Powder Metallurgy Synthesis of Heusler Alloys: Effects of Process Parameters
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
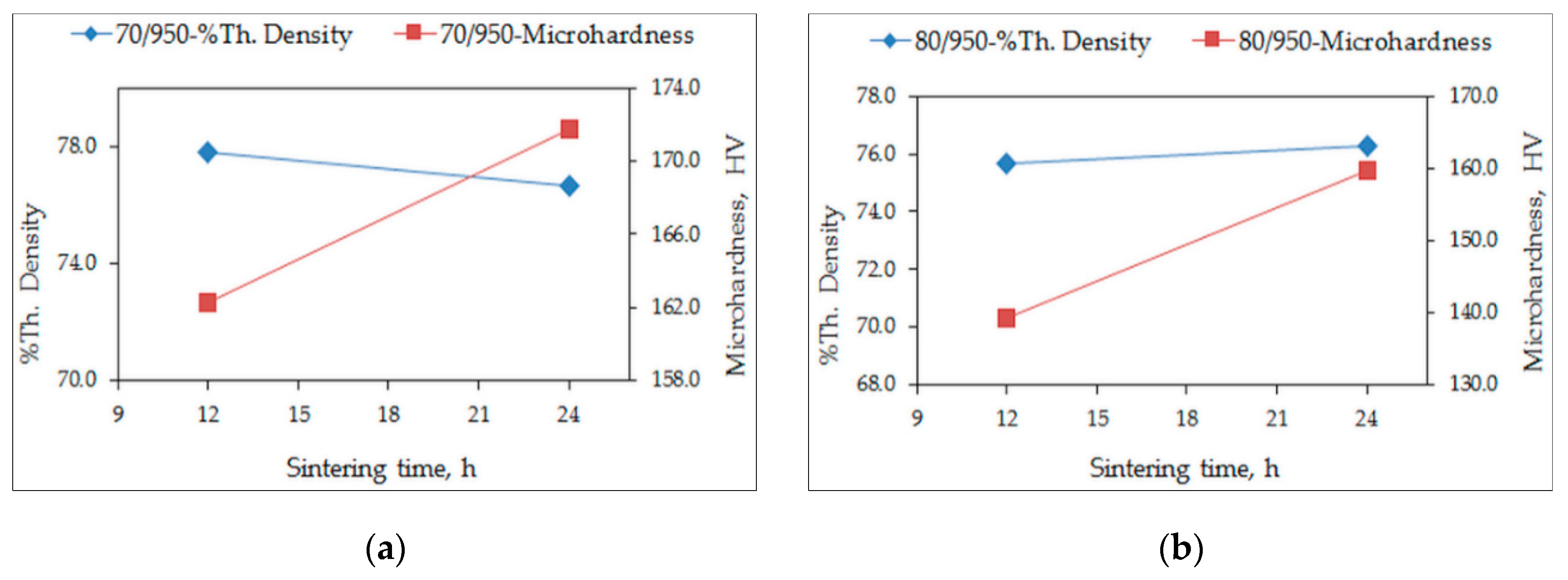
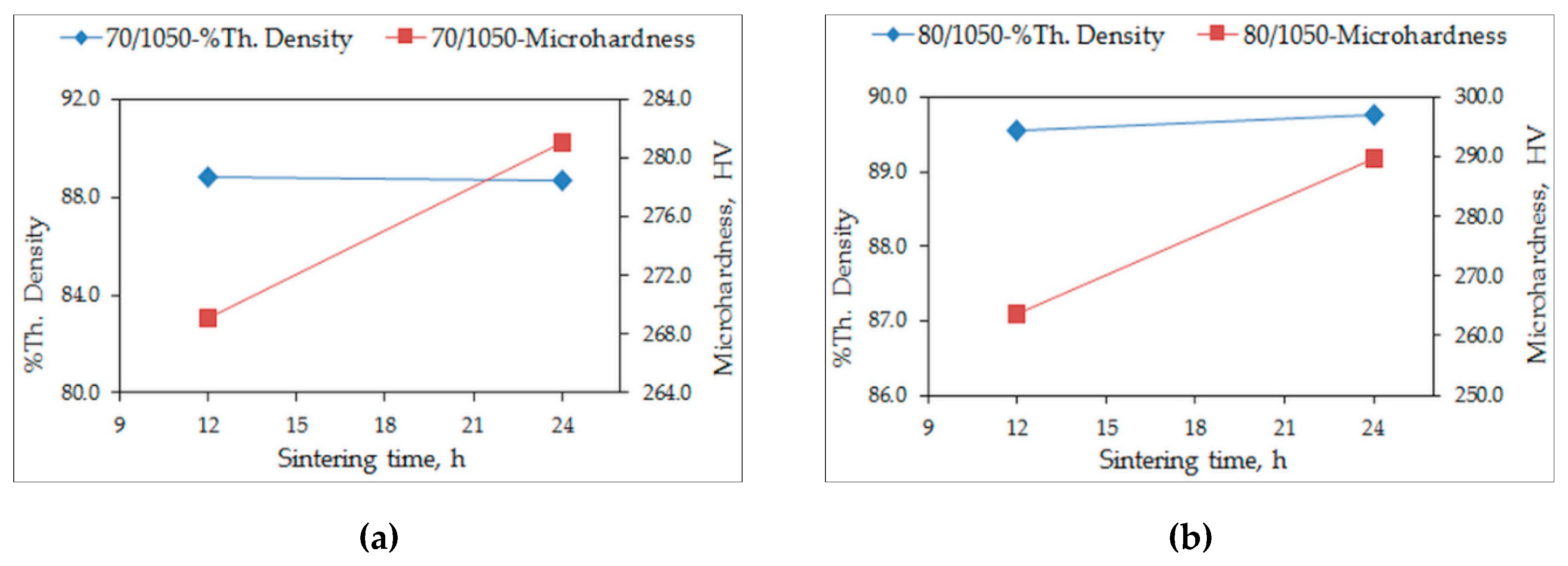
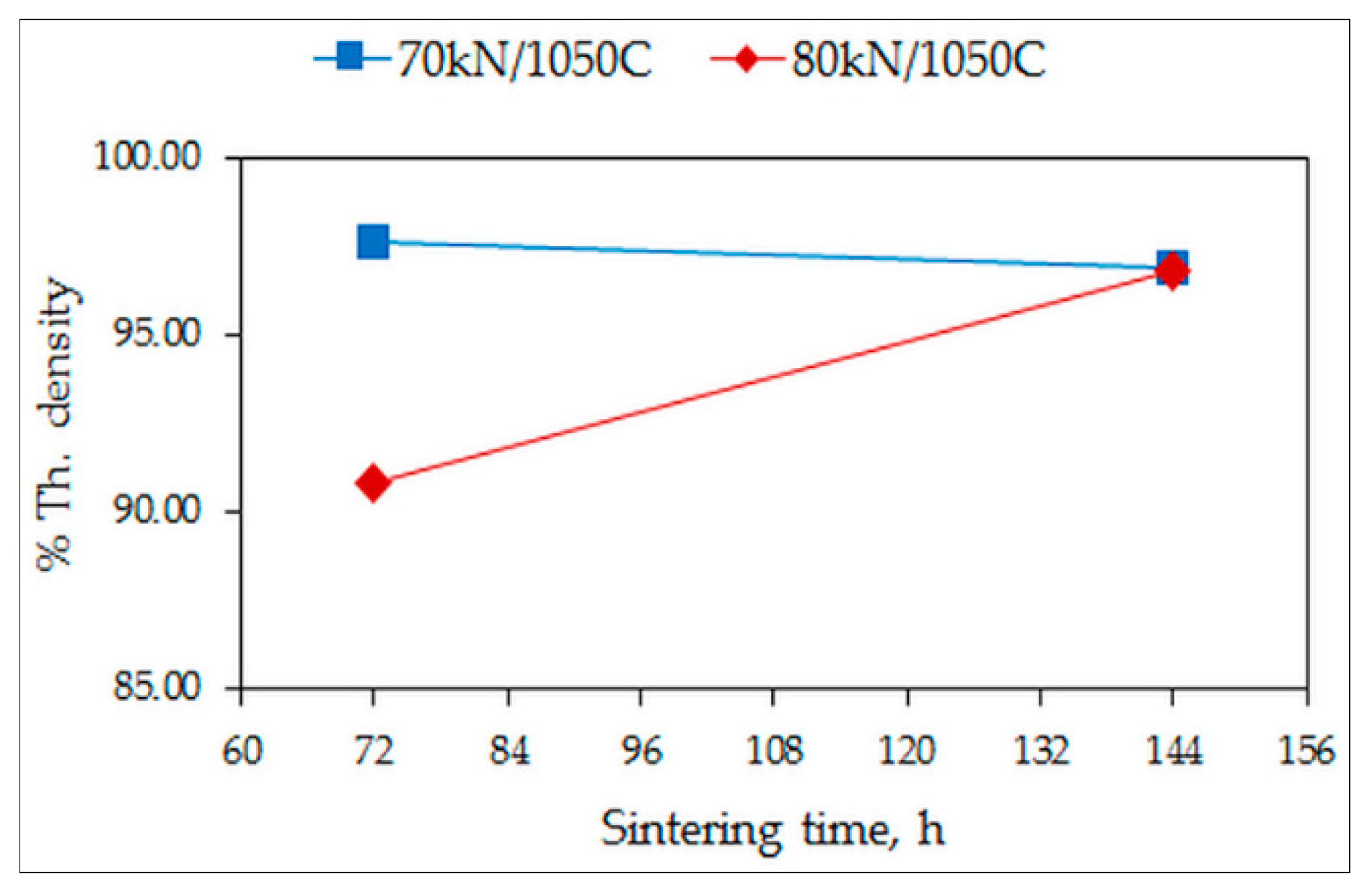
3.1. Density and Hardness
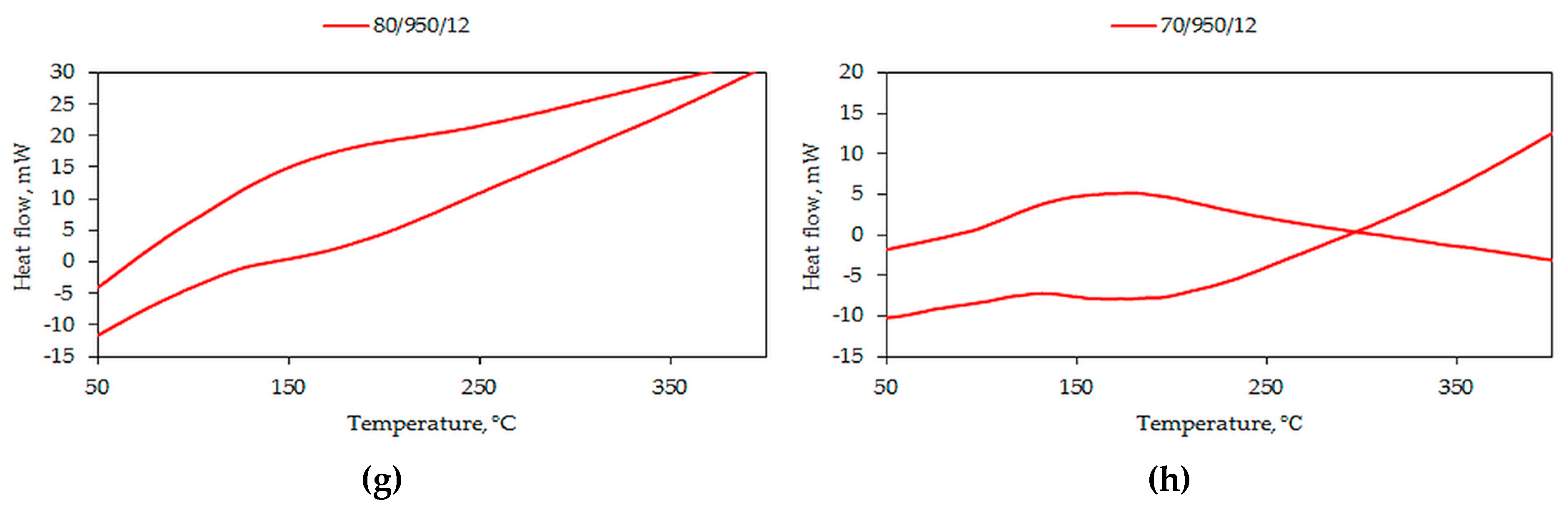
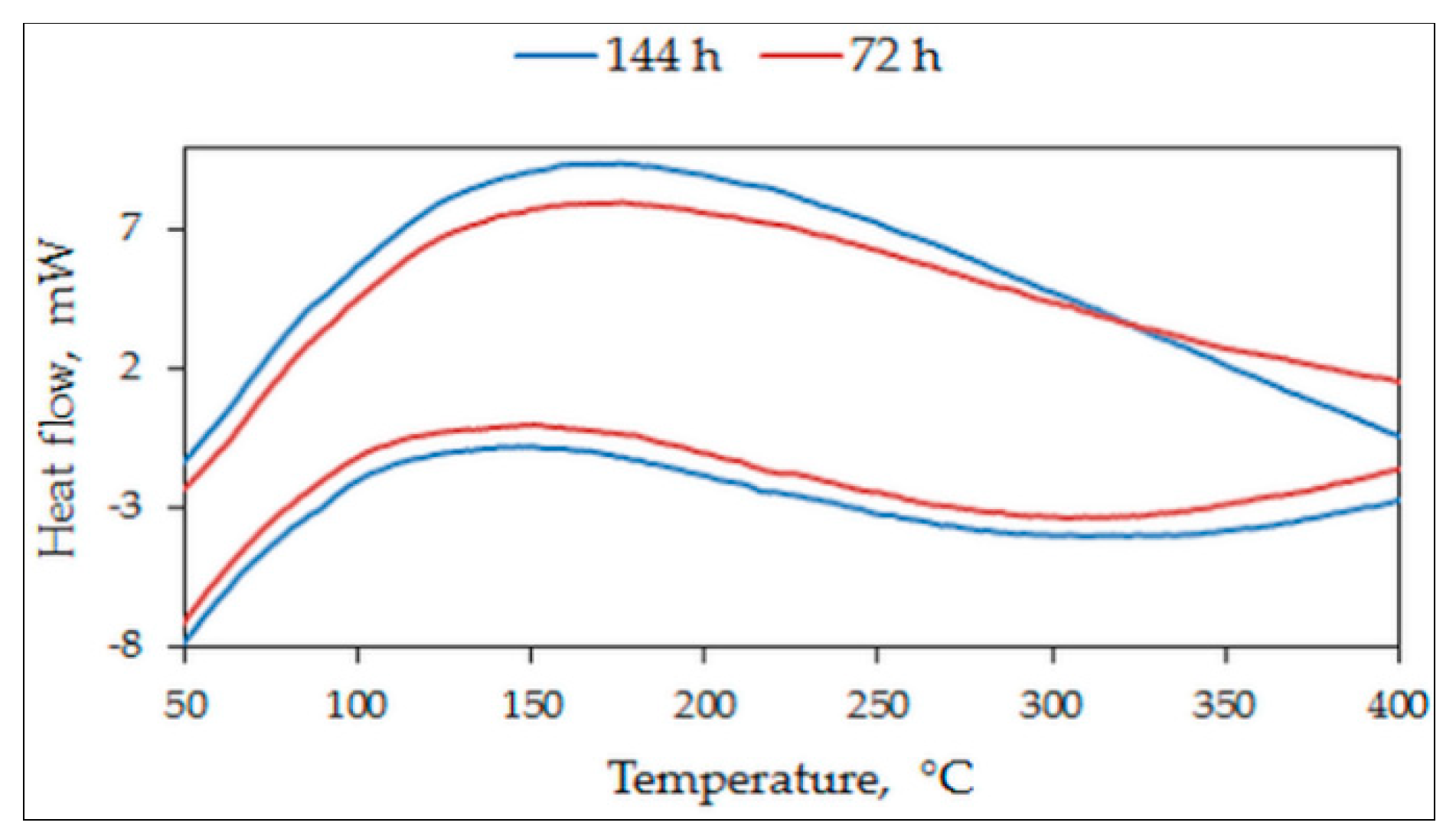
3.2. Differential Scanning Microscopy
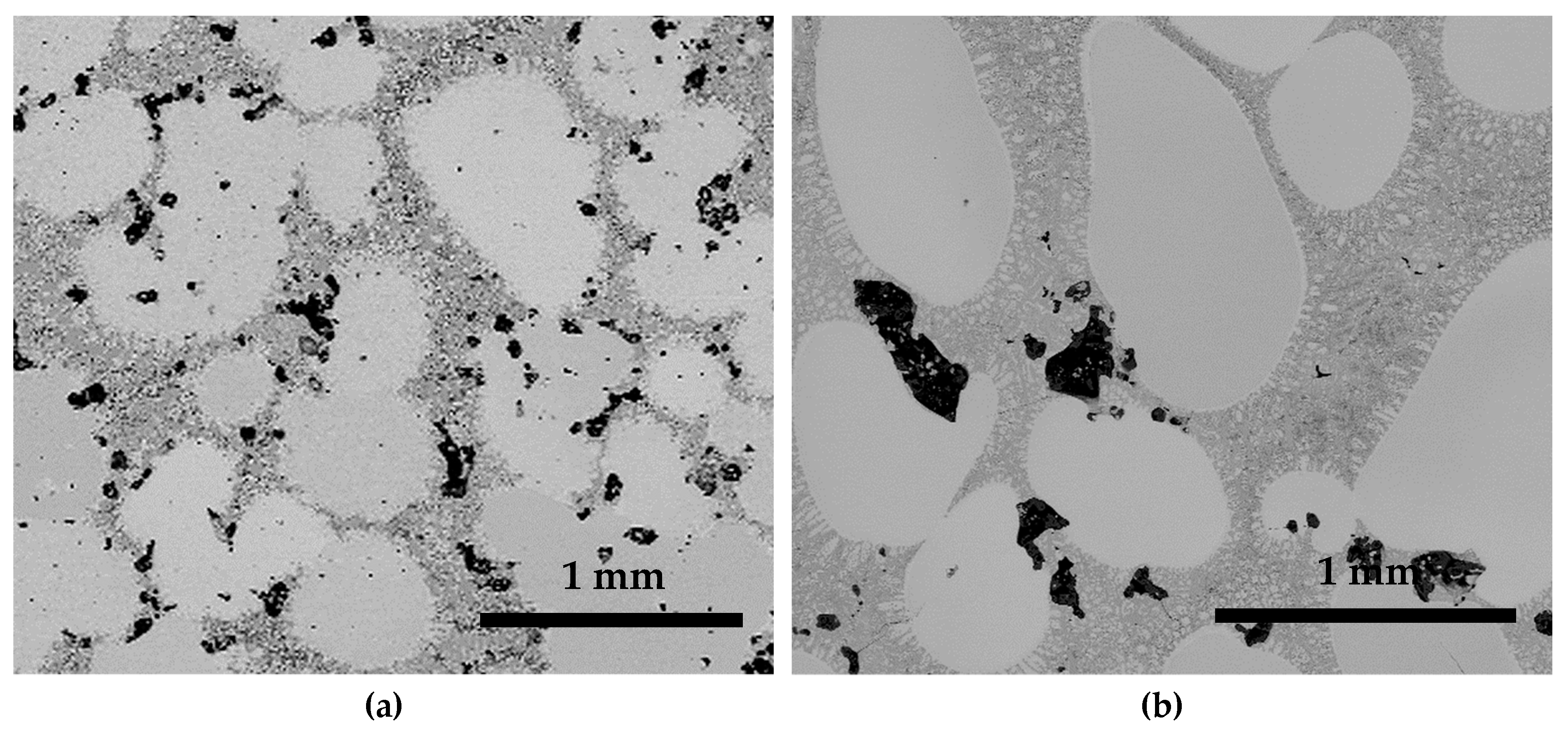
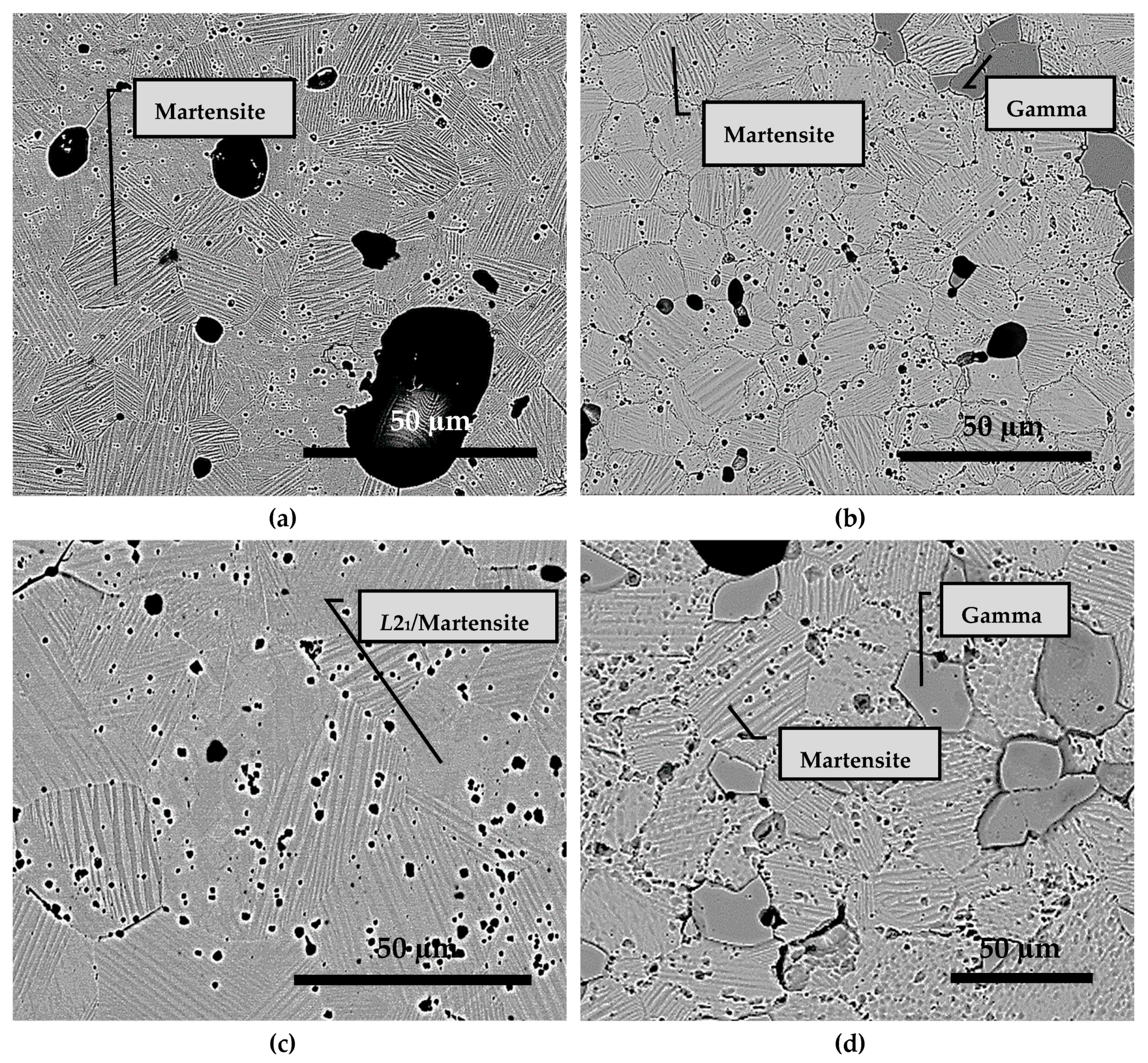
3.3. Microstructure Analysis
3.4. Magnetization Measurements
4. Conclusions
- Synthesis of Ni–Co–Mn–Sn Heusler alloys by powder processing using elemental powders is feasible;
- The effect of compaction pressure on the magnetostructural characteristics is not as significant;
- A higher sintering temperature of 1050 °C enables adequate sintering to occur, demonstrated by the decrease of the porosity and the increase of hardness;
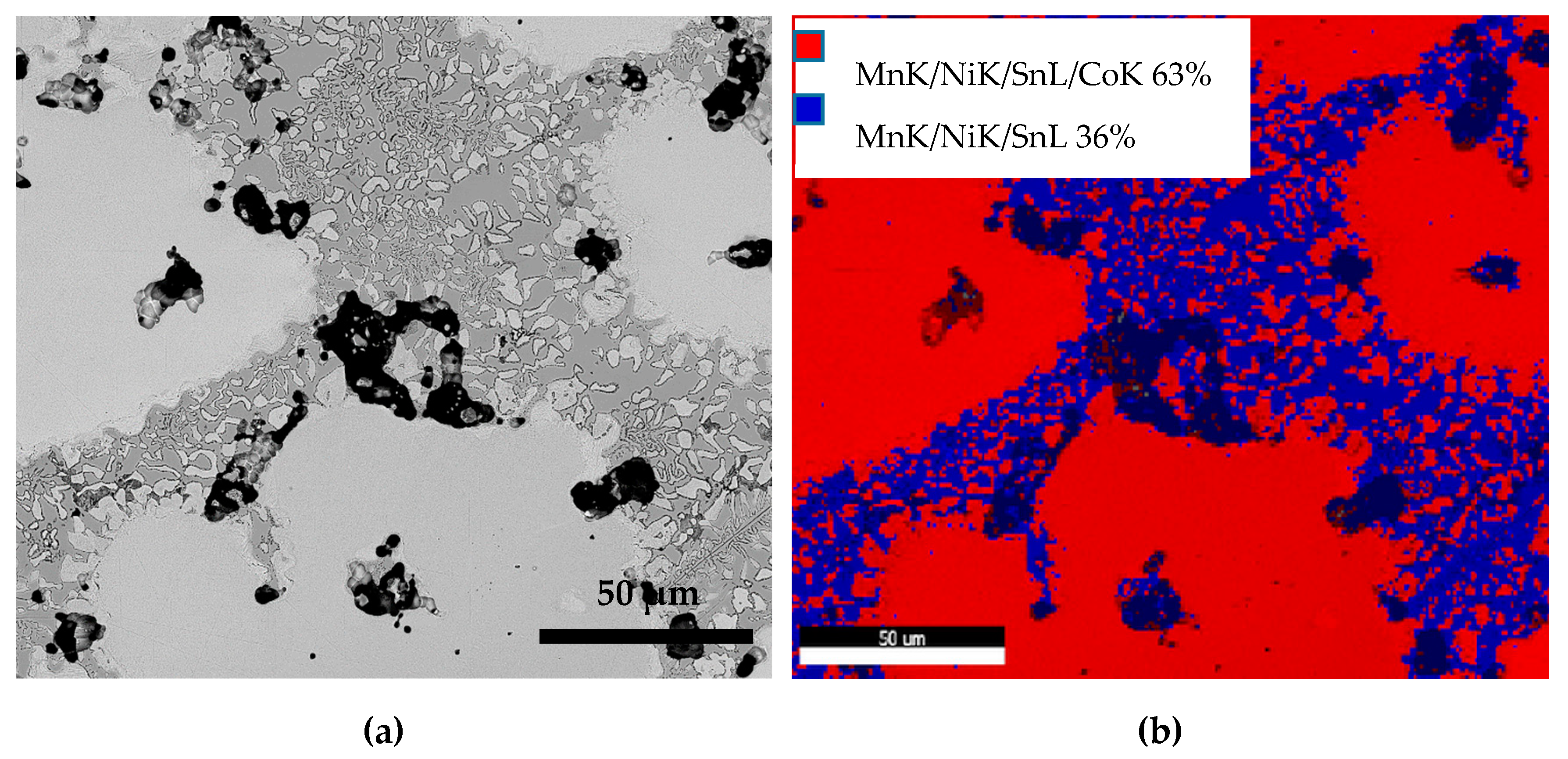
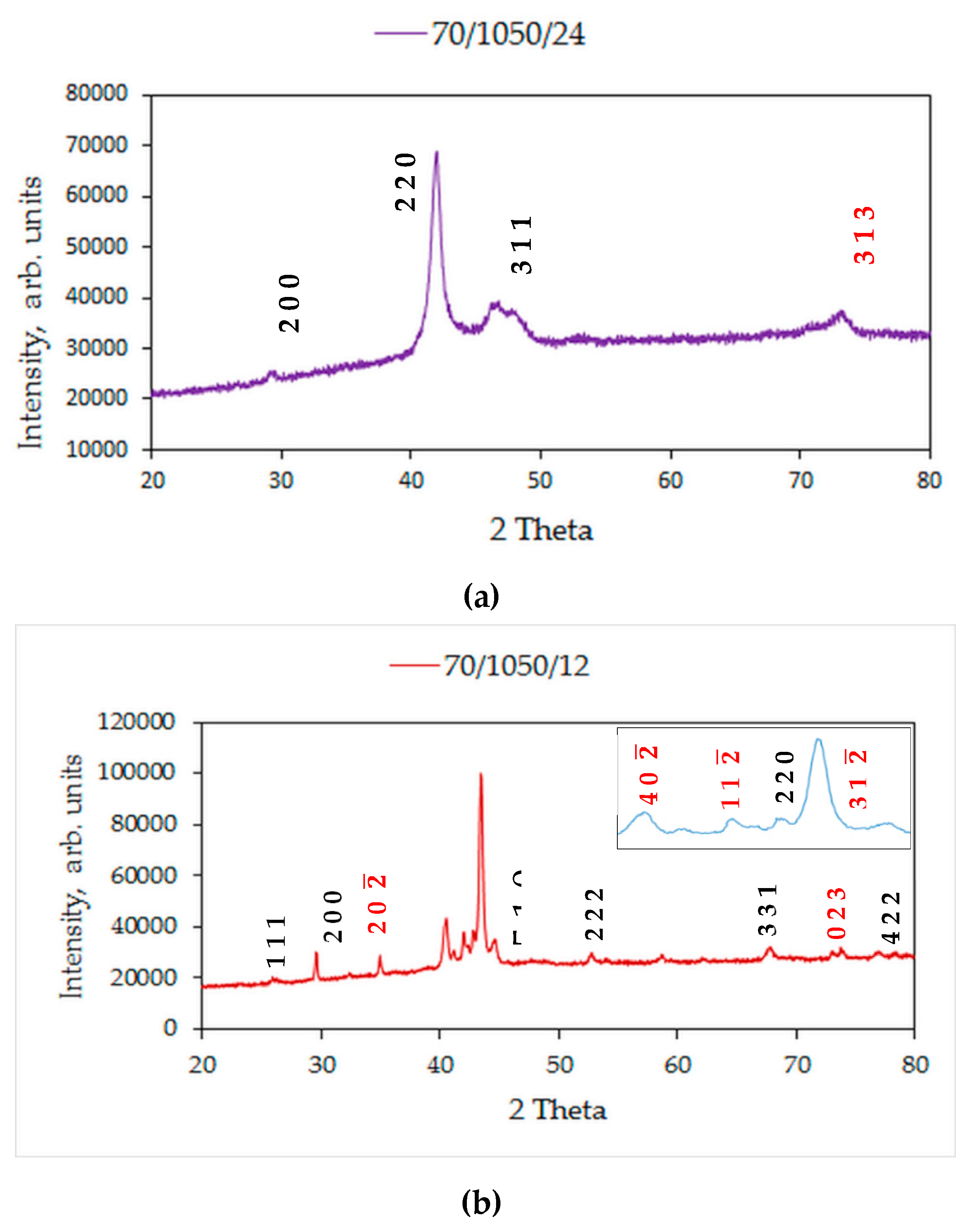
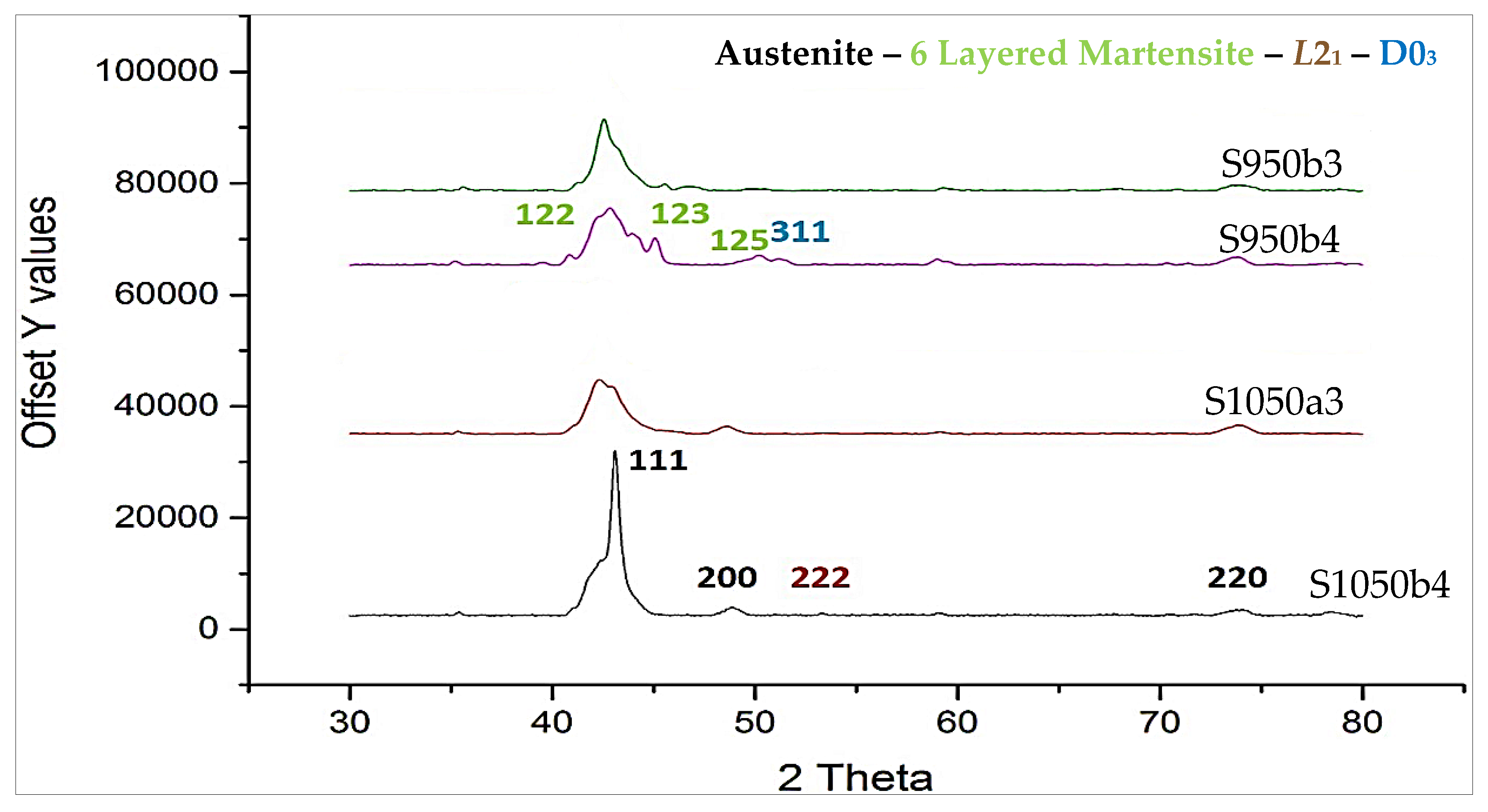
- Sintering at 1050 °C results in a predominantly single phase L21 structure and a small fraction of γ-phase. At a lesser duration of 12/24 h, the γ-phase is less and at a higher duration of 72/144 h, it stabilizes to around 36% of the alloy composition;
- At 72/144 h, the L21 solidified again into L21 and γ-phase in a eutectoid process;
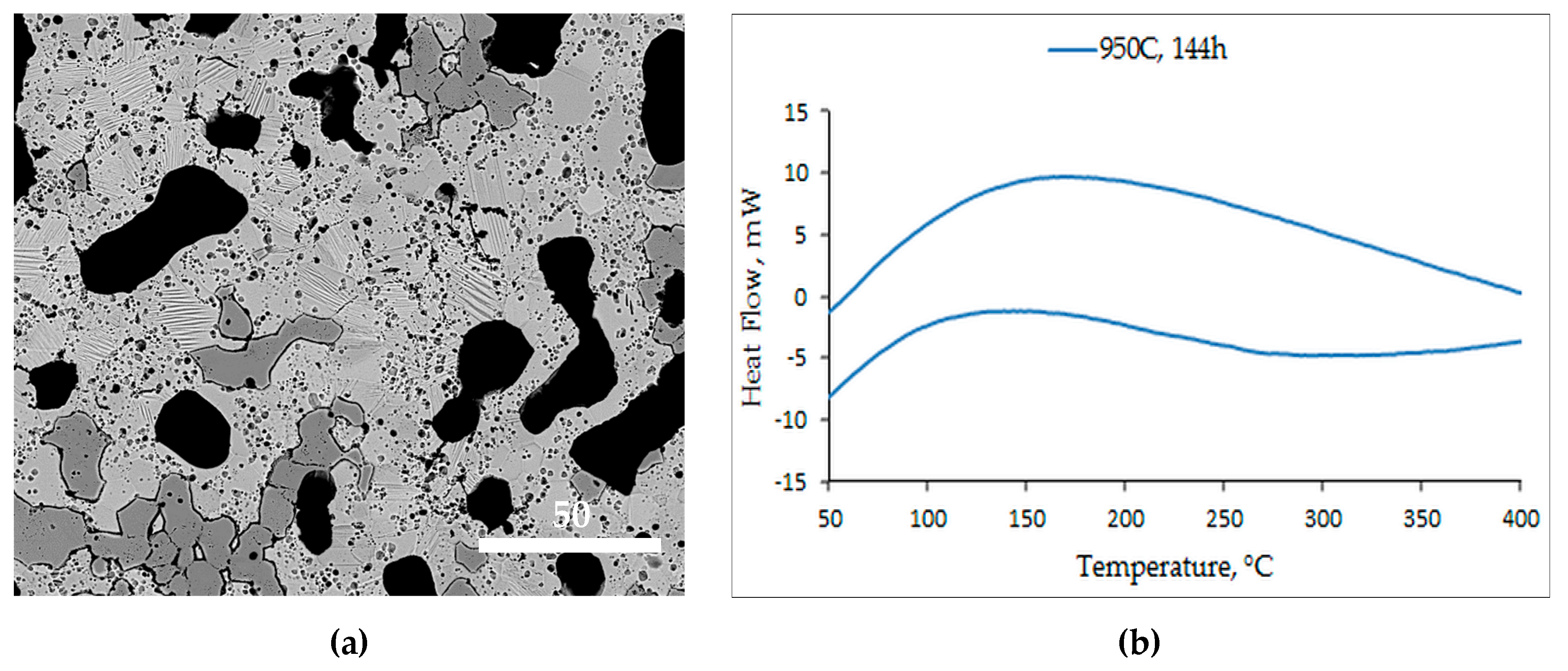
- A slightly lower process temperature of 950 °C did not ensure adequate diffusion and sintering and, consequently, no martensitic transformation, essential for magnetostructural applications. However, after 144 h, the microstructure had clear martensitic grains with grain refinement. Still, no martensitic transformation was recorded, due to the persistence of inhomogeneity;
- A processing condition of 1050 °C/24 h is favorable for synthesizing ferromagnetic Ni–Co–Mn–Sn alloys. A secondary thermomechanical procedure is necessary for the elimination of the γ-phase that masks magnetostructural behaviour.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Srivastava, V.; Bhatti, K.P. Ferromagnetic shape memory Heusler alloys. In Ferroics and Multiferroics; Hardev, S.V., Kleeman, W., Eds.; Trans Tech Publications Ltd.: Pfaffikon, Switzerland, 2012; Volume 189, pp. 189–208. [Google Scholar]
- Planes, A.; Mañosa, L.ı.; Acet, M. Magnetocaloric effect and its relation to shape-memory properties in ferromagnetic Heusler alloys. J. Phys. Condens. Matter 2009, 21, 233201. [Google Scholar] [CrossRef] [PubMed]
- Krenke, T.; Aksoy, S.; Duman, E.; Acet, M.; Moya, X.; Manosa, L.; Planes, A. Hysteresis effects in the magnetic-field-induced reverse martensitic transition in magnetic shape-memory alloys. J. Appl. Phys. 2010, 108, 043914. [Google Scholar] [CrossRef]
- Ullakko, K.; Huang, J.K.; Kantner, C.; O’Handley, R.C.; Kokorin, V.V. Large magnetic-field-induced strains in Ni2MnGa single crystals. Appl. Phys. Lett. 1996, 69, 1965–1968. [Google Scholar] [CrossRef]
- Huang, L.; Cong, D.Y.; Ma, L.; Nie, Z.H.; Wang, M.G.; Wang, Z.L.; Suo, H.L.; Ren, Y.; Wang, Y.D. Large magnetic entropy change and magnetoresistance in a Ni41Co9Mn40Sn10 magnetic shape memory alloy. J. Alloy Compd. 2015, 647, 1081–1085. [Google Scholar] [CrossRef]
- Krenke, T.; Duman, E.; Acet, M.; Wassermann, E.F.; Moya, X.; Manosa, L.; Planes, A. Inverse magnetocaloric effect in ferromagnetic Ni-Mn-Sn alloys. Nat. Mater. 2005, 4, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Moya, X.; Manosa, L.; Planes, A.; Krenke, T.; Duman, E.; Wassermann, E.F. Calorimetric study of the inverse magnetocaloric effect in ferromagnetic Ni-Mn-Sn. J. Magn. Magn. Mater. 2007, 316, e572–e574. [Google Scholar] [CrossRef]
- Pérez-Sierra, A.M.; Bruno, N.M.; Pons, J.; Cesari, E.; Karaman, I. Atomic order and martensitic transformation entropy change in Ni–Co–Mn–In metamagnetic shape memory alloys. Scr. Mater. 2016, 110, 61–64. [Google Scholar] [CrossRef]
- Barandiaran, J.M.; Chernenko, V.A.; Lazpita, P.; Gutierrez, J.; Feuchtwanger, J. Effect of martensitic transformation and magnetic field on transport properties of Ni-Mn-Ga and Ni-Fe-Ga Heusler alloys. Phys. Rev. B 2009, 80, 104404. [Google Scholar] [CrossRef]
- Koyama, K.; Okamoto, S.; Watanabe, T.; Kanomata, T.; Kainuma, R.; Ito, W.; Oikawa, K.; Ishida, K. Observation of large magnetoresistance of magnetic Heusler alloy Ni50Mn36Sn14 in high magnetic fields. Appl. Phys. Lett. 2006, 89, 182510. [Google Scholar] [CrossRef]
- Sanchez, T.; Sato Turtelli, R.; Grossinger, R.; Sanchez, M.L.; Santos, J.D.; Rosa, W.O.; Prida, V.M.; Escoda, L.; Sunol, J.J.; Koledov, V.; et al. Exchange bias behavior in Ni50.0Mn35.5In14.5 ribbons annealed at different temperatures. J. Magn. Magn. Mater. 2012, 324, 3535–3537. [Google Scholar] [CrossRef]
- Srivastava, V.; Chen, X.; James, R.D. Hysteresis and unusual magnetic properties in the singular Heusler alloy Ni45Co5Mn40Sn10. Appl. Phys. Lett. 2010, 97, 014101. [Google Scholar] [CrossRef]
- Sutou, Y.; Imano, Y.; Koeda, N.; Omori, T.; Kainuma, R.; Ishida, K.; Oikawa, K. Magnetic and martensitic transformations of NiMnX (X = In, Sn, Sb) ferromagnetic shape memory alloys. Appl. Phys. Lett. 2004, 85, 4358–4360. [Google Scholar] [CrossRef]
- Kainuma, R.; Imano, Y.; Ito, W.; Sutou, Y.; Morito, H.; Okamoto, S.; Kitakami, O.; Oikawa, K.; Fujita, A.; Kanomata, T.; et al. Magnetic-field-induced shape recovery by reverse phase transformation. Nature 2006, 439, 957–960. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, V.; Song, Y.; Bhatti, K.; James, R.D. The direct conversion of heat to electricity using multiferroic alloys. Adv. Energy Mater. 2011, 1, 97–104. [Google Scholar] [CrossRef]
- Schlagel, D.L.; McCallum, R.W.; Lograsso, T.A. Influence of solidification microstructure on the magnetic properties of Ni-Mn-Sn Heusler alloys. J. Alloy Compd. 2008, 463, 38–46. [Google Scholar] [CrossRef]
- Krenke, T.; Acet, M.; Wasserman, E.F.; Moya, X.; Manosa, L.; Planes, A. Martensitic transitions and nature of ferromagnetism in the austenitic and martensitic states of Ni-Mn-Sn alloys. Phys. Rev. B 2005, 72. [Google Scholar] [CrossRef]
- Lázpita, P.; Sasmaz, M.; Cesari, E.; Barandiarán, J.M.; Gutiérrez, J.; Chernenko, V.A. Martensitic transformation and magnetic field induced effects in Ni42Co8Mn39Sn11 metamagnetic shape memory alloy. Acta Mater. 2016, 109, 170–176. [Google Scholar] [CrossRef]
- Çakır, A.; Righi, L.; Albertini, F.; Acet, M.; Farle, M. Intermartensitic transitions and phase stability in Ni50Mn50−xSnx Heusler alloys. Acta Mater. 2015, 99, 140–149. [Google Scholar] [CrossRef]
- Pérez-Sierra, A.M.; Pons, J.; Santamarta, R.; Vermaut, P.; Ochin, P. Solidification process and effect of thermal treatments on Ni–Co–Mn–Sn metamagnetic shape memory alloys. Acta Mater. 2015, 93, 164–174. [Google Scholar] [CrossRef]
- Graf, T.; Felser, C.; Parkin, S.S.P. Simple rules for the understanding of Heusler compounds. Prog. Solid State Chem. 2011, 39, 1–50. [Google Scholar] [CrossRef]
- Chen, X.; Naik, V.B.; Mahendiran, R.; Ramanujan, R.V. Optimization of Ni-Co-Mn-Sn Heusler alloy composition for near room temperature magnetic cooling. J. Alloy Compd. 2014, 618, 187–191. [Google Scholar] [CrossRef]
- Bruno, N.M.; Yegin, C.; Karaman, I.; Chen, J.-H.; Ross, J.H.; Liu, J.; Li, J. The effect of heat treatments on Ni43Mn42Co4Sn11 meta-magnetic shape memory alloys for magnetic refrigeration. Acta Mater. 2014, 74, 66–84. [Google Scholar] [CrossRef]
- Das, R.; Saravanan, P.; Arvindha Babu, D.; Perumal, A.; Srinivasan, A. Influence of solidification rate and heat treatment on magnetic refrigerant properties of melt spun Ni51Mn34In14Si1 ribbons. J. Magn. Magn. Mater. 2013, 344, 152–157. [Google Scholar] [CrossRef]
- Sanchez Llamazares, J.L.; Sanchez, T.; Santos, J.D.; Prez, M.J.; Sanchez, M.L.; Hernando, B.; Escoda, L.; Sunol, J.J.; Varga, R. Martensitic phase transformation in rapidly solidified Mn50Ni40In10 alloy ribbons. Appl. Phys. Lett. 2008, 92, 012513. [Google Scholar] [CrossRef]
- Rajkumar, D.M.; Rao, N.V.R.; Muthu, S.E.; Arumugam, S.; Raja, M.M.; Suresh, K.G. Effect of Fe on the Martensitic Transition, Magnetic and Magnetocaloric Properties in Ni-Mn-In Melt-spun Ribbons. Def. Sci. J. 2016, 66, 403–412. [Google Scholar] [CrossRef][Green Version]
- Ma, S.C.; Shih, C.W.; Liu, J.; Yuan, J.H.; Lee, S.Y.; Lee, Y.I.; Chang, H.W.; Chang, W.C. Wheel speed-dependent martensitic transformation and magnetocaloric effect in Ni-Co-Mn-Sn ferromagnetic shape memory alloy ribbons. Acta Mater. 2015, 90, 292–302. [Google Scholar] [CrossRef]
- Chen, F.; Tong, Y.X.; Li, L.; Sánchez Llamazares, J.L.; Sánchez-Valdés, C.F.; Müllner, P. The effect of step-like martensitic transformation on the magnetic entropy change of Ni40.6Co8.5Mn40.9Sn10 unidirectional crystal grown with the Bridgman-Stockbarger technique. J. Alloy Compd. 2017, 691, 269–274. [Google Scholar] [CrossRef]
- Laudise, R.A.; Sunder, W.A.; O’Bryan, H.M.; Carlson, D.J.; Witt, A.F. Czochralski growth of single crystals of Ni3−xMnxSn. J. Cryst. Growth 1992, 118, 277–286. [Google Scholar] [CrossRef]
- Ito, K.; Ito, W.; Umetsu, R.Y.; Tajima, S.; Kawaura, H.; Kainuma, R.; Ishida, K. Metamagnetic shape memory effect in polycrystalline NiCoMnSn alloy fabricated by spark plasma sintering. Scr. Mater. 2009, 61, 504–507. [Google Scholar] [CrossRef]
- Ito, K.; Ito, W.; Umetsu, R.Y.; Karaman, I.; Ishida, K.; Kainuma, R. Mechanical and shape memory properties of Ni43Co7Mn39Sn11 alloy compacts fabricat by pressureless sintering. Scr. Mater. 2010, 63, 1236–1239. [Google Scholar] [CrossRef]
- Ito, K.; Ito, W.; Umetsu, R.Y.; Nagasako, M.; Kainuma, R.; Fujita, A.; Oikawa, K.; Ishida, K. Martensitic transformation in NiCoMnSn metamagnetic shape memory alloy powders. Mater. Trans. 2008, 49, 1915–1918. [Google Scholar] [CrossRef]
- Monroe, J.A.; Cruz-Perez, J.; Yegin, C.; Karaman, I.; Geltmacher, A.B.; Everett, R.K.; Kainuma, R. Magnetic response of porous NiCoMnSn metamagnetic shape memory alloys fabricated using solid-state replication. Scr. Mater. 2012, 67, 116–119. [Google Scholar] [CrossRef]
- Ahamed, R.; Ghomashchi, R.; Xie, Z.; Chen, L.; Munroe, P.; Xu, S. Powder processing and characterisation of a quinary Ni-Mn-Co-Sn-Cu Heusler alloy. Powder Technol. 2018, 324, 69–75. [Google Scholar] [CrossRef]
- Yuhasz, W.M.; Schlagel, D.L.; Xing, Q.; Dennis, K.W.; McCallum, R.W.; Lograsso, T.A. Influence of annealing and phase decomposition on the magnetostructural transitions in Ni50Mn39Sn11. J. Appl. Phys. 2009, 105, 07A921. [Google Scholar] [CrossRef]
- Nespoli, A.; Biffi, C.A.; Villa, E.; Tuissi, A. Effect of heating/cooling rate on martensitic transformation of NiMnGa-Co high temperature ferromagnetic shape memory alloys. J. Alloy Compd. 2017, 690, 478–484. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, W.; Xue, S.; Zhai, Q.; Frenzel, J.; Luo, Z. Composition-dependent crystal structure and martensitic transformation in Heusler Ni-Mn-Sn alloys. Acta Mater. 2013, 61, 4648–4656. [Google Scholar] [CrossRef]


| Ni (µm) | Mn (µm) | Sn (µm) | Co (µm) | |
|---|---|---|---|---|
| D (v,0.5) | 11.52 | 16.91 | 19.14 | 8.05 |
| Sample ID | Compaction Pressure (MPa) | Holding Time (h) | Temperature (°C) |
|---|---|---|---|
| S950a1 | 184 | 12 | 950 |
| S950a2 | 184 | 24 | 950 |
| S1050a1 | 184 | 12 | 1050 |
| S1050a2 | 184 | 24 | 1050 |
| S950b1 | 210 | 12 | 950 |
| S950b2 | 210 | 24 | 950 |
| S1050b1 | 210 | 12 | 1050 |
| S1050b2 | 210 | 24 | 1050 |
| S1050a3 | 184 | 72 | 1050 |
| S1050a4 | 184 | 144 | 1050 |
| S1050b3 | 210 | 72 | 1050 |
| S1050b4 | 210 | 144 | 1050 |
| Sample ID | Ni (at %) | Mn (at %) | Co (at %) | Sn (at %) | Total |
|---|---|---|---|---|---|
| S950a1 | 44.7 | 42.8 | 5.3 | 7.2 | 100.0 |
| S950a2 | 45.8 | 41.1 | 5.1 | 7.9 | 100.0 |
| S1050a1 | 44.8 | 41.8 | 5.4 | 8.0 | 100.0 |
| S1050a2 | 45.9 | 39.9 | 4.6 | 9.5 | 100.0 |
| S950b1 | 45.0 | 42.2 | 5.1 | 7.7 | 100.0 |
| S950b2 | 45.2 | 42.0 | 4.9 | 7.8 | 100.0 |
| S1050b1 | 44.9 | 41.0 | 5.0 | 8.9 | 100.0 |
| S1050b2 | 45.2 | 40.8 | 5.1 | 8.9 | 100.0 |
| S1050a3 | 45.8 | 40.3 | 5.0 | 8.9 | 100.0 |
| S1050a4 | 44.9 | 41.6 | 5.6 | 7.8 | 100.0 |
| S1050b3 | 45.3 | 40.9 | 5.9 | 7.7 | 100.0 |
| S1050b4 | 44.6 | 42.7 | 5.8 | 6.9 | 100.0 |
| Sample ID | AS (°C) | AF (°C) | AP (°C) | MS (°C) | MF (°C) | MP (°C) | (AS + MF)/2 (°C) | (AF – MS) (°C) | ΔH/Jg−1 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Heat | Cool | |||||||||
| S950a1 | 135 | – | – | – | 103 | – | – | – | – | – |
| S950a2 | 140 | 215 | 175 | 210 | 105 | 156 | 123 | 5 | 20.08 | 33.75 |
| S1050a1 | 120 | 182 | 160 | 155 | 85 | 125 | 103 | 27 | 26.45 | 25.60 |
| S1050a2 | 130 | 175 | 157 | 158 | 109 | 135 | 120 | 17 | 12.75 | 13.89 |
| S950b1 | 132 | – | – | – | – | – | – | – | – | – |
| S950b2 | 130 | 220 | 170 | 205 | 100 | 155 | 115 | 15 | 19.42 | 22.66 |
| S1050b1 | 125 | 160 | 148 | 132 | 88 | 112 | 107 | 28 | 22.27 | 19.56 |
| S1050b2 | 130 | 187 | 174 | 168 | 100 | 140 | 115 | 19 | 32.17 | 34.29 |
| S1050a3 | – | – | – | – | – | – | – | – | – | – |
| S1050a4 | – | – | – | – | – | – | – | – | – | – |
| Sample ID | Ni (at %) | Mn (at %) | Co (at %) | Sn (at %) | Total | In Figure |
|---|---|---|---|---|---|---|
| S1050a1 | 45.2 | 40.8 | 5.1 | 8.9 | 100.0 | 8a: Martensite |
| S1050a2 | 45.6 | 40.4 | 5.4 | 8.7 | 100.0 | 8b: Martensite |
| S1050a2 | 44.9 | 41.9 | 11.1 | 2.1 | 100.0 | 8b: γ-phase |
| S1050b1 | 45.0 | 41.7 | 5.1 | 8.2 | 100.0 | 8c: Martensite |
| S1050b1 | 45.5 | 41.1 | 4.6 | 8.8 | 100.0 | 8c: Non-martensite |
| S1050b2 | 45.0 | 41.0 | 5.1 | 8.9 | 100.0 | 8d: Martensite |
| S1050b2 | 42.3 | 45.2 | 11.3 | 1.2 | 100.0 | 8d: γ-phase |
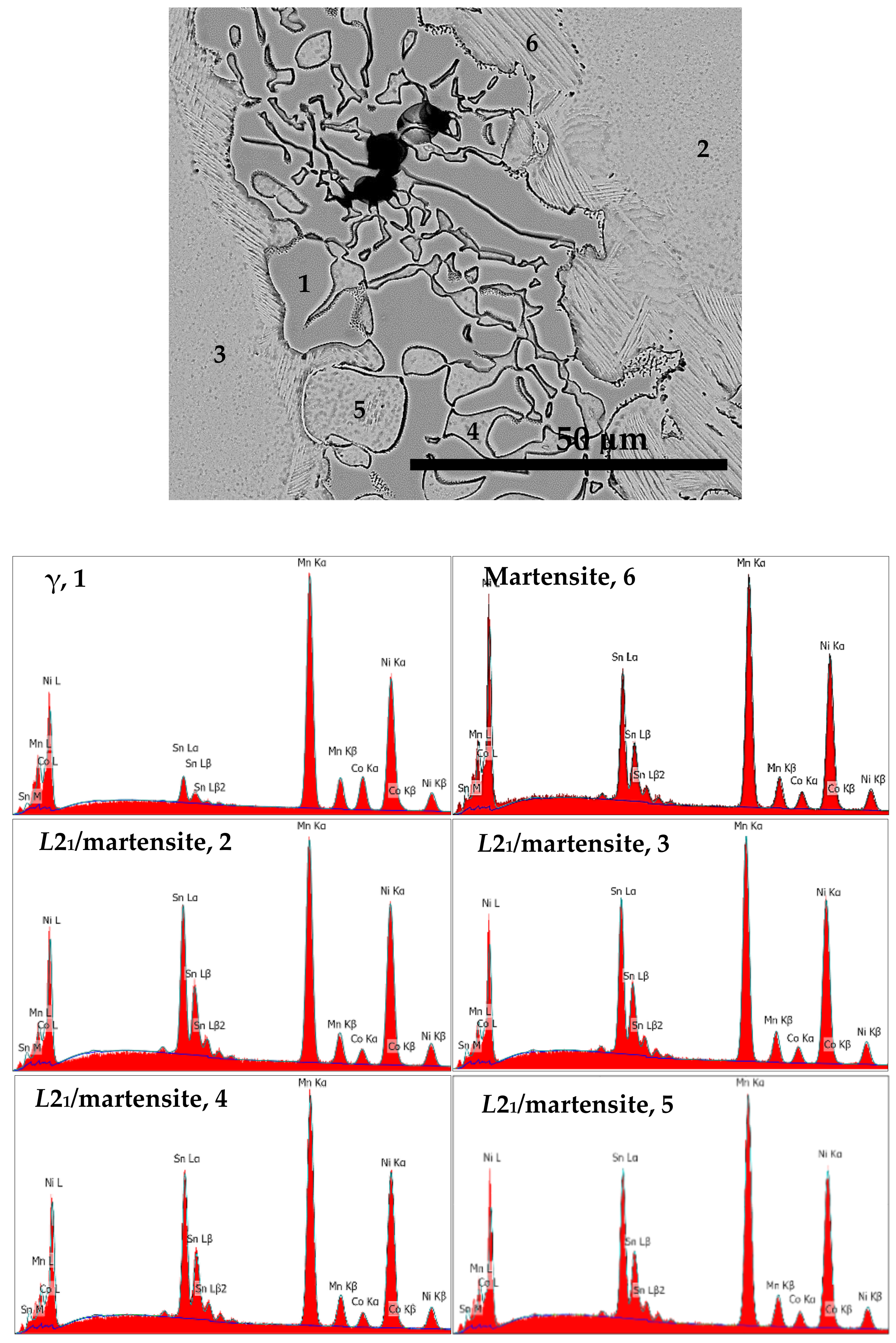
| Sample ID | Ni (at %) | Mn (at %) | Co (at %) | Sn (at %) | Total | In Figure |
|---|---|---|---|---|---|---|
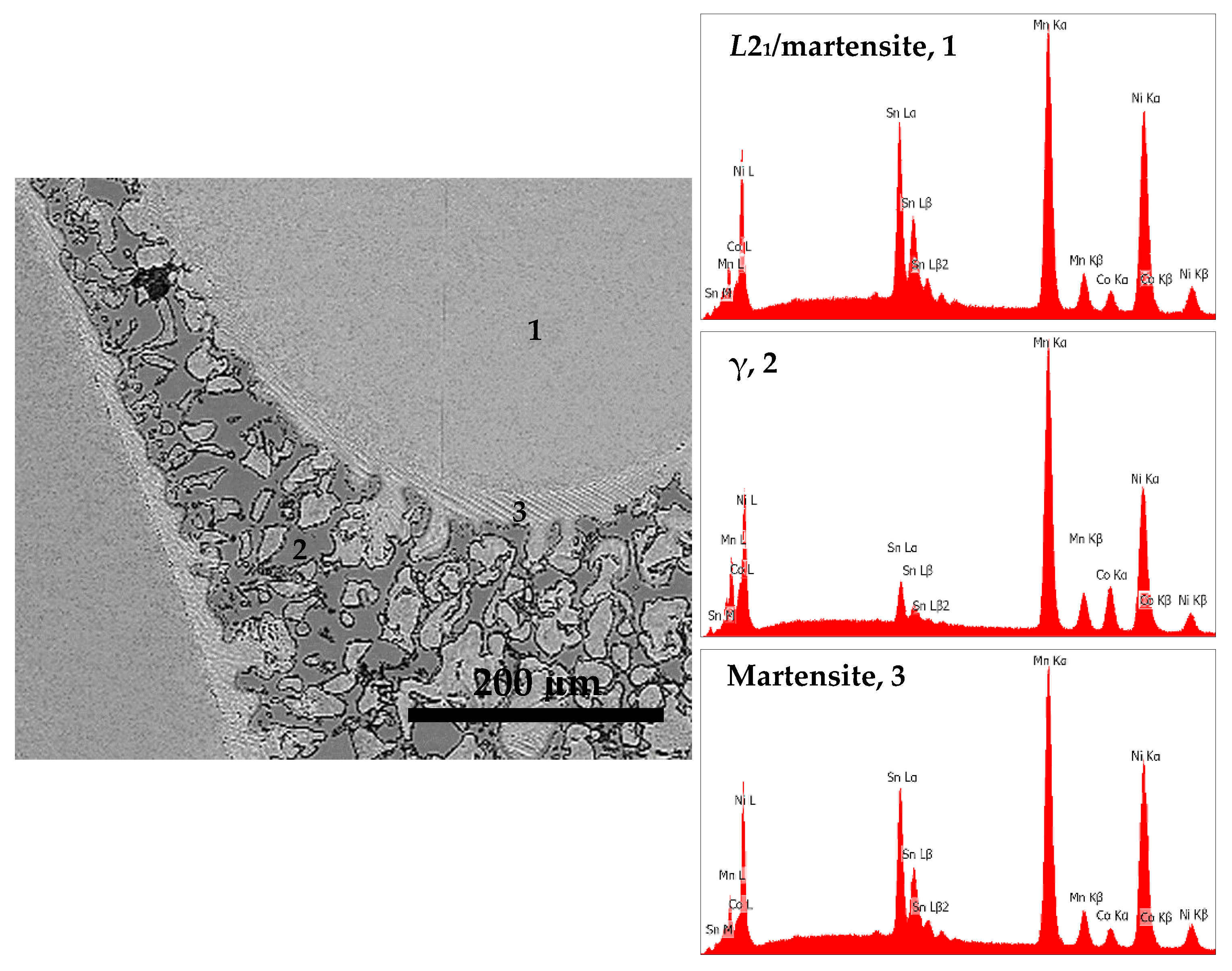
| S1050a3 | 46.0 | 40.2 | 4.4 | 9.4 | 100.0 | 9: Martensite, 6 |
| S1050a3 | 44.6 | 43.7 | 9.6 | 2.1 | 100.0 | 9: γ-phase, 1 |
| S1050a3 | 46.9 | 38.3 | 3.9 | 10.9 | 100.0 | 9: L21/martensite, 2 |
| S1050a3 | 46.9 | 38.1 | 4.0 | 11.0 | 100.0 | 9: L21/martensite, 3 |
| S1050a3 | 45.9 | 40.0 | 3.7 | 10.4 | 100.0 | 9: L21/martensite, 4 |
| S1050a3 | 45.7 | 39.6 | 4.0 | 10.7 | 100.0 | 9: L21/martensite, 5 |
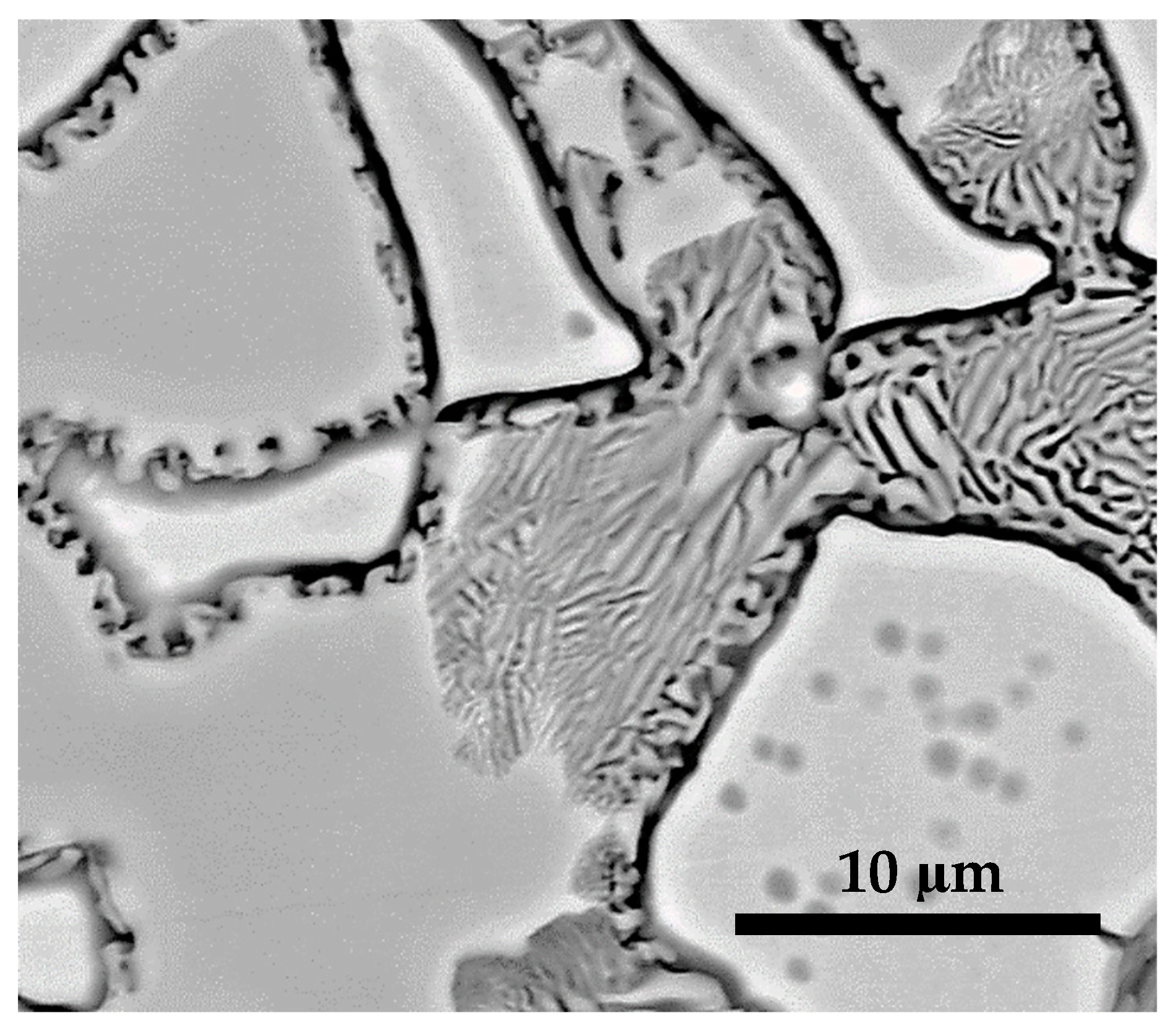
| S1050b4 | 46.6 | 39.2 | 4.5 | 9.7 | 100.0 | 10: L21/martensite, 1 |
| S1050b4 | 40.3 | 45.4 | 11.6 | 2.7 | 100.0 | 10: γ-phase, 2 |
| S1050b4 | 45.9 | 40.9 | 4.6 | 8.6 | 100.0 | 10: Martensite, 3 |
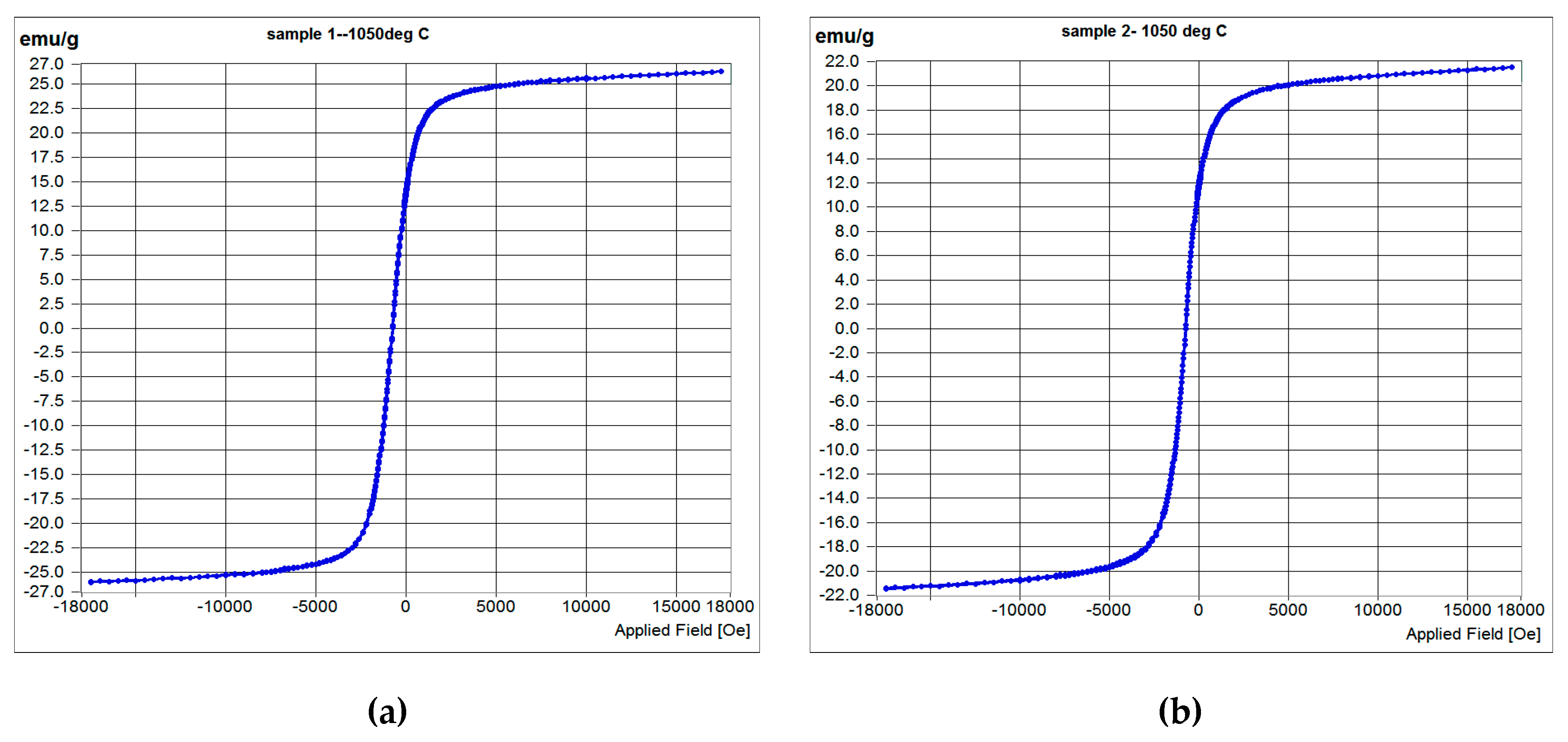
| Sample | Coercivity, HC Oe | Remanence, MR emu/g | Saturation magnetization, Ms emu/g | Squareness, MR/MS |
|---|---|---|---|---|
| S1050b4 | 4.64 | 0.09654 | 26.148 | 0.532 |
| S1050b3 | 6.596 | 0.12719 | 21.483 | 0.552 |
| S950b4 | 7.623 | 0.1566 | 12.34 | 0.593 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahamed, R.; Ghomashchi, R.; Xie, Z.; Chen, L. Powder Metallurgy Synthesis of Heusler Alloys: Effects of Process Parameters. Materials 2019, 12, 1596. https://doi.org/10.3390/ma12101596
Ahamed R, Ghomashchi R, Xie Z, Chen L. Powder Metallurgy Synthesis of Heusler Alloys: Effects of Process Parameters. Materials. 2019; 12(10):1596. https://doi.org/10.3390/ma12101596
Chicago/Turabian StyleAhamed, Riaz, Reza Ghomashchi, Zonghan Xie, and Lei Chen. 2019. "Powder Metallurgy Synthesis of Heusler Alloys: Effects of Process Parameters" Materials 12, no. 10: 1596. https://doi.org/10.3390/ma12101596
APA StyleAhamed, R., Ghomashchi, R., Xie, Z., & Chen, L. (2019). Powder Metallurgy Synthesis of Heusler Alloys: Effects of Process Parameters. Materials, 12(10), 1596. https://doi.org/10.3390/ma12101596






