Mechanosynthesis of the Whole Y1−xBixMn1−xFexO3 Perovskite System: Structural Characterization and Study of Phase Transitions
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
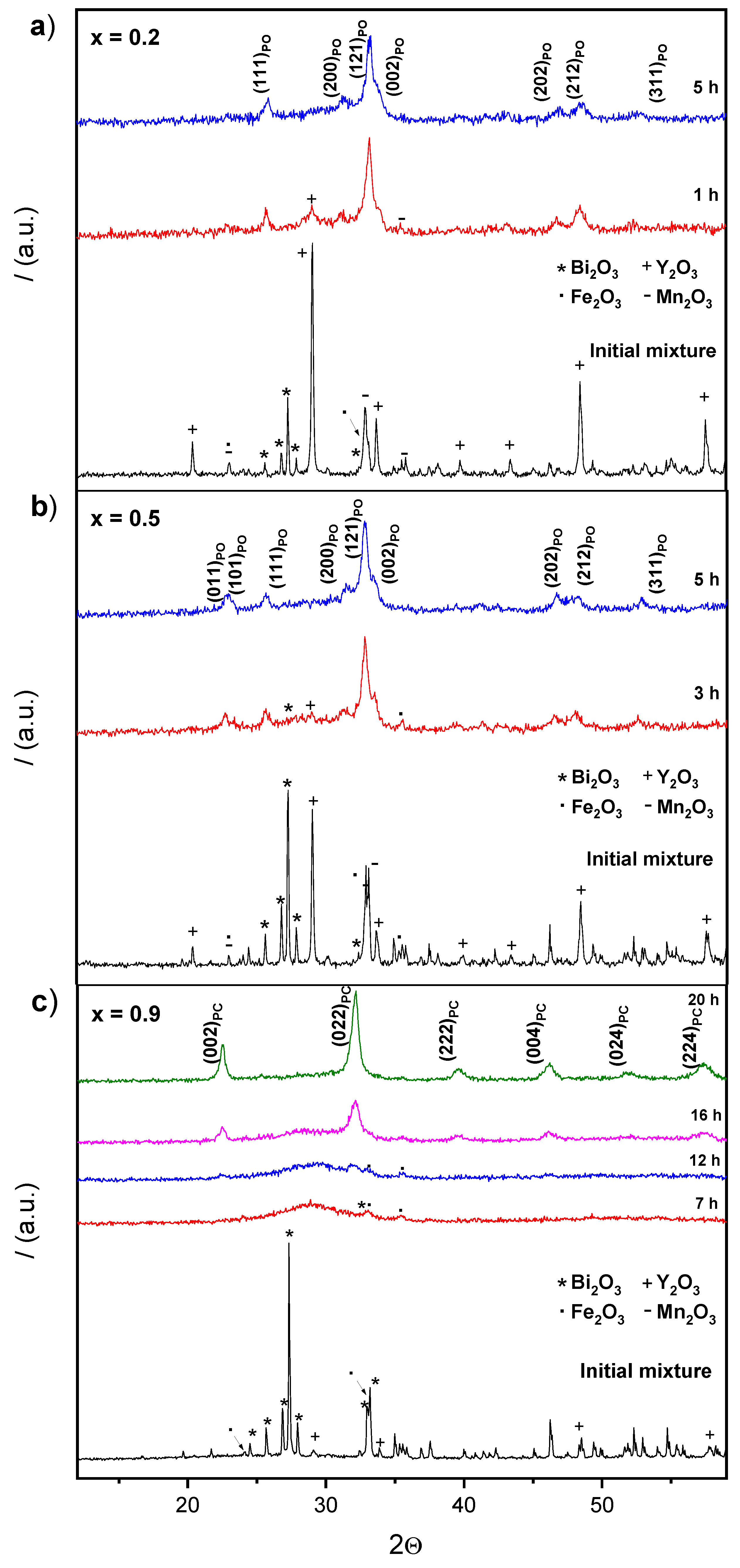
3.1. Mechanosynthesis
3.2. Structural Characterization
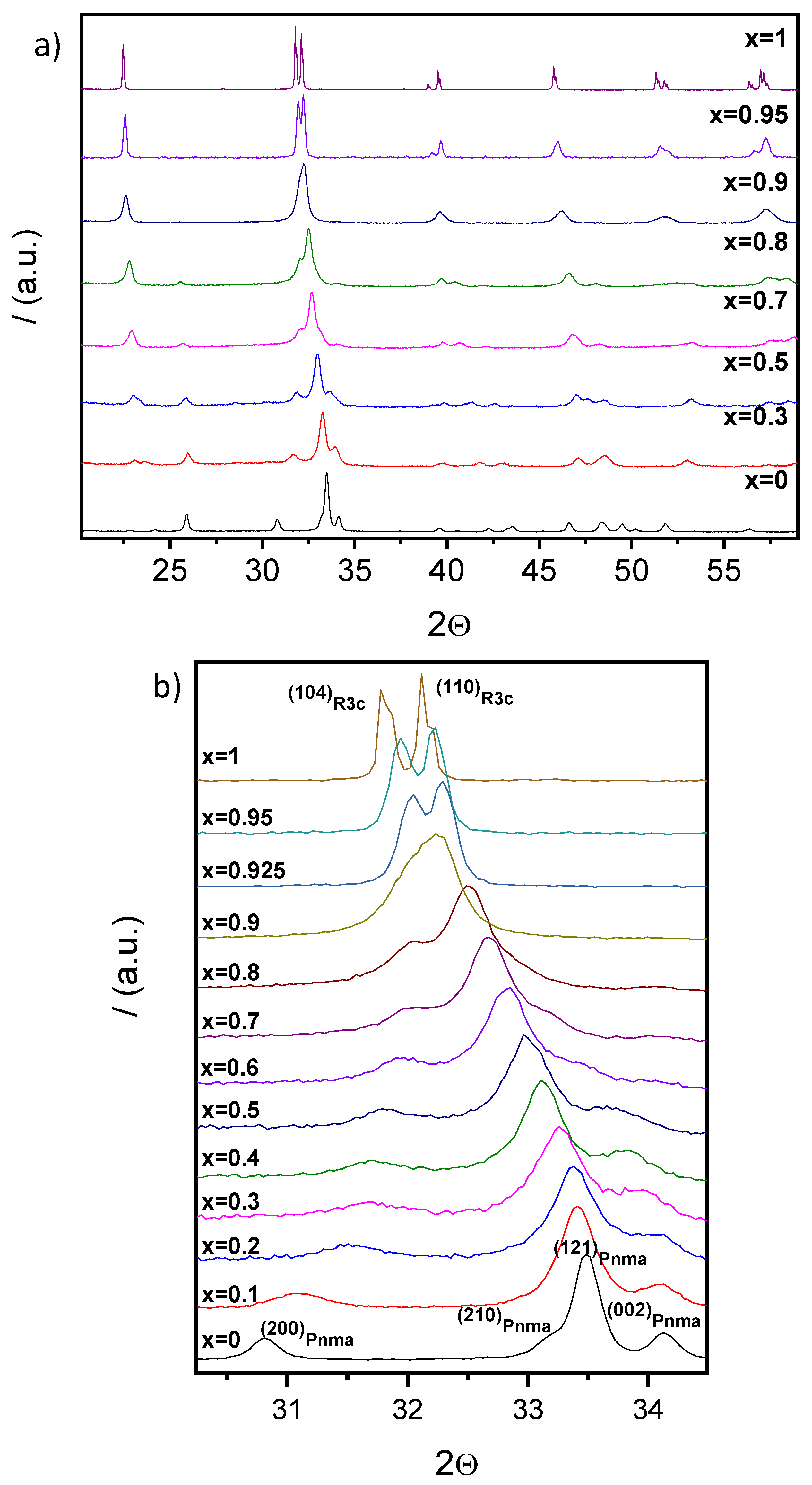
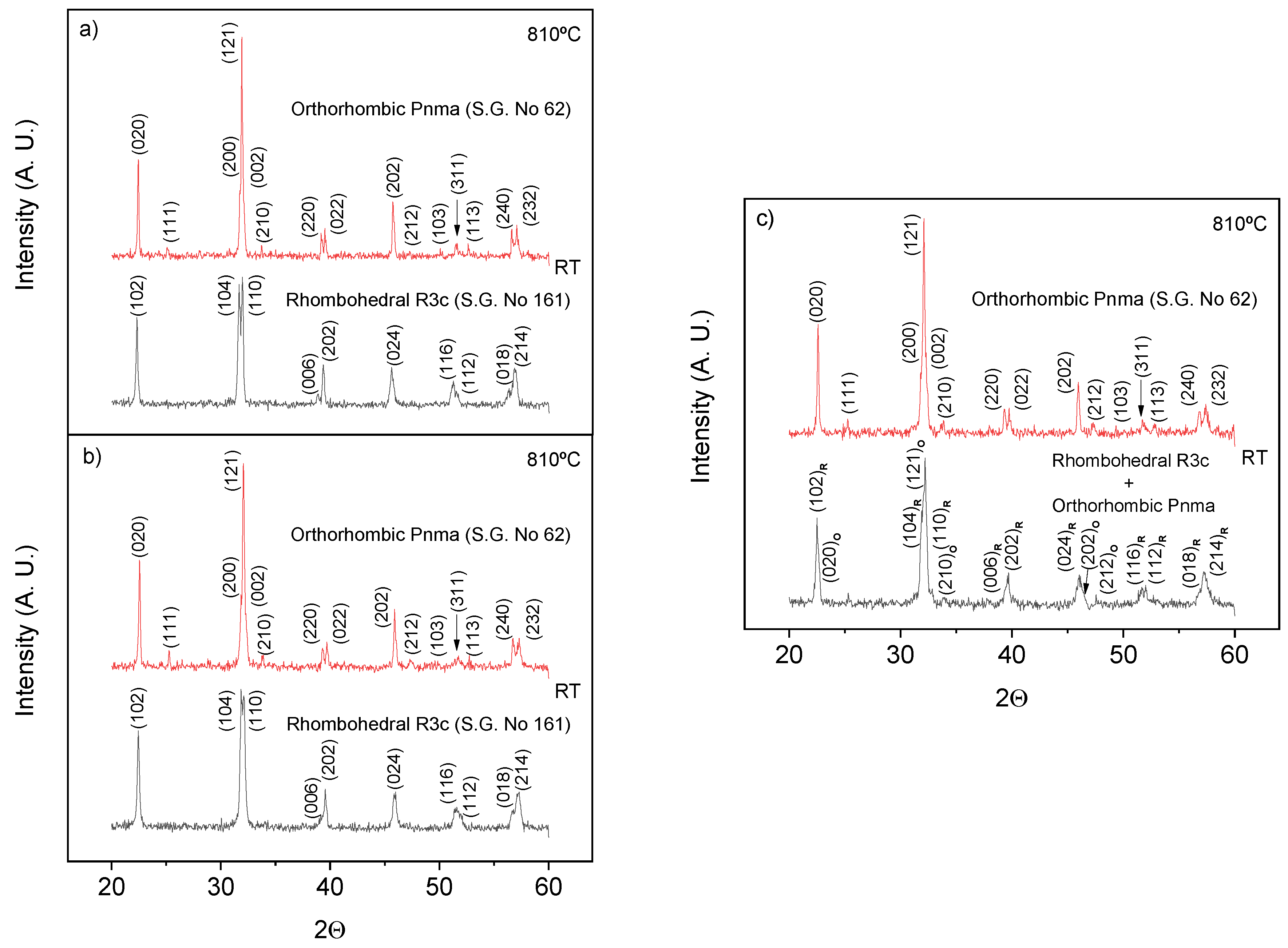
3.2.1. XRD Studies
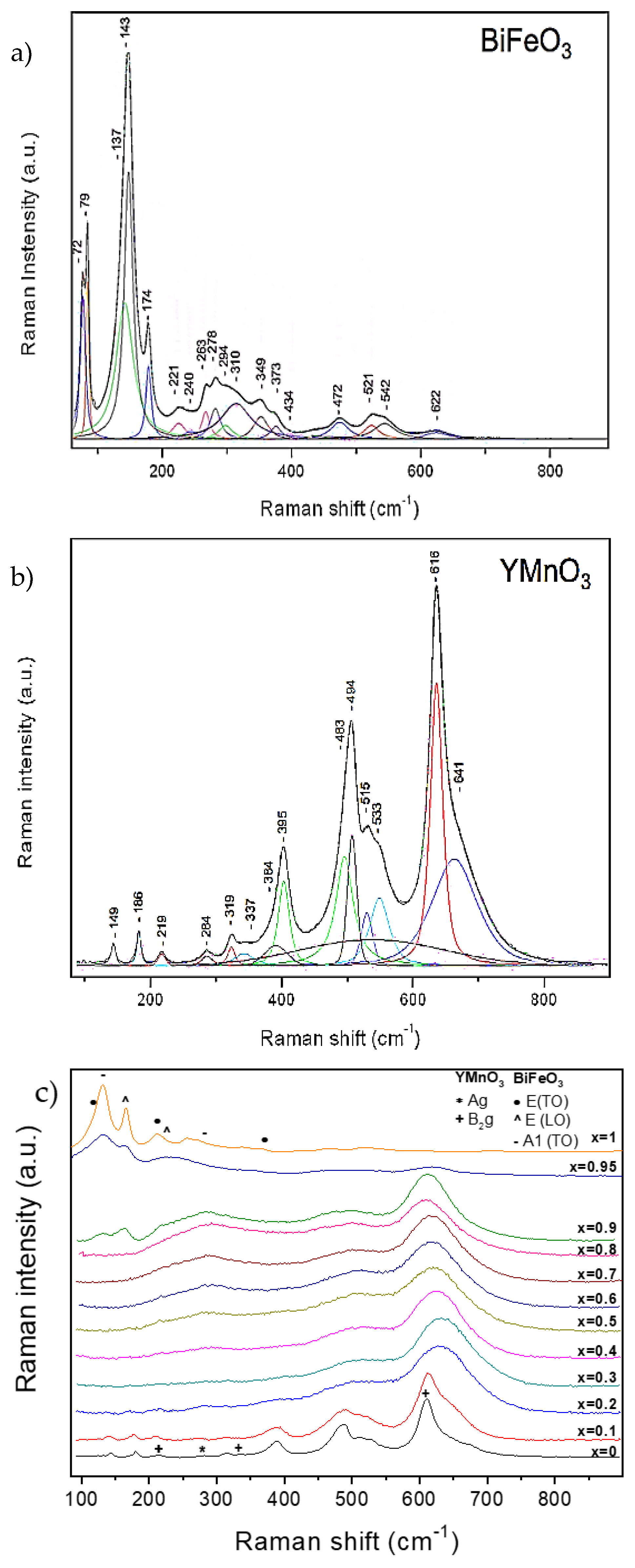
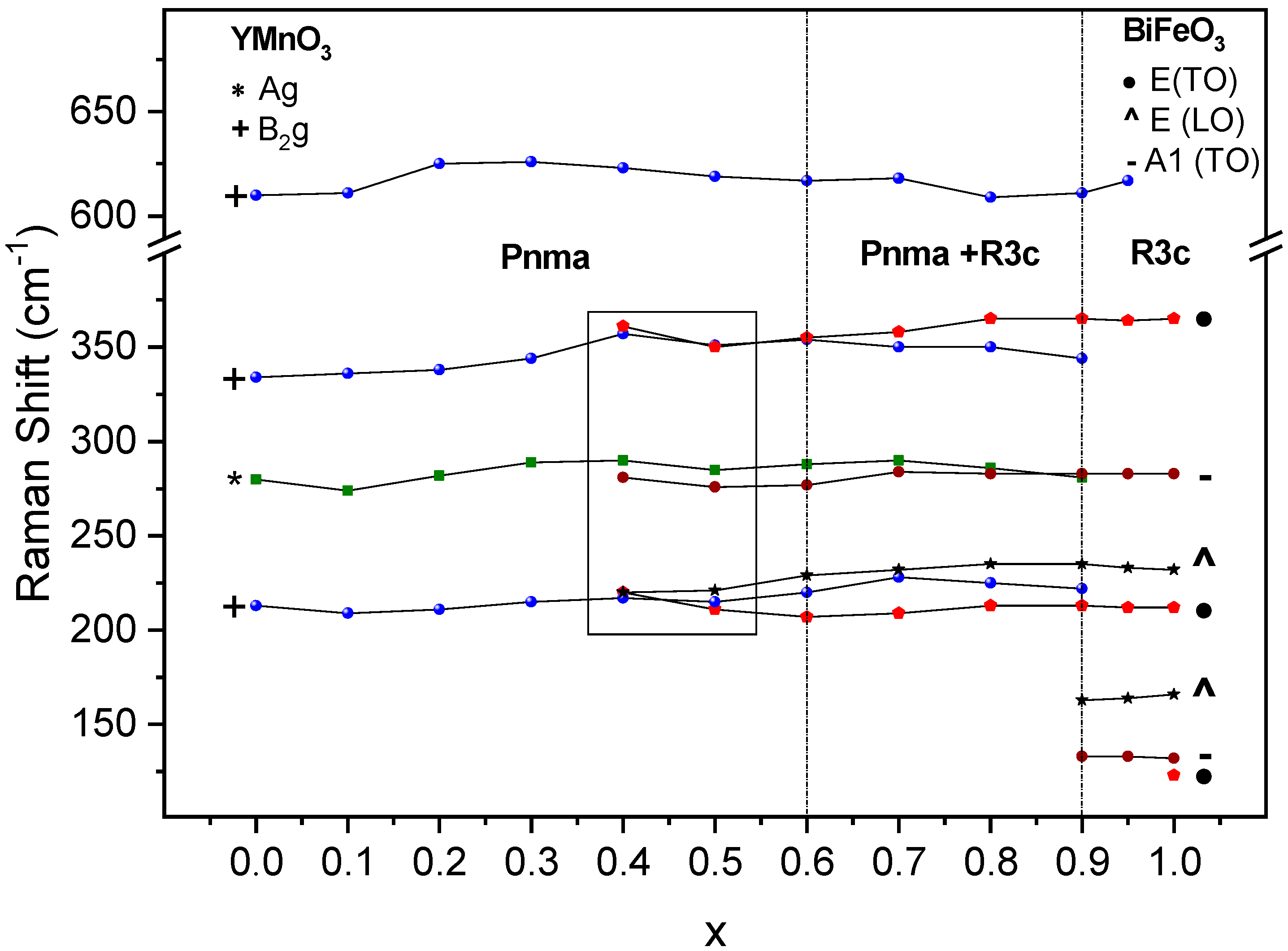
3.2.2. Raman Studies
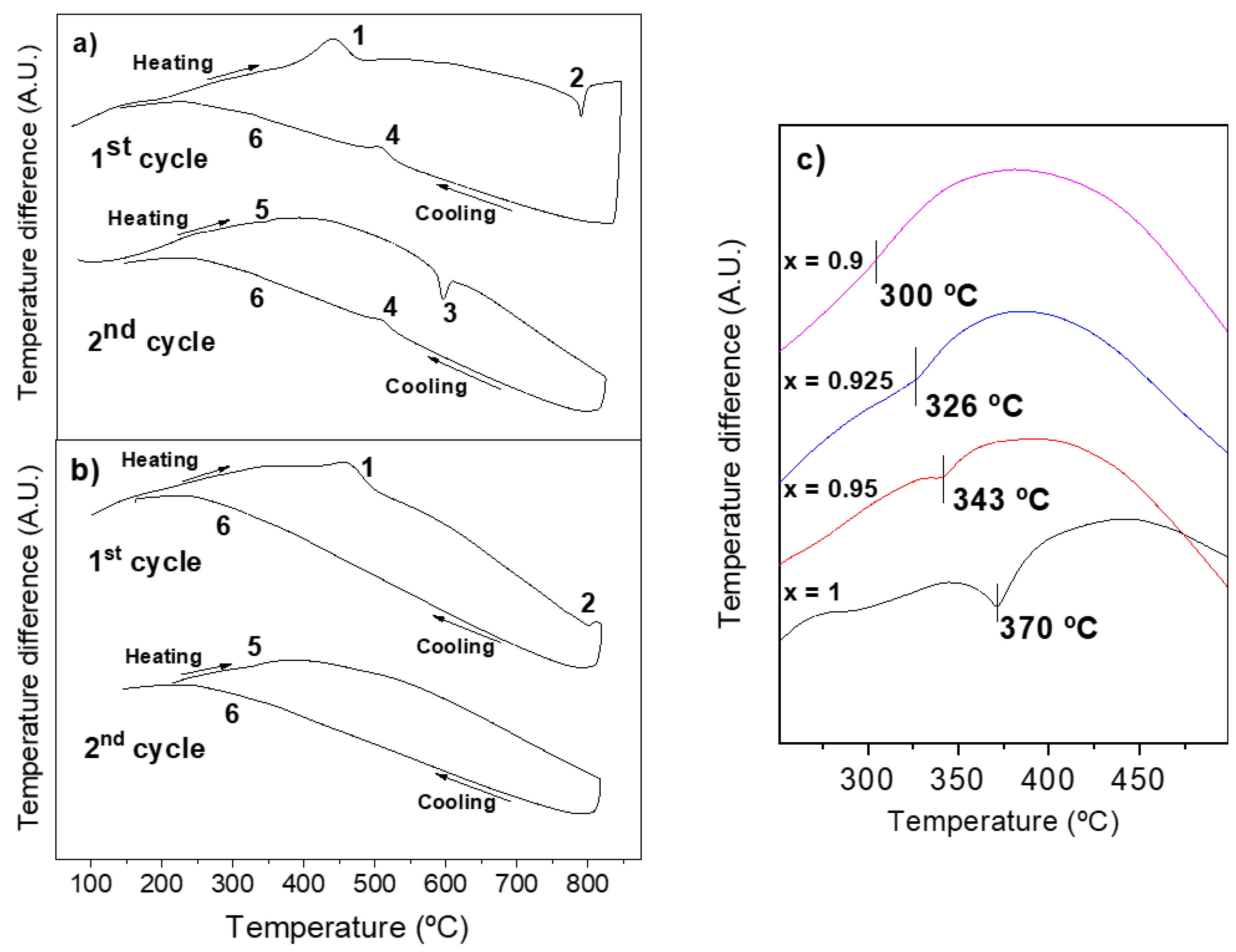
3.3. Phase Transitions and Phase Stability
3.4. Considerations about Mechanosynthesis and Thermal Stability
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Scott, J.F. Applications of magnetoelectrics. J. Mater. Chem. 2012, 22, 4567–4574. [Google Scholar] [CrossRef]
- Fusil, S.; Garcia, V.; Barth, A.; Bibes, M. Magnetoelectric Devices for Spintronics. Annu. Rev. Mater. Res. 2014, 44, 91–116. [Google Scholar] [CrossRef]
- Ishiwata, S.; Tokunaga, Y.; Taguchi, Y.; Tokura, Y. High-pressure hydrothermal crystal growth and multiferroic properties of a perovskite YMnO3. J. Am. Chem. Soc. 2011, 133, 13818–13820. [Google Scholar] [CrossRef] [PubMed]
- Yakel, H.L.; Koehler, W.C.; Bertaut, E.F.; Forrat, E.F. On the Crystal Structure of the Manganese (III) Trioxides of the Heavy Lanthanides and Yttrium. Acta Crystallogr. 1963, 16, 957–962. [Google Scholar] [CrossRef]
- Brinks, H.W.; Fjellvag, H.; Kjekshus, A. Synthesis of Metastable Perovskite-type YMnO3 and HoMnO3. J. Solid State Chem. 1997, 129, 334–340. [Google Scholar] [CrossRef]
- Uusi-esko, K.; Malm, J.; Imamura, N.; Yamauchi, H.; Karppinen, M. Characterization of RMnO3 (R = Sc, Y, Dy-Lu): High-pressure synthesized metastable perovskites and their hexagonal precursor phases. Mater. Chem. Phys. 2008, 112, 1029–1034. [Google Scholar] [CrossRef]
- Moure, A.; Hungría, T.; Castro, A.; Galy, J.; Peña, O.; Tartaj, J.; Moure, C. Doping influence on the stability of YMnO3 orthorhombic perovskite obtained by mechanosynthesis. Mater. Chem. Phys. 2012, 133, 764–771. [Google Scholar] [CrossRef]
- Fiebig, M.; Lottermoser, T.; Meier, D.; Trassin, M. The evolution of multiferroics. Nat. Rev. Mater. 2016, 1, 16046. [Google Scholar] [CrossRef]
- Catalan, G.; Scott, J.F. Physics and applications of bismuth ferrite. Adv. Mater. 2009, 21, 2463–2485. [Google Scholar] [CrossRef]
- Eerenstein, W.; Mathur, N.D.; Scott, J.F. Multiferroic and magnetoelectric materials. Nature 2006, 442, 759–765. [Google Scholar] [CrossRef]
- Palkar, V.R.; John, J.; Pinto, R. Observation of saturated polarization and dielectric anomaly in magnetoelectric BiFeO3 thin films. Appl. Phys. Lett. 2002, 80, 1628–1630. [Google Scholar] [CrossRef]
- Koizumi, H.; Niizeki, N.; Ikeda, T. An X-Ray Study on Bi2O3-Fe2O3 System. Jpn. J. Appl. Phys. 1964, 3, 495–496. [Google Scholar] [CrossRef]
- Valant, M.; Axelsson, A.; Alford, N. Peculiarities of a Solid-State Synthesis of Multiferroic Polycrystalline BiFeO3. Chem. Mater. 2007, 19, 5431–5436. [Google Scholar] [CrossRef]
- Cao, W.; Cross, L.E. Theoretical model for the morphotropic phase boundary in lead zirconate-lead titanate solid solution. Phys. Rev. B Condens. Matter Mater. Phys. 1993, 47, 4825–4830. [Google Scholar] [CrossRef]
- Fernández-Posada, C.M.; Amorín, H.; Jiménez, R.; Kiat, J.-M.; Algueró, M.; Peña, O.; Castro, A.; Porcher, F. The Polar/Antipolar Phase Boundary of BiMnO3-BiFeO3-PbTiO3: Interplay among Crystal Structure, Point Defects, and Multiferroism. Adv. Funct. Mater. 2018, 28, 1802338. [Google Scholar] [CrossRef]
- Damjanovic, D. A morphotropic phase boundary system based on polarization rotation and polarization extension. Appl. Phys. Lett. 2010, 97, 062906. [Google Scholar] [CrossRef]
- Fernández-Posada, C.M.; Castro, A.; Kiat, J.-M.; Porcher, F.; Peña, O.; Algueró, M.; Amorín, H. A novel perovskite oxide chemically designed to show multiferroic phase boundary with room-temperature magnetoelectricity. Nat. Commun. 2016, 7, 12772. [Google Scholar] [CrossRef] [PubMed]
- Hungría, T.; Castro, A. Synthesis and structural characterization of the new solid solution Ba(2-x)SrxTiO4 (0 ≤ x ≤ 1): Effect of the method of synthesis on the polymorphic phase isolated. J. Alloys Compd. 2007, 436, 266–271. [Google Scholar] [CrossRef]
- Algueró, M.; Ricote, J.; Castro, A. Mechanosynthesis and thermal stability of piezoelectric perovskite 0.92Pb(Zn1/3Nb2/3 )O3-0.08PbTiO3 powders. J. Am. Ceram. Soc. 2004, 87, 772–778. [Google Scholar] [CrossRef]
- Algueró, M.; Ricote, J.; Hungría, T.; Castro, A. High-sensitivity piezoelectric, low-tolerance-factor perovskites by mechanosynthesis. Chem. Mater. 2007, 19, 4982–4990. [Google Scholar] [CrossRef]
- Correas, C.; Hungría, T.; Castro, A. Mechanosynthesis of the whole xBiFeO3-(1−x)PbTiO3 multiferroic system: Structural characterization and study of phase transitions. J. Mater. Chem. 2011, 21, 3125–3132. [Google Scholar] [CrossRef]
- Castro, A.; Correas, C.; Peña, O.; Landa-Cánovas, Á.R.; Algueró, M.; Amorín, H.; Dollé, M.; Vila, E.; Hungría, T. Nanostructured BiMn O(3+δ) obtained at ambient pressure: Analysis of its multiferroicity. J. Mater. Chem. 2012, 22, 9928–9938. [Google Scholar] [CrossRef]
- Moure, A.; Hungría, T.; Castro, A.; Galy, J.; Peña, O.; Tartaj, J.; Moure, C. Mechanosynthesis of the orthorhombic Perovskites ErMn(1−x)NixO3 (x = 0, 0.1). Processing and characterization of nanostructured ceramics. Chem. Mater. 2010, 22, 2908–2915. [Google Scholar] [CrossRef]
- Narayan Tripathy, S.; Mishra, K.K.; Sen, S.; Mishra, B.G.; Pradhan, D.K.; Palai, R.; Pradhan, D.K. Phase transition and magneto-electric coupling of BiFeO3- YMnO3 multiferroic nanoceramics. J. Appl. Phys. 2013, 114, 144104. [Google Scholar] [CrossRef]
- Tang, P.; Kuang, D.; Yang, S.; Zhang, Y. The Structural and Magnetic Properties of Bi(1−x)YxFe(1−y)MnyO3 Particles Synthesized Via a Sol-Gel Technique. Ferroelectrics 2016, 491, 104–111. [Google Scholar] [CrossRef]
- Nazarenko, A.V.; Razumnaya, A.G.; Kupriyanov, M.F.; Kabirov, Y.V.; Rudskaya, A.G.; Teslenko, P.Y.; Kofanova, N.B. Specific Features of Structural States in the BiFeO3–YMnO3 Solid Solutions. Phys. Solid State 2011, 53, 1599–1602. [Google Scholar] [CrossRef]
- Laugier, J.; Bochu, B. Ecole Nationale Supérieure de physique de Grenoble Celref V3 Program 2003. Available online: http://www.ccp14.ac.uk/ccp/web-mirrors/lmgp-laugier-bochu/ (accessed on 8 May 2019).
- Selbach, S.M.; Tybell, T.; Einarsrud, M.; Grande, T. The Ferroic Phase Transitions of BiFeO3. Adv. Mater. 2008, 20, 3692–3696. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Crystallogr. 1976, 32A, 751–767. [Google Scholar] [CrossRef]
- Hlinka, J.; Pokorny, J.; Karimi, S.; Reaney, I.M. Angular dispersion of oblique phonon modes in BiFeO3 from micro-Raman scattering. Phys. Rev. B 2011, 83, 020101. [Google Scholar] [CrossRef]
- Iliev, M.N.; Abrashev, M.V.; Lee, H.-G.; Popov, V.N.; Sun, Y.Y.; Thomsen, C.; Meng, R.L.; Chu, C.W. Raman spectroscopy of orthorhombic perovskitelike YMnO3 and LaMnO3. Phys. Rev. B 1998, 57, 2872–2877. [Google Scholar] [CrossRef]
- Todorov, N.D.; Abrashev, M.V.; Ivanov, V.G.; Tsutsumanova, G.G.; Marinova, V.; Wang, Y.; Iliev, M.N. Comparative Raman study of isostructural YCrO3 and YMnO3: Effects of structural distortions and twinning. Phys. Rev. B 2011, 83, 224303. [Google Scholar] [CrossRef]
- Schütz, D.; Deluca, M.; Krauss, W.; Feteira, A.; Jackson, T.; Reichmann, K. Lone-Pair-Induced Covalency as the Cause of Temperature- and Field-Induced Instabilities in Bismuth Sodium Titanate. Adv. Funct. Mater. 2012, 22, 2285–2294. [Google Scholar] [CrossRef]
- Iliev, M.N.; Abrashev, M.V.; Popov, V.N.; Hadjiev, V.G. Role of Jahn-Teller disorder in Raman scattering of mixed-valence manganites. Phys. Rev. B Condens. Matter Mater. Phys. 2003, 67, 212301. [Google Scholar] [CrossRef]
- Goldschmidt, V.M. Die Gesetze der Krystallochemie. Naturwissenschaften 1926, 14, 477–485. [Google Scholar] [CrossRef]
- Eitel, R.E.; Randall, C.A.; Shrout, T.R.; Rehrig, P.W. New High Temperature Morphotropic Phase Boundary Piezoelectrics Based on Bi(Me)O3–PbTiO3 Ceramics. Jpn. J. Appl. Phys. 2001, 40, 5999–6002. [Google Scholar] [CrossRef]

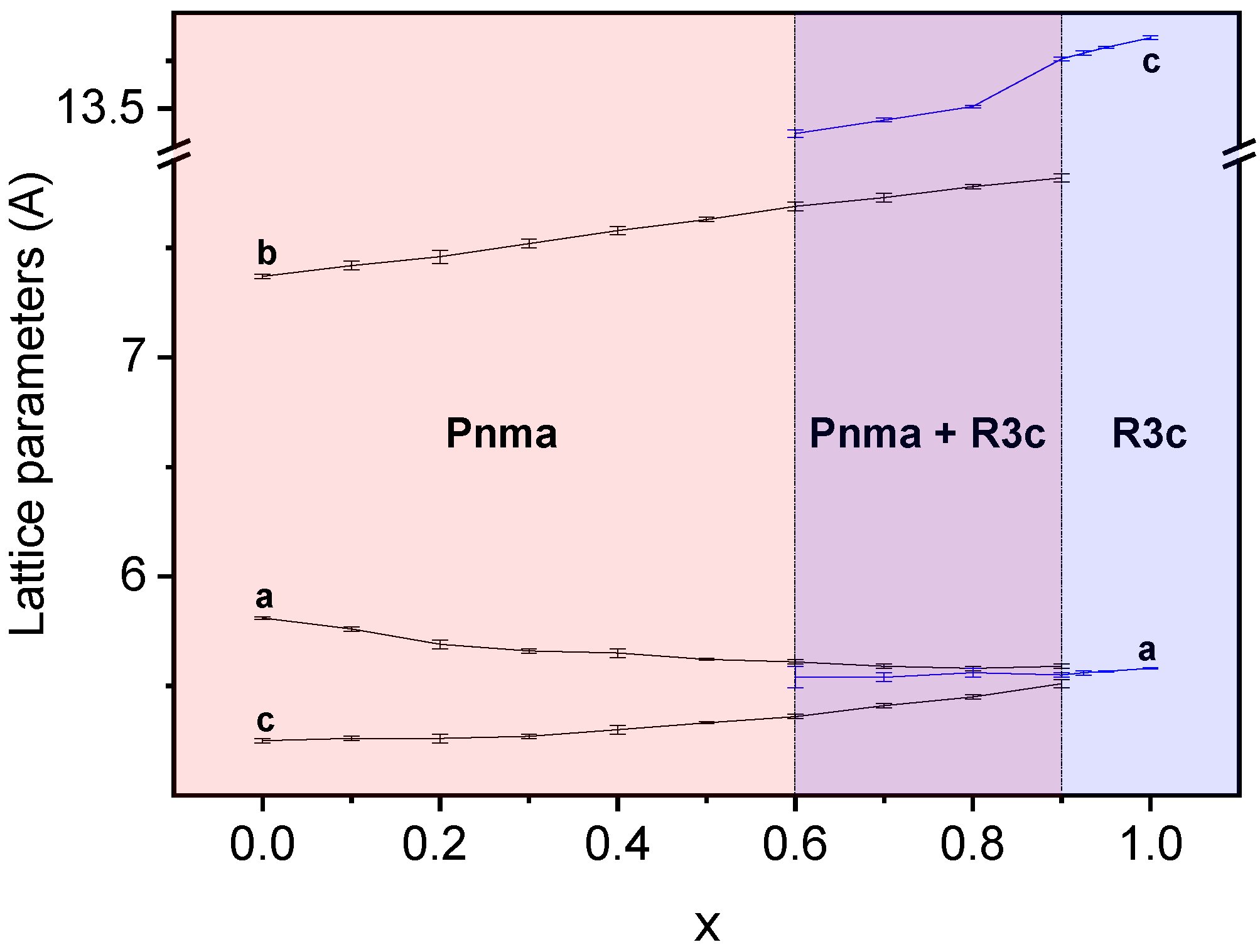
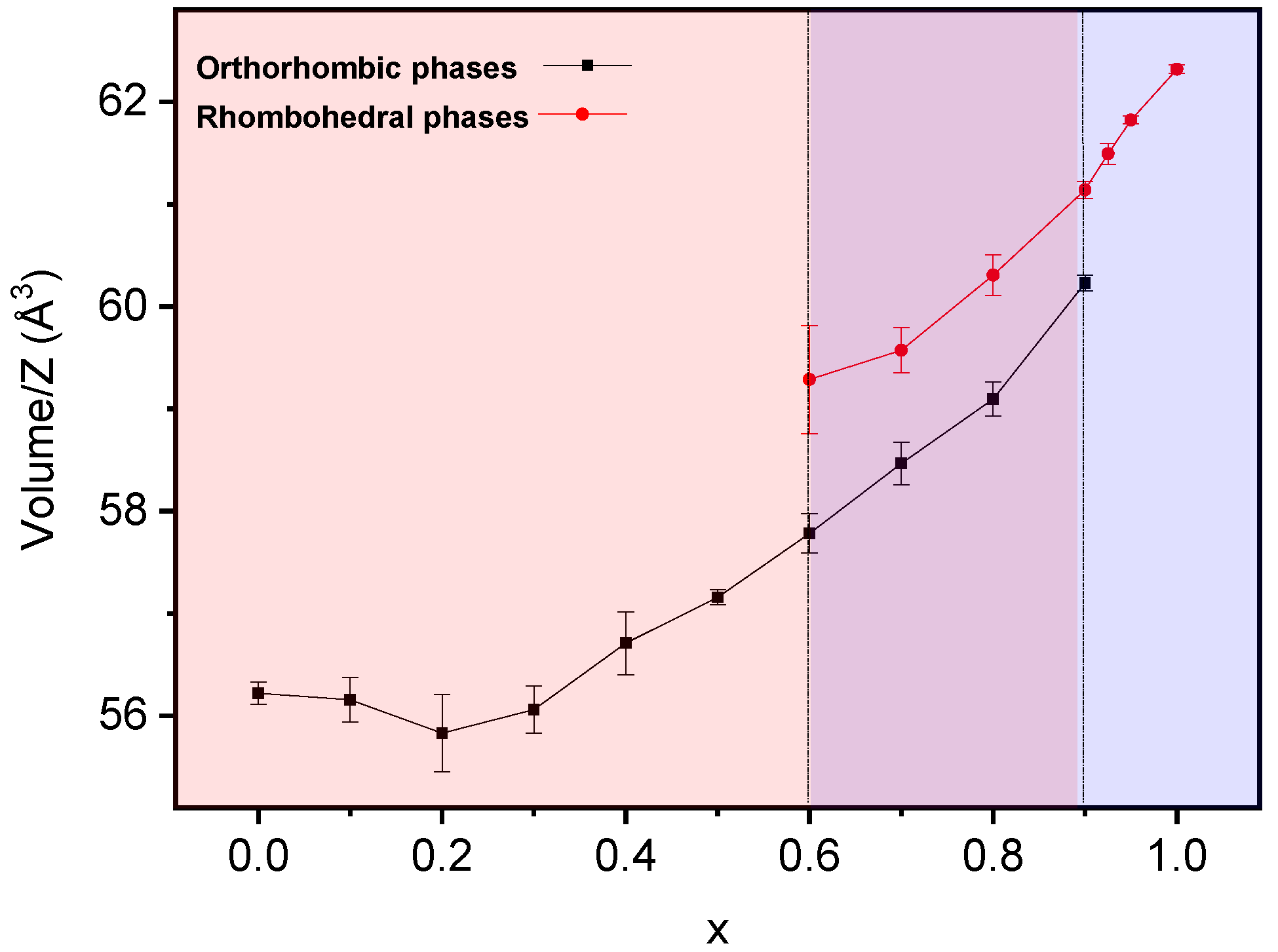
| x | Reaction Time (h) | Max. T (°C) | Orthorhombic Pnma | Rhombohedral R3c h | |||
|---|---|---|---|---|---|---|---|
| a | b | c | a | C | |||
| 0 | 8 | 900 | 5.810(6) | 7.37(1) | 5.252(6) | -- | -- |
| 0.1 | 7 | 700 | 5.76(1) | 7.42(2) | 5.26(2) | -- | -- |
| 0.2 | 5 | 600 | 5.69(2) | 7.46(3) | 5.26(2) | -- | -- |
| 0.3 | 4 | 550 | 5.66(2) | 7.52(2) | 5.27(1) | -- | -- |
| 0.4 | 3 | 550 | 5.65(2) | 7.58(2) | 5.30(2) | -- | -- |
| 0.5 | 5 | 550 | 5.621(3) | 7.629(6) | 5.332(3) | -- | -- |
| 0.6 | 7 | 550 | 5.61(1) | 7.69(2) | 5.36(1) | 5.54(6) | 13.37(2) |
| 0.7 | 9 | 600 | 5.59(1) | 7.73(2) | 5.41(1) | 5.54(2) | 13.44(1) |
| 0.8 | 11 | 700 | 5.58(1) | 7.78(1) | 5.45(1) | 5.56(2) | 13.510(6) |
| 0.9 | 20 | 750 | 5.59(1) | 7.82(2) | 5.51(2) | 5,55(2) | 13.761(6) |
| 0.925 | 18 | 825 | -- | -- | -- | 5.56(1) | 13.79(1) |
| 0.95 | 17 | 825 | -- | -- | -- | 5.567(3) | 13.820(6) |
| 1 | 14 | 750 | -- | -- | -- | 5.580(3) | 13.87(1) |
| Exp. | Ref. [30] | Exp. | Ref. [30] | Exp. | Ref. [30] | Exp. | Ref. [30] |
|---|---|---|---|---|---|---|---|
| Eg (TO) 1 | Eg (TO) 1 | Eg (LO) 2 | Eg (LO) 2 | A1g (TO) 1 | A1g (TO) 1 | A1g (LO) 2 | A1g (LO) 2 |
| 72 | 74 | 79 | 81 | 143 | 149 | - | 178 |
| 137 | 132 | 174 | 175 | 221 | 223 | - | 229 |
| 240 | 240 | - | 242 | 310 | 310 | - | 502 |
| 263 | 265 | 294 | 276 | 542 | 557 | - | 591 |
| 278 | 278 | - | 346 | ||||
| 349 | 351 | - | 368 | ||||
| 373 | 374 | - | 430 | ||||
| 434 | 441 | 472 | 468 | ||||
| 521 | 523 | 622 | 616 |
| Symmetry | Exp. | Ref. [31] | Ref. [32] | Calc. (Ref. [32]) |
|---|---|---|---|---|
| Ag | 149 | 151 | 150 | 165 |
| Ag | 186 | 188 | 187 | 217 |
| Ag | 284 | 288 | 289 | 265 |
| Ag | 319 | 323 | 324 | 302 |
| Ag | 395 | 396 | 398 | 373 |
| Ag | 494 | 497 | 497 | 483 |
| Ag | 515 | 518 | 519 | 498 |
| B2g | 149 | 151 | 152 | 179 |
| B2g | 219 | 220 | 222 | 234 |
| B2g | 319 | 317 | 318 | 297 |
| B2g | 338 | 341 | 342 | 351 |
| B2g | 483 | 481 | 481 | 497 |
| B2g | 533 | 537 | 539 | 523 |
| B2g | 616 | 616 | 618 | 618 |
| B1g | - | 205 | 205 | 226 |
| B1g | - | 284 | 285 | 269 |
| B1g | 384 | 383 | 382 | 327 |
| B1g | - | - | - | 414 |
| B1g | - | - | - | 647 |
| B3g | - | 178 | 179 | 184 |
| B3g | - | 336 | 337 | 332 |
| B3g | - | - | - | 410 |
| B3g | - | - | - | 491 |
| B3g | - | - | - | 642 |
| 641 | Defect origin |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quintana-Cilleruelo, J.Á.; K. Veerapandiyan, V.; Deluca, M.; Algueró, M.; Castro, A. Mechanosynthesis of the Whole Y1−xBixMn1−xFexO3 Perovskite System: Structural Characterization and Study of Phase Transitions. Materials 2019, 12, 1515. https://doi.org/10.3390/ma12091515
Quintana-Cilleruelo JÁ, K. Veerapandiyan V, Deluca M, Algueró M, Castro A. Mechanosynthesis of the Whole Y1−xBixMn1−xFexO3 Perovskite System: Structural Characterization and Study of Phase Transitions. Materials. 2019; 12(9):1515. https://doi.org/10.3390/ma12091515
Chicago/Turabian StyleQuintana-Cilleruelo, Jose Ángel, Vignaswaran K. Veerapandiyan, Marco Deluca, Miguel Algueró, and Alicia Castro. 2019. "Mechanosynthesis of the Whole Y1−xBixMn1−xFexO3 Perovskite System: Structural Characterization and Study of Phase Transitions" Materials 12, no. 9: 1515. https://doi.org/10.3390/ma12091515
APA StyleQuintana-Cilleruelo, J. Á., K. Veerapandiyan, V., Deluca, M., Algueró, M., & Castro, A. (2019). Mechanosynthesis of the Whole Y1−xBixMn1−xFexO3 Perovskite System: Structural Characterization and Study of Phase Transitions. Materials, 12(9), 1515. https://doi.org/10.3390/ma12091515






