Thermal Properties of Composite Polymer Electrolytes Poly(Ethylene Oxide)/Sodium Trifluoroacetate/Aluminum Oxide (PEO)10CF3COONa + x wt.% Al2O3
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mindemark, J.; Lacey, M.J.; Bowden, T.; Brandell, D. Beyond PEO—Alternative host materials for Li+-conducting solid polymer electrolytes. Prog. Polym. Sci. 2018, 81, 114–143. [Google Scholar] [CrossRef]
- Judez, X.; Zhang, H.; Li, C.; Eshetu, G.G.; González-Marcos, J.A.; Armand, M.; Rodriguez-Martinez, L.M. Solid Electrolytes for Safe and High Energy Density Lithium-Sulfur Batteries: Promises and Challenges. J. Electrochem. Soc. 2018, 165, A6008–A6016. [Google Scholar] [CrossRef]
- Jinisha, B.; Anilkumar, K.M.; Manoj, M.; Abhilash, A.; Pradeep, V.S.; Jayalekshmi, S. Poly (ethylene oxide) (PEO)-based, sodium ion-conducting‚ solid polymer electrolyte films, dispersed with Al2O3 filler, for applications in sodium ion cells. Ionics 2018, 24, 1675–1683. [Google Scholar] [CrossRef]
- Delgado, I.; Castillo, J.; Chacón, M.; Vargas, R.A. Ionic conductivity in the polymer electrolytes PEO/CF3COONa. Phys. Status Solidi B 2000, 220, 625–629. [Google Scholar] [CrossRef]
- ElBellihi, A.A.; Bayoumy, W.A.; Masoud, E.M.; Mousa, M.A. Preparation, Characterizations and Conductivity of Composite Polymer Electrolytes. Polym. Nano Compos. Electrolytes 2012, 33, 1–6. [Google Scholar]
- Liang, B.; Tang, S.; Jiang, Q.; Chen, C.; Chen, X.; Li, S.; Yan, X. Preparation and characterization of PEO-PMMA polymer composite electrolytes doped with nano-Al2O3. Electrochim. Acta 2015, 169, 334–341. [Google Scholar] [CrossRef]
- Klongkan, S.; Pumchusak, J. Effects of Nano Alumina and Plasticizers on Morphology, Ionic Conductivity, Thermal and Mechanical Properties of PEO-LiCF3SO3 Solid Polymer Electrolyte. Electrochim. Acta 2015, 161, 171–176. [Google Scholar] [CrossRef]
- Agrawal, A.; Satapathy, A. Thermal and dielectric behaviour of polypropylene composites reinforced with ceramic fillers. J. Mater. Sci. Mater. Electron. 2015, 26, 103–112. [Google Scholar] [CrossRef]
- Rybalko, V.P.; Nikityuk, A.I.; Pisarenko, E.I.; Kuznetsova, T.I.; D’yachenko, P.B.; Guseinov, S.L.; Malashin, A.S.; Korchmarek, A.S.; Kireev, V.V. Effect of inorganic nanopowders on properties of acrylic composites. Russ. J. Appl. Chem. 2015, 88, 826–832. [Google Scholar] [CrossRef]
- Jurado-Meneses, N.M.; Delgado-Rosero, M.I.; Meléndez-Lira, M.A. Structural and vibrational studies on composites polymer electrolytes (PEO)10CF3COONa + x wt.% Al2O3. Rev. Fac. Ing. Univ. Antioquia 2017, 83, 43–49. [Google Scholar] [CrossRef]
- Jurado Meneses, N.M.; Delgado Rosero, M.I.; Vargas Zapata, R.A. Conductividad iónica en nuevos compositos (PEO)10 (CF3COONa)-X % Al2O3. Univ. Sci. 2013, 18, 173–180. [Google Scholar] [CrossRef]
- Ahn, J.H.; Wang, G.X.; Liu, H.K.; Dou, S.X. Nanoparticle-dispersed PEO polymer electrolytes for Li batteries. J. Power Sources 2003, 119, 422–426. [Google Scholar] [CrossRef]
- Köster, T.K.J.; van Wüllen, L. Cation-anion coordination, ion mobility and the effect of Al2O3 addition in PEO based polymer electrolytes. Solid State Ionics 2010, 181, 489–495. [Google Scholar] [CrossRef]
- Vijayalekshmi, V.; Khastgir, D. Chitosan/partially sulfonated poly(vinylidene fluoride) blends as polymer electrolyte membranes for direct methanol fuel cell applications. Cellulose 2018, 25, 661–681. [Google Scholar] [CrossRef]
- Yang, R.; Zhang, S.; Zhang, L.; Bi, X. Effects of LiClO4 on the Characteristics and Ionic Conductivity of the Solid Polymer Electrolytes Composed of PEO, LiClO4 and PLiAA. Mater. Sci. Forum 2013, 743, 53–58. [Google Scholar] [CrossRef]
- Gurusiddappa, J.; Madhuri, W.; Suvarna, R.P.; Dasan, K.P. Studies on the morphology and conductivity of PEO/LiClO4. Mater. Today Proc. 2016, 3, 1451–1459. [Google Scholar] [CrossRef]
- Martinez-cisneros, C.S.; Levenfeld, B.; Varez, A.; Sanchez, J.Y. Development of sodium-conducting polymer electrolytes : comparison between fi lm-casting and fi lms obtained via green processes. Electrochim. Acta 2016, 192, 456–466. [Google Scholar] [CrossRef]
- Delgado, M.I.; Jurado, N.M.; Vargas, R.A. Phase diagram of polymer electrolyte: (x)(PEO)–(1−x)CF3COOLi. Rev. Fac. Ing. Univ. Antioquia 2012, 62, 77–82. [Google Scholar]
- Wu, X.L.; Xin, S.; Seo, H.H.; Kim, J.; Guo, Y.G.; Lee, J.S. Enhanced Li+ conductivity in PEO–LiBOB polymer electrolytes by using succinonitrile as a plasticizer. Solid State Ionics 2011, 186, 1–6. [Google Scholar] [CrossRef]
- Polu, A.R.; Rhee, H.W. Nanocomposite solid polymer electrolytes based on poly(ethylene oxide)/POSS-PEG (n = 13.3) hybrid nanoparticles for lithium ion batteries. J. Ind. Eng. Chem. 2015, 31, 323–329. [Google Scholar] [CrossRef]
- Langer, F.; Bardenhagen, I.; Glenneberg, J.; Kun, R. Microstructure and temperature dependent lithium ion transport of ceramic–polymer composite electrolyte for solid-state lithium ion batteries based on garnet-type Li7La3Zr2O. Solid State Ionics 2016, 291, 8–13. [Google Scholar] [CrossRef]
- Polu, A.R.; Kumar, R. Mg2+-ion conducting poly(ethylene glycol)-TiO2 composite polymer electrolytes for solid-state batteries. Mater. Express 2014, 4, 79–84. [Google Scholar] [CrossRef]
- Rajesh Kumar, S.; Juan, C.H.; Liao, G.M.; Lin, J.S.; Yang, C.C.; Ma, W.T.; You, J.H.; Jessie Lue, S. Fumed Silica Nanoparticles Incorporated in Quaternized Poly(Vinyl Alcohol) Nanocomposite Membrane for Enhanced Power Densities in Direct Alcohol Alkaline Fuel Cells. Energies 2016, 9, 15. [Google Scholar] [CrossRef]
- Saikia, D.; Chen-Yang, Y.W.; Chen, Y.T.; Li, Y.K.; Lin, S.I. Investigation of ionic conductivity of composite gel polymer electrolyte membranes based on P(VDF-HFP), LiClO4 and silica aerogel for lithium ion battery. Desalination 2008, 234, 24–32. [Google Scholar] [CrossRef]
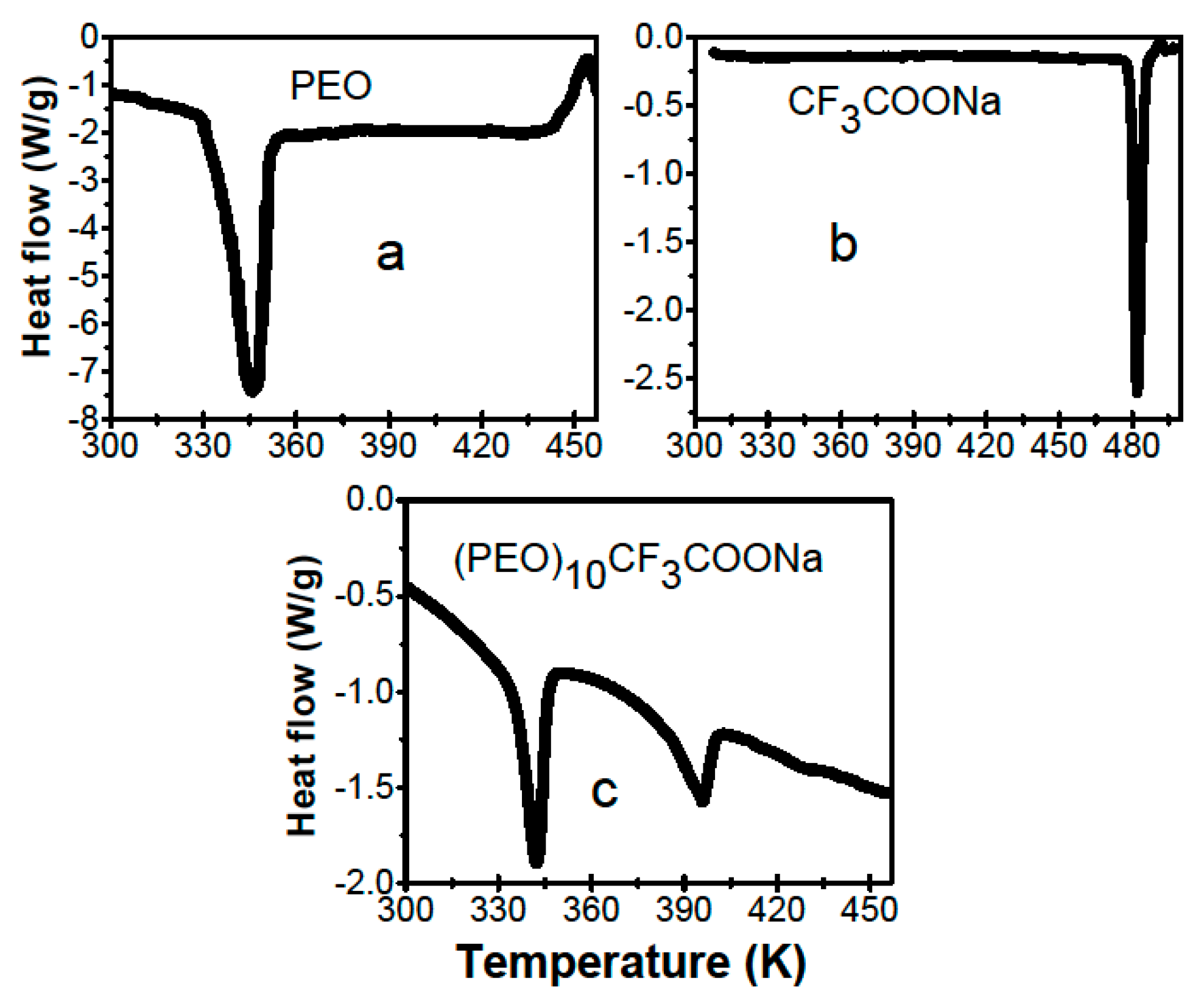
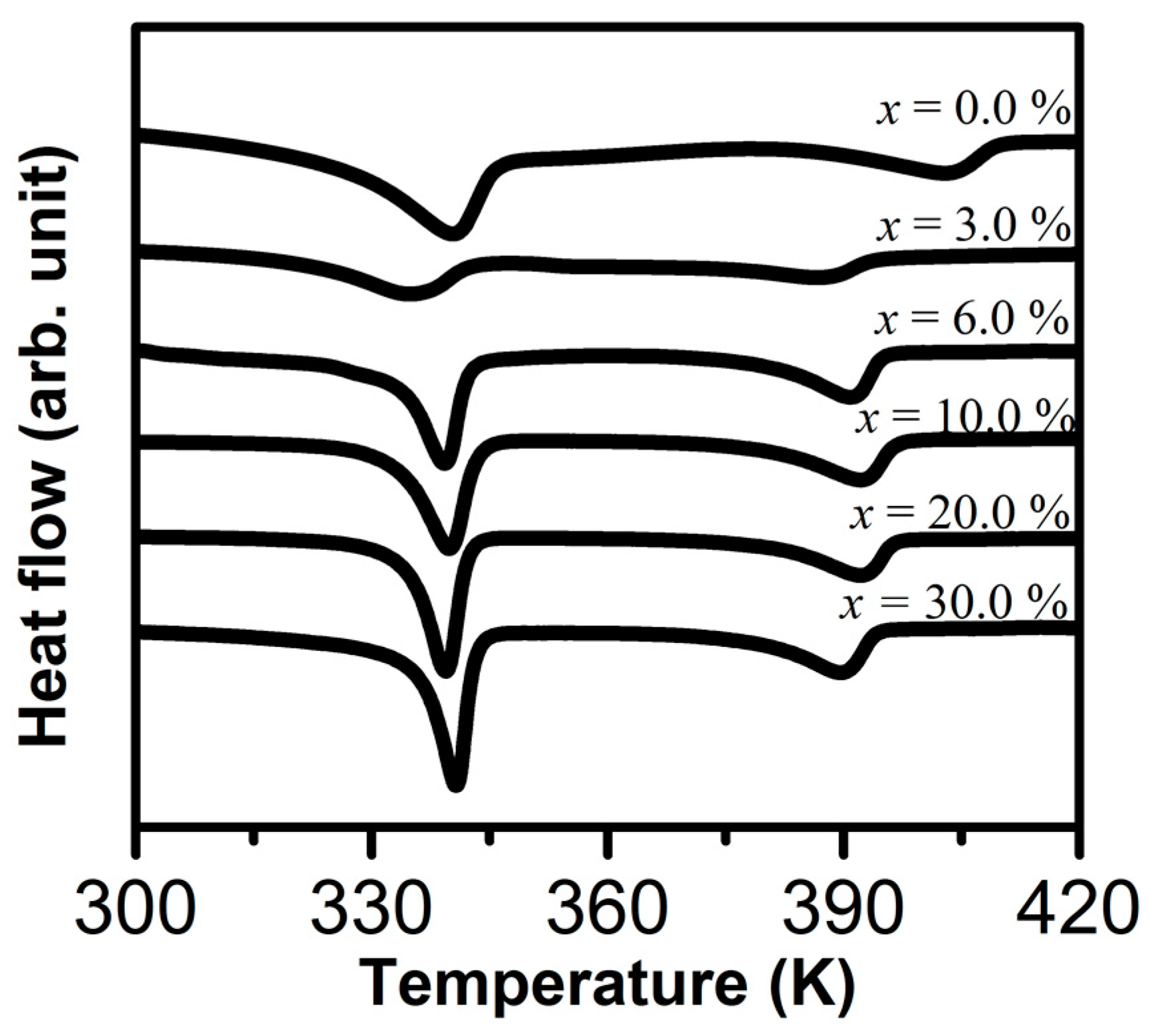


| x wt.% Al2O3 | Melting Temperature Tm (K) | Enthalpy ΔHm (J/g) | Crystallinity χc (%) |
|---|---|---|---|
| 0.0 | 319.54 | 67.70 | 33.3 |
| 3.0 | 320.30 | 26.61 | 13.1 |
| 6.0 | 332.19 | 53.68 | 26.4 |
| 10.0 | 332.06 | 56.42 | 27.8 |
| 20.0 | 332.10 | 58.71 | 28.9 |
| 30.0 | 335.28 | 62.43 | 30.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delgado Rosero, M.I.; Jurado Meneses, N.M.; Uribe Kaffure, R. Thermal Properties of Composite Polymer Electrolytes Poly(Ethylene Oxide)/Sodium Trifluoroacetate/Aluminum Oxide (PEO)10CF3COONa + x wt.% Al2O3. Materials 2019, 12, 1464. https://doi.org/10.3390/ma12091464
Delgado Rosero MI, Jurado Meneses NM, Uribe Kaffure R. Thermal Properties of Composite Polymer Electrolytes Poly(Ethylene Oxide)/Sodium Trifluoroacetate/Aluminum Oxide (PEO)10CF3COONa + x wt.% Al2O3. Materials. 2019; 12(9):1464. https://doi.org/10.3390/ma12091464
Chicago/Turabian StyleDelgado Rosero, Miguel I., Nori M. Jurado Meneses, and Ramiro Uribe Kaffure. 2019. "Thermal Properties of Composite Polymer Electrolytes Poly(Ethylene Oxide)/Sodium Trifluoroacetate/Aluminum Oxide (PEO)10CF3COONa + x wt.% Al2O3" Materials 12, no. 9: 1464. https://doi.org/10.3390/ma12091464
APA StyleDelgado Rosero, M. I., Jurado Meneses, N. M., & Uribe Kaffure, R. (2019). Thermal Properties of Composite Polymer Electrolytes Poly(Ethylene Oxide)/Sodium Trifluoroacetate/Aluminum Oxide (PEO)10CF3COONa + x wt.% Al2O3. Materials, 12(9), 1464. https://doi.org/10.3390/ma12091464





