Use of Superelastic Nitinol and Highly-Stretchable Latex to Develop a Tongue Prosthetic Assist Device and Facilitate Swallowing for Dysphagia Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Superelastic Nitinol and Acrylic Resin
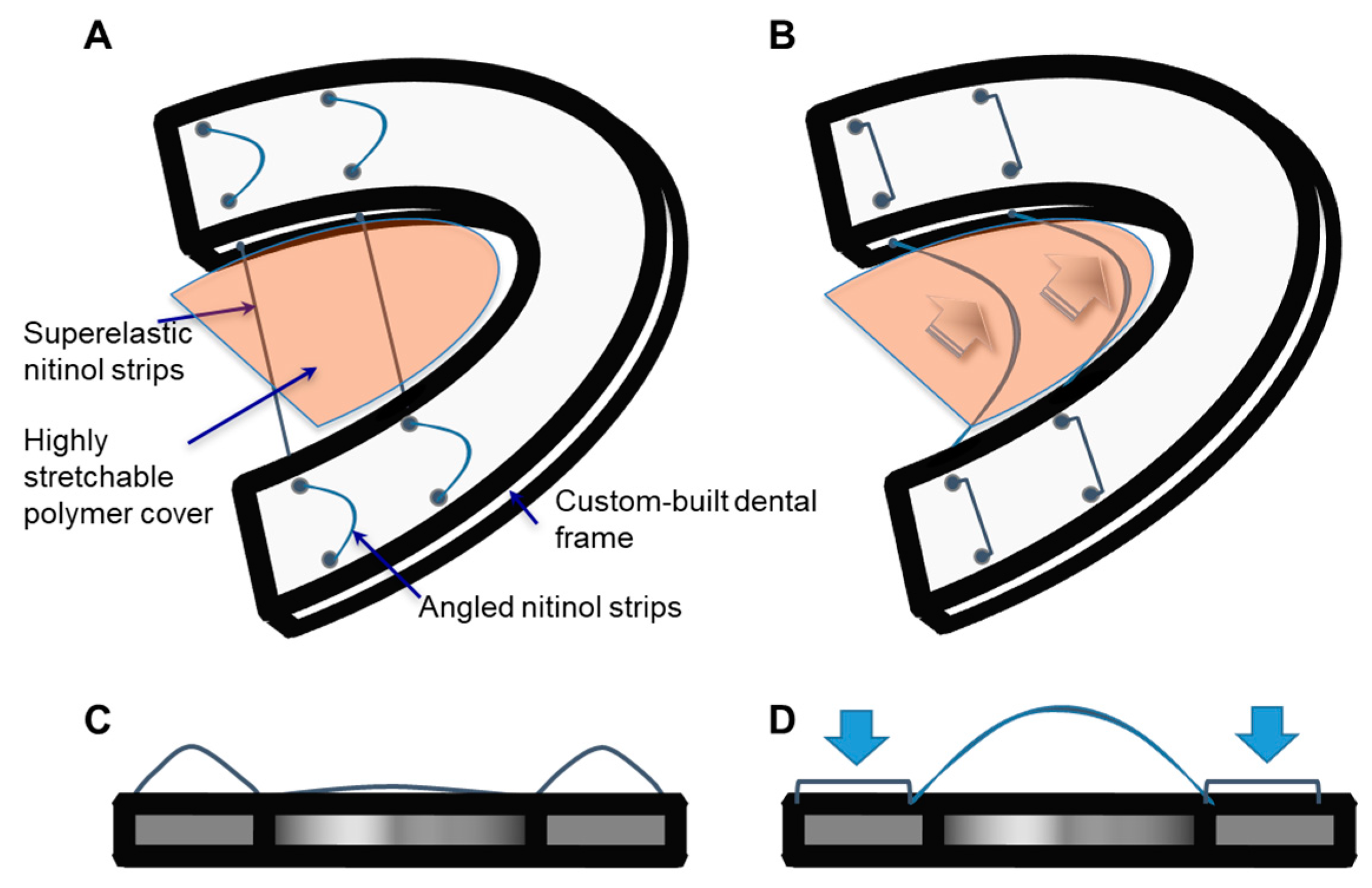
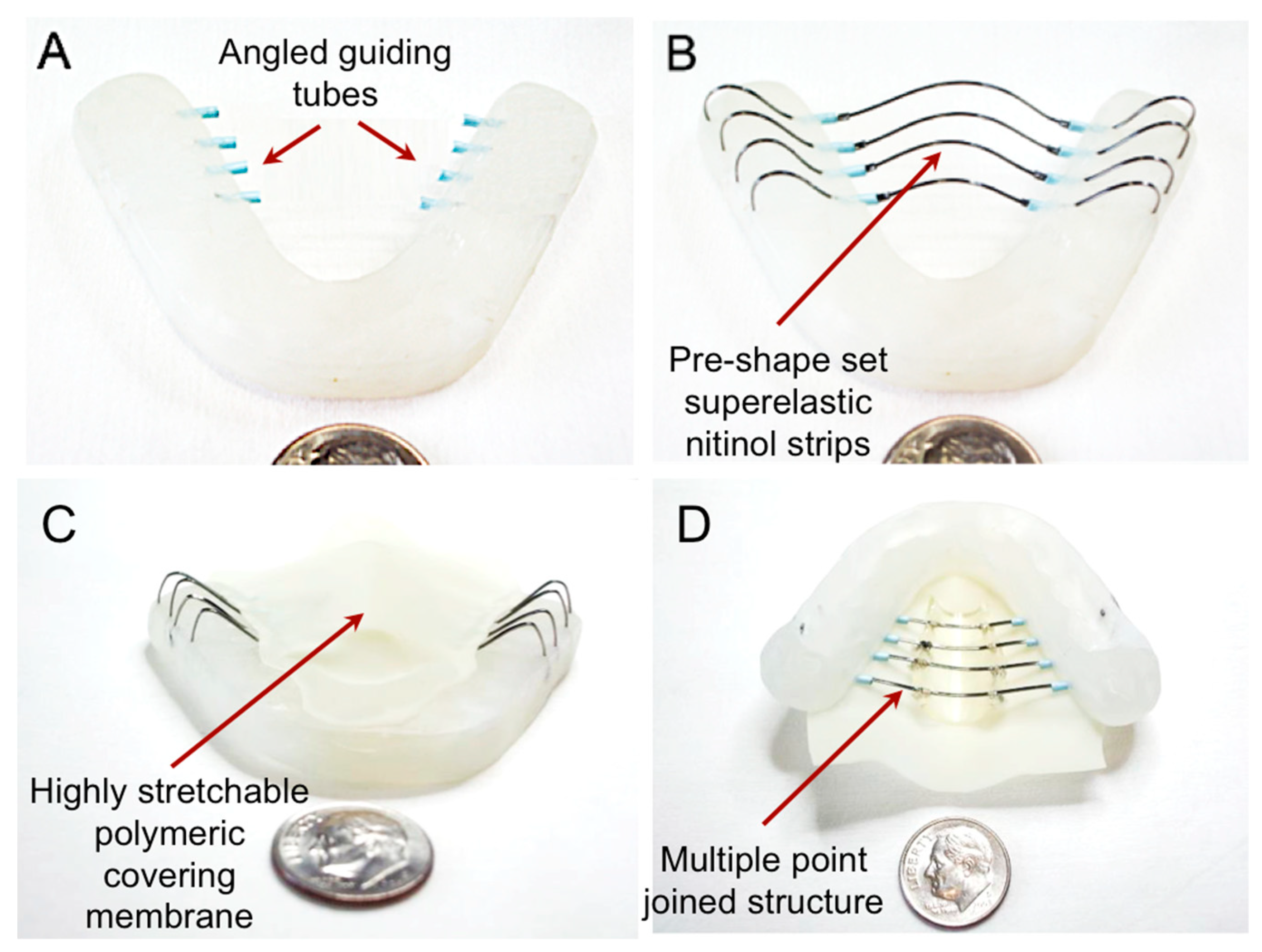
2.2. Fabrication Processes of a TPAD Prototype
2.3. In Vitro Mechanical Property Measurements
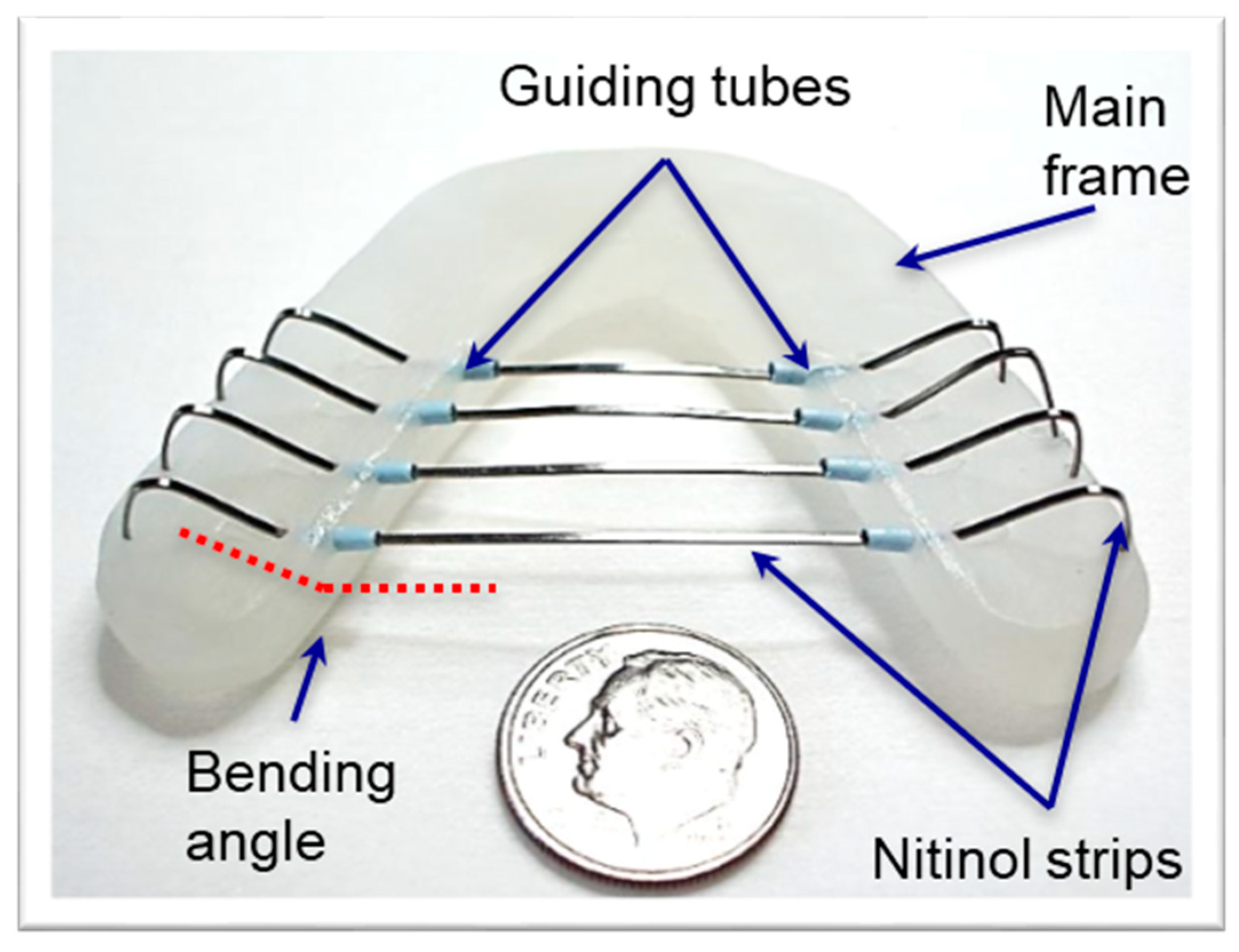
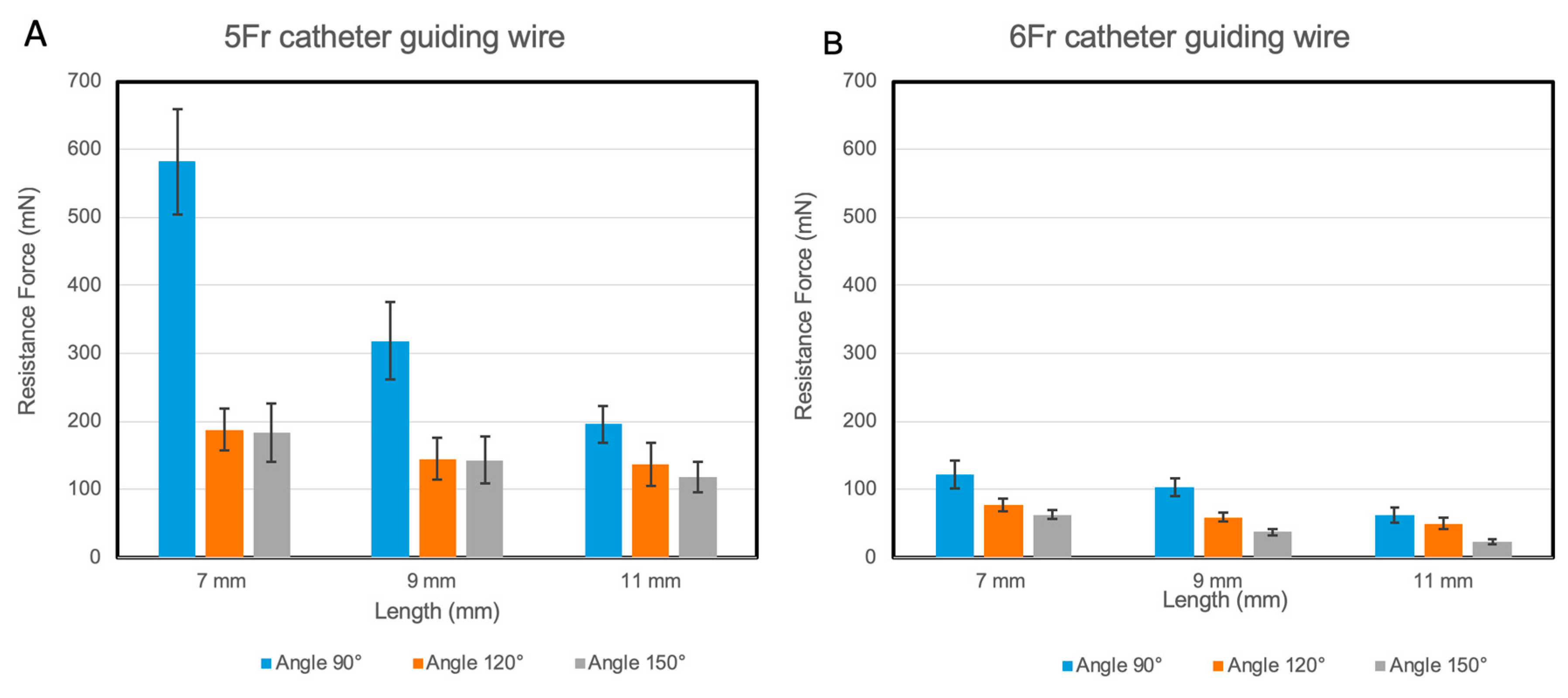
2.3.1. Resistance Measurement between Nitinol Strip and Guiding Tubes
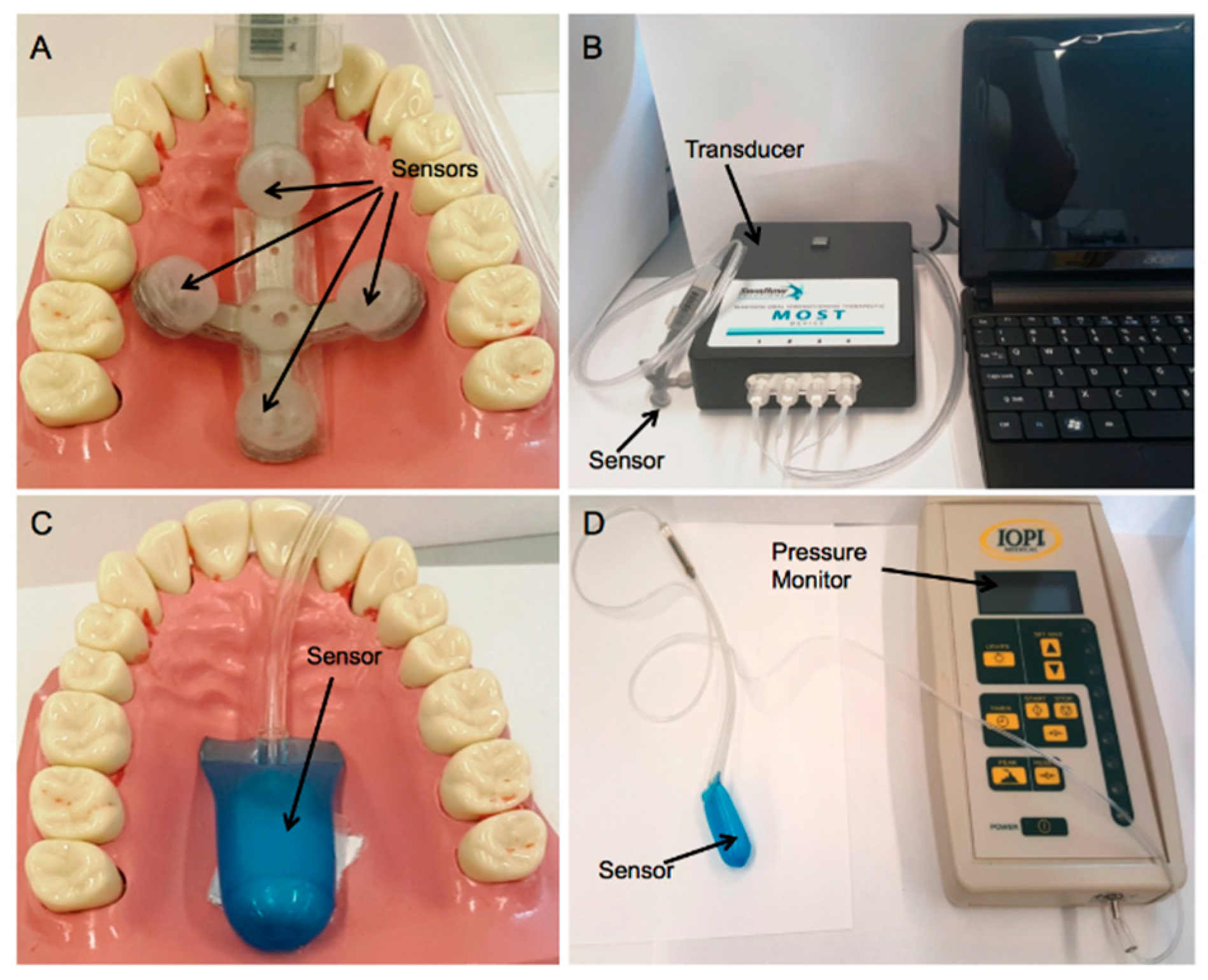
2.3.2. Tongue Pressure Measurements
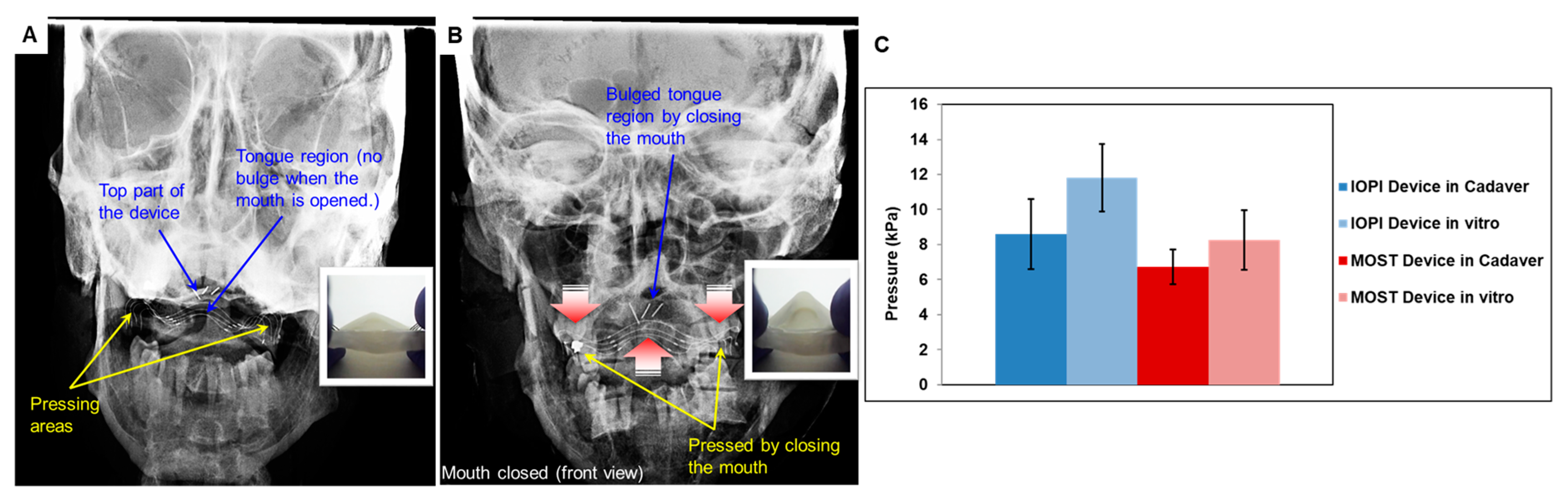
2.4. In Vitro Cadaver Test
2.5. Statistical Analysis
3. Results
3.1. Resistance in the Guiding Tubes
3.2. Prototype Fabrication
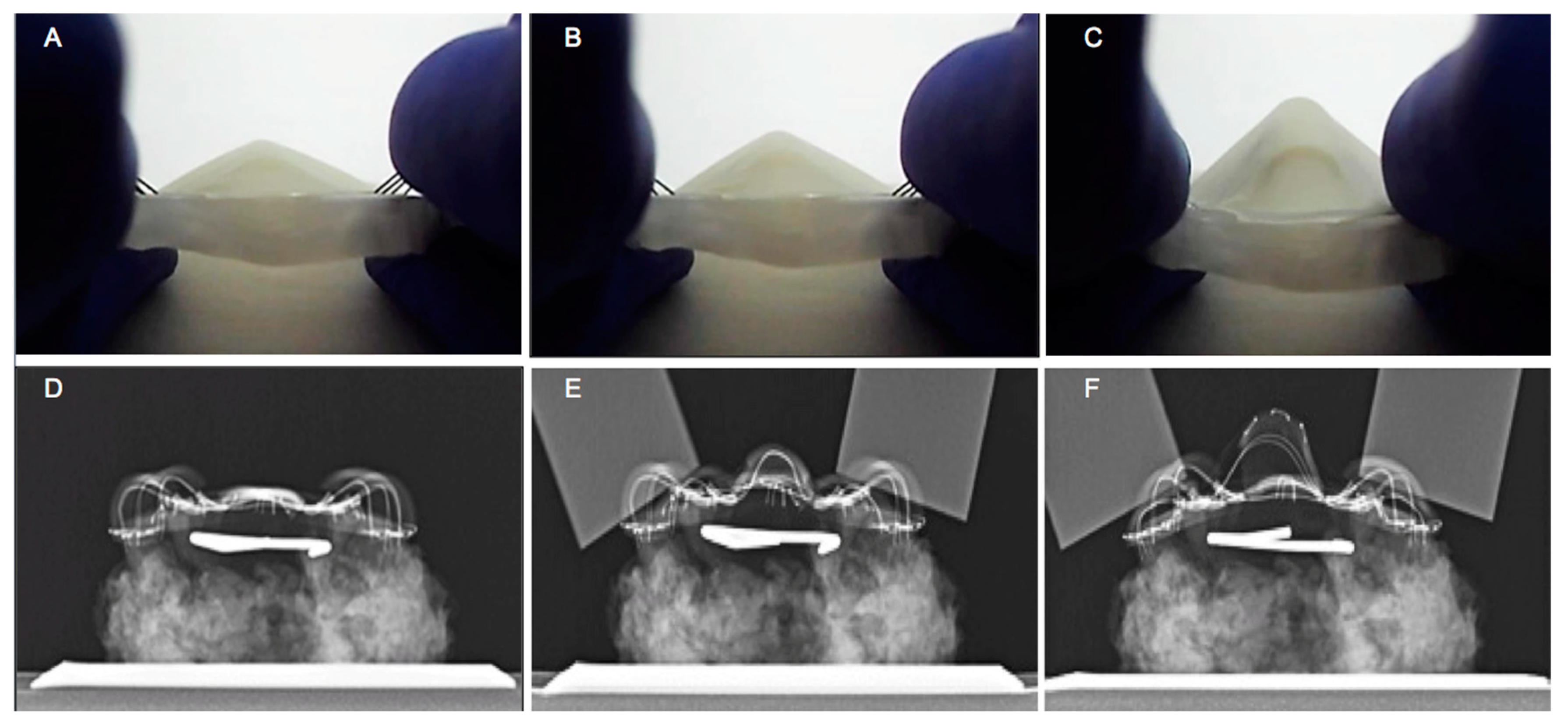
3.3. In Vitro Assessment of the Mechanical Performance of TPAD
3.4. TPAD Performance Evaluation using Cadaveric Head Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Crary, M.A.; Groher, M.E. Introduction to adult swallowing disorders; Elsevier Science; Butterworth Heinemann: Oxford, UK, 2003. [Google Scholar]
- Smith, B.S.; Adams, M. Dysphagia: Risk Factors, Diagnosis and Treatment; Nova Biomedical/Nova Science: New York, NY, USA, 2012. [Google Scholar]
- Rofes, L.; Arreola, V.; Almirall, J.; Cabré, M.; Campins, L.; García-Peris, P.; Speyer, R.; Clavé, P. Diagnosis and management of oropharyngeal dysphagia and its nutritional and respiratory complications in the elderly. Gastroenterol. Res. Pract. 2010, 2011, 818979. [Google Scholar] [CrossRef]
- Hewitt, A.; Hind, J.; Kays, S.; Nicosia, M.; Doyle, J.; Tompkins, W.; Gangnon, R.; Robbins, J. Standardized instrument for lingual pressure measurement. Dysphagia 2008, 23, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Bogaardt, H.; Grolman, W.; Fokkens, W. The use of biofeedback in the treatment of chronic dysphagia in stroke patients. Folia Phoniatr. Logop. 2009, 61, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Nicholls, B.; Lee, D.S.; Chen, Y.; Chun, Y.; Ang, C.S.; Yeo, W.-H. Soft electronics enabled ergonomic human-computer interaction for swallowing training. Sci. Rep. 2017, 7, 46697. [Google Scholar] [CrossRef] [PubMed]
- Kikutani, T.; Tamura, F.; Nishiwaki, K. Case presentation: Dental treatment with pap for als patient. Int. J. Orofac. Myol. 2006, 32, 32–35. [Google Scholar]
- Duerig, T.; Pelton, A.; Stöckel, D. An overview of nitinol medical applications. Mater. Sci. Eng. A 1999, 273, 149–160. [Google Scholar] [CrossRef]
- Youmans, S.R.; Stierwalt, J.A. Measures of tongue function related to normal swallowing. Dysphagia 2006, 21, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Logemann, J.A. Evaluation and Treatment of Swallowing Disorders; College-Hill Press: San Diego, CA, USA, 1983. [Google Scholar]
- Lazarus, C.L.; Logemann, J.A.; Pauloski, B.R.; Rademaker, A.W.; Larson, C.R.; Mittal, B.B.; Pierce, M. Swallowing and tongue function following treatment for oral and oropharyngeal cancer. J. Speech Lang. Hear. Res. 2000, 43, 1011–1023. [Google Scholar] [CrossRef] [PubMed]
- Koc, D.; Dogan, A.; Bek, B. Bite force and influential factors on bite force measurements: A literature review. Eur. J. Dent. 2010, 4, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Gay, T.; Rendell, J.; Majoureau, A.; Maloney, F.T. Estimating human incisal bite forces from the electromyogram/bite-force function. Arch. Oral Biol. 1994, 39, 111–115. [Google Scholar] [CrossRef]
- Braun, S.; Bantleon, H.-P.; Hnat, W.P.; Freudenthaler, J.W.; Marcotte, M.R.; Johnson, B.E. A study of bite force, part 1: Relationship to various physical characteristics. Angle Orthod. 1995, 65, 367–372. [Google Scholar] [PubMed]
- Ferrario, V.F.; Sforza, C.; Zanotti, G.; Tartaglia, G.M. Maximal bite forces in healthy young adults as predicted by surface electromyography. J. Dent. 2004, 32, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Vanderwegen, J.; Guns, C.; Van Nuffelen, G.; Elen, R.; De Bodt, M. The influence of age, sex, bulb position, visual feedback, and the order of testing on maximum anterior and posterior tongue strength and endurance in healthy belgian adults. Dysphagia 2012, 28, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Braun, M.M.; Osecheck, M.; Joyce, N.C. Nutrition assessment and management in amyotrophic lateral sclerosis. Phys. Med. Rehabil. Clin. N Am. 2012, 23, 751–771. [Google Scholar] [CrossRef] [PubMed]
- Balasubramaniam, M.K.; Chidambaranathan, A.S.; Shanmugam, G.; Tah, R. Rehabilitation of glossectomy cases with tongue prosthesis: A literature review. J. Clin. Diagn. Res. 2016, 10, ZE01–ZE04. [Google Scholar] [CrossRef] [PubMed]
- Hathaway, B.; Baumann, B.; Byers, S.; Wasserman-Wincko, T.; Badhwar, V.; Johnson, J. Handgrip strength and dysphagia assessment following cardiac surgery. Laryngoscope 2015, 125, 2330–2332. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Values Tested |
|---|---|
| Tube Bending Angle | 90°, 120° and 150° |
| Tube Length | 7, 9 and 11 mm |
| Tube Diameter | 5 and 6Fr |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shayan, M.; Gildener-Leapman, N.; Elsisy, M.; Hastings, J.T.; Kwon, S.; Yeo, W.-H.; Kim, J.-H.; Shridhar, P.; Salazar, G.; Chun, Y. Use of Superelastic Nitinol and Highly-Stretchable Latex to Develop a Tongue Prosthetic Assist Device and Facilitate Swallowing for Dysphagia Patients. Materials 2019, 12, 3555. https://doi.org/10.3390/ma12213555
Shayan M, Gildener-Leapman N, Elsisy M, Hastings JT, Kwon S, Yeo W-H, Kim J-H, Shridhar P, Salazar G, Chun Y. Use of Superelastic Nitinol and Highly-Stretchable Latex to Develop a Tongue Prosthetic Assist Device and Facilitate Swallowing for Dysphagia Patients. Materials. 2019; 12(21):3555. https://doi.org/10.3390/ma12213555
Chicago/Turabian StyleShayan, Mahdis, Neil Gildener-Leapman, Moataz Elsisy, Jack T. Hastings, Shinjae Kwon, Woon-Hong Yeo, Jee-Hong Kim, Puneeth Shridhar, Gabrielle Salazar, and Youngjae Chun. 2019. "Use of Superelastic Nitinol and Highly-Stretchable Latex to Develop a Tongue Prosthetic Assist Device and Facilitate Swallowing for Dysphagia Patients" Materials 12, no. 21: 3555. https://doi.org/10.3390/ma12213555
APA StyleShayan, M., Gildener-Leapman, N., Elsisy, M., Hastings, J. T., Kwon, S., Yeo, W.-H., Kim, J.-H., Shridhar, P., Salazar, G., & Chun, Y. (2019). Use of Superelastic Nitinol and Highly-Stretchable Latex to Develop a Tongue Prosthetic Assist Device and Facilitate Swallowing for Dysphagia Patients. Materials, 12(21), 3555. https://doi.org/10.3390/ma12213555






