A Soft Polydimethylsiloxane Liquid Metal Interdigitated Capacitor Sensor and Its Integration in a Flexible Hybrid System for On-Body Respiratory Sensing
Abstract
1. Introduction
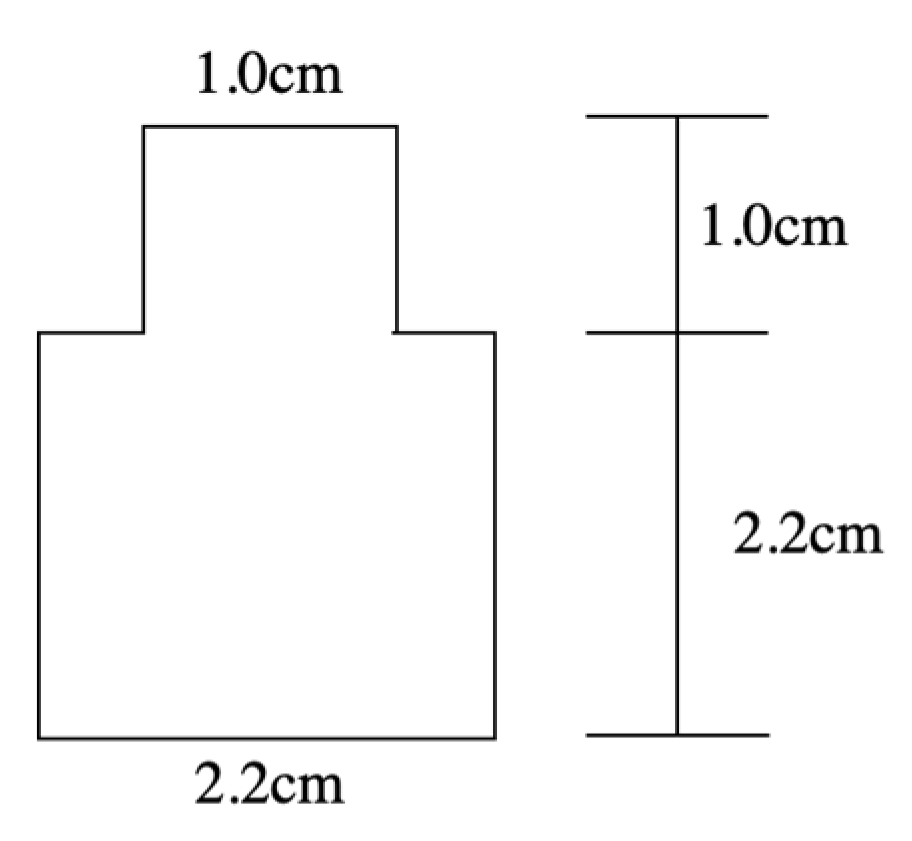
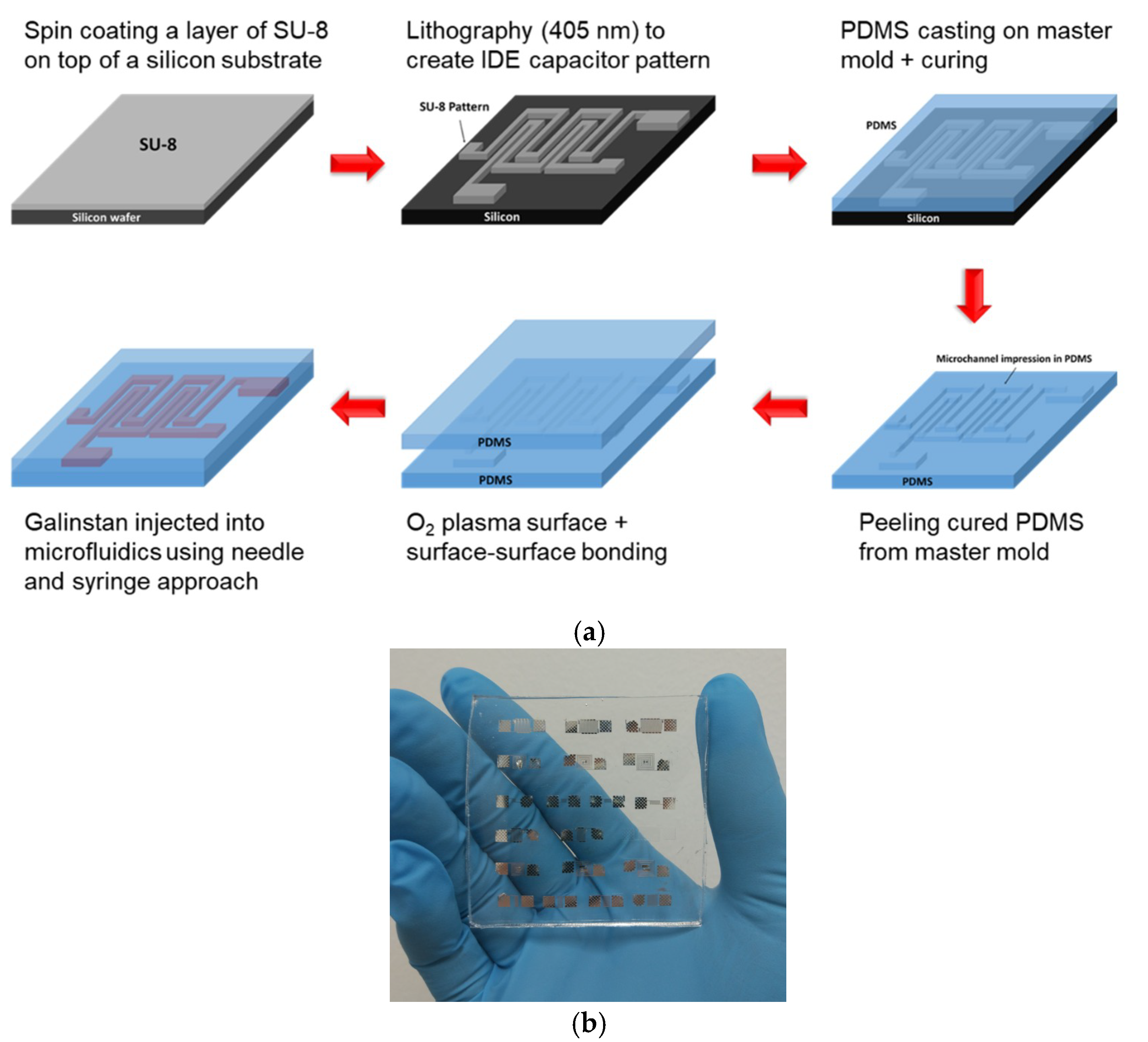
2. Fabrication Process of Soft PDMS-Liquid Metal IDE Capacitor
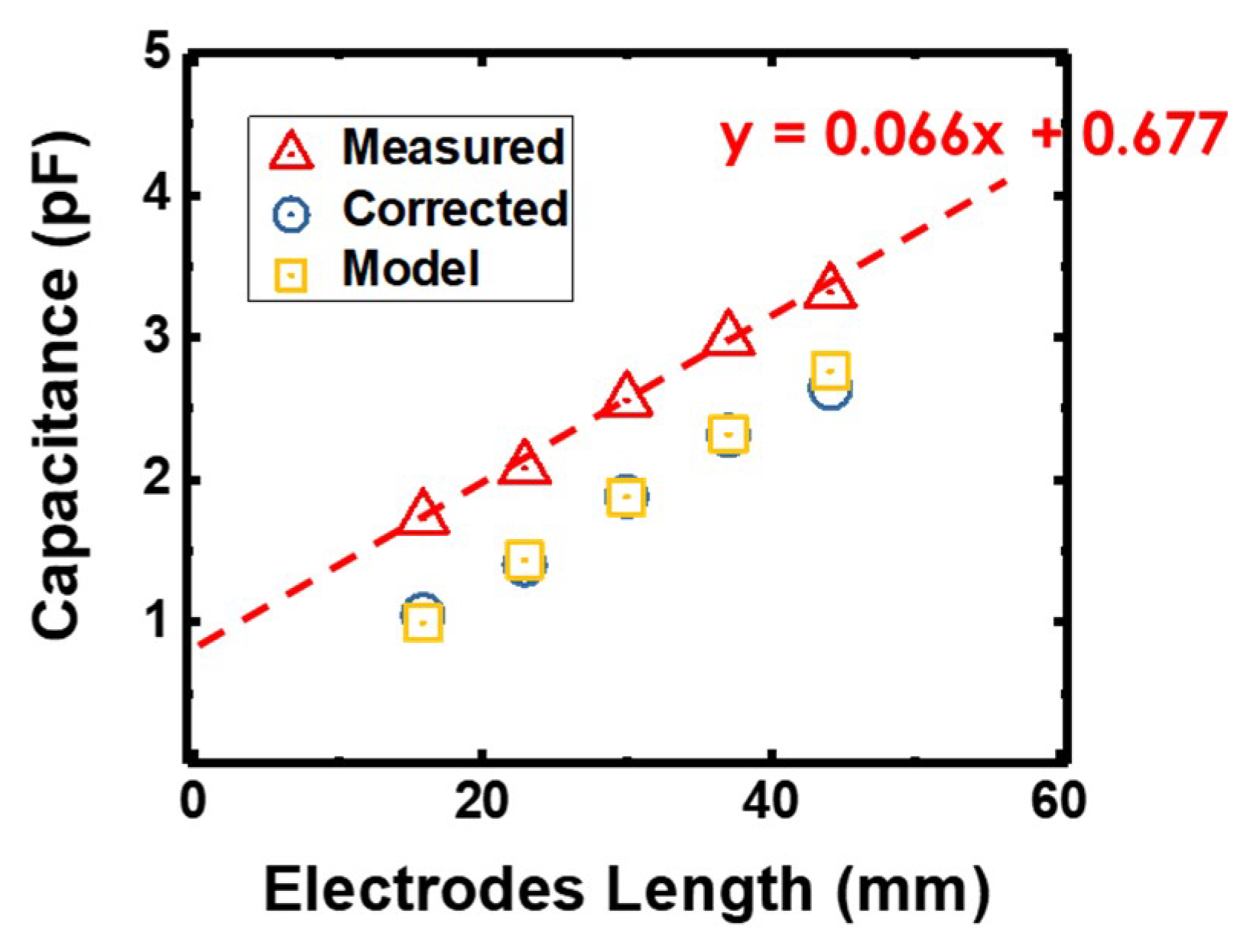
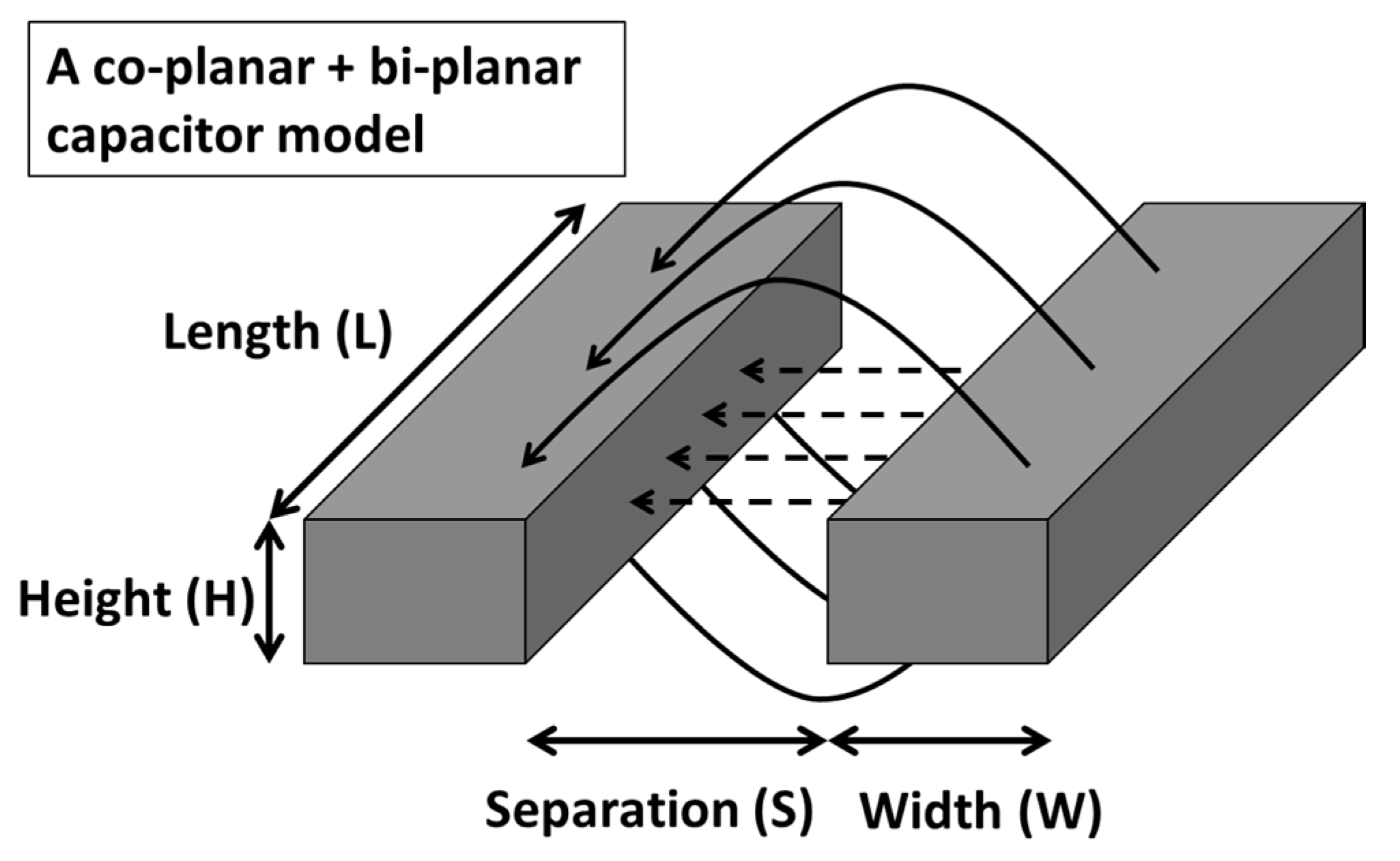
3. Electrical Characterization and Modelling of a Static IDE Capacitor
3.1. Functional Test—Strain Effect
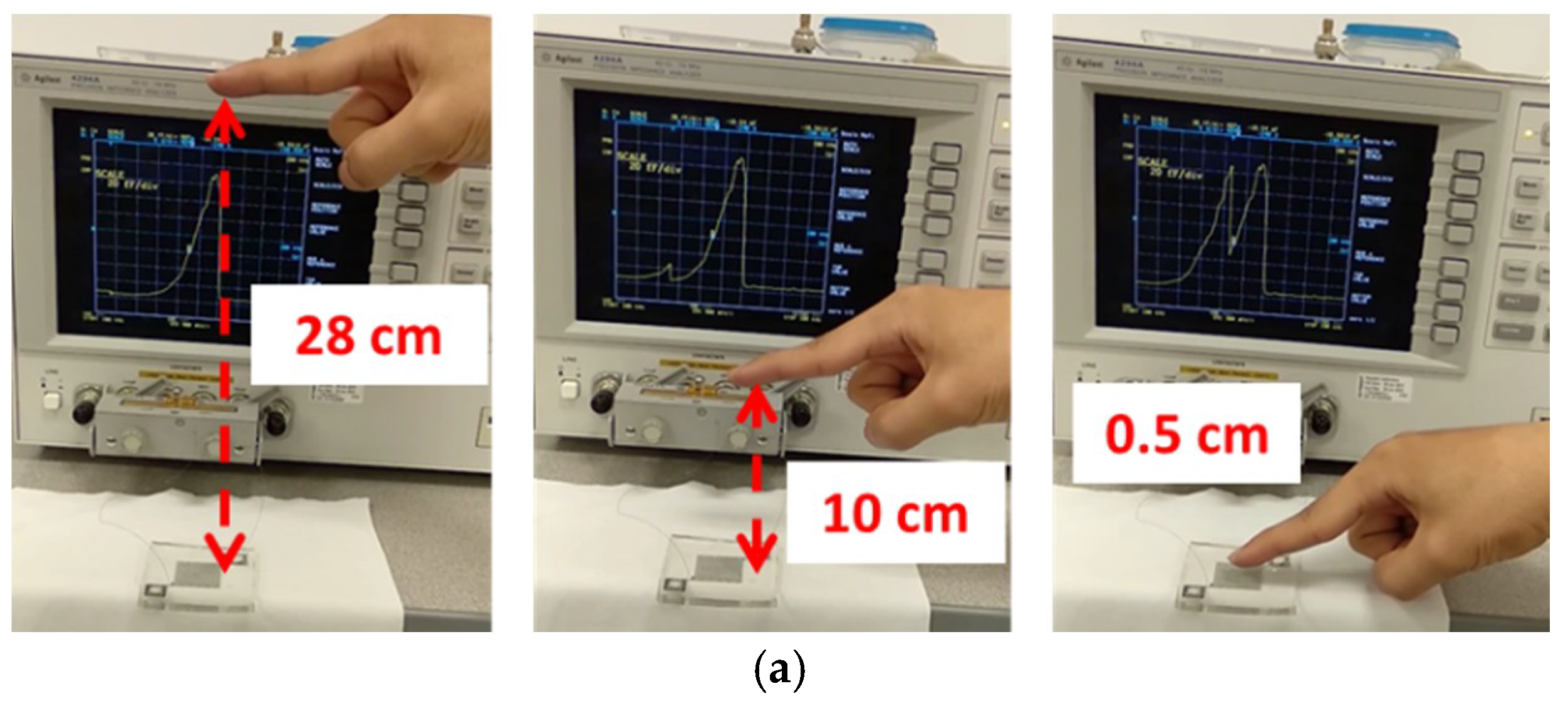
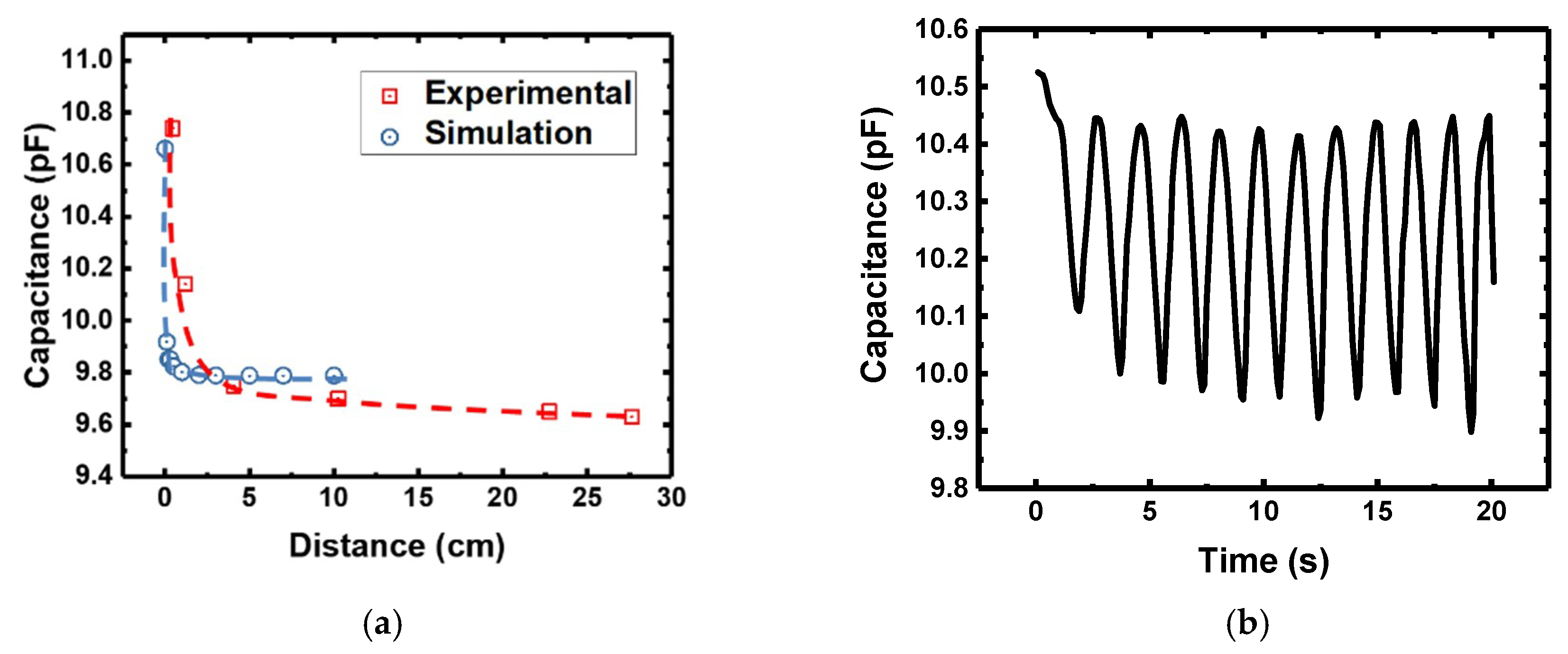
3.2. Proximity Effect
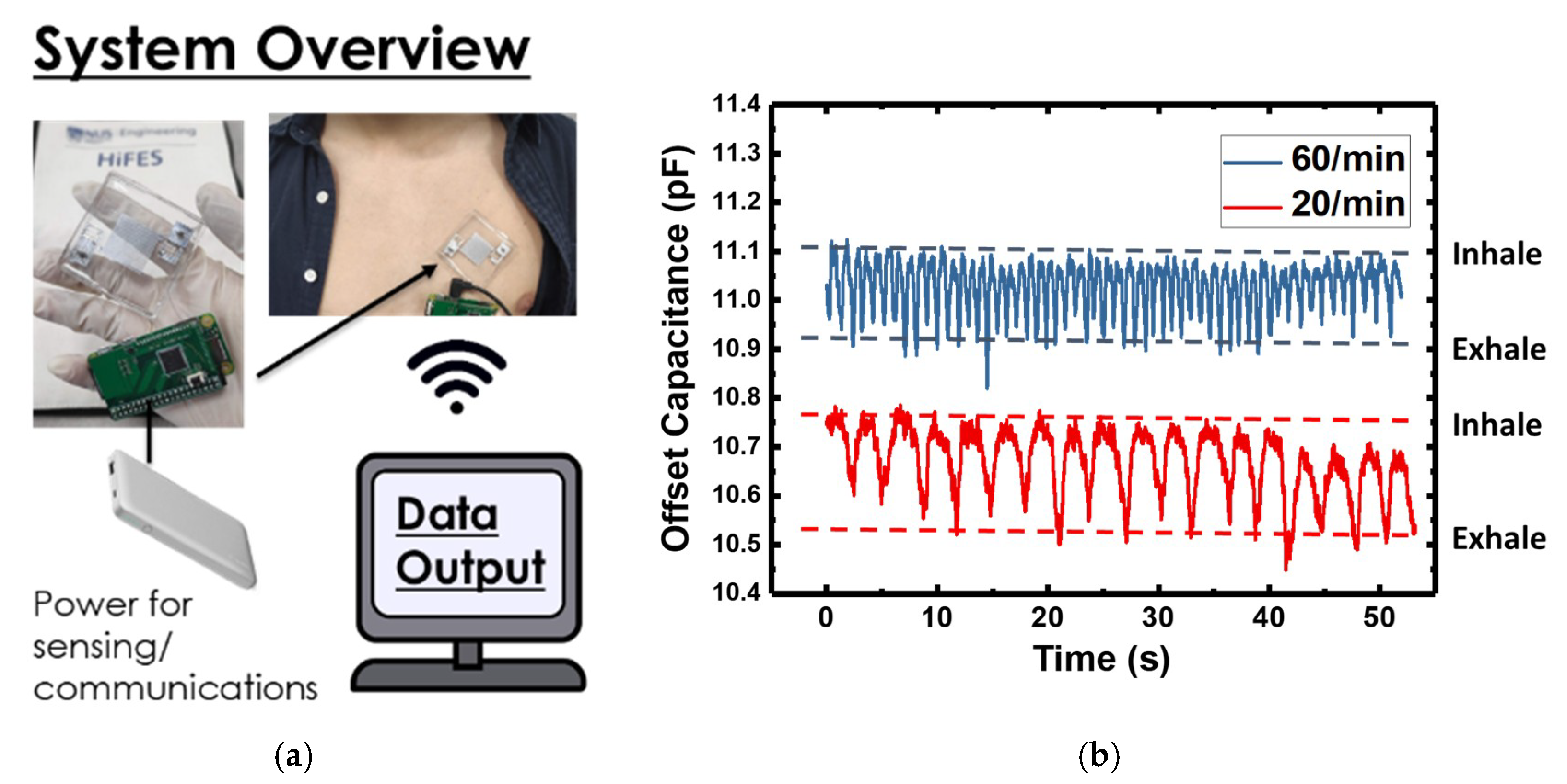
4. Demonstration of an IDE Capacitor Based Flexible Hybrid Respiratory System
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
References
- So, J.-H.; Thelen, J.; Qusba, A.; Hayes, G.J.; Lazzi, G.; Dickey, M.D. Reversibly deformable and mechanically tunable fluidic antennas. Adv. Funct. Mater. 2009, 19, 3632–3637. [Google Scholar] [CrossRef]
- Kim, J.; Banks, A.; Xie, Z.; Heo, S.Y.; Gutruf, P.; Lee, J.W.; Xu, S.; Jang, K.-I.; Liu, F.; Brown, G.; et al. Miniaturized flexible electronic systems with wireless power and near-field communication capabilities. Adv. Funct. Mater. 2015, 25, 4761–4767. [Google Scholar] [CrossRef]
- Roberts, P.; Damian, D.D.; Shan, W.; Lu, T.; Majidi, C. Soft-matter capacitive sensor for measuring shear and pressure deformation. In Proceedings of the 2013 IEEE International Conference on Robotics and Automation, Karlsruhe, Germany, 6–10 May 2013; pp. 3529–3534. [Google Scholar]
- Miyamoto, A.; Lee, S.; Cooray, N.F.; Lee, S.; Mori, M.; Matsuhisa, N.; Jin, H.; Yoda, L.; Yokota, T.; Itoh, A.; et al. Inflammation-free, gas-permeable, lightweight, stretchable on-skin electronics with nanomeshes. Nat. Nanotechnol. 2017, 12, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhang, Y.; Jia, L.; Mathewson, K.E.; Jang, K.-I.; Kim, J.; Fu, H.; Huang, X.; Chava, P.; Wang, R.; et al. Soft microfluidic assemblies of sensors, circuits, and radios for the skin. Science 2014, 344, 70–74. [Google Scholar] [CrossRef]
- Rogers, J.; Malliaras, G.; Someya, T. Biomedical devices go wild. Science Advances 2018, 4, eaav1889. [Google Scholar] [CrossRef] [PubMed]
- Khan, Y.; Garg, M.; Gui, Q.; Schadt, M.; Gaikwad, A.; Han, D.; Yamamoto, N.A.D.; Hart, P.; Welte, R.; Wilson, W.; et al. Flexible hybrid electronics: Direct interfacing of soft and hard electronics for wearable health monitoring. Adv. Funct. Mater. 2016, 26, 8764–8775. [Google Scholar] [CrossRef]
- Sheng, L.; Teo, S.; Liu, J. Liquid-metal-painted stretchable capacitor sensors for wearable healthcare electronics. J. Med Boil. Eng. 2016, 36, 265–272. [Google Scholar] [CrossRef]
- Li, Y.; Luo, Y.; Nayak, S.; Liu, Z.; Chichvarina, O.; Zamburg, E.; Zhang, X.; Liu, Y.; Heng, C.H.; Thean, A.V.-Y. A stretchable-hybrid low-power monolithic ecg patch with microfluidic liquid-metal interconnects and stretchable carbon-black nanocomposite electrodes for wearable heart monitoring. Adv. Electron. Mater. 2019, 5, 1800463. [Google Scholar] [CrossRef]
- Shay, T.; Velev, O.D.; Dickey, M.D. Soft electrodes combining hydrogel and liquid metal. Soft Matter 2018, 14, 3296–3303. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Matsuhisa, N.; Lee, S.; Abbas, M.; Yokota, T.; Someya, T. Enhancing the performance of stretchable conductors for e-textiles by controlled ink permeation. Adv. Mater. 2017, 29, 1605848. [Google Scholar] [CrossRef]
- Cooper, C.B.; Arutselvan, K.; Liu, Y.; Armstrong, D.; Lin, Y.; Khan, M.R.; Genzer, J.; Dickey, M.D. Stretchable capacitive sensors of torsion, strain, and touch using double helix liquid metal fibers. Adv. Funct. Mater. 2017, 27, 1605630. [Google Scholar] [CrossRef]
- Gao, W.; Emaminejad, S.; Nyein, H.Y.Y.; Challa, S.; Chen, K.; Peck, A.; Fahad, H.M.; Ota, H.; Shiraki, H.; Kiriya, D.; et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 2016, 529, 509–514. [Google Scholar] [CrossRef]
- Liu, Y.; Pharr, M.; Salvatore, G.A. Lab-on-skin: A review of flexible and stretchable electronics for wearable health monitoring. ACS Nano 2017, 11, 9614–9635. [Google Scholar] [CrossRef] [PubMed]
- Herbert, R.; Kim, J.-H.; Kim, Y.S.; Lee, H.M.; Yeo, W.-H. Soft material-enabled, flexible hybrid electronics for medicine, healthcare, and human-machine interfaces. Materials 2018, 11, 187. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.E.; Rivnay, J.; Whiting, G.L.; Mei, P.; Zhang, Y.; Krusor, B.; Kor, S.; Daniel, G.; Ready, S.E.; Veres, J.; et al. Flexible hybrid electronic circuits and systems. IEEE J. Emerg. Sel. Top. Circuits Syst. 2017, 7, 27–37. [Google Scholar] [CrossRef]
- Sears, N.C.; Berrigan, J.D.; Buskohl, P.R.; Harne, R.L. Dynamic response of flexible hybrid electronic material systems. Compos. Struct. 2019, 208, 377–384. [Google Scholar] [CrossRef]
- Tong, G.; Jia, Z.; Chang, J. Flexible hybrid electronics: Review and challenges. In Proceedings of the 2018 IEEE International Symposium on Circuits and Systems (ISCAS), Florence, Italy, 27–30 May 2018; pp. 1–5. [Google Scholar]
- Abu-Khalaf, J.M.; Al-Ghussain, L.; Al-Halhouli, A.a. Fabrication of stretchable circuits on polydimethylsiloxane (pdms) pre-stretched substrates by inkjet printing silver nanoparticles. Materials 2018, 11, 2377. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.A.; Someya, T.; Huang, Y. Materials and mechanics for stretchable electronics. Science 2010, 327, 1603–1607. [Google Scholar] [CrossRef] [PubMed]
- Matsuhisa, N.; Inoue, D.; Zalar, P.; Jin, H.; Matsuba, Y.; Itoh, A.; Yokota, T.; Hashizume, D.; Someya, T. Printable elastic conductors by in situ formation of silver nanoparticles from silver flakes. Nat. Mater. 2017, 16, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, N.; Meyer, C.D.; Bedair, S.S.; Nochetto, H.; Kierzewski, I.M. Multilayer liquid metal stretchable inductors. Smart Mater. Struct. 2014, 23, 085036. [Google Scholar] [CrossRef]
- Fassler, A.; Majidi, C. Soft-matter capacitors and inductors for hyperelastic strain sensing and stretchable electronics. Smart Mater. Struct. 2013, 22, 055023. [Google Scholar] [CrossRef]
- Staginus, J.; Aerts, I.M.; Chang, Z.-y.; Meijer, G.C.M.; de Smet, L.C.P.M.; Sudhölter, E.J.R. Capacitive response of pdms-coated ide platforms directly exposed to aqueous solutions containing volatile organic compounds. Sensors Actuators B: Chem. 2013, 184, 130–142. [Google Scholar] [CrossRef]
- Ma, M.; Wang, Y.; Liu, F.; Zhang, F.; Liu, Z.; Li, Y. Passive wireless LC proximity sensor based on LTCC technology. Sensors 2019, 19, 1110. [Google Scholar] [CrossRef]
- Smith, J.; White, T.; Dodge, C.; Paradiso, J.; Gershenfeld, N.; Allport, D. Electric field sensing for graphical interfaces. IEEE Comput. Graph. Appl. 1998, 18, 54–60. [Google Scholar] [CrossRef]
- Dickey, M.D. Stretchable and soft electronics using liquid metals. Adv. Mater. 2017, 29, 1606425. [Google Scholar] [CrossRef]
- Zhang, B.; Dong, Q.; Korman, C.E.; Li, Z.; Zaghloul, M.E. Flexible packaging of solid-state integrated circuit chips with elastomeric microfluidics. Sci. Rep. 2013, 3, 1098. [Google Scholar] [CrossRef]
- Luo, Y.; Heng, C. An 8.2 µw 0.14 mm2 16-channel cdma-like period modulation capacitance-to-digital converter with reduced data throughput. In Proceedings of the 2018 IEEE Symposium on VLSI Circuits, Honolulu, HI, USA, 18–22 June 2018; pp. 165–166. [Google Scholar]
- Green Marques, D.; Alhais Lopes, P.; de Almeida, A.; Majidi, C.; Tavakoli, M. Reliable interfaces for egain multi-layer stretchable circuits and microelectronics. Lab Chip 2019, 19, 897–906. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Nayak, S.; Luo, Y.; Liu, Y.; Salila Vijayalal Mohan, H.K.; Pan, J.; Liu, Z.; Heng, C.H.; Thean, A.V.-Y. A Soft Polydimethylsiloxane Liquid Metal Interdigitated Capacitor Sensor and Its Integration in a Flexible Hybrid System for On-Body Respiratory Sensing. Materials 2019, 12, 1458. https://doi.org/10.3390/ma12091458
Li Y, Nayak S, Luo Y, Liu Y, Salila Vijayalal Mohan HK, Pan J, Liu Z, Heng CH, Thean AV-Y. A Soft Polydimethylsiloxane Liquid Metal Interdigitated Capacitor Sensor and Its Integration in a Flexible Hybrid System for On-Body Respiratory Sensing. Materials. 2019; 12(9):1458. https://doi.org/10.3390/ma12091458
Chicago/Turabian StyleLi, Yida, Suryakanta Nayak, Yuxuan Luo, Yijie Liu, Hari Krishna Salila Vijayalal Mohan, Jieming Pan, Zhuangjian Liu, Chun Huat Heng, and Aaron Voon-Yew Thean. 2019. "A Soft Polydimethylsiloxane Liquid Metal Interdigitated Capacitor Sensor and Its Integration in a Flexible Hybrid System for On-Body Respiratory Sensing" Materials 12, no. 9: 1458. https://doi.org/10.3390/ma12091458
APA StyleLi, Y., Nayak, S., Luo, Y., Liu, Y., Salila Vijayalal Mohan, H. K., Pan, J., Liu, Z., Heng, C. H., & Thean, A. V.-Y. (2019). A Soft Polydimethylsiloxane Liquid Metal Interdigitated Capacitor Sensor and Its Integration in a Flexible Hybrid System for On-Body Respiratory Sensing. Materials, 12(9), 1458. https://doi.org/10.3390/ma12091458





