Effects of Citric Acid on Structures and Properties of Thermoplastic Hydroxypropyl Amylomaize Starch Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Hydroxypropyl High Amylomaize Starch (HPAS)
2.3. Preparation of HPAS-CA Films
2.4. Degree of Diesterification of HPAS-CA Films
2.5. Thickness
2.6. Mechanical Properties
2.7. Attenuated Total Reflectance Fourier-Transform Infrared Analysis
2.8. 13C Solid-State Nuclear Magnetic Resonance
2.9. Molecular Weight of HPAS-CA Films
2.10. Scanning Electron Microscopy
2.11. X-Ray Diffraction
2.12. Statistical Analysis
3. Results and Discussion
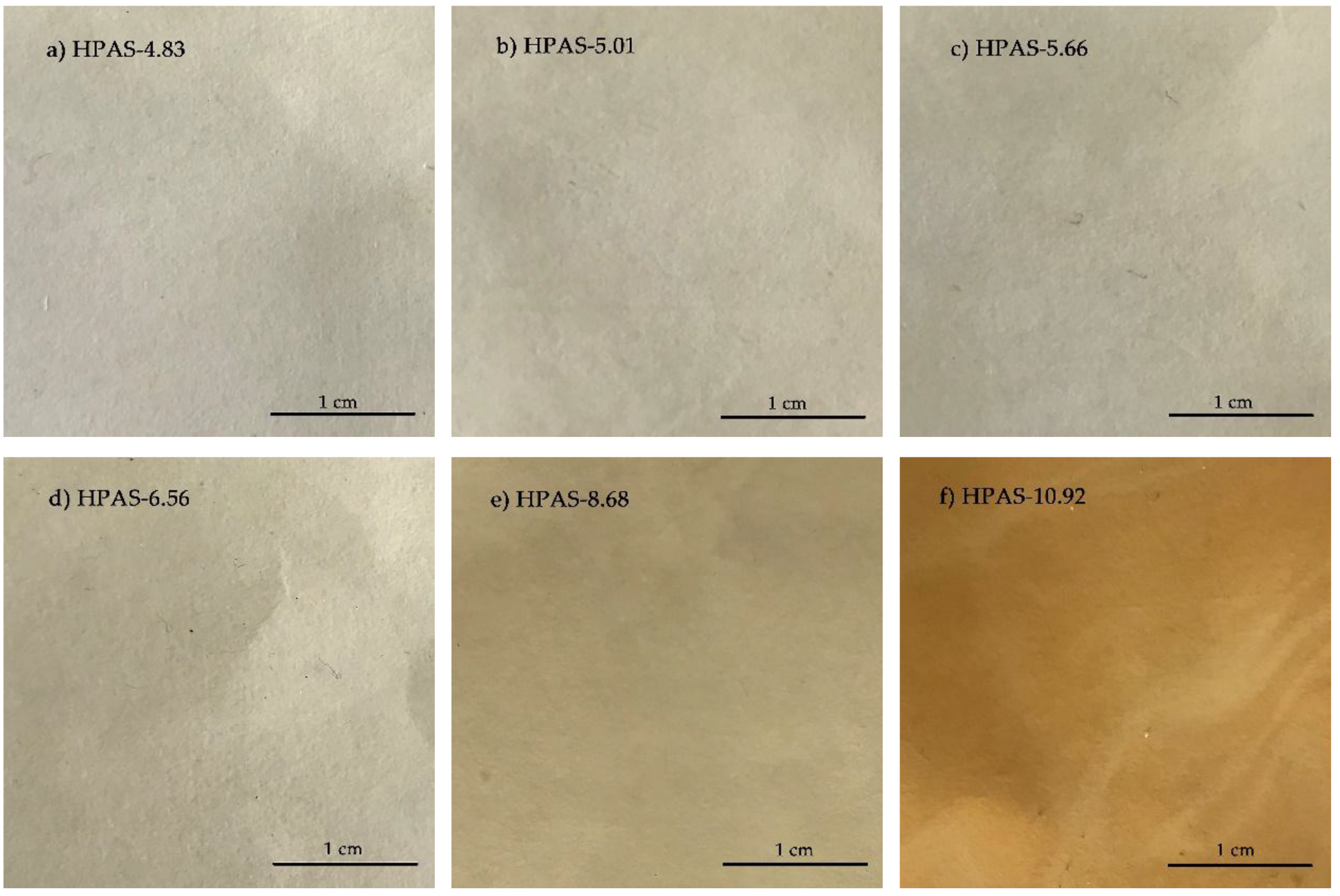
3.1. Appearance of HPAS-CA Films
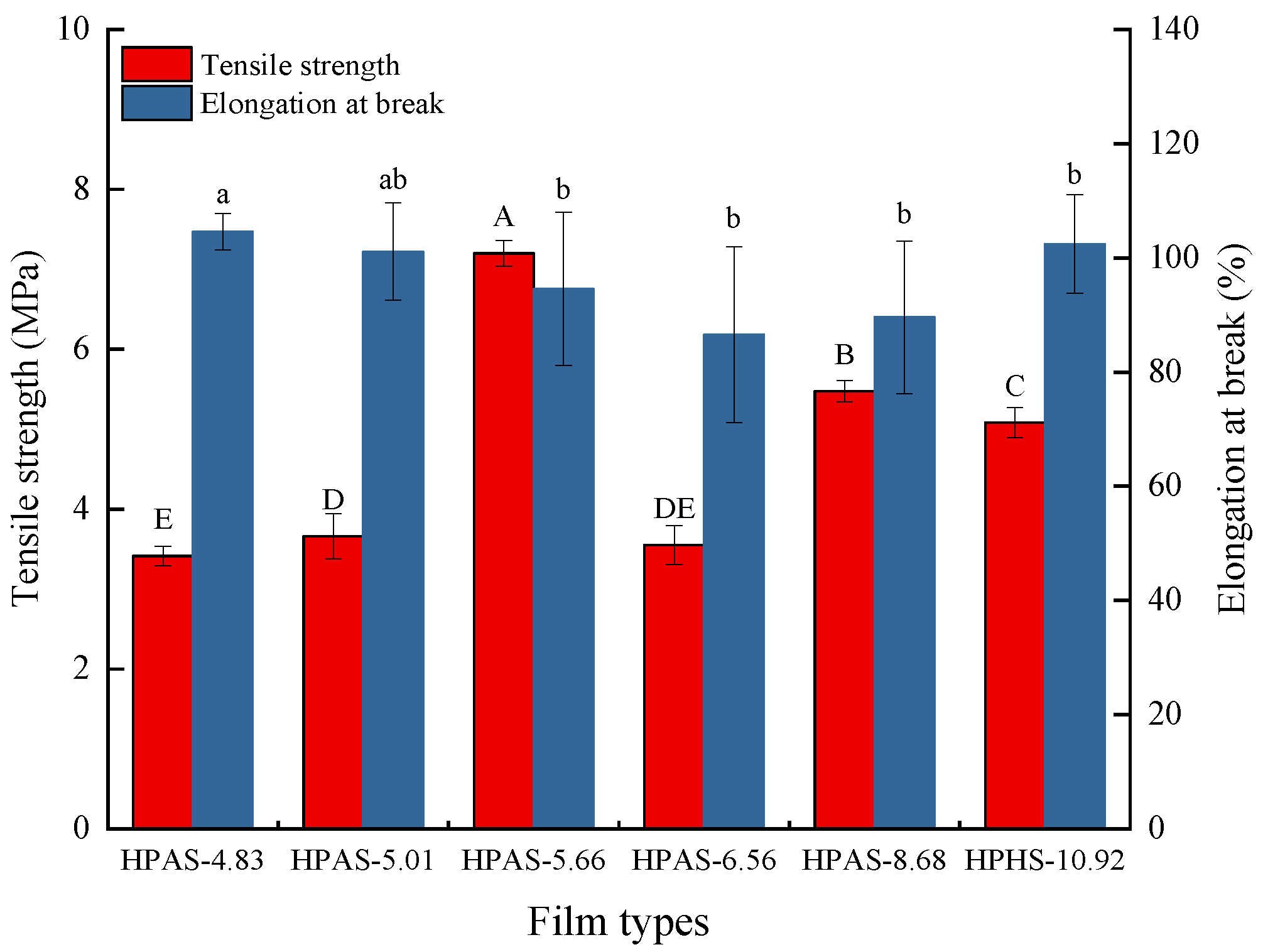
3.2. Mechanical Properties of HPAS-CA Films
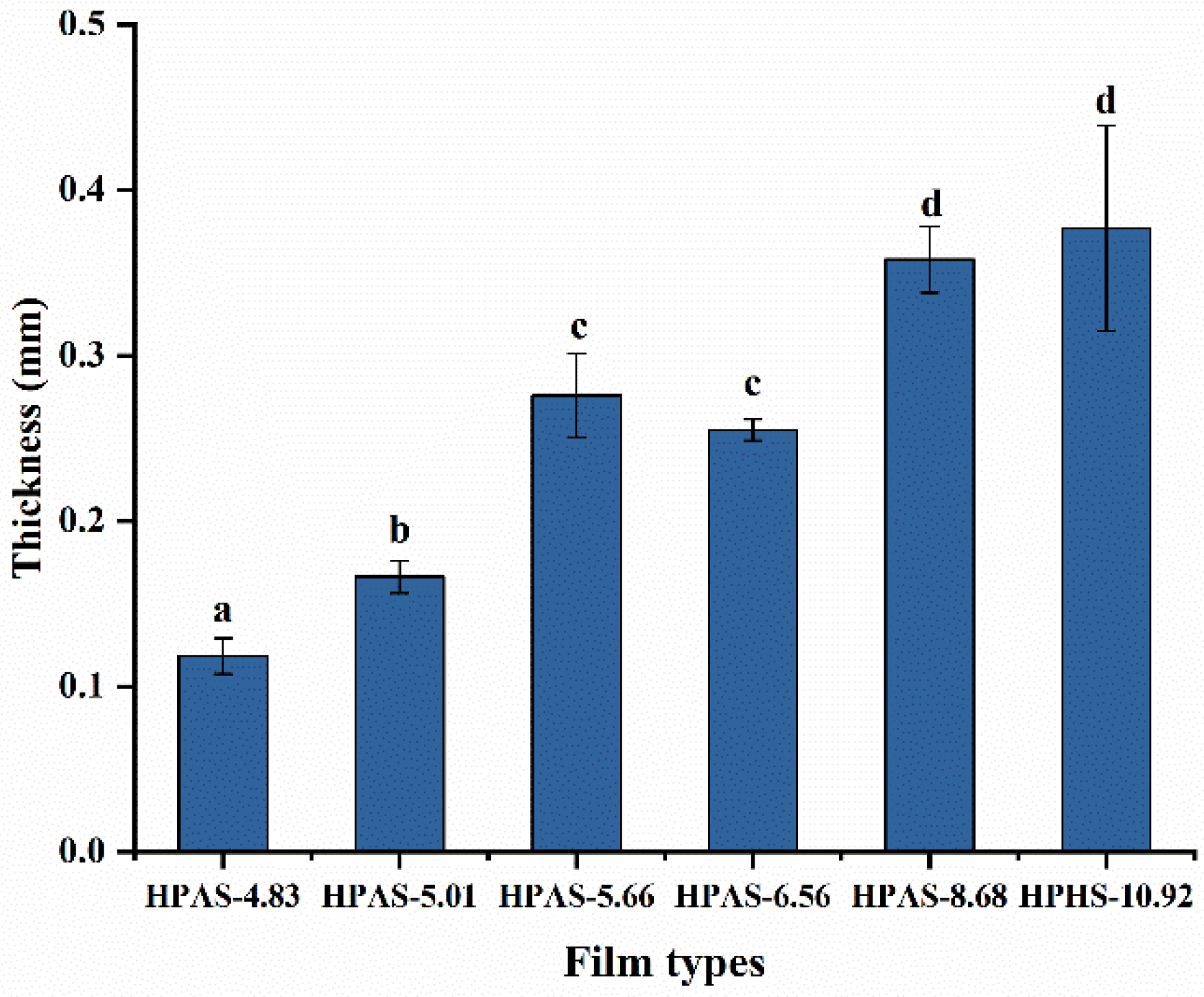
3.3. Thickness of HPAS-CA Films
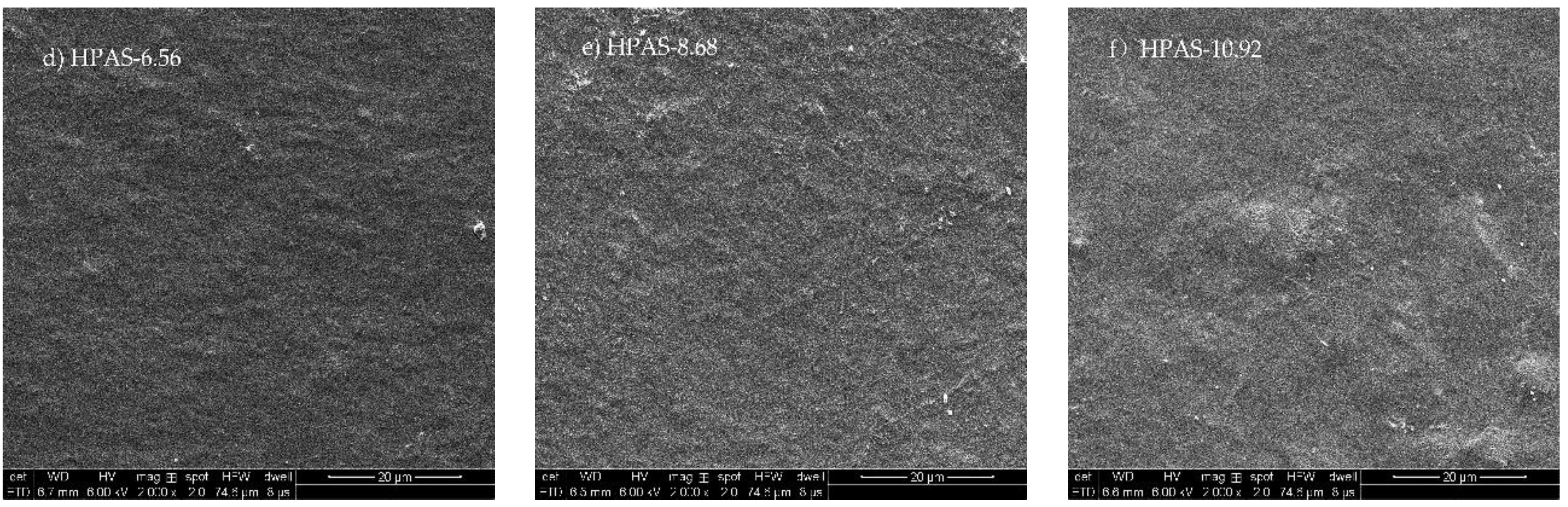
3.4. Morphology of HPAS-CA Films
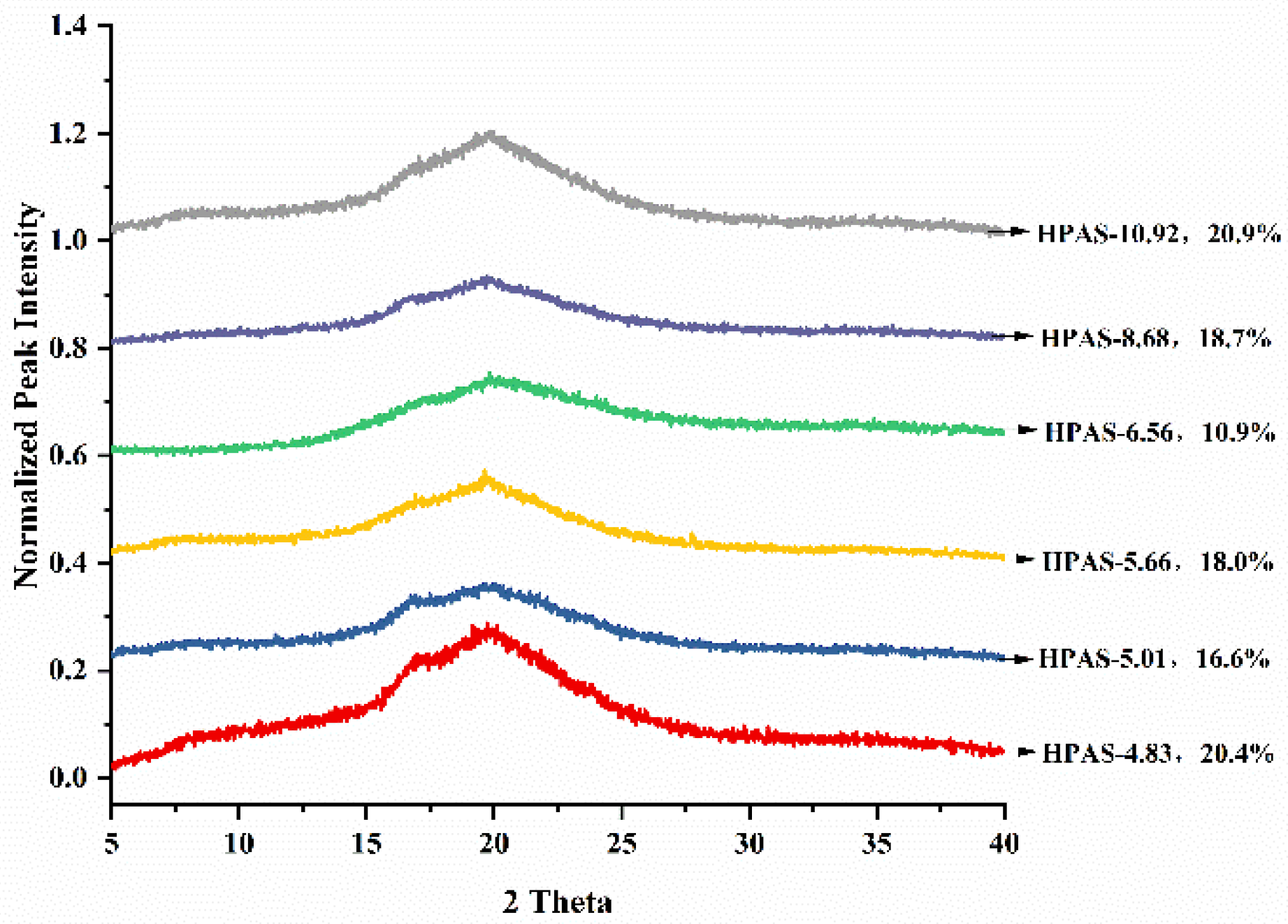
3.5. Crystalline Structure of HPAS-CA Films
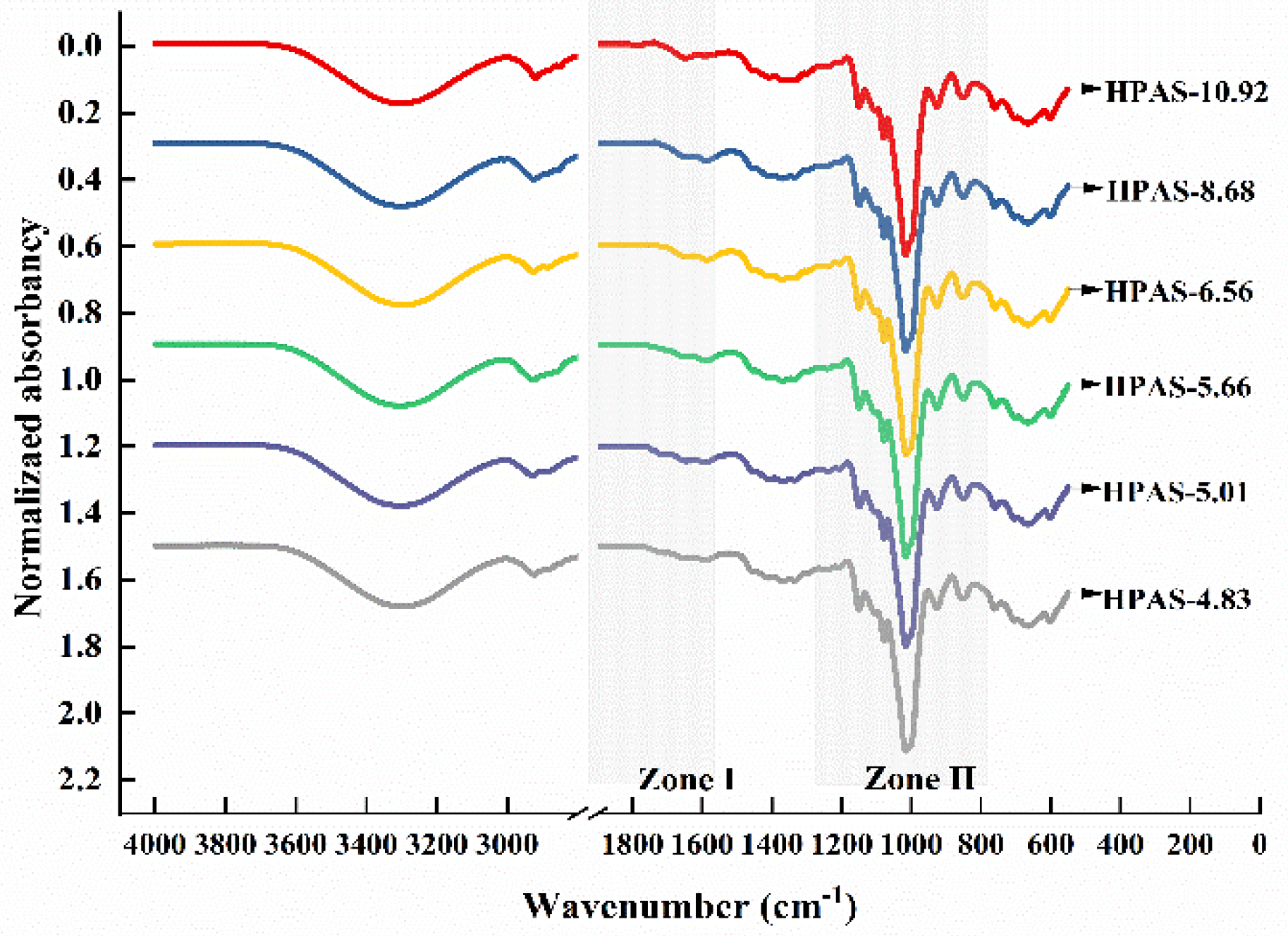
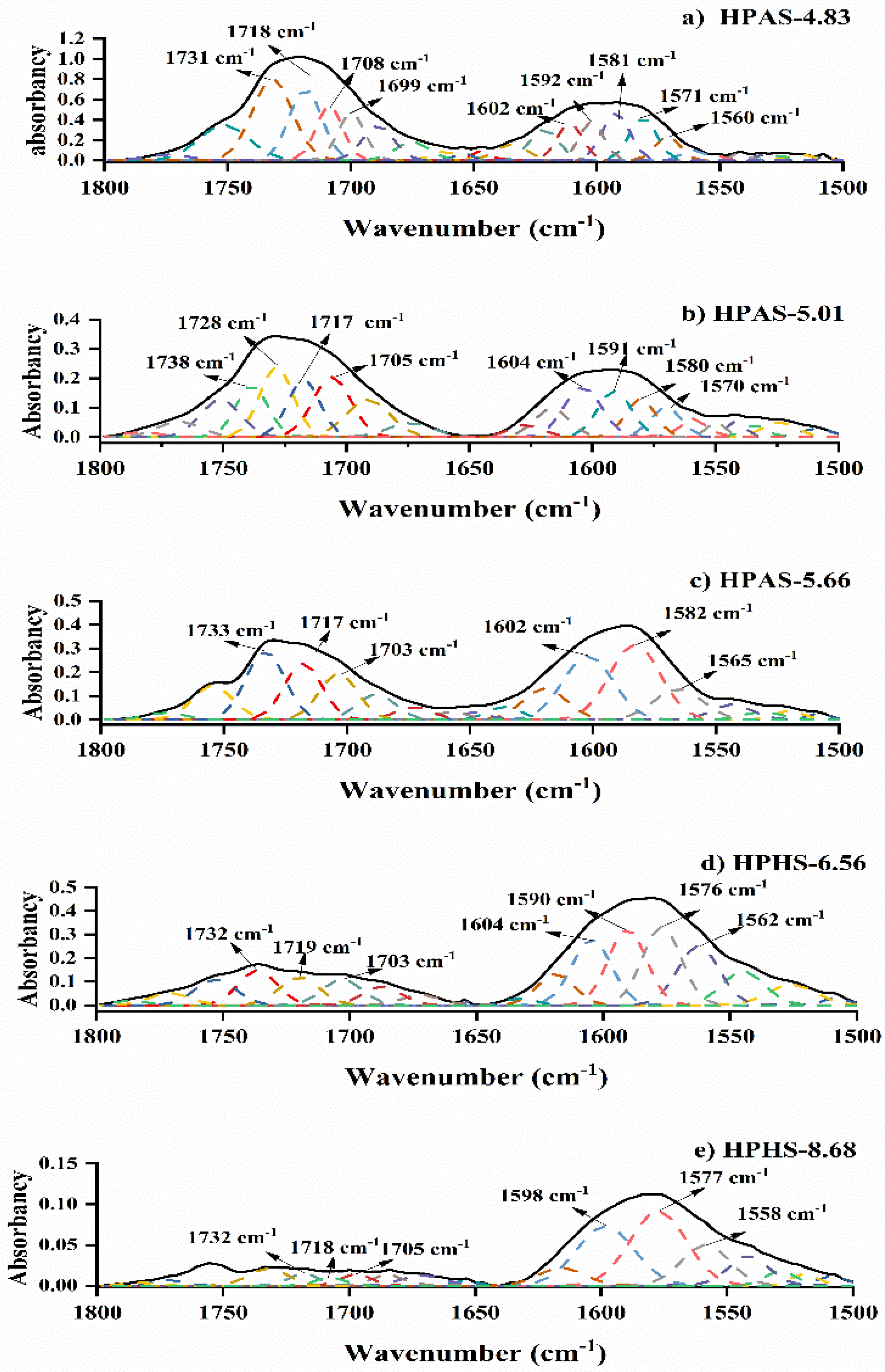
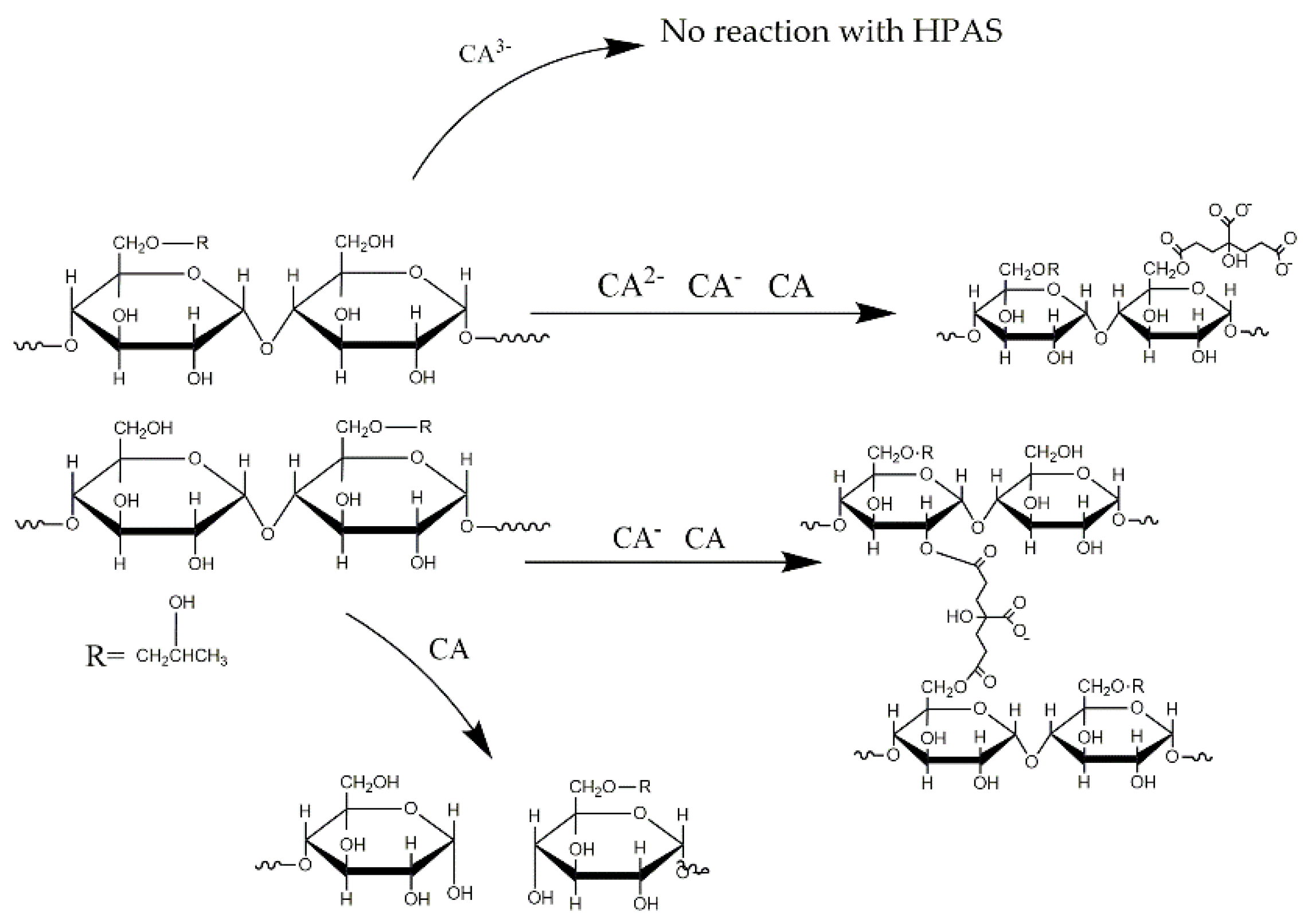
3.6. Molecular Interaction between Hydroxypropyl High Amylomaize Starch and Citric Acid
3.7. Complexometric Titration of Citric Acid with Copper (II)-Sulfate
3.8. Weight Average Molecular Weight of HPAS-CA Films
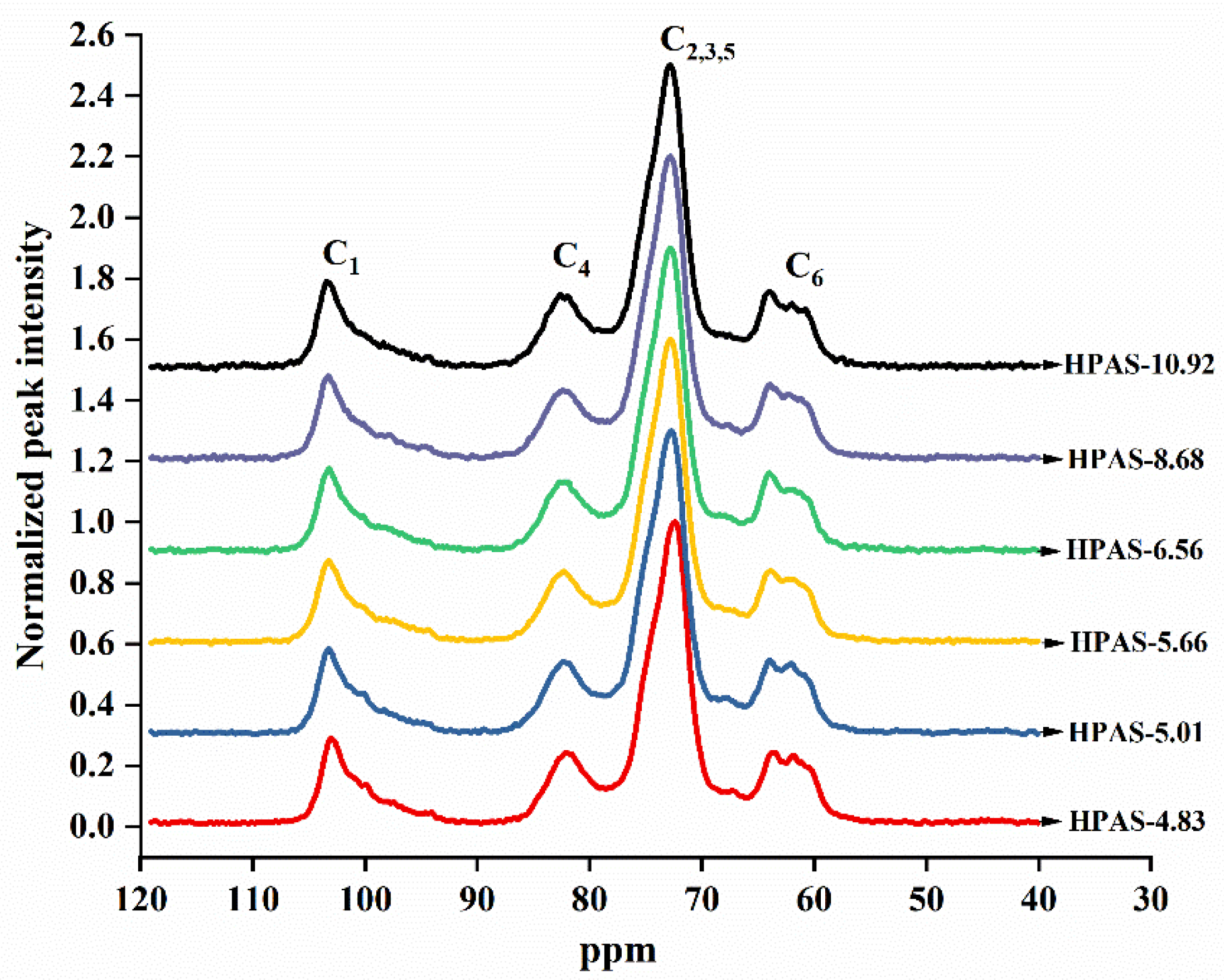
3.9. Short-Ordered Structure of HPAS-CA Films
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhou, J.; Tong, J.; Su, X.; Ren, L. Hydrophobic starch nanocrystals preparations through crosslinking modification using citric acid. Int. J. Biol. Macromol. 2016, 91, 1186–1193. [Google Scholar] [CrossRef]
- Wang, W. Preparation, Film-Forming Mechanism and Application of Starch-Based Nanocomposite Films. Ph.D. Thesis, Shandong Agricultural University, Tai’an, China, 14 June 2016. [Google Scholar]
- Li, M.; Liu, P.; Zou, W.; Yu, L.; Xie, F.; Pu, H.; Liu, H.; Chen, L. Extrusion processing and characterization of edible starch films with different amylose contents. J. Food. Eng. 2011, 106, 95–101. [Google Scholar] [CrossRef]
- Nafchi, A.M.; Moradpour, M.; Saeidi, M.; Alias, A.K. Thermoplastic starches: Properties, challenges, and prospects. Starch-Stärke 2013, 65, 61–72. [Google Scholar] [CrossRef]
- Zdrahala, R.J. Thermoplastic starch revisited. Structure/property relationship for “dialed-in” biodegradability. Macromol. Symp. 1997, 123, 113–121. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Jane, J.-L.; Lamsal, B. Hydroxypropylation improves film properties of high amylose corn starch. Ind. Crop. Prod. 2017, 95, 175–183. [Google Scholar] [CrossRef]
- Muscat, D.; Adhikari, B.; Adhikari, R.; Chaudhary, D. Comparative study of film forming behaviour of low and high amylose starches using glycerol and xylitol as plasticizers. J. Food. Eng. 2012, 109, 189–201. [Google Scholar] [CrossRef]
- Thuwall, M.; Boldizar, A.; Rigdahl, M. Extrusion processing of high amylose potato starch materials. Carbohydr. Polym. 2006, 65, 441–446. [Google Scholar] [CrossRef]
- Wootton, M.; Manatsathit, A. The Influence of Molar Substitution on the Water Binding Capacity of Hydroxypropyl Maize Starches. Starch-Stärke 1983, 35, 92–94. [Google Scholar] [CrossRef]
- Gunaratne, A.; Corke, H. Effect of hydroxypropylation and alkaline treatment in hydroxypropylation on some structural and physicochemical properties of heat-moisture treated wheat, potato and waxy maize starches. Carbohydr. Polym. 2007, 68, 305–313. [Google Scholar] [CrossRef]
- Lee, H.; Yoo, B. Effect of hydroxypropylation on physical and rheological properties of sweet potato starch. LWT-Food Sci. Technol. 2011, 44, 765–770. [Google Scholar] [CrossRef]
- Tessler, M.M.; Jarowenko, W.; Amitrano, R.A. Hydroxypropylated, Inhibited High Amylose Retort Starches. U.S. Patent No. 3,904,601, 9 September 1975. [Google Scholar]
- Yu, J.; Wang, N.; Ma, X. The effects of citric acid on the properties of thermoplastic starch plasticized by glycerol. Starch-Stärke 2005, 57, 494–504. [Google Scholar]
- Wang, N.; Yu, J.; Han, C. Influence of citric acid on the properties of glycerol-plasticised cornstarch extrusion blends. Polym. Polym. Compos. 2007, 15, 545–552. [Google Scholar]
- Olsson, E.; Menzel, C.; Johansson, C.; Andersson, R.; Koch, K.; Järnström, L. The effect of pH on hydrolysis, cross-linking and barrier properties of starch barriers containing citric acid. Carbohydr. Polym. 2013, 98, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Menzel, C.; Olsson, E.; Plivelic, T.S.; Andersson, R.; Johansson, C.; Kuktaite, R.; Järnström, L.; Koch, K. Molecular structure of citric acid cross-linked starch films. Carbohydr. Polym. 2013, 96, 270–276. [Google Scholar] [CrossRef] [PubMed]
- ASTM. D882-12 Standard Test Method for Tensile Properties of Thin Plastic Sheeting; ASTM International: West Conshohocken, PA, USA, 2012; Volume D882, p. 12. [Google Scholar]
- Golova, O.P.; Nosova, N.I. Degradation of Cellulose by Alkaline Oxidation. Russ. Chem. Rev. 1973, 42, 327–338. [Google Scholar] [CrossRef]
- Tang, M.; Wen, S.; Liu, D. Effects of heating- or caustic-digested starch on its flocculation on hematite. Miner. Process. Extr. Metall. Rev. 2016, 37, 49–57. [Google Scholar] [CrossRef]
- Reddy, N.; Yang, Y. Citric acid cross-linking of starch films. Food Chem. 2010, 118, 702–711. [Google Scholar] [CrossRef] [Green Version]
- Ortega-Toro, R.; Collazo-Bigliardi, S.; Talens, P.; Chiralt, A. Influence of citric acid on the properties and stability of starch-polycaprolactone based films. J. Appl. Polym. Sci. 2016, 133, 42220. [Google Scholar] [CrossRef]
- Morales, N.J.; Candal, R.; Famá, L.; Goyanes, S.; Rubiolo, G.H. Improving the physical properties of starch using a new kind of water dispersible nano-hybrid reinforcement. Carbohydr. Polym. 2015, 127, 291–299. [Google Scholar] [CrossRef]
- Mei, J.-Q.; Zhou, D.-N.; Jin, Z.-Y.; Xu, X.-M.; Chen, H.-Q. Effects of citric acid esterification on digestibility, structural and physicochemical properties of cassava starch. Food Chem. 2015, 187, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-N.; Xie, Y.; Chen, H.-Q.; Zhang, B. Effects of heat-moisture treatment after citric acid esterification on structural properties and digestibility of wheat starch, A- and B-type starch granules. Food Chem. 2019, 272, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Xie, F.; Zhao, L.; Qiao, Q.; Liu, X. Effect of acid hydrolysis on the multi-scale structure change of starch with different amylose content. Food Hydrocoll. 2017, 69, 359–368. [Google Scholar] [CrossRef]
- Sun, X.; Yu, J.; Liu, Y. Study of acid hydrolysis course and properties of different starches. Fine Chem. 2004, 3, 202–205. [Google Scholar]
- Ramos, M.E.; Huertas, F.J. Adsorption of lactate and citrate on montmorillonite in aqueous solutions. Appl. Clay Sci. 2014, 90, 27–34. [Google Scholar] [CrossRef]
- Shi, R.; Bi, J.; Zhang, Z.; Zhu, A.; Chen, D.; Zhou, X.; Zhang, L.; Tian, W. The effect of citric acid on the structural properties and cytotoxicity of the polyvinyl alcohol/starch films when molding at high temperature. Carbohydr. Polym. 2008, 74, 763–770. [Google Scholar] [CrossRef]
- Strathmann, T.J.; Myneni, S.C. Speciation of aqueous Ni(II)-carboxylate and Ni(II)-fulvic acid solutions: Combined ATR-FTIR and XAFS analysis. Geochim. Cosmochim. Acta 2004, 68, 3441–3458. [Google Scholar] [CrossRef]
- Van Soest, J.J.; Tournois, H.; De Wit, D.; Vliegenthart, J.F. Short-range structure in (partially) crystalline potato starch determined with attenuated total reflectance Fourier-transform IR spectroscopy. Carbohydr. Res. 1995, 279, 201–214. [Google Scholar] [CrossRef] [Green Version]
- Spiridon, I.; Teaca, C.-A.; Bodirlau, R. Preparation and characterization of adipic acid-modified starch microparticles/plasticized starch composite films reinforced by lignin. J. Mater. Sci. 2011, 46, 3241–3251. [Google Scholar] [CrossRef]
- Véchambre, C.; Buléon, A.; Chaunier, L.; Jamme, F.; Lourdin, D. Macromolecular Orientation in Glassy Starch Materials That Exhibit Shape Memory Behavior. Macromolecules 2010, 43, 9854–9858. [Google Scholar] [CrossRef]
- Lawal, O.S. Starch hydroxyalkylation: Physicochemical properties and enzymatic digestibility of native and hydroxypropylated finger millet (Eleusine coracana) starch. Food Hydrocoll. 2009, 23, 415–425. [Google Scholar] [CrossRef]
- Wang, S.; Copeland, L. Effect of alkali treatment on structure and function of pea starch granules. Food Chem. 2012, 135, 1635–1642. [Google Scholar] [CrossRef] [PubMed]
- El-Tahlawy, K.; Venditti, R.A.; Pawlak, J.J. Aspects of the preparation of starch microcellular foam particles crosslinked with glutaraldehyde using a solvent exchange technique. Carbohydr. Polym. 2007, 67, 319–331. [Google Scholar] [CrossRef]
- Tan, I.; Flanagan, B.M.; Halley, P.J.; Whittaker, A.K.; Gidley, M.J. A Method for Estimating the Nature and Relative Proportions of Amorphous, Single, and Double-Helical Components in Starch Granules by13C CP/MAS NMR. Biomacromolecules 2007, 8, 885–891. [Google Scholar] [CrossRef] [PubMed]

| Sample Codes | HPAS/g | Glycerol/g | Citric Acid/g | pH of 1% HPAS-CA Suspension |
|---|---|---|---|---|
| HPAS-4.83 | 2000 | 600 | 120 | 4.83 |
| HPAS-5.01 | 2000 | 600 | 100 | 5.01 |
| HPAS-5.66 | 2000 | 600 | 80 | 5.66 |
| HPAS-6.56 | 2000 | 600 | 45 | 6.56 |
| HPAS-8.68 | 2000 | 600 | 40 | 8.68 |
| HPAS-10.92 | 2000 | 600 | 0 | 10.92 |
| Sample | R1039/1018 | Proportion of Diester to Total CA/% | DDE of Starch/×10−3 | Mw /×105 g·mol−1 |
|---|---|---|---|---|
| HPAS-4.83 | 0.544 | 17.78 ± 3.14 | 7.0 ± 1.22 | 3.18 ± 0.15 |
| HPAS-5.01 | 0.544 | 18.63 ± 3.20 | 6.0 ± 1.04 | 3.44 ± 0.09 |
| HPAS-5.66 | 0.637 | 21.45 ± 5.99 | 5.6 ± 1.55 | 4.17 ± 0.23 |
| HPAS-6.56 | 0.610 | 15.60 ± 1.04 | 2.3 ± 0.15 | 4.12 ± 0.16 |
| HPAS-8.68 | 0.562 | 2.57 ± 0.20 | 0.3 ± 0.03 | 3.81 ± 0.14 |
| HPAS-10.92 | 0.548 | 0 | 0 | 3.79 ± 0.18 |
| Sample | C1 | C4 | |
|---|---|---|---|
| Single Helix Area/% | Double Helix Area/% | Amorphous Area/% | |
| HPAS-4.83 | 24.33 | 24.79 | 50.88 |
| HPAS-5.01 | 33.08 | 19.23 | 47.69 |
| HPAS-5.66 | 34.43 | 15.43 | 50.14 |
| HPAS-6.56 | 27.04 | 20.26 | 52.70 |
| HPAS-8.68 | 23.89 | 23.54 | 52.57 |
| HPAS-10.92 | 27.69 | 21.10 | 51.21 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, Y.; Wang, W.; Zhang, H.; Dai, Y.; Hou, H.; Dong, H. Effects of Citric Acid on Structures and Properties of Thermoplastic Hydroxypropyl Amylomaize Starch Films. Materials 2019, 12, 1565. https://doi.org/10.3390/ma12091565
Qin Y, Wang W, Zhang H, Dai Y, Hou H, Dong H. Effects of Citric Acid on Structures and Properties of Thermoplastic Hydroxypropyl Amylomaize Starch Films. Materials. 2019; 12(9):1565. https://doi.org/10.3390/ma12091565
Chicago/Turabian StyleQin, Yang, Wentao Wang, Hui Zhang, Yangyong Dai, Hanxue Hou, and Haizhou Dong. 2019. "Effects of Citric Acid on Structures and Properties of Thermoplastic Hydroxypropyl Amylomaize Starch Films" Materials 12, no. 9: 1565. https://doi.org/10.3390/ma12091565
APA StyleQin, Y., Wang, W., Zhang, H., Dai, Y., Hou, H., & Dong, H. (2019). Effects of Citric Acid on Structures and Properties of Thermoplastic Hydroxypropyl Amylomaize Starch Films. Materials, 12(9), 1565. https://doi.org/10.3390/ma12091565




