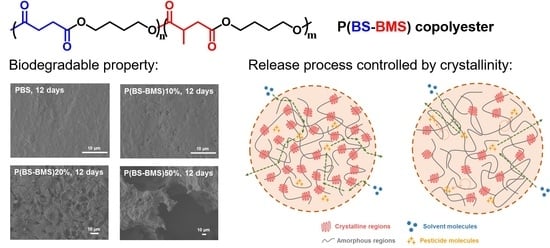
Synthesis, Properties of Biodegradable Poly(Butylene Succinate-co-Butylene 2-Methylsuccinate) and Application for Sustainable Release
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
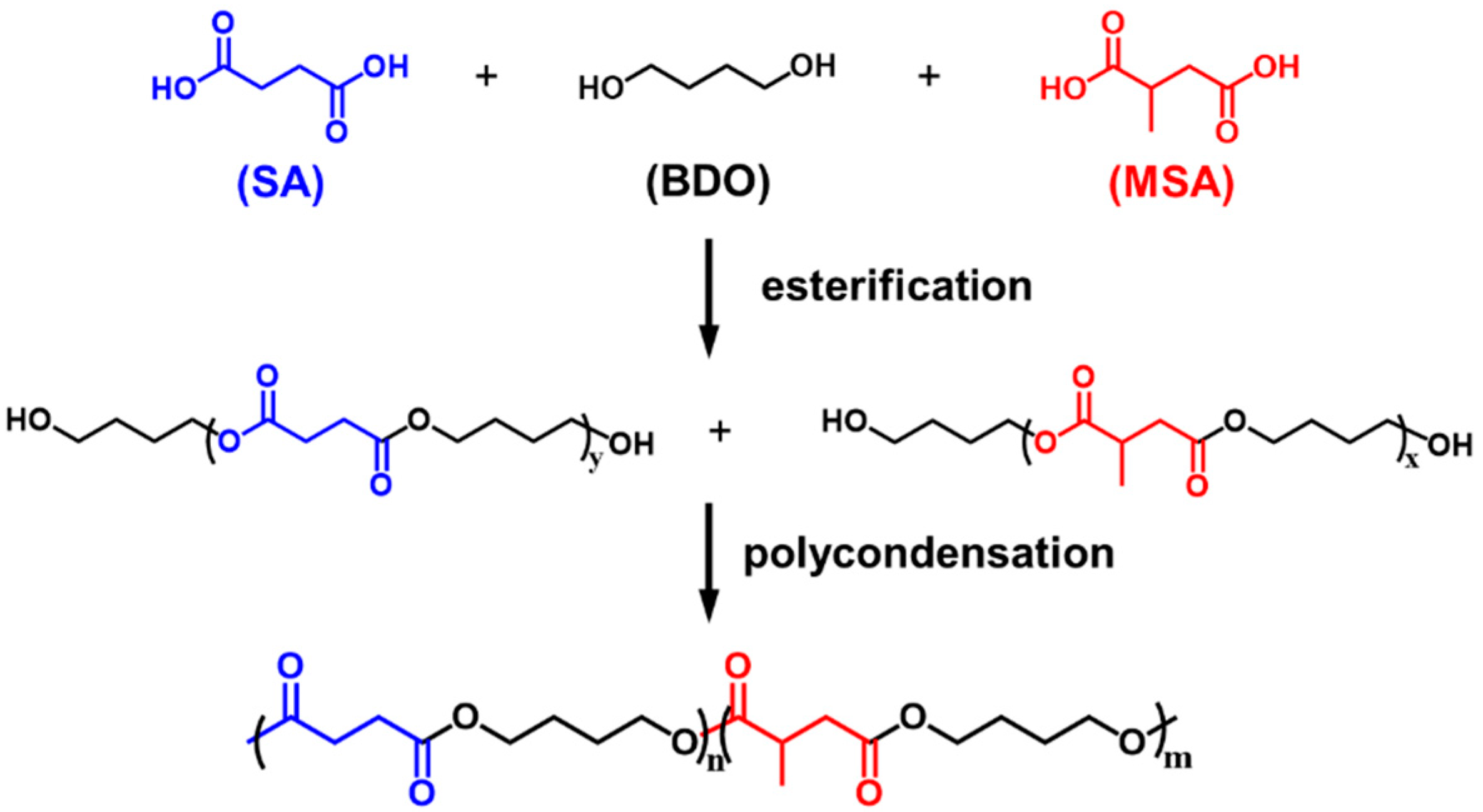
2.2. Synthesis of P(BS-BMS) Copolyesters
2.3. Preparation of the Av-Loaded P(BS-BMS) Microspheres
2.4. Polymer Characterizations
2.5. In Vitro Degradation Test
2.6. Characterizations of Av-Loaded Microspheres
2.7. Release Kinetics of Av
3. Results and Discussion
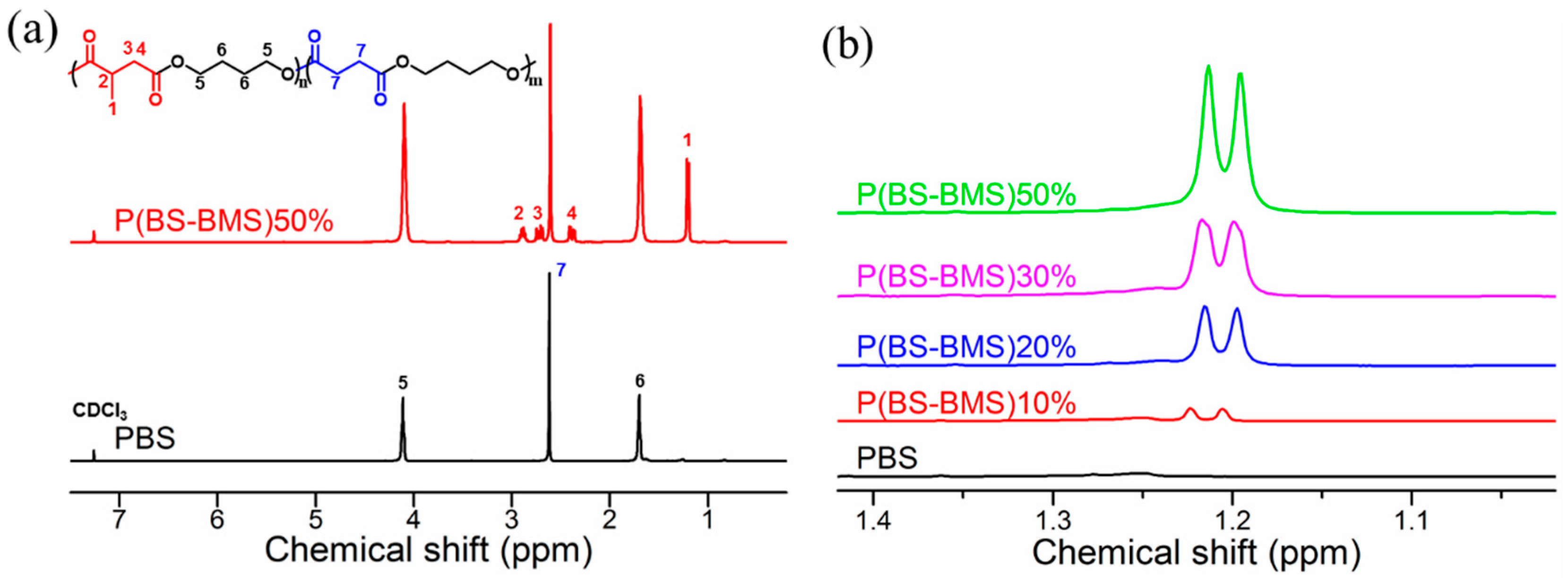
3.1. Synthesis and Characterization of P(BS-BMS) Copolymers
3.2. Thermal Properties and Crystal Structures of P(BS-BMS) Copolymers
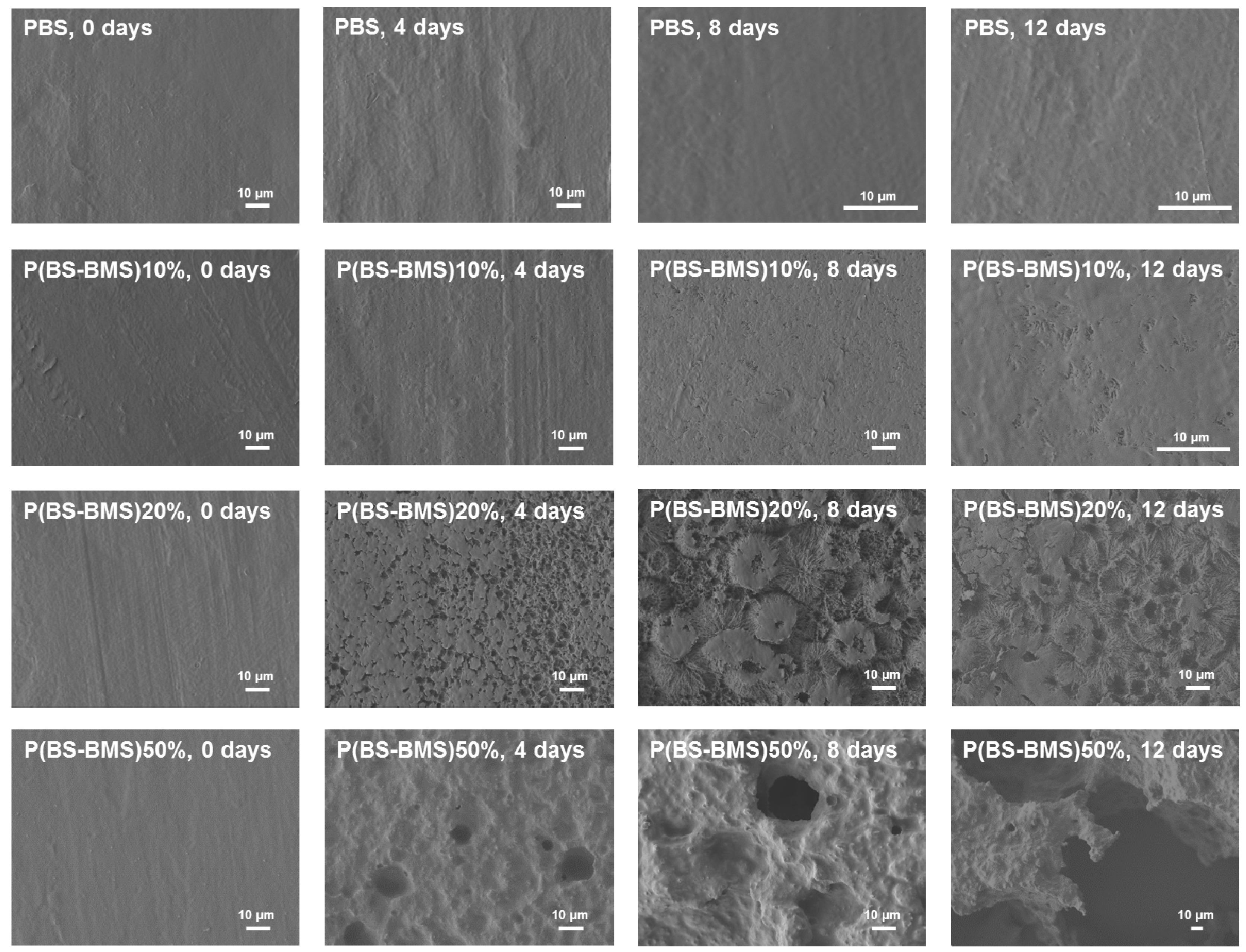
3.3. Thermal Stability and Biodegradation of P(BS-BMS) Copolymers
3.4. Preparation of the Av-Loaded Delivery Systems Based on P(BS-BMS) Copolymers
3.5. Av entrapment Efficiency and Encapsulation State
3.6. Control Release of Av
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kong, X.; Qi, H.; Curtis, J.M. Synthesis and characterization of high-molecular weight aliphatic polyesters from monomers derived from renewable resources. J. Appl. Sci. 2014, 131, 4401–4404. [Google Scholar] [CrossRef]
- Dai, J.; Ma, S.; Teng, N.; Dai, X.; Shen, X.; Wang, S.; Liu, X.; Zhu, J. 2,5-Furandicarboxylic Acid- and Itaconic Acid-Derived Fully Biobased Unsaturated Polyesters and Their Cross-Linked Networks. Ind. Eng. Chem. 2017, 56, 2650–2657. [Google Scholar] [CrossRef]
- Trotta, J.T.; Watts, A.; Wong, A.R.; LaPointe, A.M.; Hillmyer, M.A.; Fors, B.P. Renewable Thermosets and Thermoplastics from Itaconic Acid. ACS Sustainable Chem. Eng. 2019, 7, 2691–2701. [Google Scholar] [CrossRef]
- Bastioli, C. Handbook of Biodegradable Polymers; Rapra Technology Limited: Shropshire, UK, 2005. [Google Scholar]
- Tachibana, Y.; Yamahata, M.; Kimura, S.; Kasuya, K.I. Synthesis, physical properties, and biodegradability of Bio-based Poly(butylene succinate-co-butylene oxabicyclate). ACS Sustain. Chem. Eng. 2018, 6, 10806–10814. [Google Scholar] [CrossRef]
- Liu, T.; Hao, C.; Wang, L.; Li, Y.; Liu, W.; Xin, J.; Zhang, J. Eugenol-Derived Biobased Epoxy: Shape Memory, Repairing, and Recyclability. Macromolecules 2017, 50, 8588–8597. [Google Scholar] [CrossRef]
- Liu, T.; Guo, X.; Liu, W.; Hao, C.; Wang, L.; Hiscox, W.C.; Liu, C.; Jin, C.; Xin, J.; Zhang, J. Selective cleavage of ester linkages of anhydride-cured epoxy using a benign method and reuse of the decomposed polymer in new epoxy preparation. Green Chem. 2017, 19, 4364–4372. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, T.; Hao, C.; Wang, L.; Han, J.; Liu, H.; Zhang, J. Preparation of a lignin-based vitrimer material and its potential use for recoverable adhesives. Green Chem. 2018, 20, 2995–3000. [Google Scholar] [CrossRef]
- Altuna, F.I.; Pettarin, V.; Williams, R.J. Self-healable polymer networks based on the cross-linking of epoxidised soybean oil by an aqueous citric acid solution. Green Chem. 2013, 15, 3360–3366. [Google Scholar] [CrossRef]
- Shi, K.; Bai, Z.; Su, T.; Wang, Z. Selective enzymatic degradation and porous morphology of poly(butylene succinate)/poly(lactic acid) blends. Int. J. Biol. Macromol. 2019, 126, 436–442. [Google Scholar] [CrossRef]
- Kitamoto, H.; Yoshida, S.; Koitabashi, M.; Yamamoto-Tamura, K.; Ueda, H.; Yarimizu, T.; Sameshima-Yamashita, Y. Enzymatic degradation of poly-butylene succinate-co-adipate film in rice husks by yeast Pseudozyma antarctica in indoor conditions. J. Biosci. Bioeng. 2018, 125, 199–204. [Google Scholar] [CrossRef]
- Abe, M.; Kobayashi, K.; Honma, N.; Nakasaki, K. Microbial degradation of poly(butylene succinate) by Fusarium solani in soil environments. Degrad. Stab. 2010, 95, 138–143. [Google Scholar] [CrossRef]
- Shinozaki, Y.; Morita, T.; Cao, X.H.; Yoshida, S.; Koitabashi, M.; Watanabe, T.; Kitamoto, H.K. Biodegradable plastic-degrading enzyme from Pseudozyma antarctica: Cloning, sequencing, and characterization. Appl. Microbiol. Biotechnol. 2013, 97, 2951–2959. [Google Scholar] [CrossRef]
- Huang, Z.; Qian, L.; Yin, Q.; Yu, N.; Liu, T.; Tian, D. Biodegradability studies of poly(butylene succinate) composites filled with sugarcane rind fiber. Polym. Test. 2018, 66, 319–326. [Google Scholar] [CrossRef]
- Shi, K.; Liu, Y.; Hu, X.; Su, T.; Li, P.; Wang, Z. Preparation, characterization, and biodegradation of poly(butylene succinate)/cellulose triacetate blends. Int. J. Boil. Macromol. 2018, 114, 373–380. [Google Scholar] [CrossRef]
- Wang, D.; Lu, X.; Qu, J. Role of In situ thermal-reduced graphene oxide on the morphology and properties of biodegradable poly(Lactic acid)/poly(butylene succinate) blends. Polym. Compos. 2017, 39, 3057–3065. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, X.; Yang, B.; Xu, Y.; Zhang, W.; Zhang, Y.; Ji, J. Synthesis, physical properties and enzymatic degradation of bio-based poly(butylene adipate-co-butylene furandicarboxylate) copolyesters. Degrad. Stab. 2013, 98, 2177–2183. [Google Scholar] [CrossRef]
- Tachibana, Y.; Masuda, T.; Funabashi, M.; Kunioka, M. Chemical Synthesis of Fully Biomass-Based Poly(butylene succinate) from Inedible-Biomass-Based Furfural and Evaluation of Its Biomass Carbon Ratio. Biomacromolecules 2010, 11, 2760–2765. [Google Scholar] [CrossRef]
- Tserki, V.; Matzinos, P.; Pavlidou, E.; Vachliotis, D.; Panayiotou, C. Biodegradable aliphatic polyesters. Part I. Properties and biodegradation of poly(butylene succinate-co-butylene adipate). Polym. Degrad. Stab. 2006, 91, 367–376. [Google Scholar] [CrossRef]
- Puchalski, M.; Szparaga, G.; Biela, T.; Gutowska, A.; Sztajnowski, S.; Krucińska, I. Molecular and Supramolecular Changes in Polybutylene Succinate (PBS) and Polybutylene Succinate Adipate (PBSA) Copolymer during Degradation in Various Environmental Conditions. Polymers 2018, 10, 251. [Google Scholar] [CrossRef]
- Hongsriphan, N.; Pinpueng, A. Properties of Agricultural Films Prepared from Biodegradable Poly(Butylene Succinate) Adding Natural Sorbent and Fertilizer. J. Polym. Environ. 2019, 27, 434–443. [Google Scholar] [CrossRef]
- Siwek, P.; Libik, A.; Kalisz, A.; Zawiska, I. The effect of biodegradable nonwoven direct covers on yield and quality of winter leek. Folia Hort. 2013, 25, 61–65. [Google Scholar] [CrossRef]
- Khalil, H.A.; Banerjee, A.; Saurabh, C.K.; Tye, Y.Y.; Suriani, A.B.; Mohamed, A.; Paridah, M.T. Biodegradable Films for Fruits and Vegetables Packaging Application: Preparation and Properties. Food Eng. Rev. 2018, 10, 139–153. [Google Scholar] [CrossRef]
- Chen, S.; Ma, C.; Zhang, G. Biodegradable polymers for marine antibiofouling: Poly(ε-caprolactone)/poly(butylene succinate) blend as controlled release system of organic antifoulant. Polymer 2016, 90, 215–221. [Google Scholar] [CrossRef]
- Bi, S.; Tan, B.; Soule, J.L.; Sobkowicz, M.J. Enzymatic degradation of poly (butylene succinate-co-hexamethylene succinate). Degrad. Stab. 2018, 155, 9–14. [Google Scholar] [CrossRef]
- Li, F.; Xu, X.; Hao, Q.; Li, Q.; Yu, J.; Cao, A. Effects of comonomer sequential structure on thermal and crystallization behaviors of biodegradable poly(butylene succinate-co-butylene terephthalate)s. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 1635–1644. [Google Scholar] [CrossRef]
- Heidarzadeh, N.; Rafizadeh, M.; Taromi, F.A.; Del Valle, L.J.; Franco, L.; Puiggalí, J. Biodegradability and biocompatibility of copoly(butylene sebacate-co-terephthalate)s. Degrad. Stab. 2017, 135, 18–30. [Google Scholar] [CrossRef]
- Zeng, J.-B.; Huang, C.-L.; Jiao, L.; Lu, X.; Wang, Y.-Z.; Wang, X.-L. Synthesis and Properties of Biodegradable Poly(butylene succinate-co-diethylene glycol succinate) Copolymers. Ind. Eng. Chem. 2012, 51, 12258–12265. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, J.; Wu, S.; Qi, Z.; Xu, J.; Guo, B. Synthesis, physical properties and photodegradation of functional poly(butylene succinate) covalently linking UV stabilizing moieties in molecular chains. Colloids Surfaces A: Physicochem. Eng. Asp. 2017, 524, 160–168. [Google Scholar] [CrossRef]
- Nikolic, M.S.; Poleti, D.; Djonlagic, J. Synthesis and characterization of biodegradable poly(butylene succinate-co-butylene fumarate)s. Eur. Polym. J. 2003, 39, 2183–2192. [Google Scholar] [CrossRef]
- Li, C.; Xiao, Y.; Guan, G.; Zheng, L.; Wang, Z.; Wang, J.; Zhang, D. Novel Biodegradable and Double Crystalline Multiblock Copolymers Comprising of Poly(butylene succinate) and Poly(ε-caprolactone): Synthesis, Characterization, and Properties. Ind. Eng. Chem. 2012, 51, 7264–7272. [Google Scholar]
- Wu, S.; Zhang, Y.; Han, J.; Xie, Z.; Xu, J.; Guo, B. Copolymerization with Polyether Segments Improves the Mechanical Properties of Biodegradable Polyesters. ACS Omega 2017, 2, 2639–2648. [Google Scholar] [CrossRef]
- Zhang, Y.; Dai, Z.-H.; Han, J.; Li, T.; Xu, J.; Guo, B. Interplay between crystallization and the Diels–Alder reaction in biobased multiblock copolyesters possessing dynamic covalent bonds. Polym. Chem. 2017, 8, 4280–4289. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, Z.; Wu, S.; Li, C.; Zhang, D.; Xiao, Y. Novel Poly(butylene fumarate) and Poly(butylene succinate) Multiblock Copolymers Bearing Reactive Carbon–Carbon Double Bonds: Synthesis, Characterization, Cocrystallization, and Properties. Ind. Eng. Chem. 2013, 52, 6147–6155. [Google Scholar] [CrossRef]
- Dai, Z.; Yang, Z.; Chen, Z.; Zhao, Z.; Lou, Y.; Zhang, Y.; Liu, T.; Fu, F.; Fu, Y.; Liu, X. Fully Biobased Composites of an Itaconic Acid Derived Unsaturated Polyester Reinforced with Cotton Fabrics. ACS Sustain. Chem. Eng. 2018, 6, 15056–15063. [Google Scholar] [CrossRef]
- Panic, V.V.; Seslija, S.I.; Popovic, I.G.; Spasojevic, V.D.; Popovic, A.R.; Nikolic, V.B.; Spasojevic, P.M. Simple One-Pot Synthesis of Fully Biobased Unsaturated Polyester Resins Based on Itaconic Acid. Biomacromolecules 2017, 18, 3881–3891. [Google Scholar] [CrossRef] [PubMed]
- Da Cruz, J.C.; de Castro, A.M.; Sérvulo, E.F.C. World market and biotechnological production of itaconic acid. Biotech 2018, 8, 138. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Gao, C.; Wang, C.; Shen, S.; Wu, Y. Application of Poly(butylenes 2-methylsuccinate) as Migration Resistant Plasticizer for Poly(vinyl chloride). Polym. Technol. Eng. 2014, 53, 465–471. [Google Scholar] [CrossRef]
- Wu, Y.; Xie, Q.; Gao, C.; Wang, T.; Wang, C. Synthesis and Characterization of a Novel Aliphatic Polyester Based on Itaconic Acid. Polym. Eng. Sci. 2015, 54, 2515–2521. [Google Scholar] [CrossRef]
- Li, T.; Lü, S.; Ji, Y.; Qi, T.; Liu, M. A biodegradable Fe-fertilizer with high mechanical property and sustainable release for potential agriculture and horticulture applications. New J. Chem. 2018, 42, 19129–19136. [Google Scholar] [CrossRef]
- Tanan, W.; Panichpakdee, J.; Saengsuwan, S. Novel biodegradable hydrogel based on natural polymers: Synthesis, characterization, swelling/reswelling and biodegradability. Eur. J. 2019, 112, 678–687. [Google Scholar] [CrossRef]
- Feng, D.; Bai, B.; Wang, H.; Suo, Y. Novel Fabrication of Biodegradable Superabsorbent Microspheres with Diffusion Barrier through Thermo-Chemical Modification and Their Potential Agriculture Applications for Water Holding and Sustained Release of Fertilizer. J. Agric. Food Chem. 2017, 65, 5896–5907. [Google Scholar] [CrossRef]
- Geicu-Cristea, M.; Popa, M.E.; Miteluţ, A.C.; Dănăilă-Guidea, S.M.; Neaţă, G.; Burnichi, R.; Tănase, E.E.; Drăghici, M.C.; Popescu, P.A. Research on Agriculture Applications of Some Newly Developed Biobased Products. Agric. Agric. Sci. Procedia 2016, 10, 485–493. [Google Scholar] [CrossRef]
- Puoci, F.; Iemma, F.; Spizzirri, U.G.; Cirillo, G.; Curcio, M.; Picci, N. Polymer in Agriculture: A Review. Am. J. Agric. Biol. Sci. 2008, 3, 299–314. [Google Scholar] [CrossRef]
- K, S.; Ngouajio, M. Polyethylene and biodegradable mulches for agricultural applications: A review. Agron. Sustain. Dev. 2012, 32, 501–529. [Google Scholar]
- Touchaleaume, F.; Martin-Closas, L.; Angellier-Coussy, H.; Chevillard, A.; Cesar, G.; Gontard, N.; Gastaldi, E. Performance and environmental impact of biodegradable polymers as agricultural mulching films. Chemosphere 2016, 144, 433–439. [Google Scholar] [CrossRef]
- Lubkowski, K.; Smorowska, A.; Grzmil, B.; Kozłowska, A. Controlled-Release Fertilizer Prepared Using a Biodegradable Aliphatic Copolyester of Poly(butylene succinate) and Dimerized Fatty Acid. J. Agric. Food Chem. 2015, 63, 2597–2605. [Google Scholar] [CrossRef]
- Yusoff, S.N.M.; Kamari, A.; Aljafree, N.F.A. A review of materials used as carrier agents in pesticide formulations. Int. J. Environ. Sci. Technol. 2016, 13, 2977–2994. [Google Scholar] [CrossRef]
- Ding, K.; Yi, Y.; Shi, L.; Zhang, L.; Zeng, T.; Yin, Y. Synthesis of photoresponsive polymeric propesticide micelles based on PEG for the controlled release of a herbicide. Polym. Chem. 2016, 7, 899–904. [Google Scholar] [CrossRef]
- Guan, W.; Zhang, W.; Tang, L.; Wang, Y.; Cui, H. Fabrication of novel avermectin nanoemulsion using polyurethane emulsifier with cleavable disulfide bonds. J. Agric. Food Chem. 2018, 66, 6569–6577. [Google Scholar] [CrossRef]
- Nuruzzaman, M.; Rahman, M.M.; Liu, Y.; Naidu, R. Nanoencapsulation, Nano-guard for Pesticides: A New Window for Safe Application. J. Agric. Food Chem. 2016, 64, 1447–1483. [Google Scholar] [CrossRef]
- Fenner, K.; Canonica, S.; Wackett, L.P.; Elsner, M. Evaluating pesticide degradation in the environment: Blind spots and emerging opportunities. Science 2013, 341, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Sheng, W.-B.; Li, W.; Tong, Y.-B.; Liu, Z.-Y.; Zhou, F. Adhesive Polydopamine Coated Avermectin Microcapsules for Prolonging Foliar Pesticide Retention. ACS Appl. Mater. Interfaces 2014, 6, 19552–19558. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, B.; Yang, F.; Wang, X.; Shen, H.; Wu, D. Preparation of uniform starch microcapsules by premix membrane emulsion for controlled release of avermectin. Carbohydr. Polym. 2016, 136, 341–349. [Google Scholar] [CrossRef]
- Nazir, A.; Schroën, K.; Boom, R. Premix emulsification: A review. J. Membr. Sci. 2010, 362, 1–11. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, Y.; Wang, L.; Hao, D.; Ma, G. Fabrication strategy for amphiphilic microcapsules with narrow size distribution by premix membrane emulsification. Colloids Surf. B Biointerfaces 2011, 87, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Kou, X.; Li, Q.; Lei, J.; Geng, L.; Deng, H.; Zhang, G.; Ma, G.; Su, Z.; Jiang, Q. Preparation of molecularly imprinted nanospheres by premix membrane emulsification technique. J. Membr. Sci. 2012, 417, 87–95. [Google Scholar] [CrossRef]
- Wei, Q.; Wei, W.; Lai, B.; Wang, L.-Y.; Wang, Y.-X.; Su, Z.-G.; Ma, G.-H. Uniform-sized PLA nanoparticles: Preparation by premix membrane emulsification. Int. J. Pharm. 2008, 359, 294–297. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, T.; Wang, Q.; Zhang, G.; Huo, J.; Huang, J.; Wang, L. Fabrication of uniform alginate-agarose microcapsules loading FeSO4 using water-oil-water-oil multiple emulsions system combined with premix membrane emulsification technique. Colloids Surfaces A Physicochem. Eng. Asp. 2016, 498, 128–138. [Google Scholar] [CrossRef]
- Wang, Y.; Ono, H.; Ikeda, A.; Hori, N.; Takemura, A.; Yamada, T.; Tsukatani, T. 1H NMR and 13C NMR investigations of sequence distribution and tacticity in poly(vinyl alcohol-co-vinyl levulinate). Polymer 2006, 47, 7827–7834. [Google Scholar] [CrossRef]
- Matsuda, H.; Asakura, T.; Miki, T. Triad Sequence Analysis of Poly(ethylene/butylene terephthalate) Copolymer Using1H NMR. Macromolecules 2002, 35, 4664–4668. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, Z.; Feng, Q.; Cui, F. The influence of soft segment length on the properties of poly(butylene terephthalate-co-succinate)-b-poly(ethylene glycol) segmented random copolymers. Eur. J. 2004, 40, 1297–1308. [Google Scholar] [CrossRef]
- Debuissy, T.; Pollet, E.; Avérous, L. Synthesis and characterization of biobased poly(butylene succinate- ran -butylene adipate). Analysis of the composition-dependent physicochemical properties. Eur. J. 2017, 87, 84–98. [Google Scholar] [CrossRef]
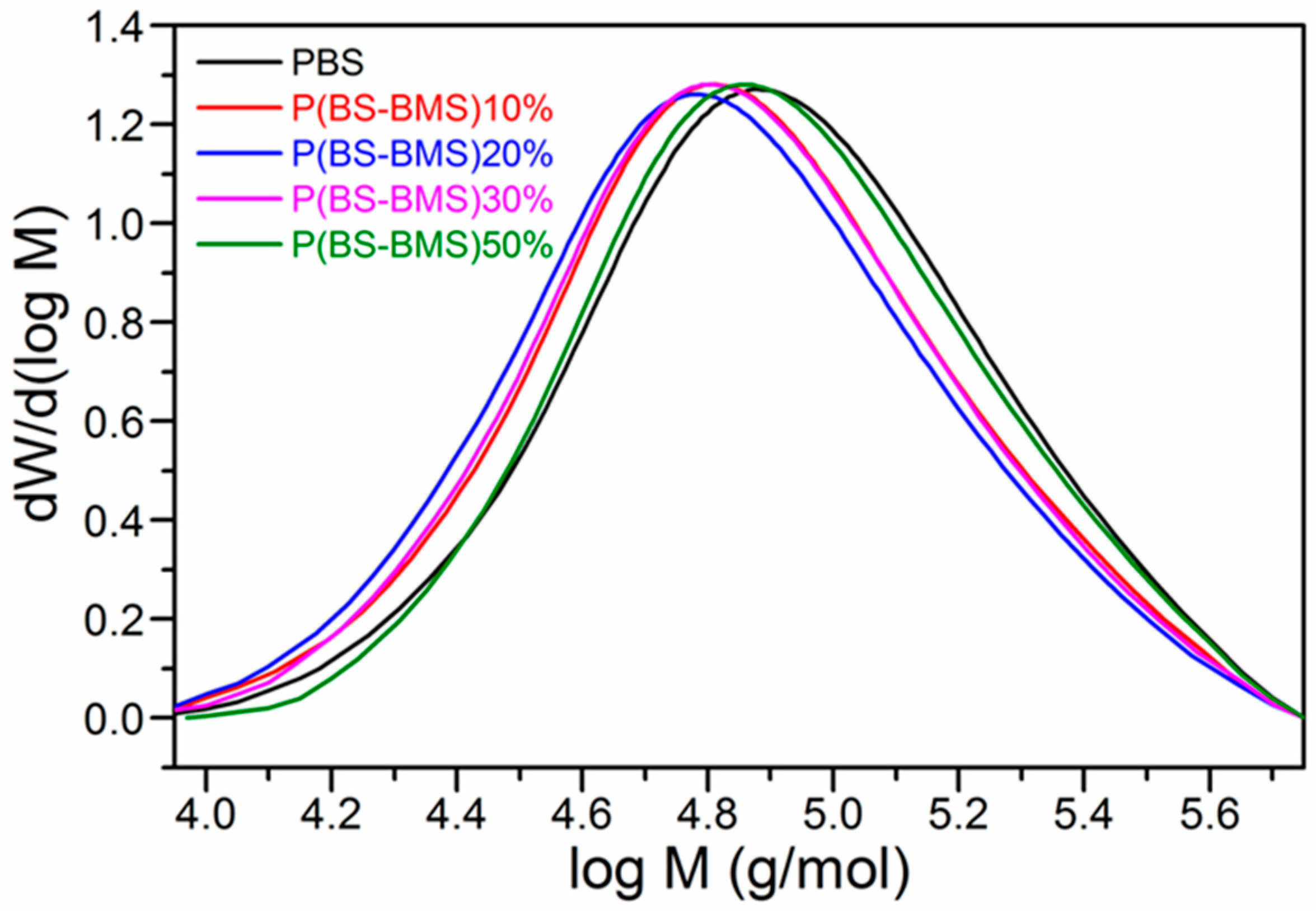





| Polymer | MSA (mol) | SA (mol) | BDO (mol) | Theoretical Content of PBMS (%) | Experimental Content of PBMS (%) | Mn (10−4 g/mol) | Mw (10−4 g/mol) | Đ |
|---|---|---|---|---|---|---|---|---|
| PBS | - | 0.2 | 0.24 | 0 | 0 | 6.3 | 10.7 | 1.70 |
| P(BS-BMS)10% | 0.02 | 0.18 | 0.24 | 10.0 | 9.5 | 5.5 | 9.5 | 1.73 |
| P(BS-BMS)20% | 0.04 | 0.16 | 0.24 | 20.0 | 20.3 | 5.1 | 9.0 | 1.76 |
| P(BS-BMS)30% | 0.06 | 0.14 | 0.24 | 30.0 | 29.1 | 5.5 | 9.4 | 1.71 |
| P(BS-BMS)50% | 0.1 | 0.1 | 0.24 | 50.0 | 48.5 | 6.4 | 10.5 | 1.64 |
| Copolymer | fSS (%) | fSMS (%) | fMSMS (%) | Ln,S-BDO | Ln,MS-BDO | R |
|---|---|---|---|---|---|---|
| P(BS-BMS)10% | 77.3 | 19.4 | 3.3 | 9.0 | 1.3 | 0.88 |
| P(BS-BMS)20% | 53.7 | 36.6 | 9.7 | 3.9 | 1.5 | 0.92 |
| P(BS-BMS)30% | 43.9 | 40.3 | 15.9 | 3.2 | 1.8 | 0.87 |
| P(BS-BMS)50% | 32.2 | 47.6 | 20.2 | 2.3 | 1.9 | 0.96 |
| Sample | Tm (°C) | ΔHm (J/g) | Tc (°C) | Xc (%)a | Thermal Degradation (°C) | |
|---|---|---|---|---|---|---|
| Td,5 | Td,max | |||||
| PBS | 112.0 | 64.3 | 79.4 | 58.2 | 337.7 | 398.3 |
| P(BS-BMS)10% | 102.8 | 54.2 | 64.6 | 49.1 | 340.0 | 397.6 |
| P(BS-BMS)20% | 91.6 | 48.0 | 43.4 | 43.5 | 339.9 | 399.4 |
| P(BS-BMS)30% | 82.4 | 41.0 | 35.4 | 37.1 | 339.5 | 398.1 |
| P(BS-BMS)50% | 58.3 | 0.2 | - | 0.2 | 337.1 | 399.1 |
| Sample | Size (μm) | PDI | Theoretical Loading Content (%) | Measured Loading Content (%) | Entrapment Efficiency (%) | n | r2 |
|---|---|---|---|---|---|---|---|
| PBS/Av-2/1 | 1.84 | 1.24 | 33.3 | 26.6 | 79.9 | 0.62 | 0.99 |
| P(BS-BMS)10%/Av-2/1 | 1.32 | 1.27 | 33.3 | 28.2 | 84.7 | 0.40 | 0.98 |
| P(BS-BMS)20%/Av-2/1 | 1.31 | 1.24 | 33.3 | 29.5 | 88.5 | 0.42 | 0.99 |
| P(BS-BMS)30%/Av-2/1 | 1.54 | 1.12 | 33.3 | 26.2 | 78.7 | 0.50 | 0.99 |
| P(BS-BMS)50%/Av-2/1 | 1.61 | 1.21 | 33.3 | 22.5 | 67.6 | 0.45 | 0.97 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, J.; Shi, J.; Xie, Z.; Xu, J.; Guo, B. Synthesis, Properties of Biodegradable Poly(Butylene Succinate-co-Butylene 2-Methylsuccinate) and Application for Sustainable Release. Materials 2019, 12, 1507. https://doi.org/10.3390/ma12091507
Han J, Shi J, Xie Z, Xu J, Guo B. Synthesis, Properties of Biodegradable Poly(Butylene Succinate-co-Butylene 2-Methylsuccinate) and Application for Sustainable Release. Materials. 2019; 12(9):1507. https://doi.org/10.3390/ma12091507
Chicago/Turabian StyleHan, Jiarui, Jiaxin Shi, Zhining Xie, Jun Xu, and Baohua Guo. 2019. "Synthesis, Properties of Biodegradable Poly(Butylene Succinate-co-Butylene 2-Methylsuccinate) and Application for Sustainable Release" Materials 12, no. 9: 1507. https://doi.org/10.3390/ma12091507
APA StyleHan, J., Shi, J., Xie, Z., Xu, J., & Guo, B. (2019). Synthesis, Properties of Biodegradable Poly(Butylene Succinate-co-Butylene 2-Methylsuccinate) and Application for Sustainable Release. Materials, 12(9), 1507. https://doi.org/10.3390/ma12091507