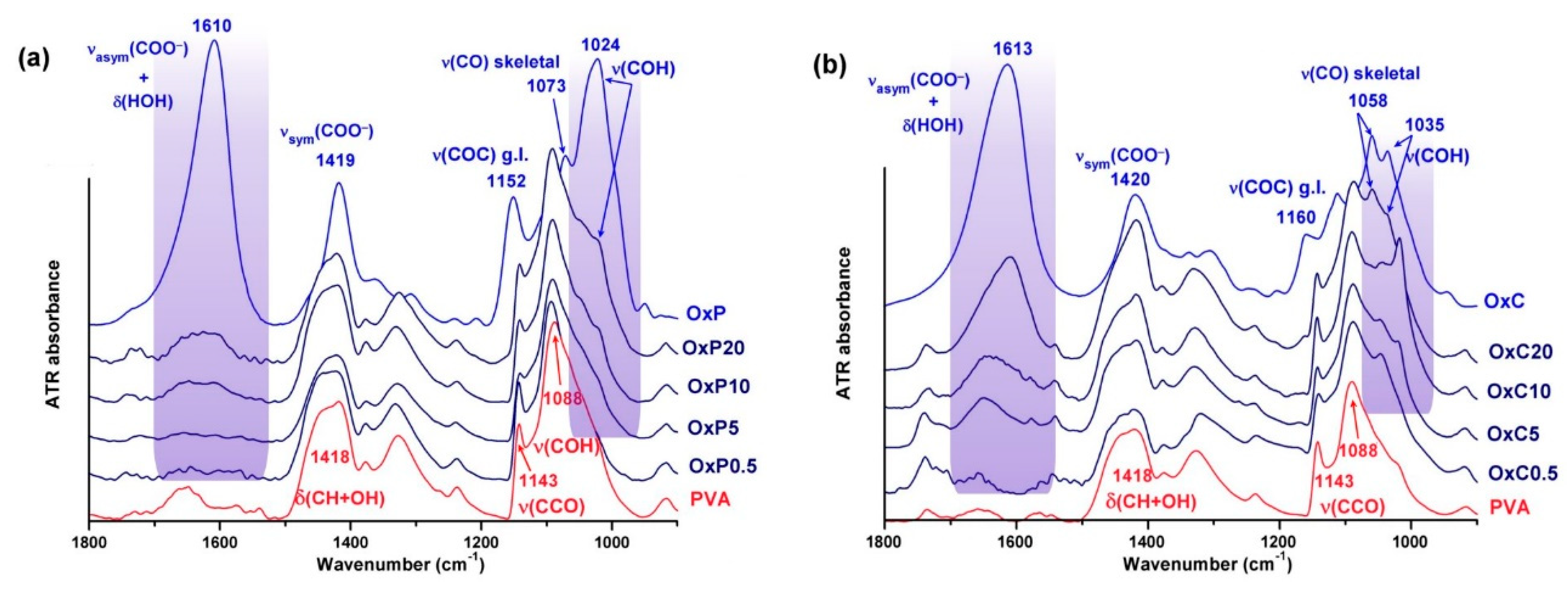
3.2. FTIR Spectra of the Composite Hydrogels
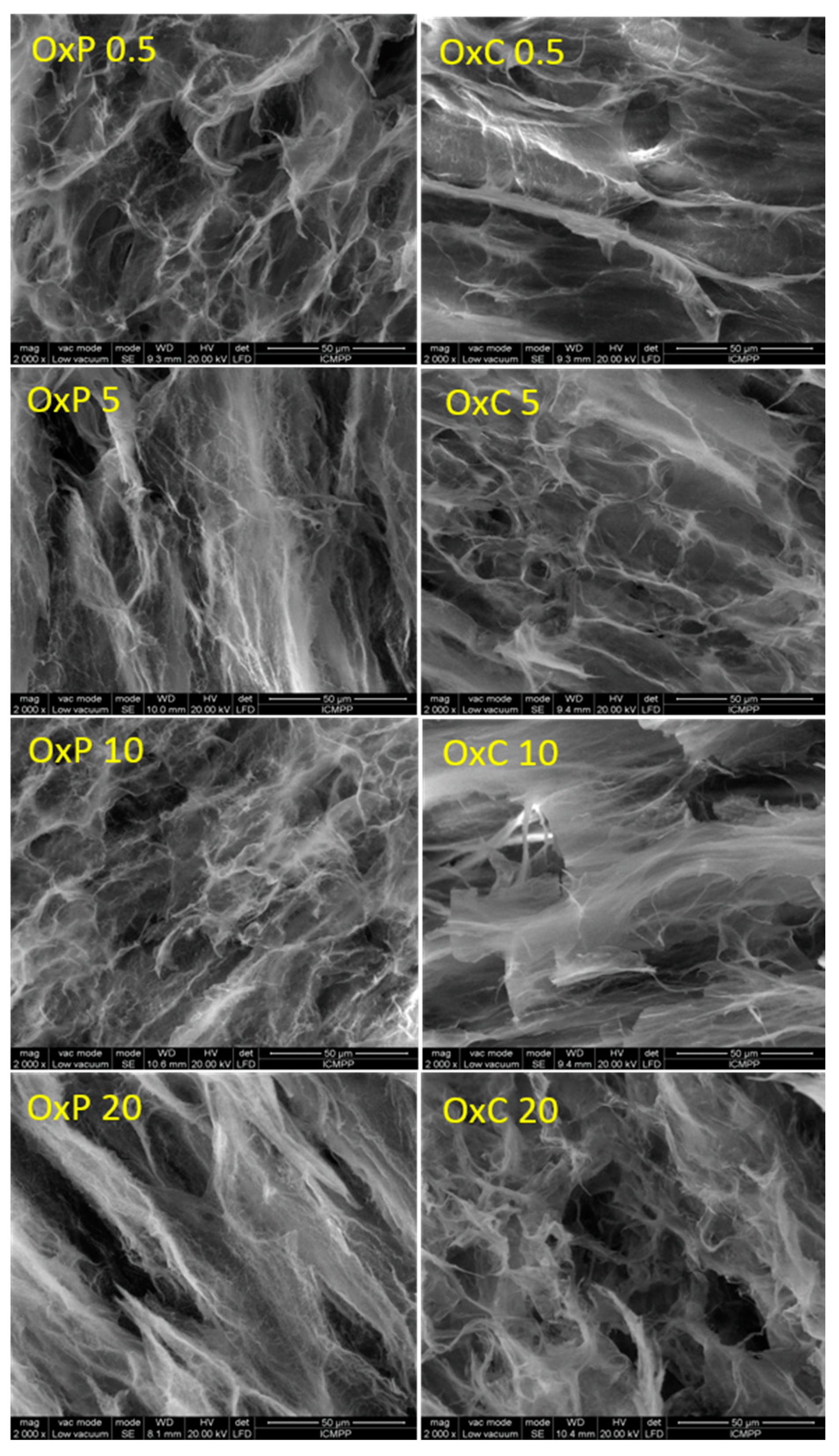
3.3. Morphology of Hydrogels
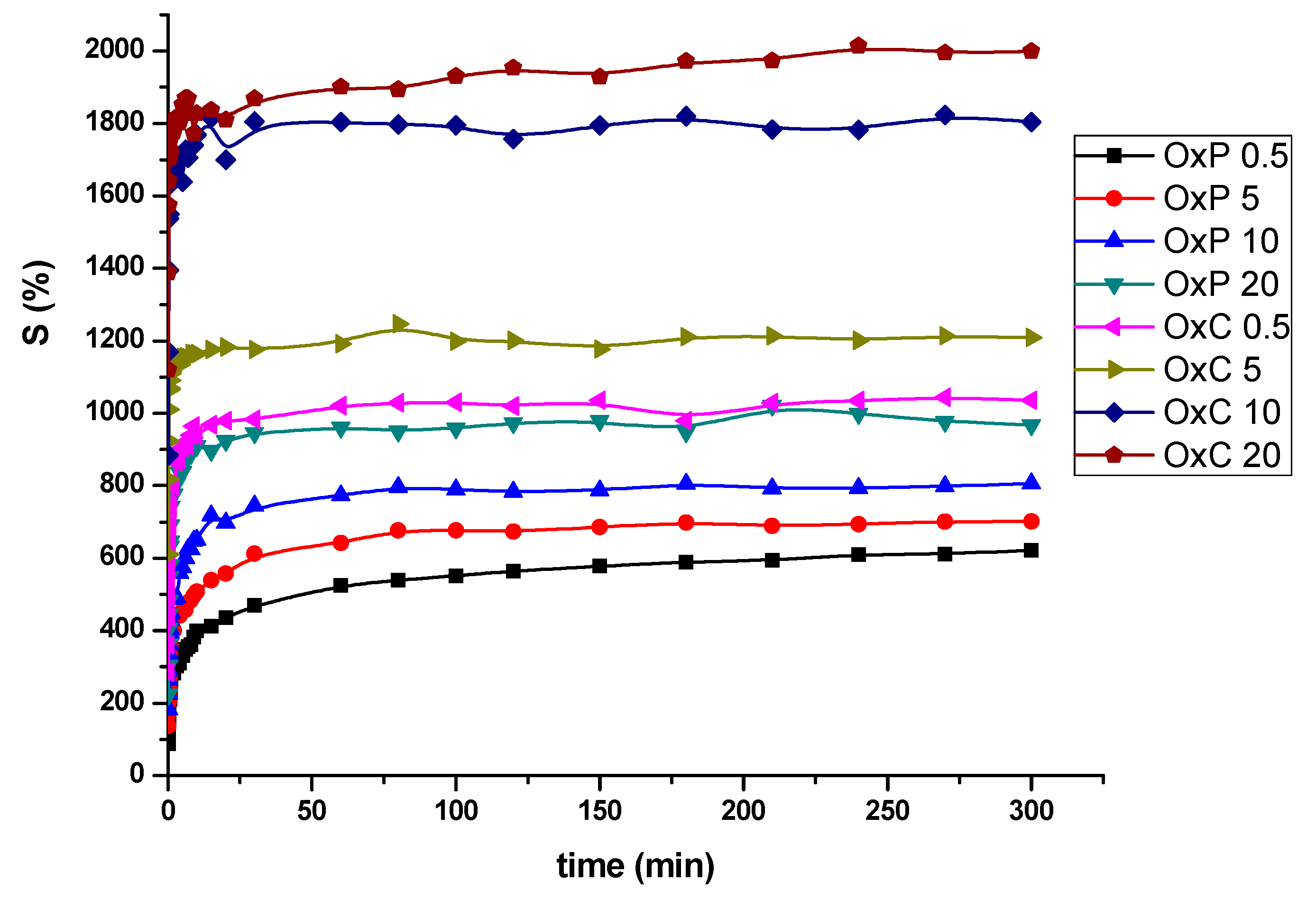
3.4. Swelling Behavior
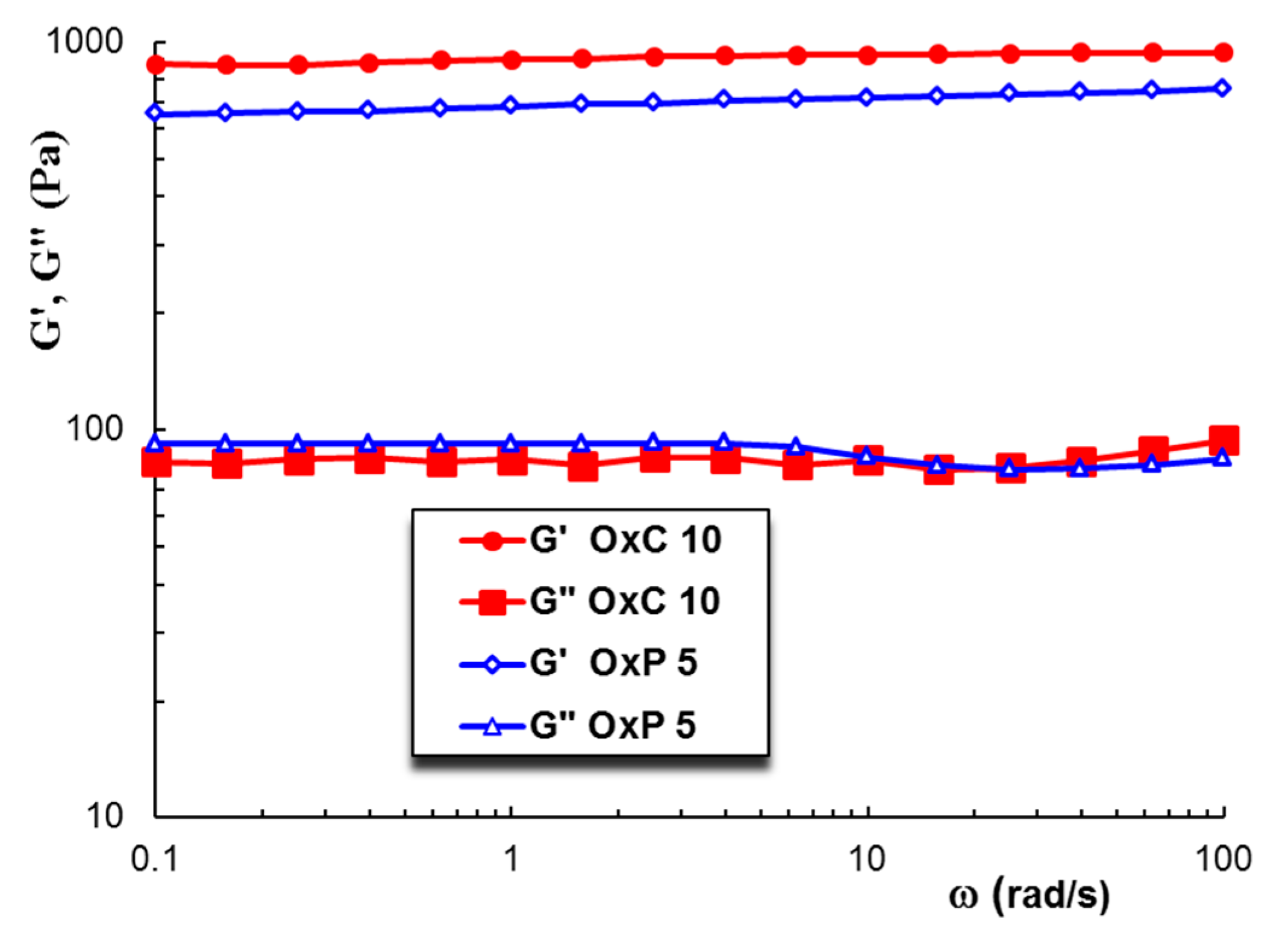
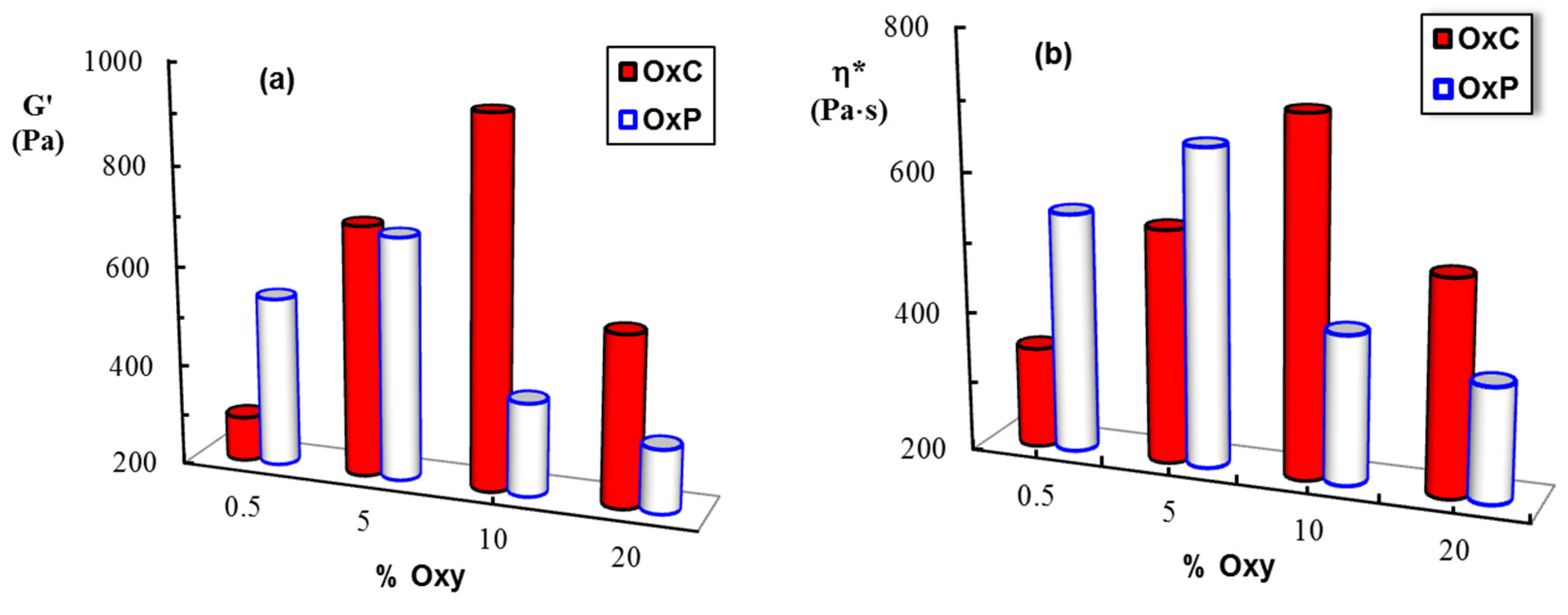
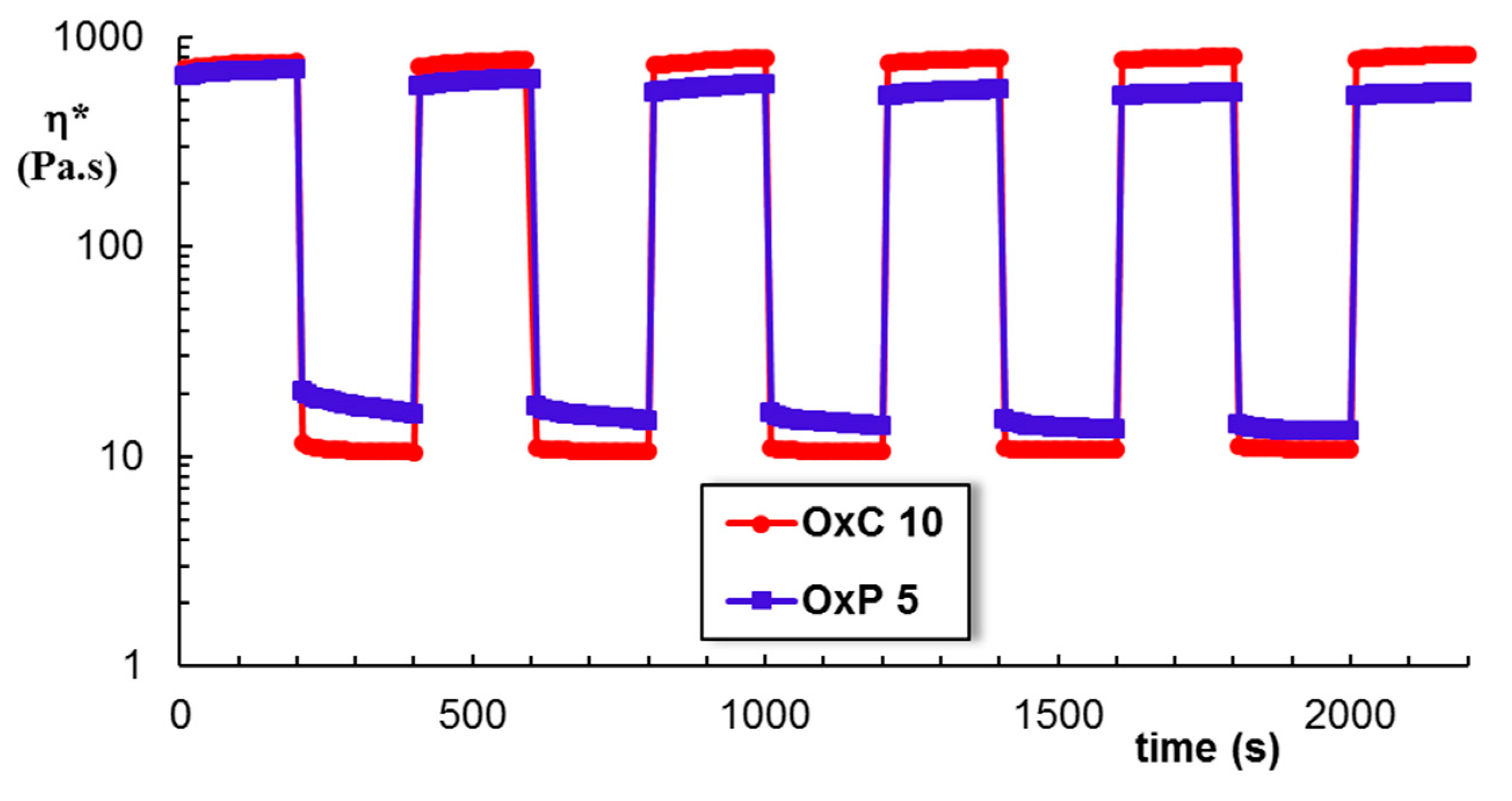
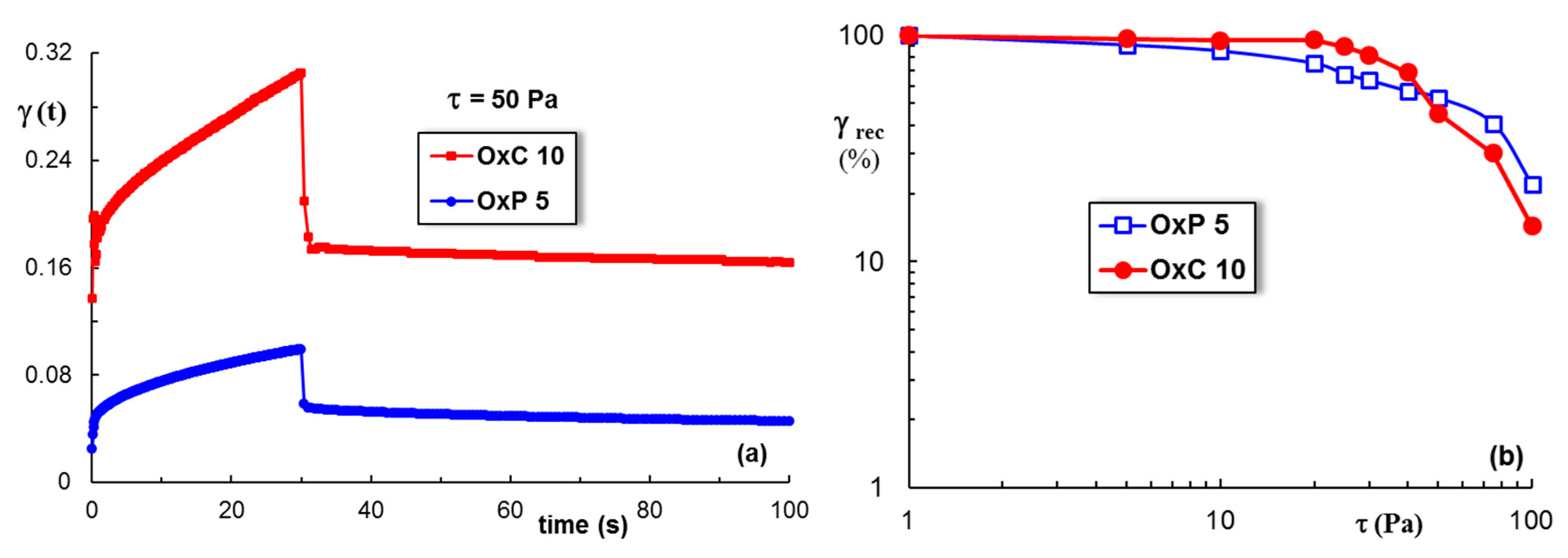
3.5. Rheological Behavior
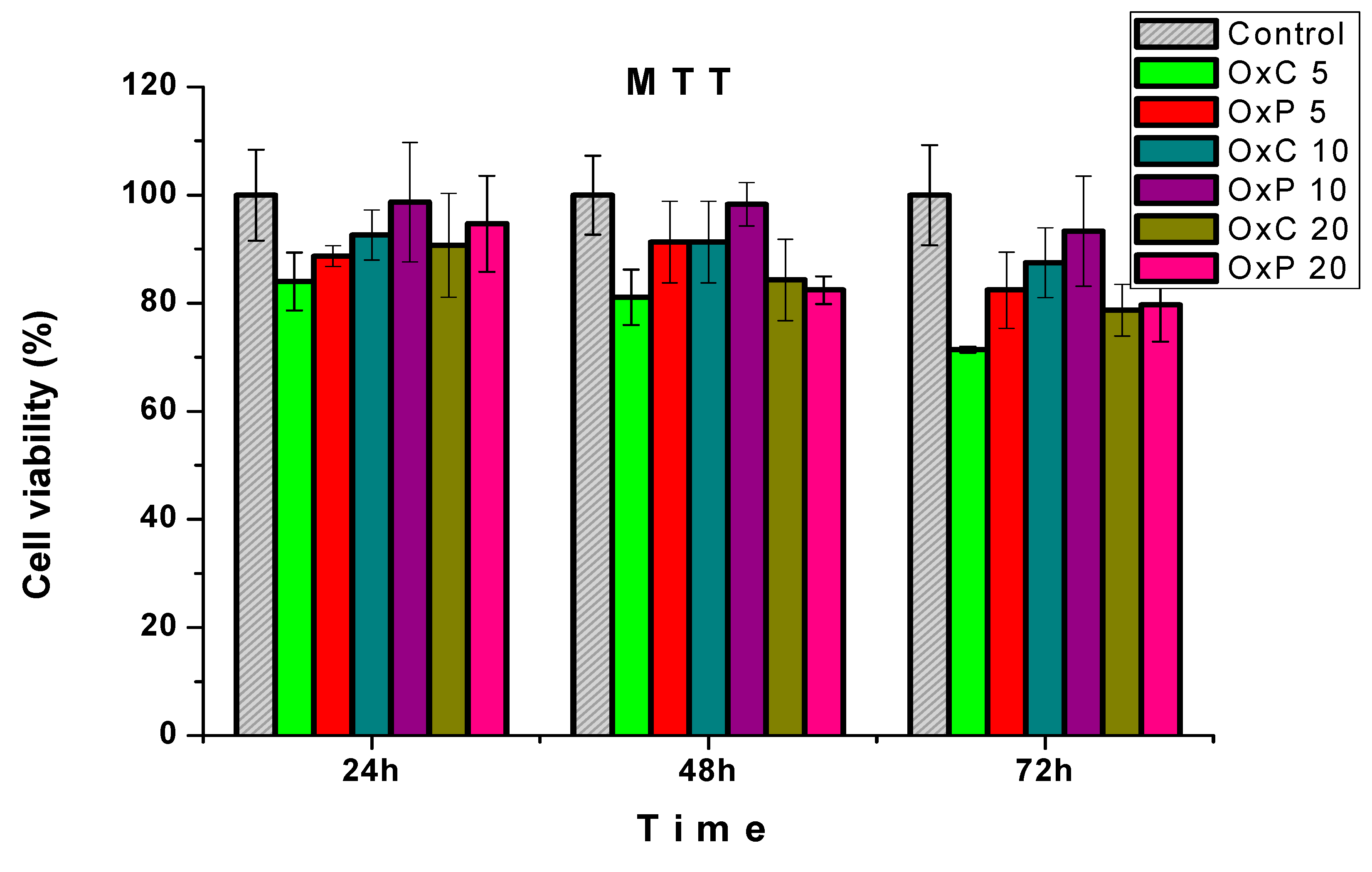
3.6. Cytotoxicity Assays
3.7. Adsorption and Release of L-Arginine
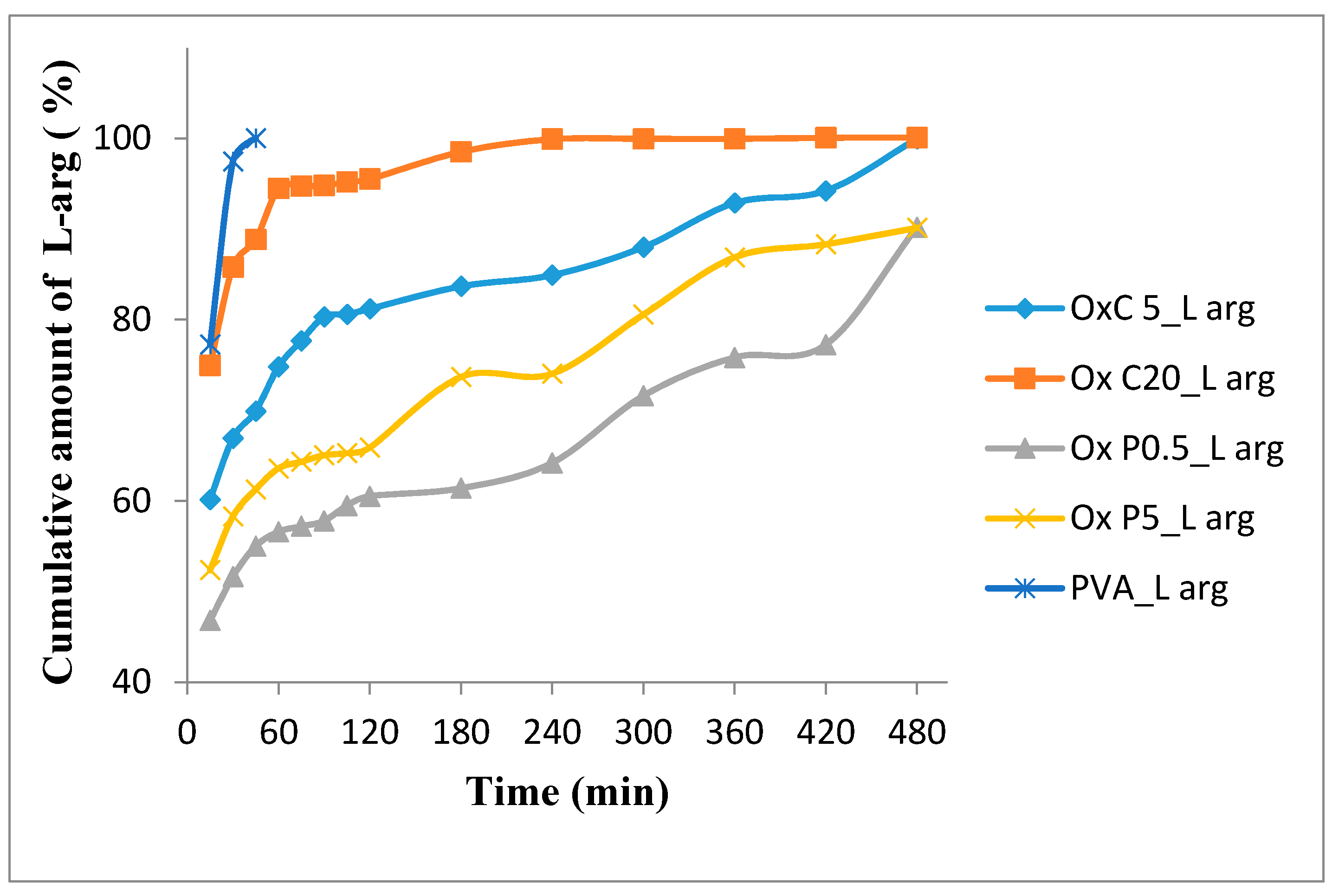
3.7.1. In Vitro L-Arginine Release Studies
3.7.2. Analysis of In Vitro Drug Release Kinetics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Apopei Loghin, D.F.; Biliuta, G.; Coseri, S.; Dragan, E.S. Preparation and characterization of oxidized starch/poly(N,N-dimethylaminoethyl methacrylate) semi-IPN cryogels and in vitro controlled release evaluation of indomethacin. Int. J. Biol. Macromol. 2017, 96, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Biliuta, G.; Coseri, S. Magnetic cellulosic materials based on TEMPO-oxidized viscose fibers. Cellulose 2016, 23, 3407–3415. [Google Scholar] [CrossRef]
- Coseri, S.; Bercea, M.; Harabagiu, V.; Budtova, T. Oxidation vs. degradation in polysaccharides: Pullulan—A case study. Eur. Polym. J. 2016, 85, 82–91. [Google Scholar] [CrossRef]
- Coseri, S.; Spatareanu, A.; Sacarescu, L.; Socoliuc, V.; Stratulat, I.S.; Harabagiu, V. Pullulan: A versatile coating agent for superparamagnetic iron oxide nanoparticles. J. Appl. Polym. Sci. 2016, 133, 42926. [Google Scholar] [CrossRef]
- Biliuta, G.; Coseri, S. Cellulose: A ubiquitous platform for ecofriendly metal nanoparticles preparation. Coord. Chem. Rev. 2019, 383, 155–173. [Google Scholar] [CrossRef]
- Biliuta, G.; Suteu, D.; Malutan, T.; Chirculescu, A.I.; Nica, I.; Coseri, S. Valorization of TEMPO-oxidized cellulosic fractions for efficient dye removal from wastewaters. Cellul. Chem. Technol. 2018, 52, 609–618. [Google Scholar]
- Biliuta, G.; Sacarescu, L.; Socoliuc, V.; Iacob, M.; Gheorghe, L.; Negru, D.; Coseri, S. Carboxylated Polysaccharides Decorated with Ultrasmall Magnetic Nanoparticles with Antibacterial and MRI Properties. Macromol. Chem. Phys. 2017, 218, 1700062. [Google Scholar] [CrossRef]
- Coseri, S.; Spatareanu, A.; Sacarescu, L.; Rimbu, C.; Suteu, D.; Spirk, S.; Harabagiu, V. Green synthesis of the silver nanoparticles mediated by pullulan and 6-carboxypullulan. Carbohydr. Polym. 2015, 116, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Bercea, M.; Biliuta, G.; Avadanei, M.; Baron, R.I.; Butnaru, M.; Coseri, S. Self-healing hydrogels of oxidized pullulan and poly(vinyl alcohol). Carbohydr. Polym. 2019, 206, 210–219. [Google Scholar] [CrossRef]
- Baron, R.I.; Bercea, M.; Avadanei, M.; Lisa, G.; Biliuta, G.; Coseri, S. Green route to produce self-healable hydrogels based on tricarboxy cellulose and poly (vinyl alcohol). Int. J. Biol. Macromol. 2019, 123, 744–751. [Google Scholar] [CrossRef]
- Alexander, J.W.; Supp, D.M. Role of arginine and omega-3 fatty acids in wound healing and infection. Adv. Wound Care (New Rochelle) 2014, 3, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Miguel, S.P.; Moreira, A.F.; Correia, I.J. Chitosan based-asymmetric membranes for wound healing: A review. Int. J. Biol. Macromol. 2019, 127, 460–475. [Google Scholar] [CrossRef] [PubMed]
- Zeng, T.; Zhang, Y.; Yan, Q.; Huang, Z.; Zhang, L.; Yi, X.; Chen, J.; He, G.; Yin, Y. Construction and in vitro evaluation of enzyme nanoreactors based on carboxymethyl chitosan for arginine deprivation in cancer therapy. Carbohydr. Polym. 2017, 162, 35–41. [Google Scholar] [CrossRef]
- Macocinschi, D.; Filip, D.; Vlad, S.; Butnaru, M.; Knieling, L. Evaluation of polyurethane based on cellulose derivative-ketoprofen biosystem for implant biomedical devices. Int. J. Biol. Macromol. 2013, 52, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Rai, S.B. Spectroscopic studies of L-arginine molecule. Indian J. Pure Appl. Phys. 2010, 48, 251–255. [Google Scholar]
- Ipate, A.M.; Hamciuc, C.; Kalvachev, Y.; Gherman, S.; Ochiuz, L. New cryogels based on polymers and zeolite L for controlled Enalapril maleate release. J. Drug. Deliv. Sci. Tech. 2018, 44, 505–512. [Google Scholar] [CrossRef]
- Gohel, M.C.; Sarvaiya, K.G.; Shah, A.R.; Brahmbhatt, B.K. Mathematical approach for the assessment of similarity factor using a new scheme for calculating weight. Indian. J. Pharm. Sci. 2009, 71, 142–144. [Google Scholar] [CrossRef]
- Shaikh, H.K.; Kshirsagar, R.V.; Patil, S.G. Mathematical models for drug release characterization: A review. World. J. Pharm. Pharm. Sci. 2015, 4, 324–338. [Google Scholar]
- Coseri, S.; Biliuta, G.; Zemljic, L.F.; Srndovic, J.S.; Larsson, T.; Strnad, S.; Kreze, T.; Naderi, A.; Lindstrom, T. One-shot carboxylation of microcrystalline cellulose in the presence of nitroxyl radicals and sodium periodate. RSC Adv. 2015, 5, 85889–85897. [Google Scholar] [CrossRef]
- Spatareanu, A.; Bercea, M.; Budtova, T.; Harabagiu, V.; Sacarescu, L.; Coseri, S. Synthesis, characterization and solution behaviour of oxidized pullulan. Carbohydr. Polym. 2014, 111, 63–71. [Google Scholar] [CrossRef]
- Peppas, N.A. Infrared spectroscopy of semicrystalline poly(viny1 alcohol) networks. Makromol. Chem. 1977, 178, 595–601. [Google Scholar] [CrossRef]
- Shingel, K.I. Determination of structural peculiarities of dextran, pullulan and gamma-irradiated pullulan by Fourier-transform IR spectroscopy. Carbohydr. Res. 2002, 337, 1445–1451. [Google Scholar] [CrossRef]
- Heimbuck, A.M.; Priddy-Arringtona, T.R.; Sawyerb, B.J.; Caldorera-Moorea, M.E. Effects of post-processing methods on chitosan-genipin hydrogel properties. Mater. Sci. Eng. C 2019, 98, 612–618. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Kenawy, E.S.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res 2017, 8, 217–233. [Google Scholar] [CrossRef]
- Krerlow, J.; Mikos, A. From Material to Tissue: Biomaterial Development, Scaffold Fabrication, and Tissue Engineering. AIChE J. 2008, 54, 3048–3067. [Google Scholar] [CrossRef]
- Gupta, S.; Webster, T.J.; Sinba, A. Evolution of PVA gels prepared without crosslinking agents as a cell adhesive surface. J. Mater. Sci. Mater. Med. 2011, 22, 1763–1772. [Google Scholar] [CrossRef]
- Narayanaswamy, R.; Torchilin, V.P. Hydrogels and Their Applications in Targeted Drug Delivery. Molecules 2019, 24, 603. [Google Scholar] [CrossRef]
- Vishal Gupta, N.; Shivakumar, H.G. Investigation of Swelling Behavior and Mechanical Properties of a pH-Sensitive Superporous Hydrogel Composite. Iran. J. Pharm. Res. 2012, 11, 481–493. [Google Scholar]
- Zarzycki, R.; Modrzejewska, Z.; Nawrotek, K. Drug release from hydrogel matrices. Ecol. Chem. Eng. 2010, 17, 117–135. [Google Scholar]
- Costa, P.; Sousa Lobo, J.M. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Kumari, S.; Ram, B.; Kumar, D.; Ranote, S.; Chauhan, G.S. Nanoparticles of oxidized-cellulose synthesized by green method. Mat. Sci. Eng. Technol. 2018, 1, 22–28. [Google Scholar] [CrossRef]
| Sample | mhydrogel, mg | mL-arg, mg | % Pu ± SD | mL-arg Loaded, mg |
|---|---|---|---|---|
| OxC0.5 | 142 | 142 | 12.92 ± 0.51 | 18.34 ± 0.72 |
| OxC5 | 193 | 193 | 15.32 ± 0.09 | 29.57 ± 0.18 |
| OxC10 | 166 | 166 | 13.40 ± 0.21 | 22.25 ± 0.34 |
| OxC20 | 201 | 201 | 16.36 ± 0.42 | 32.89 ± 0.84 |
| OxP0.5 | 170 | 170 | 18.44 ± 0.57 | 31.35 ± 0.96 |
| OxP5 | 178 | 178 | 15.36 ± 0.32 | 28.72 ± 0.59 |
| OxP10 | 200 | 200 | 5.84 ± 0.40 | 11.69 ± 0.79 |
| OxP20 | 198 | 198 | 12.48 ± 0.47 | 24.70 ± 0.94 |
| PVA | 205 | 205 | 11.64 ± 0.32 | 23.86 ± 0.65 |
| Kinetic Model | Model Coefficients | Modified Release Sample | ||||
|---|---|---|---|---|---|---|
| OxC5_L-arg | OxC_20_L-arg | OxP0.5_L-arg | OxP5_L-arg | PVA | ||
| Zero-order | K0 | 6.1162 | 6.7557 | 5.2270 | 5.8099 | 50.12 |
| R2 | 0.2554 | 0.0842 | 0.4616 | 0.3465 | 0.8344 | |
| First-order | K0 | 1.5336 | 3.3439 | 0.5627 | 0.8223 | 8.8848 |
| R2 | 0.6919 | 0.9576 | 0.1221 | 0.3992 | 0.9789 | |
| Higuchi | K0 | 43.682 | 53.335 | 33.340 | 39.559 | 29.76 |
| R2 | 0.5259 | 0.2799 | 0.7224 | 0.6266 | 0.9664 | |
| Korsmeyer-Peppas | n | 0.52 | 0.49 | 0.57 | 0.65 | 0.7 |
| K0 | 63.40 | 84.61 | 47.31 | 55.47 | 31.5 | |
| R2 | 0.987 | 0.9600 | 0.9865 | 0.9832 | 0.9998 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baron, R.I.; Culica, M.E.; Biliuta, G.; Bercea, M.; Gherman, S.; Zavastin, D.; Ochiuz, L.; Avadanei, M.; Coseri, S. Physical Hydrogels of Oxidized Polysaccharides and Poly(Vinyl Alcohol) for Wound Dressing Applications. Materials 2019, 12, 1569. https://doi.org/10.3390/ma12091569
Baron RI, Culica ME, Biliuta G, Bercea M, Gherman S, Zavastin D, Ochiuz L, Avadanei M, Coseri S. Physical Hydrogels of Oxidized Polysaccharides and Poly(Vinyl Alcohol) for Wound Dressing Applications. Materials. 2019; 12(9):1569. https://doi.org/10.3390/ma12091569
Chicago/Turabian StyleBaron, Raluca Ioana, Madalina Elena Culica, Gabriela Biliuta, Maria Bercea, Simona Gherman, Daniela Zavastin, Lacramioara Ochiuz, Mihaela Avadanei, and Sergiu Coseri. 2019. "Physical Hydrogels of Oxidized Polysaccharides and Poly(Vinyl Alcohol) for Wound Dressing Applications" Materials 12, no. 9: 1569. https://doi.org/10.3390/ma12091569
APA StyleBaron, R. I., Culica, M. E., Biliuta, G., Bercea, M., Gherman, S., Zavastin, D., Ochiuz, L., Avadanei, M., & Coseri, S. (2019). Physical Hydrogels of Oxidized Polysaccharides and Poly(Vinyl Alcohol) for Wound Dressing Applications. Materials, 12(9), 1569. https://doi.org/10.3390/ma12091569








