Thermo-Plasmonic Killing of Escherichia coli TG1 Bacteria
Abstract
1. Introduction
Biomedical Applications of Gold Nanoparticles
2. Materials and Methods
2.1. Synthesis and Characterization of Gold Nanorods
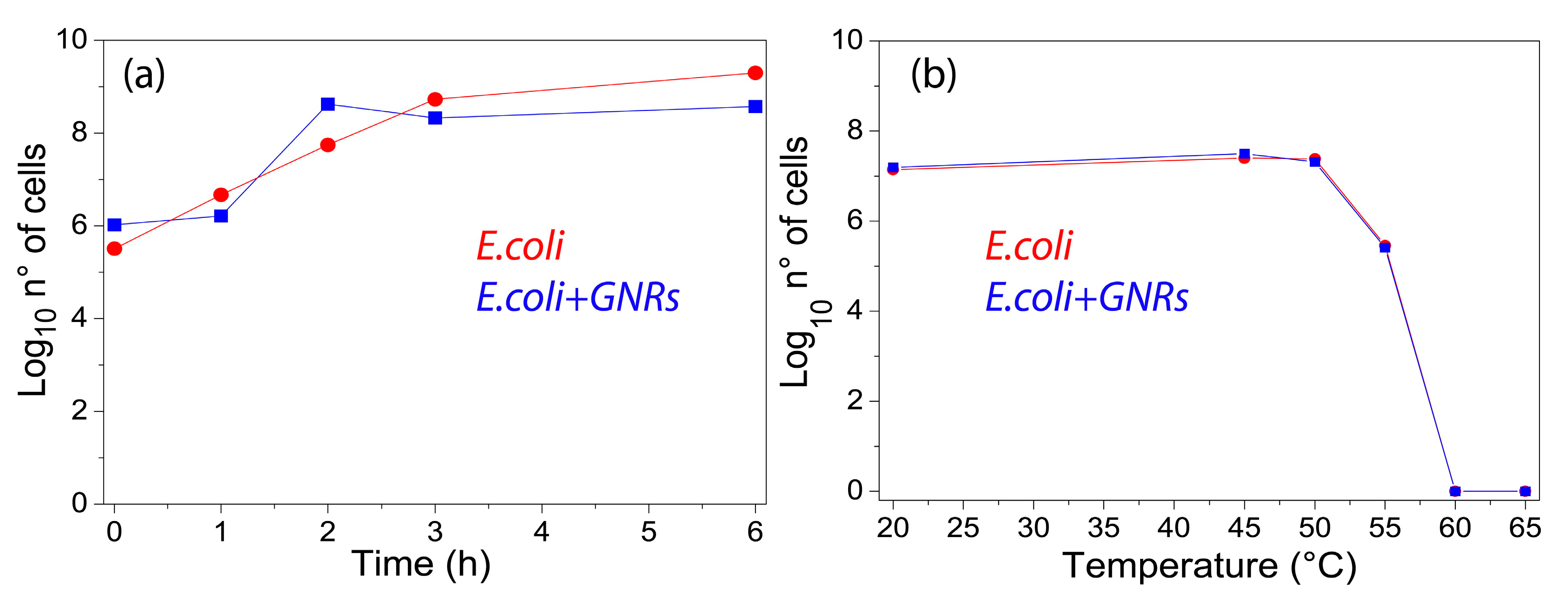
2.2. Effect of Gold Nanorods on Escherichia coli TG1 Growth
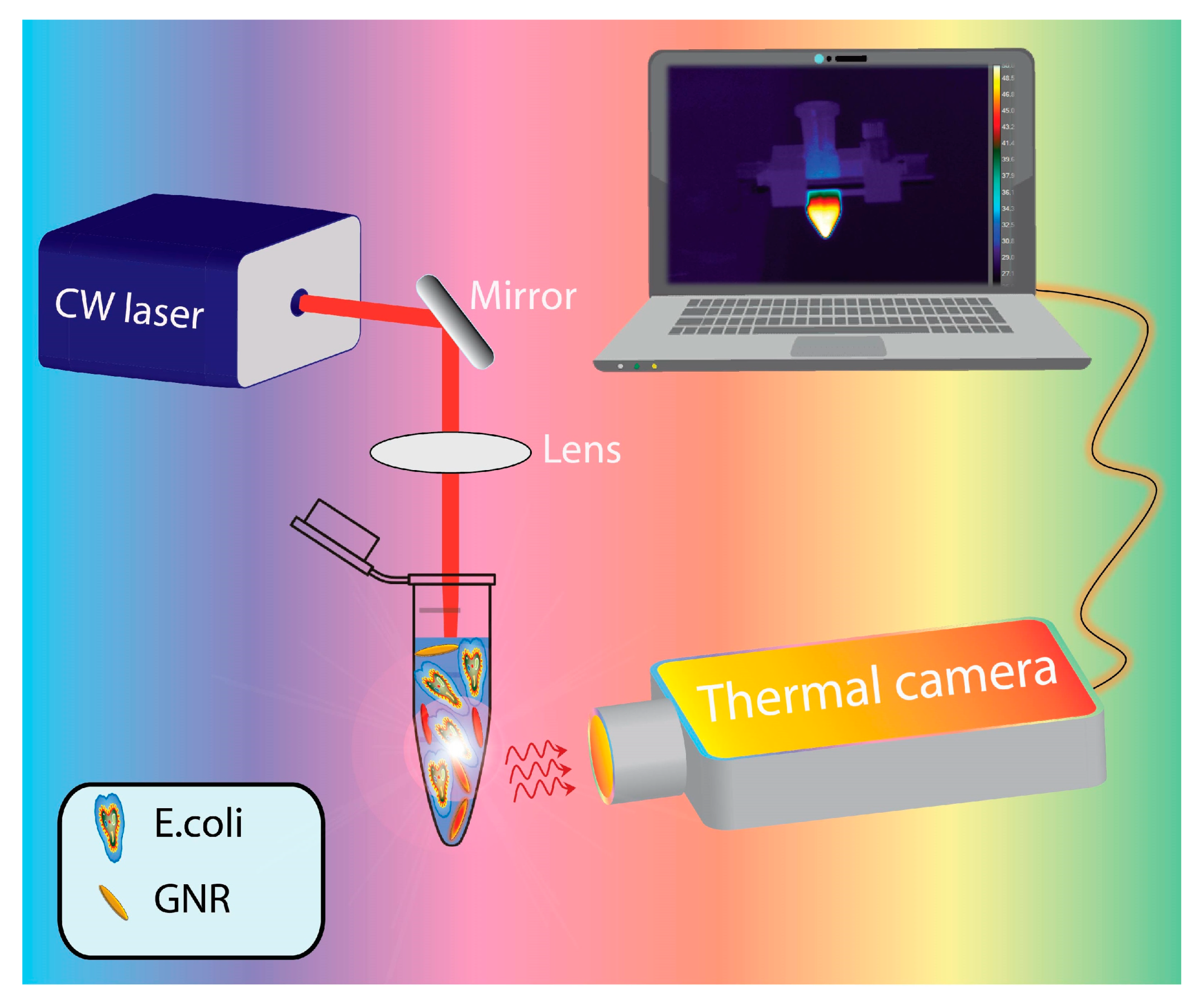
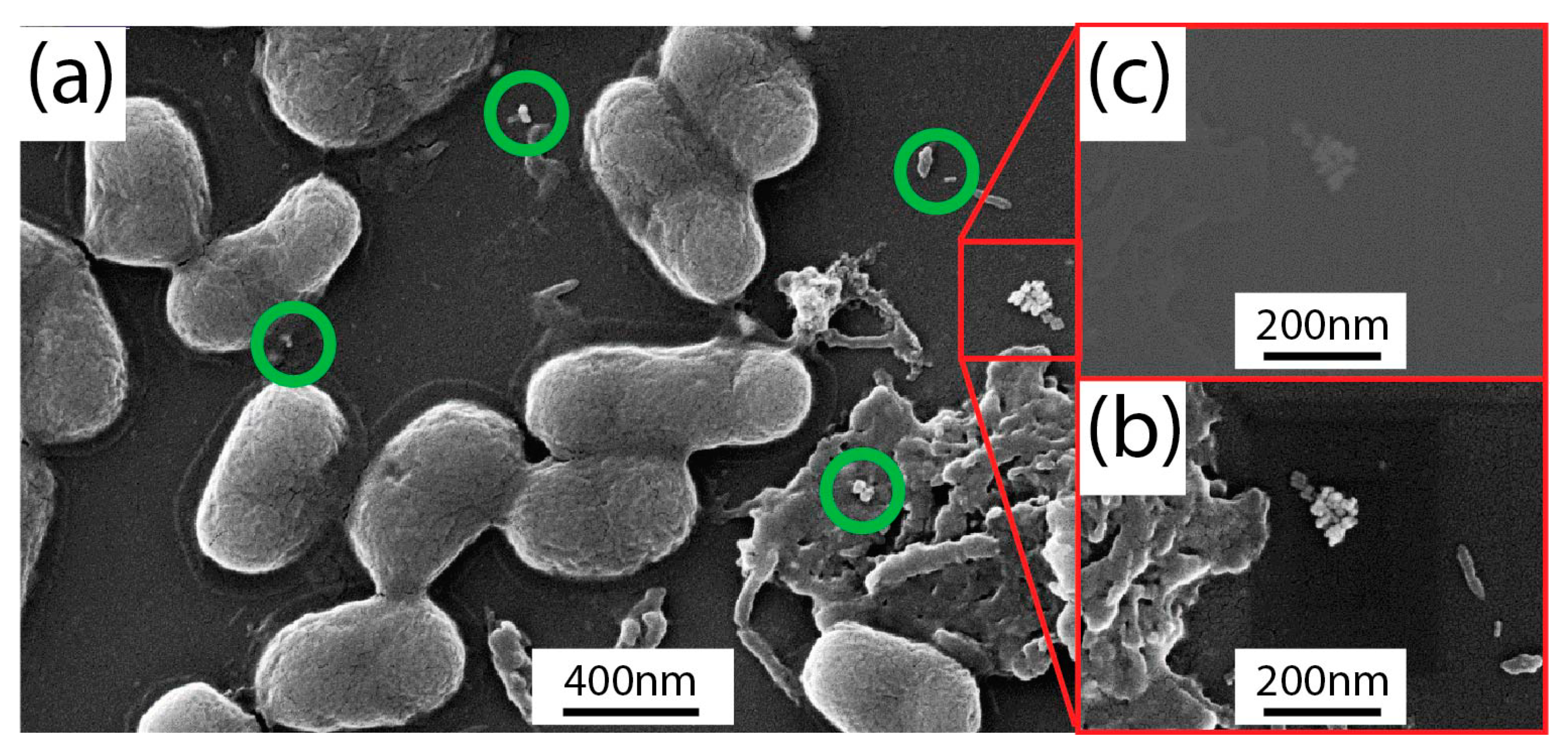
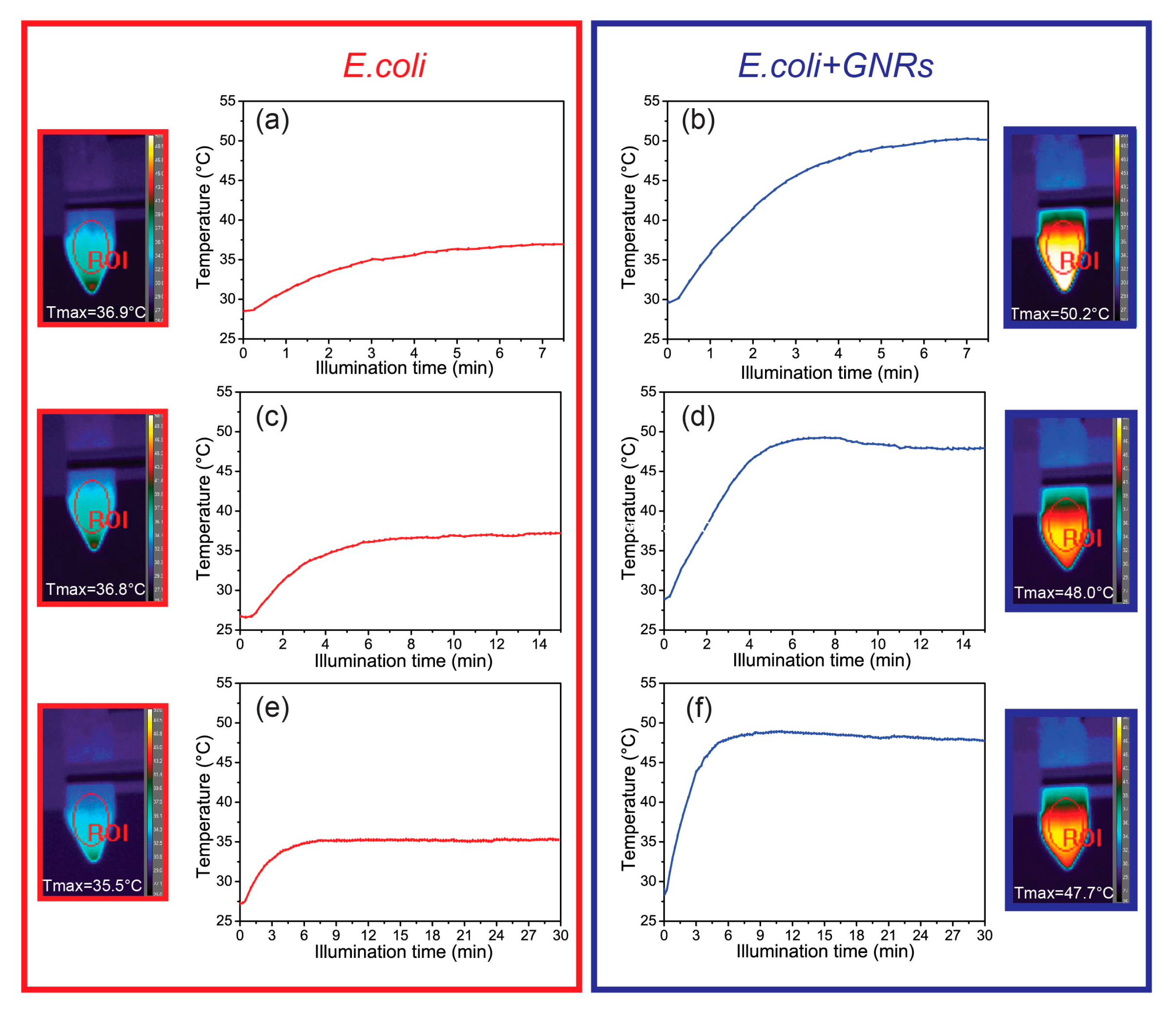
2.3. Photo-Thermal and Morphological Characterization of Escherichia Coli TG1/Gold Nanorods Solutions
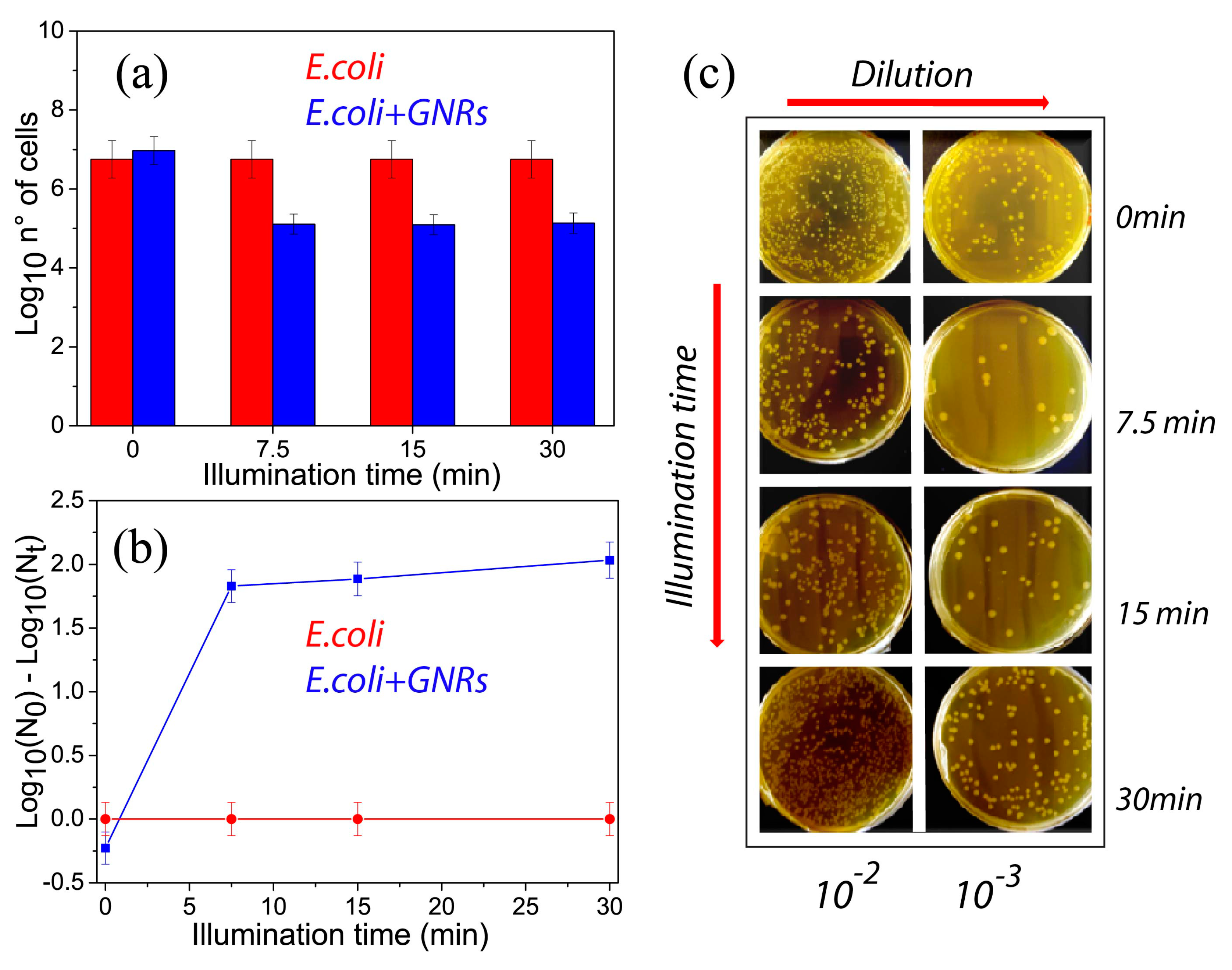
3. Experimental Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PPTT | Plasmonic photo-thermal therapy |
| NPs | Gold nanoparticles |
| PPTT | Plasmonic photo-thermal therapy |
| GNRs | Gold nanorods |
| LPR | Localized plasmonic resonance |
| MIC | Minimum inhibitory concentration |
| MBC | Minimum bactericide concentration |
| NIR | Near infrared |
| E. coli | Escherichia coli TG1 |
References
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95. [Google Scholar] [CrossRef] [PubMed]
- Witte, W. International dissemination of antibiotic resistant strains of bacterial pathogens. Infect. Genet. Evol. 2004, 4, 187–191. [Google Scholar] [CrossRef]
- Blair, J.M.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015, 13, 42. [Google Scholar] [CrossRef] [PubMed]
- Wistrand, C.; Söderquist, B.; Magnusson, A.; Nilsson, U. The effect of preheated versus room-temperature skin disinfection on bacterial colonization during pacemaker device implantation: A randomized controlled non-inferiority trial. Antimicrob. Resist. Infect. Control 2015, 4, 44. [Google Scholar] [CrossRef]
- Brigger, I.; Dubernet, C.; Couvreur, P. Nanoparticles in cancer therapy and diagnosis. Adv. Drug Deliv. Rev. 2012, 64, 24–36. [Google Scholar] [CrossRef]
- Lim, Z.-Z.J.; Li, J.-E.J.; Ng, C.-T.; Yung, L.-Y.L.; Bay, B.-H. Gold nanoparticles in cancer therapy. Acta Pharmacol. Sin. 2011, 32, 983. [Google Scholar] [CrossRef] [PubMed]
- Goodman, A.M.; Neumann, O.; Nørregaard, K.; Henderson, L.; Choi, M.-R.; Clare, S.E.; Halas, N.J. Near-infrared remotely triggered drug-release strategies for cancer treatment. Proc. Natl. Acad. Sci. USA 2017, 114, 12419–12424. [Google Scholar] [CrossRef]
- Teng, C.P.; Zhou, T.; Ye, E.; Liu, S.; Koh, L.D.; Low, M.; Loh, X.J.; Win, K.Y.; Zhang, L.; Han, M.Y. Effective Targeted Photothermal Ablation of Multidrug Resistant Bacteria and Their Biofilms with NIR-Absorbing Gold Nanocrosses. Adv. Healthc. Mater. 2016, 5, 2122–2130. [Google Scholar] [CrossRef]
- Meeker, D.G.; Jenkins, S.V.; Miller, E.K.; Beenken, K.E.; Loughran, A.J.; Powless, A.; Muldoon, T.J.; Galanzha, E.I.; Zharov, V.P.; Smeltzer, M.S. Synergistic photothermal and antibiotic killing of biofilm-associated Staphylococcus aureus using targeted antibiotic-loaded gold nanoconstructs. ACS Infect. Dis. 2016, 2, 241–250. [Google Scholar] [CrossRef]
- Carvalho, F.; George, J.; Sheikh, H.M.A.; Selvin, R. Advances in Screening, Detection and Enumeration of Escherichia coli Using Nanotechnology-Based Methods: A Review. J. Biomed. Nanotechnol. 2018, 14, 829–846. [Google Scholar] [CrossRef]
- Petronella, F.; Rtimi, S.; Comparelli, R.; Sanjines, R.; Pulgarin, C.; Curri, M.L.; Kiwi, J. Uniform TiO2/In2O3 surface films effective in bacterial inactivation under visible light. J. Photochem. Photobiol. A Chem. 2014, 279, 1–7. [Google Scholar] [CrossRef]
- Liz-Marzan, L.M. Nanometals formation and color. Mater. Today 2004, 7, 26–31. [Google Scholar]
- Baffou, G.; Quidant, R. Thermo-plasmonics: Using metallic nanostructures as nano-sources of heat. Laser Photonics Rev. 2013, 7, 171–187. [Google Scholar] [CrossRef]
- De Sio, L.; Placido, T.; Comparelli, R.; Curri, M.L.; Striccoli, M.; Tabiryan, N.; Bunning, T.J. Next-generation thermo-plasmonic technologies and plasmonic nanoparticles in optoelectronics. Prog. Quantum Electron. 2015, 41, 23–70. [Google Scholar] [CrossRef]
- Huang, X.H.; Neretina, S.; El-Sayed, M.A. Gold Nanorods: From Synthesis and Properties to Biological and Biomedical Applications. Adv. Mater. 2009, 21, 4880–4910. [Google Scholar] [CrossRef] [PubMed]
- Turcheniuk, K.; Turcheniuk, V.; Hage, C.-H.; Dumych, T.; Bilyy, R.; Bouckaert, J.; Héliot, L.; Zaitsev, V.; Boukherroub, R.; Szunerits, S. Highly effective photodynamic inactivation of E. coli using gold nanorods/SiO2 core–shell nanostructures with embedded verteporfin. Chem. Commun. 2015, 51, 16365–16368. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Liu, J.; Shi, J. Nuclear-Targeting Gold Nanorods for Extremely Low NIR Activated Photothermal Therapy. ACS Appl. Mater. Interfaces 2017, 9, 15952–15961. [Google Scholar] [CrossRef] [PubMed]
- Dreaden, E.C.; Mackey, M.A.; Huang, X.H.; Kang, B.; El-Sayed, M.A. Beating cancer in multiple ways using nanogold. Chem. Soc. Rev. 2011, 40, 3391–3404. [Google Scholar] [CrossRef]
- O’Neal, D.P.; Hirsch, L.R.; Halas, N.J.; Payne, J.D.; West, J.L. Photo-thermal tumor ablation in mice using near infrared-absorbing nanoparticles. Cancer Lett. 2004, 209, 171–176. [Google Scholar] [CrossRef]
- Joensen, J.; Demmink, J.H.; Johnson, M.I.; Iversen, V.V.; Lopes-Martins, R.A.; Bjordal, J.M. The thermal effects of therapeutic lasers with 810 and 904 nm wavelengths on human skin. Photomed. Laser Surg. 2011, 29, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Norman, R.S.; Stone, J.W.; Gole, A.; Murphy, C.J.; Sabo-Attwood, T.L. Targeted photothermal lysis of the pathogenic bacteria, Pseudomonas aeruginosa, with gold nanorods. Nano Lett. 2008, 8, 302–306. [Google Scholar] [CrossRef]
- Castillo-Martinez, J.C.; Martinez-Castanon, G.A.; Martinez-Gutierrez, F.; Zavala-Alonso, N.V.; Patino-Marin, N.; Nino-Martinez, N.; Zaragoza-Magana, V.; Cabral-Romero, C. Antibacterial and Antibiofilm Activities of the Photothermal Therapy Using Gold Nanorods against Seven Different Bacterial Strains. J. Nanomater. 2015, 2015, 783671. [Google Scholar] [CrossRef]
- Santos, G.M.; Ferrara, F.I.D.; Zhao, F.S.; Rodrigues, D.F.; Shih, W.C. Photothermal inactivation of heat-resistant bacteria on nanoporous gold disk arrays. Opt. Mater. Express 2016, 6, 1217–1229. [Google Scholar] [CrossRef]
- Pihl, M.; Bruzell, E.; Andersson, M. Bacterial biofilm elimination using gold nanorod localised surface plasmon resonance generated heat. Mater. Sci. Eng. C 2017, 80, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Abreu, A.C.; Tavares, R.R.; Borges, A.; Mergulhao, F.; Simoes, M. Current and emergent strategies for disinfection of hospital environments. J. Antimicrob. Chemother. 2013, 68, 2718–2732. [Google Scholar] [CrossRef]
- Placido, T.; Aragay, G.; Pons, J.; Comparelli, R.; Curri, M.L.; Merkoci, A. Ion-Directed Assembly of Gold Nanorods: A Strategy for Mercury Detection. ACS Appl. Mater. Interfaces 2013, 5, 1084–1092. [Google Scholar] [CrossRef] [PubMed]
- De Sio, L.; Placido, T.; Serak, S.; Comparelli, R.; Tamborra, M.; Tabiryan, N.; Curri, M.L.; Bartolino, R.; Umeton, C.; Bunning, T. Nano-Localized Heating Source for Photonics and Plasmonics. Adv. Opt. Mater. 2013, 1, 899–904. [Google Scholar] [CrossRef]
- Wang, Y.; Wan, J.; Miron, R.J.; Zhao, Y.; Zhang, Y. Antibacterial properties and mechanisms of gold–silver nanocages. Nanoscale 2016, 11143–11152. [Google Scholar] [CrossRef] [PubMed]
- Baharoglu, Z.; Mazel, D. SOS, the formidable strategy of bacteria against aggressions. FEMS Microbiol. Rev. 2014, 38, 1126–1145. [Google Scholar] [CrossRef]
- Pankey, G.A.; Sabath, L.D. Clinical Relevance of Bacteriostatic versus Bactericidal Mechanisms of Action in the Treatment of Gram-Positive Bacterial Infections. Clin. Infect. Dis. 2004, 38, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, V.; Lee, T. Nanotechnology-based flexible electronics. Nanotechnology 2012, 23, 340201. [Google Scholar] [CrossRef]
- De Sio, L.; Cataldi, U.; Guglielmelli, A.; Burgi, T.; Tabiryan, N.; Bunning, T.J. Dynamic optical properties of gold nanoparticles/cholesteric liquid crystal arrays. MRS Commun. 2018, 8, 550–555. [Google Scholar] [CrossRef]
- Madhumitha, G.F.J.; Roopan, S.M. Nanoparticles for Agriculture: Synthesis, Classification and Characterization. In Nanoscience in Food and Agriculture 3; Springer: Cham, Switzerland, 2016; pp. 99–127. [Google Scholar]
- Hofmann-Amtenbrink, M.; Grainger, D.W.; Hofmann, H. Nanoparticles in medicine: Current challenges facing inorganic nanoparticle toxicity assessments and standardizations. Nanomedicine 2015, 11, 1689–1694. [Google Scholar] [CrossRef]
- Petronella, F.; Pagliarulo, A.; Striccoli, M.; Calia, A.; Lettieri, M.; Colangiuli, D.; Curri, M.L.; Comparelli, R. Colloidal Nanocrystalline Semiconductor Materials as Photocatalysts for Environmental Protection of Architectural Stone. Crystals 2017, 7, 30. [Google Scholar] [CrossRef]
- Isomaa, B.; Reuter, J.; Djupsund, B.M. The subacute and chronic toxicity of cetyltrimethylammonium bromide (CTAB), a cationic surfactant, in the rat. Arch. Toxicol. 1976, 35, 91–96. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Annesi, F.; Pane, A.; Losso, M.A.; Guglielmelli, A.; Lucente, F.; Petronella, F.; Placido, T.; Comparelli, R.; Guzzo, M.G.; Curri, M.L.; et al. Thermo-Plasmonic Killing of Escherichia coli TG1 Bacteria. Materials 2019, 12, 1530. https://doi.org/10.3390/ma12091530
Annesi F, Pane A, Losso MA, Guglielmelli A, Lucente F, Petronella F, Placido T, Comparelli R, Guzzo MG, Curri ML, et al. Thermo-Plasmonic Killing of Escherichia coli TG1 Bacteria. Materials. 2019; 12(9):1530. https://doi.org/10.3390/ma12091530
Chicago/Turabian StyleAnnesi, Ferdinanda, Alfredo Pane, Maria Adele Losso, Alexa Guglielmelli, Fabrizio Lucente, Francesca Petronella, Tiziana Placido, Roberto Comparelli, Maria Grazia Guzzo, Maria Lucia Curri, and et al. 2019. "Thermo-Plasmonic Killing of Escherichia coli TG1 Bacteria" Materials 12, no. 9: 1530. https://doi.org/10.3390/ma12091530
APA StyleAnnesi, F., Pane, A., Losso, M. A., Guglielmelli, A., Lucente, F., Petronella, F., Placido, T., Comparelli, R., Guzzo, M. G., Curri, M. L., Bartolino, R., & De Sio, L. (2019). Thermo-Plasmonic Killing of Escherichia coli TG1 Bacteria. Materials, 12(9), 1530. https://doi.org/10.3390/ma12091530








