Enhanced High-Temperature Mechanical Properties of Al–Cu–Li Alloy through T1 Coarsening Inhibition and Ce-Containing Intermetallic Refinement
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
- (1)
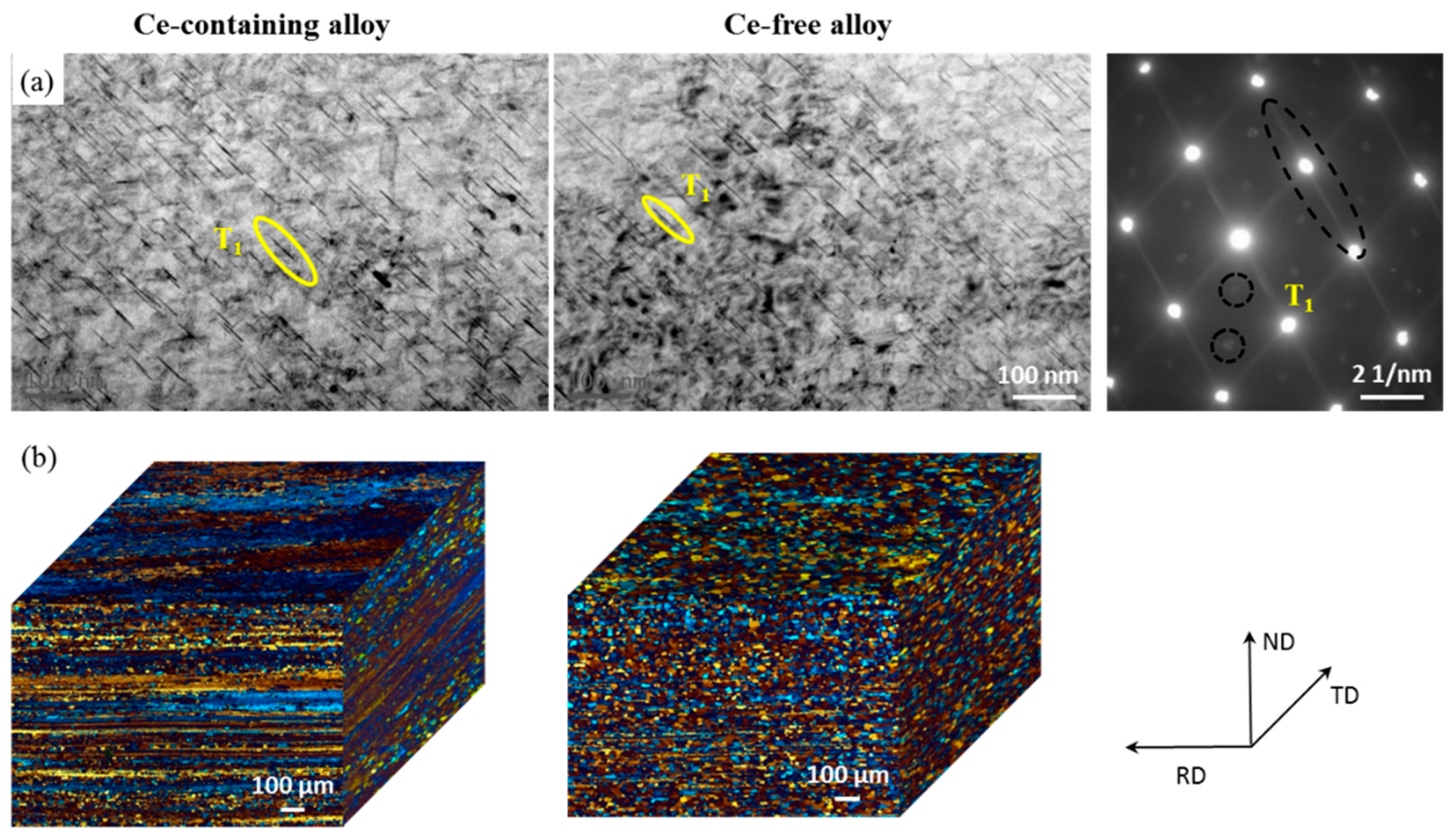
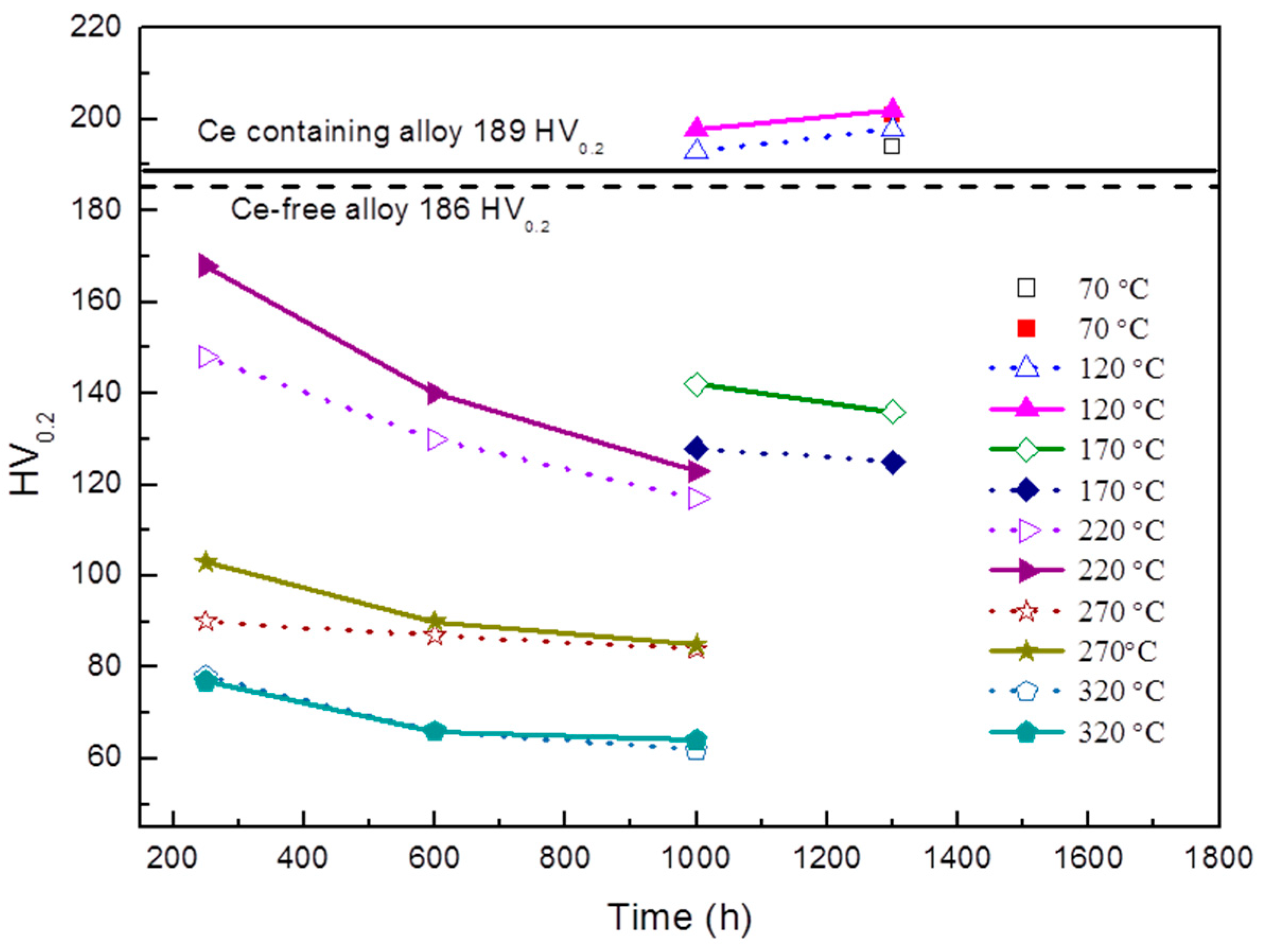
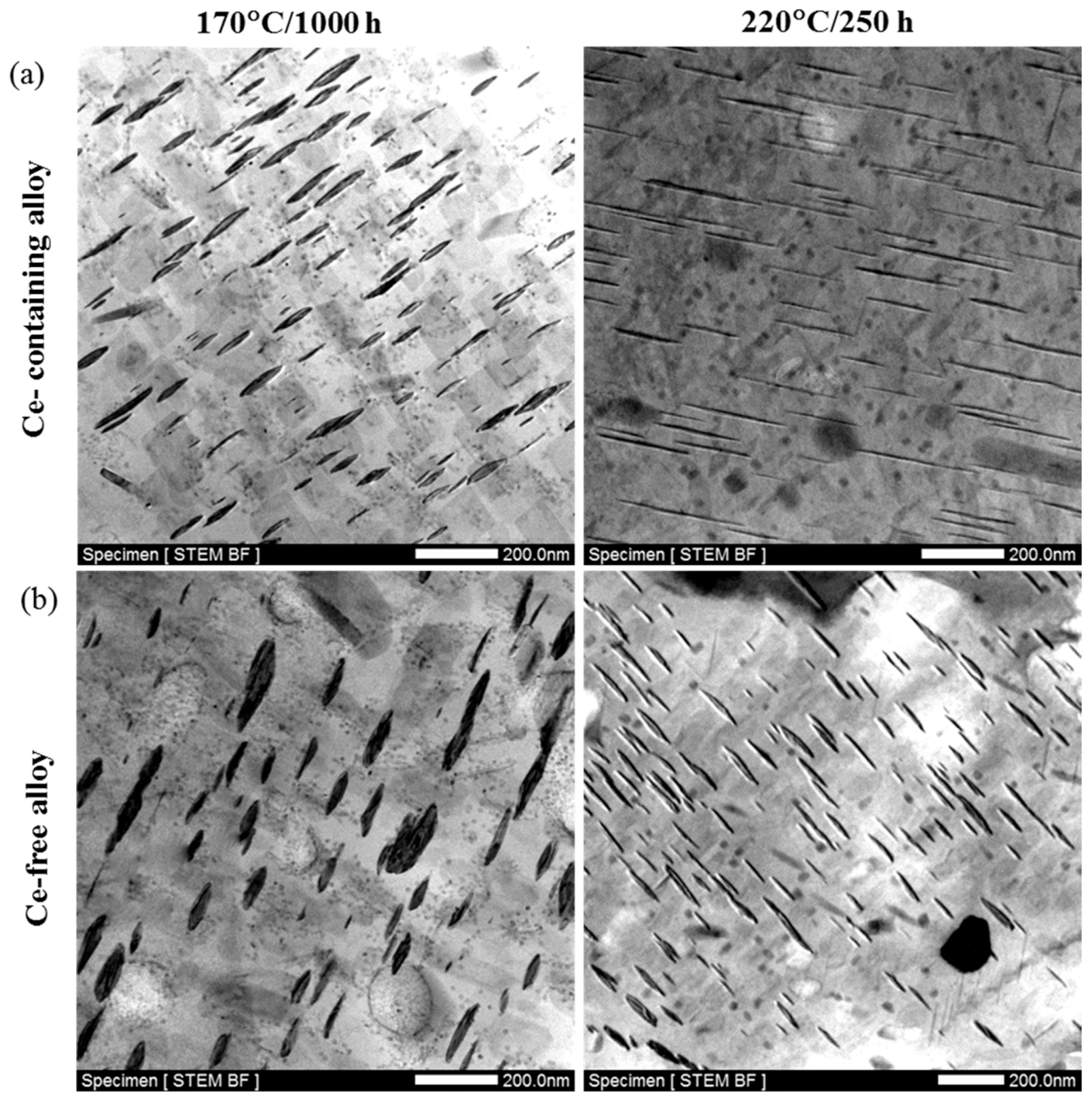
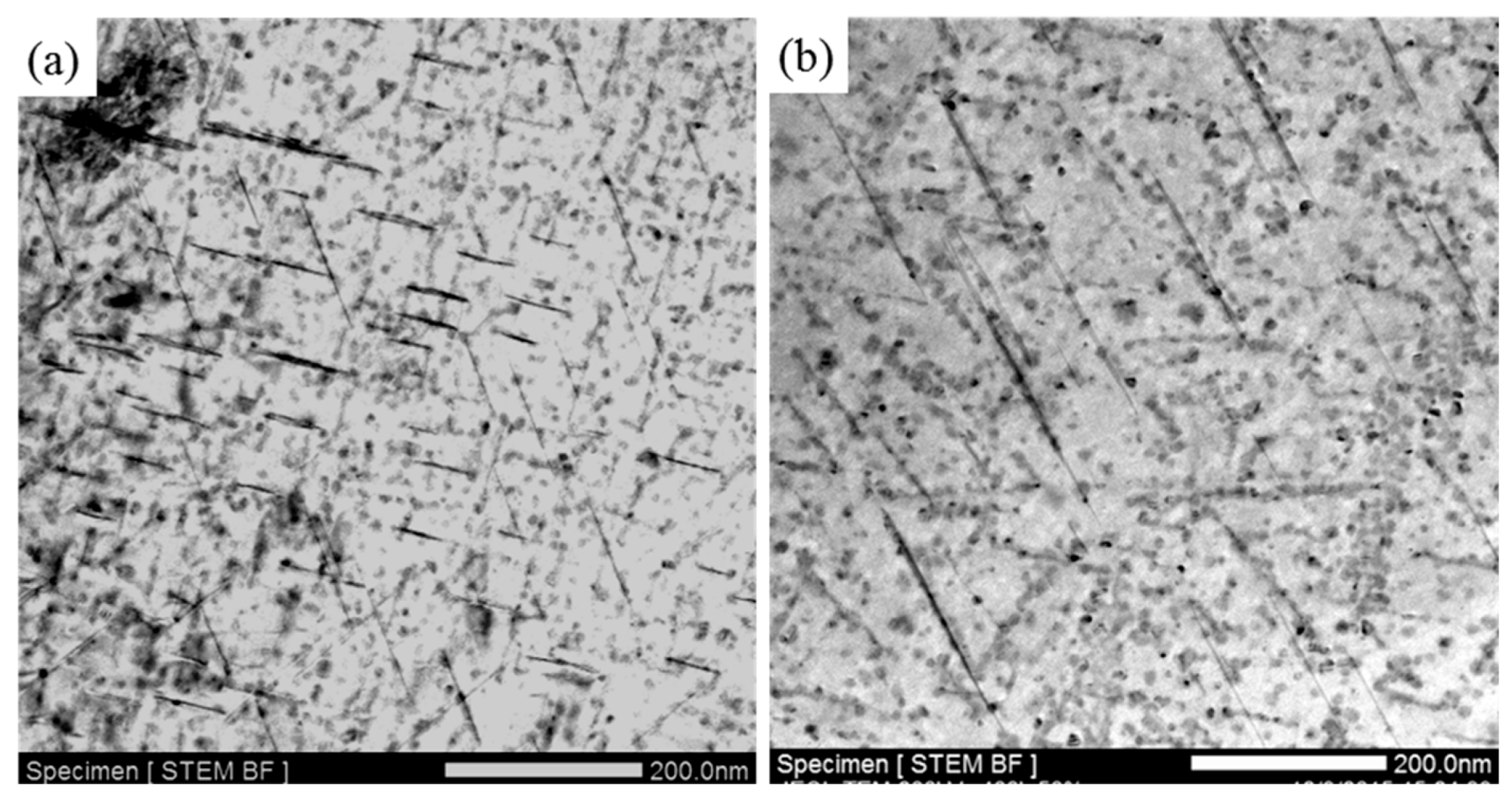
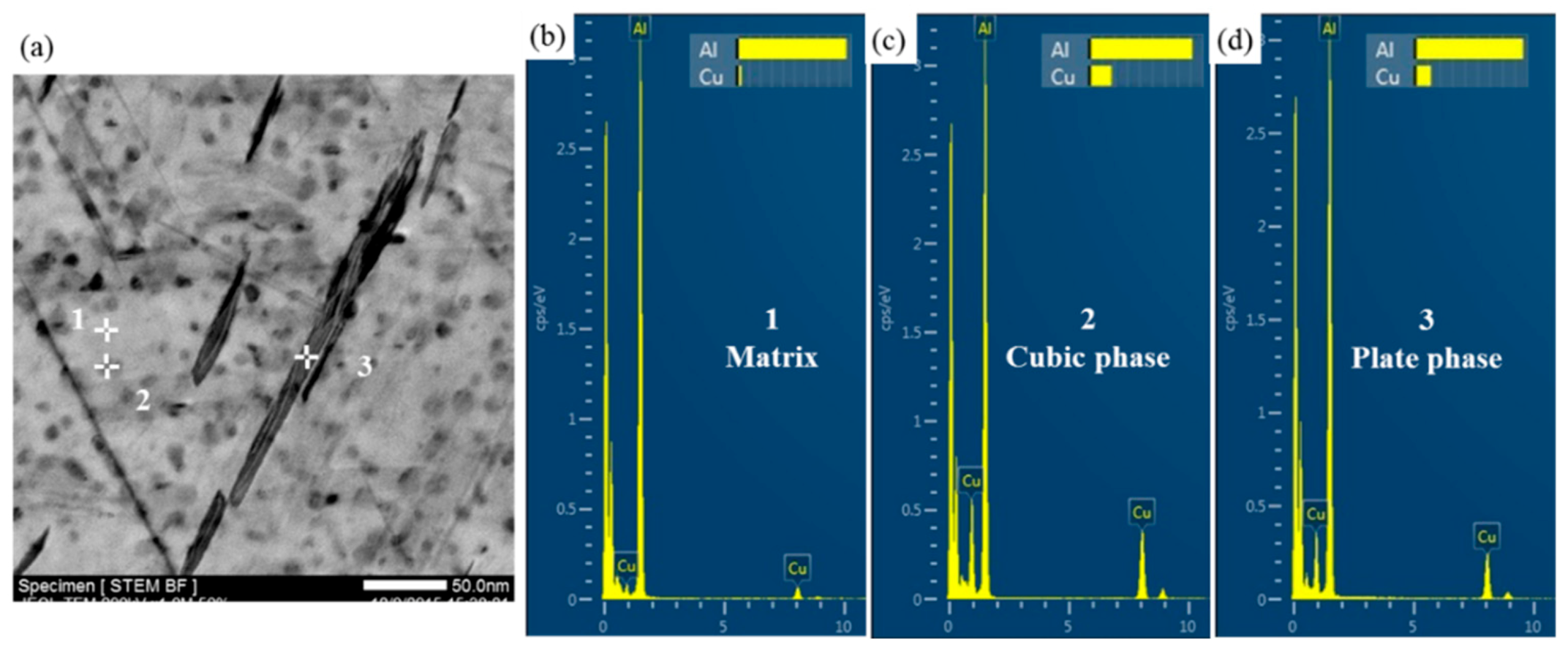
- The thermal stability of Al–Cu–Li alloy is substantiality improved by Ce addition during thermal exposure at the medium-high-temperature stage (170 °C to 270 °C). The enhanced thermal stability of Ce-containing alloy can be attributed to the coarsening inhibition of T1 through impeding the diffusion of Cu. Ce segregates at the interface of the T1 phase can restrict its lateral growth, thereby inhibiting its coarsening.
- (2)
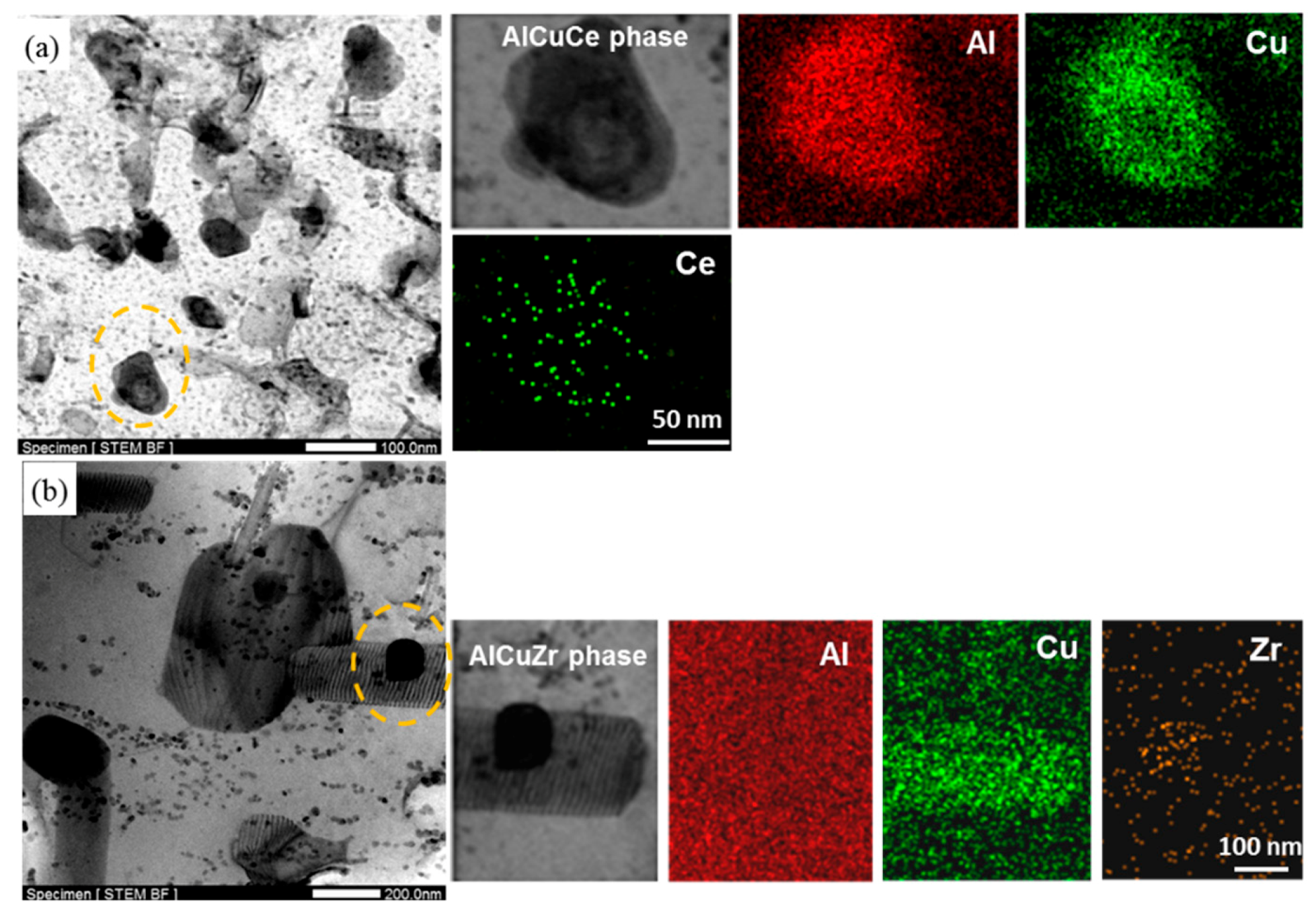
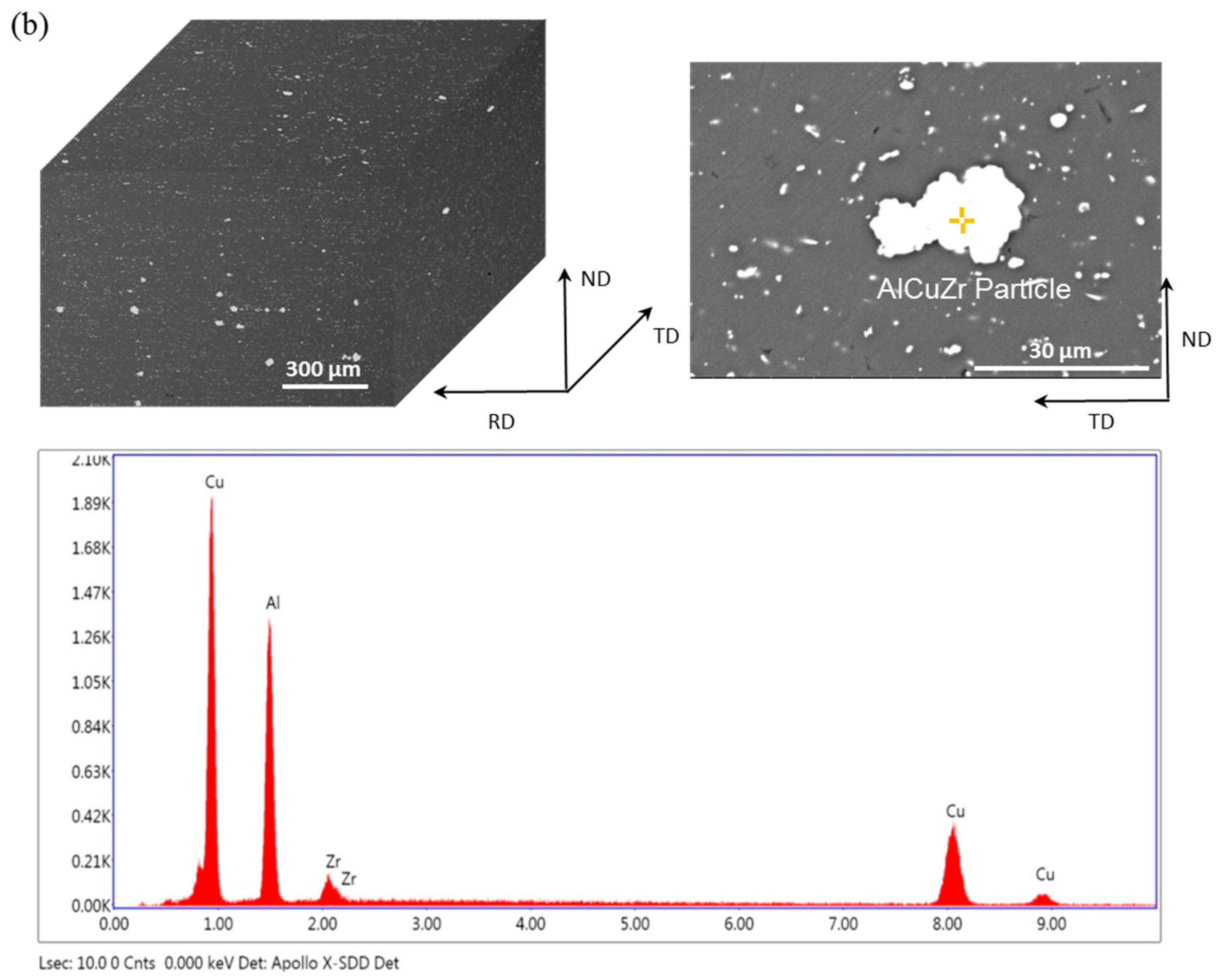
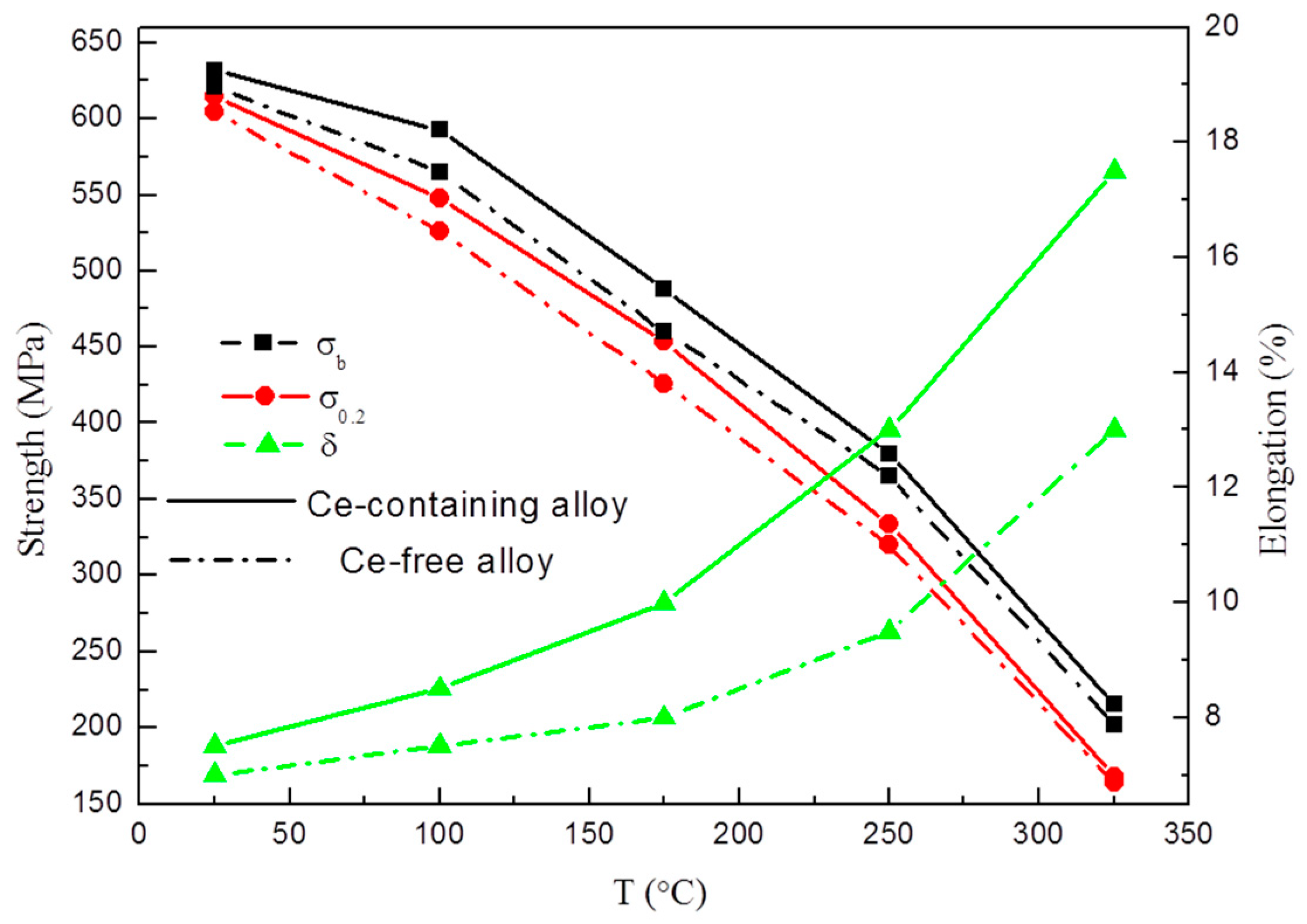
- As the test temperature increases from room temperature to 325 °C, the elongation of Ce-containing alloy increased rapidly from 7.5% to 17.5%, while that of Ce-free alloy only increased from 7% to 13%. The high tensile strength of Ce-containing alloy is always higher than that of Ce-free alloy. The high-temperature stable small Al8Cu4Ce phase in Ce-containing alloy can effectively pin dislocations and grain boundaries, which is beneficial to the improvement of the ductility and strength of the alloy in the high-temperature tensile test.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yu, X.X.; Dai, H.; Li, Z.T.; Sun, J.; Zhao, J.F.; Li, C.Q.; Liu, W.W. Improved recrystallization resistance of Al–Cu–Li–Zr alloy through Ce addition. Metals 2018, 8, 1035. [Google Scholar] [CrossRef]
- Rodgers, B.I.; Prangnell, P.B. Quantification of the influence of increased pre-stretching on microstructure strength relationships in the Al–Cu–Li alloy AA2195. Acta Mater. 2016, 108, 55–67. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, X.; Thompson, G.E.; Curioni, M.; Skeldon, P.; Zhang, X.; Sun, Z.; Luo, C.; Tang, Z.; Lu, F. Anodic film growth on Al-Li-Cu alloy AA2099-T8. Electrochim. Acta 2012, 80, 148–159. [Google Scholar] [CrossRef]
- Rioja, R.J.; Liu, J. The evolution of Al-Li base products for aerospace and space applications. Metall. Mater. Trans. A 2012, 43, 3325–3337. [Google Scholar] [CrossRef]
- Polmear, I.J.; Pons, G.; Barbaux, Y.; Octor, H.; Sanchez, C.; Morton, A.J.; Borbidge, W.E.; Rogers, S. After concorde: Evaluation of creep resistant Al-Cu-Mg-Ag alloys. J. Mater. Sci. Technol. 2013, 15, 861–868. [Google Scholar] [CrossRef]
- Ortiz, D.; Brown, J.; Abdelshehid, M.; DeLeon, P.; Dalton, R.; Mendez, L.; Soltero, J.; Pereira, M.; Hahn, M.; Lee, E.; et al. The effects of prolonged thermal exposure on the mechanical properties and fracture toughness of C458 aluminum-lithium alloy. Eng. Fail. Anal. 2006, 13, 170–180. [Google Scholar] [CrossRef]
- Jabra, J.; Romios, M.; Lai, J.; Lee, E.; Setiawan, M.; Lee, E.W.; Witters, J.; Abourialy, N.; Ogren, J.R.; Clark, R.; et al. The effect of thermal exposure on the mechanical properties of 2099-T6 die forgings, 2099-T83 extrusions, 7075-T7651 plate, 7085-T7452 die forgings, 7085-T7651 plate, and 2397-T87 plate aluminum alloys. J. Mater. Eng. Perform. 2006, 15, 601–607. [Google Scholar] [CrossRef]
- Deschamps, A.; Garcia, M.; Chevy, J.; Davo, B.; De Geuser, F. Influence of Mg and Li content on the microstructure evolution of Al-Cu-Li alloys during long-term ageing. Acta Mater. 2017, 122, 32–46. [Google Scholar] [CrossRef]
- Balducci, E.; Ceschini, L.; Messieri, S.; Wenner, S.; Holmestad, R. Thermal stability of the lightweight 2099 Al-Cu-Li alloy: Tensile tests and microstructural investigations after overaging. Mater. Des. 2017, 119, 54–64. [Google Scholar] [CrossRef]
- Decreus, B.; Deschamps, A.; De Geuser, F.; Donnadieu, P.; Sigli, C.; Weyland, M. The influence of Cu/Li ratio on precipitation in Al-Cu-Li-x alloys. Acta Mater. 2013, 61, 2207–2218. [Google Scholar] [CrossRef]
- Dorin, T.; Deschamps, A.; De Geuser, F.; Sigli, C. Quantification and modelling of the microstructure/strength relationship by tailoring the morphological parameters of the T1 phase in an Al–Cu–Li alloy. Acta Mater. 2014, 75, 134–146. [Google Scholar] [CrossRef]
- Chen, Z.; Zheng, Z. Microstructural evolution and ageing behavior of the low Cu:Mg ratio Al-Cu-Mg Alloys containing Scandium and Lithium. Scr. Mater. 2004, 50, 1067–1071. [Google Scholar] [CrossRef]
- Wang, J.; Yi, D.; Lu, B.; Liu, S.; Cao, Y. Effect of yttrium on microstructure and properties of 2618 Aluminum alloy. J. Chin. Rare Earth Soc. 2002, 20, 150–153. [Google Scholar]
- Yao, D.; Zhao, W.; Zhao, H.; Qiu, F.; Jiang, Q. High creep resistance behavior of the casting Al–Cu alloy modified by La. Scr. Mater. 2009, 61, 1153–1155. [Google Scholar] [CrossRef]
- Yang, J.; Nie, Z.; Jin, T.; Xu, G.; Fu, J.; Ruan, H.; Zuo, T. Effect of trace rare earth element Er on high pure Al-Zn-Mg alloy. Trans. Nonferrous Met. Soc. China 2003, 13, 1035–1039. [Google Scholar]
- Yu, X.X.; Yin, D.F.; Yu, Z.M. Effects of cerium and zirconium microalloying addition on the microstructures and tensile properties of novel Al–Cu–Li alloys. Rare Met. Mater. Eng. 2016, 45, 1917–1923. [Google Scholar]
- Vončina, M.; Kores, S.; Mrvar, P.; Medved, J. Effect of Ce on solidification and mechanical properties of A360 alloy. J. Alloys Compd. 2011, 509, 7349–7355. [Google Scholar] [CrossRef]
- Meng, L.; Zhang, B. Effect of Ce modification on Cu diffusion coefficient in diffusion system of alloy 2090–Al. Acta Metall. Sin.-Engl. 1993, 29, 57–61. [Google Scholar]
- Cabibbo, M.; Evangelista, E.; Vedani, M. Influence of severe plastic deformations on secondary phase precipitation in a 6082 Al–Mg–Si alloy. Metall. Mat. Trans. A 2005, 36, 1353–1364. [Google Scholar] [CrossRef]
- Chen, X.; Zheng, Z.; Li, S.; Wei, X.; Chen, Z. Trace element effects on microstructure and mechanical properties in Al–Cu–Li alloy after thermal exposure. Rare Met. Mater. Eng. 2007, 36, 1024–1028. [Google Scholar]
- Chaubey, A.K.; Mohapatra, S.; Jayasankar, K.; Pradhan, S.K.; Satpati, B.; Sahay, S.S.; Mishra, B.K.; Mukherjee, P.S. Effect of cerium addition on microstructure and mechanical properties of Al–Zn–Mg–Cu alloy. Trans. Indian Inst. Met. 2009, 62, 539–543. [Google Scholar] [CrossRef]
- Lai, J.P.; Jiang, R.P.; Liu, H.S.; Dun, X.L.; Li, Y.F.; Li, X.Q. Influence of cerium on microstructures and mechanical properties of Al-Zn-Mg-Cu alloys. J. Cent. South Univ. Technol. 2012, 19, 869–874. [Google Scholar] [CrossRef]
- Yu, X.X.; Yin, D.F.; Yu, Z.M.; Zhang, Y.R.; Li, S.F. Solidification behaviour and the effects of cerium on the intermetallic structure of a novel Al–Cu–Li alloy. Rare Met. Mater. Eng. 2016, 45, 1423–1429. [Google Scholar]
- Yu, X.X.; Yin, D.F.; Yu, Z.M.; Zhang, Y.R.; Li, S.F. Microstructure evolution of a novel Al–Cu–Li–Ce alloy during homogenization. Rare Met. Mater. Eng. 2016, 45, 1687–1694. [Google Scholar]
- Yu, X.X.; Sun, J.; Li, Z.T.; Dai, H.; Fang, H.J.; Zhang, J.F.; Yin, D.F. Solidification behavior and elimination of undissolved Al2CuMg phase during homogenization in Ce-modified Al–Zn–Mg–Cu alloy. Rare Met. 2018. [Google Scholar] [CrossRef]
- Gain, A.K.; Fouzder, T.; Chan, Y.C.; Yung, W.K.C. Microstructure, kinetic analysis and hardness of Sn–Ag–Cu–1wt% nano-ZrO2 composite solder on OSP-Cu pads. J. Alloys Compd. 2011, 509, 3319–3325. [Google Scholar] [CrossRef]
- Yu, X.X.; Zhang, Y.R.; Yin, D.F.; Yu, Z.M.; Li, S.F. Characterization of hot deformation behavior of a novel Al–Cu–Li Alloy using processing maps. Acta Metall. Sin. 2015, 28, 817–825. [Google Scholar] [CrossRef]
- Gable, B.M.; Zhu, A.W.; Csontos, A.A.; Starke, E.A., Jr. The role of plastic deformation on the competitive microstructural evolution and mechanical properties of a novel Al–Li–Cu–X alloy. J. Light Met. 2001, 1, 1–14. [Google Scholar] [CrossRef]
- Tsivoulas, D.; Robson, J.D.; Sigli, C.; Prangnell, P.B. Interactions between zirconium and manganese dispersoid-forming elements on their combined addition in Al–Cu–Li alloys. Acta Mater. 2012, 60, 5245–5259. [Google Scholar] [CrossRef]
- Khan, I.N.; Starink, M.J.; Yan, J.L. A model for precipitation kinetics and strengthening in Al–Cu–Mg alloys. Mater. Sci. Eng. A 2008, 472, 66–74. [Google Scholar] [CrossRef]
- Hirosawa, S.; Sato, T.; Kamio, A.; Flower, H.M. Classification of the role of microalloying elements in phase decomposition of Al based alloys. Acta Mater. 2014, 48, 1797–1806. [Google Scholar] [CrossRef]
- Pérez-Bustamante, R.; Reyna-Cruz, A.; Acosta-Peña, D.C.; Santillán-Rodríguez, C.R.; Matutes-Aquino, J.A.; Pérez-Bustamante, F.; Maldonado-Orozco, M.C.; Aguilar-Santillán, J.; Martínez-Sánchez, R. Effect of cerium/lanthanum addition on microstructure and mechanical properties of Al7075 alloy via mechanical alloying and sintering. J. Rare Earth. 2016, 34, 420–427. [Google Scholar] [CrossRef]
- Tsivoulas, D.; Robson, J.D. Heterogeneous Zr solute segregation and Al3Zr dispersoid distributions in Al-Cu-Li alloys. Acta Mater. 2015, 93, 73–86. [Google Scholar] [CrossRef]
- Jiang, W.; Fan, Z.; Dai, Y.; Li, C. Effects of rare earth elements addition on microstructures, tensile properties and fractography of A357 alloy. Mater. Sci. Eng. A 2014, 597, 237–244. [Google Scholar] [CrossRef]
- Govindaraju, H.K.; Jayaraj, T.; Sadanandarao, P.R. Evaluation of mechanical properties of as-cast Al–Zn–Ce alloy. Mater. Des. 2010, 31, S24–S29. [Google Scholar] [CrossRef]
- Hernandez-Sandoval, J.; Garza-Elizondo, G.H.; Samuel, A.M.; Valtiierra, S.; Samuel, F.H. The ambient and high temperature deformation behavior of Al-Si-Cu-Mg alloy with minor Ti, Zr, Ni additions. Mater. Des. 2014, 58, 89–101. [Google Scholar] [CrossRef]
- Cavaliere, P.; Marco, P.P.D. Friction stir processing of a Zr-modified 2014 aluminium alloy. Mater. Sci. Eng. A 2007, 462, 206–210. [Google Scholar] [CrossRef]
- Spigarelli, S.; Cabibbo, M.; Evangelista, E.; Bidulská, J. A study of the hot formability of an Al–Cu–Mg–Zr alloy. J. Mater. Sci. 2003, 38, 81–88. [Google Scholar] [CrossRef]
- Emami, A.R.; Begum, S.; Chen, D.L.; Skszek, T.; Niu, X.P.; Zhang, Y. Cyclic deformation behavior of a cast aluminum alloy. Mater. Sci. Eng. A 2009, 516, 31–41. [Google Scholar] [CrossRef]
- Ibrahim, M.F.; Samuel, E.; Samuel, A.M.; Al-Ahmari, A.M.A.; Samuel, F.H. Metallurgical parameters controlling the microstructure and hardness of Al–Si–Cu–Mg base alloys. Mater. Des. 2011, 32, 2130–2142. [Google Scholar] [CrossRef]
- Banerjee, S.; Robi, P.S.; Srinivasan, A.; Kumar Lakavath, P. Effect of trace additions of Sn on microstructure and mechanical properties of Al–Cu–Mg alloys. Mater. Des. 2010, 31, 4007–4015. [Google Scholar] [CrossRef]


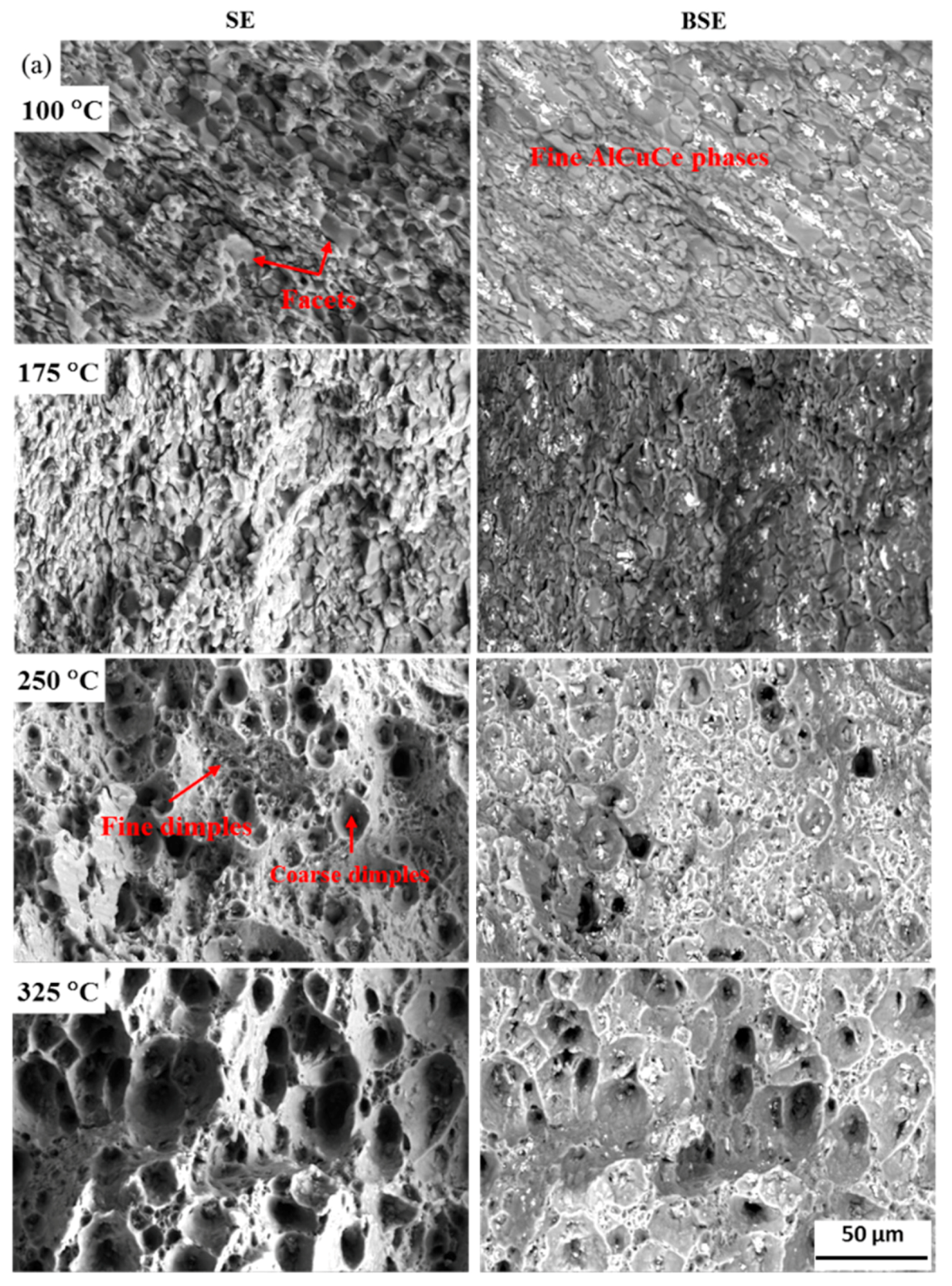
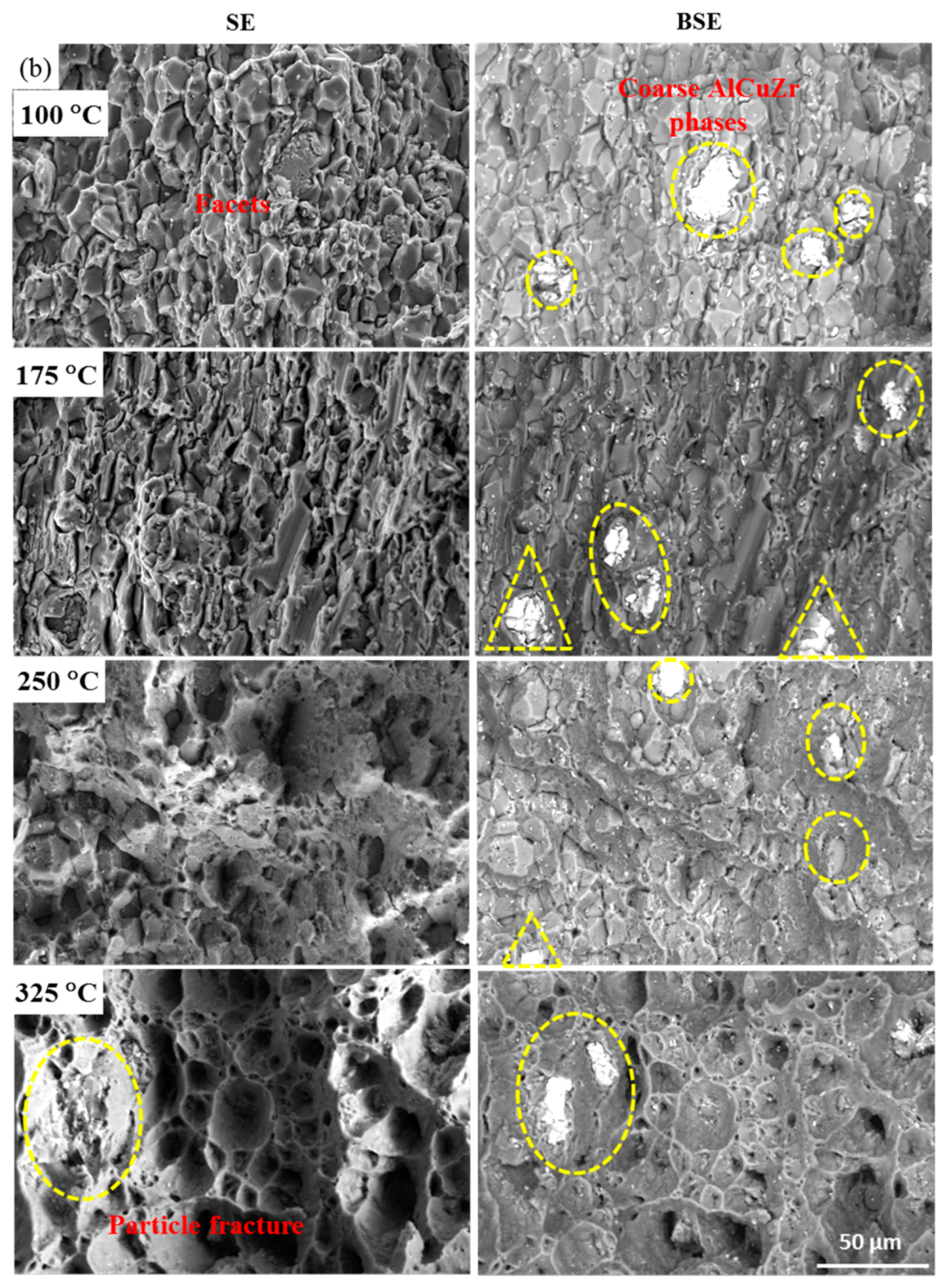
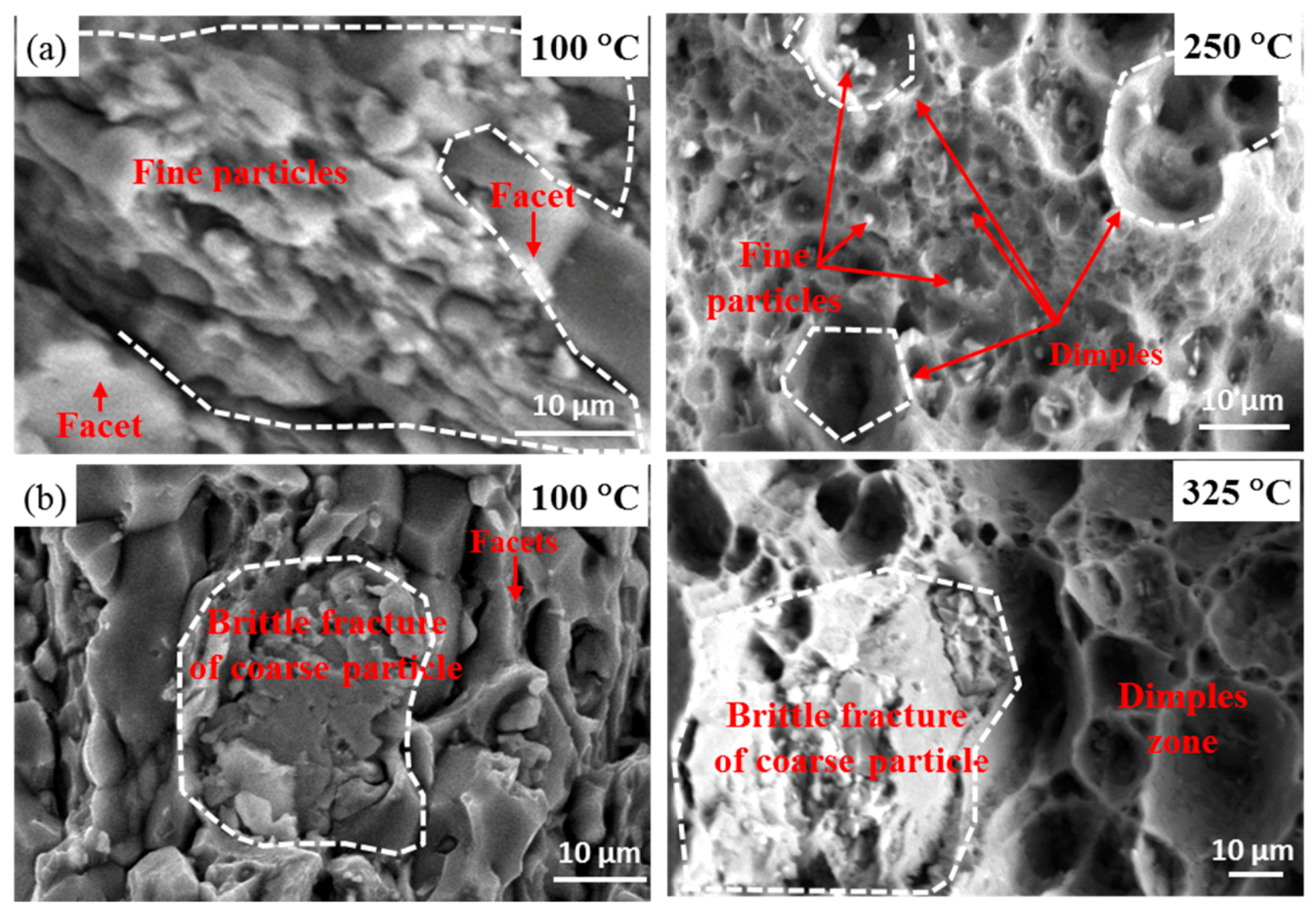
| Alloys | Li | Cu | Ag | Mg | Zr | Ce |
|---|---|---|---|---|---|---|
| Ce-free alloy | 1.29 | 4.62 | 0.41 | 0.39 | 0.14 | – |
| Ce-containing alloy | 1.31 | 4.63 | 0.38 | 0.40 | 0.13 | 0.29 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Zhao, Z.; Shi, D.; Dai, H.; Sun, J.; Dong, X. Enhanced High-Temperature Mechanical Properties of Al–Cu–Li Alloy through T1 Coarsening Inhibition and Ce-Containing Intermetallic Refinement. Materials 2019, 12, 1521. https://doi.org/10.3390/ma12091521
Yu X, Zhao Z, Shi D, Dai H, Sun J, Dong X. Enhanced High-Temperature Mechanical Properties of Al–Cu–Li Alloy through T1 Coarsening Inhibition and Ce-Containing Intermetallic Refinement. Materials. 2019; 12(9):1521. https://doi.org/10.3390/ma12091521
Chicago/Turabian StyleYu, Xinxiang, Zhiguo Zhao, Dandan Shi, Han Dai, Jie Sun, and Xiaoyan Dong. 2019. "Enhanced High-Temperature Mechanical Properties of Al–Cu–Li Alloy through T1 Coarsening Inhibition and Ce-Containing Intermetallic Refinement" Materials 12, no. 9: 1521. https://doi.org/10.3390/ma12091521
APA StyleYu, X., Zhao, Z., Shi, D., Dai, H., Sun, J., & Dong, X. (2019). Enhanced High-Temperature Mechanical Properties of Al–Cu–Li Alloy through T1 Coarsening Inhibition and Ce-Containing Intermetallic Refinement. Materials, 12(9), 1521. https://doi.org/10.3390/ma12091521





