1. Introduction
The new global standards of modern engineering technologies, continuously requiring more energy-saving and environment-friendly infrastructure [
1,
2], are driving the development of recycled construction materials. Traditional concrete, as one of the largest construction materials worldwide, entails a significant consumption of both energy and raw materials and, consequently, causes serious environmental pollution [
3]. Therefore, it is imperative to minimize these negative impacts on the environment. Recently, increasing attention has been paid to researches on the use of supplementary cementitious materials [
4,
5,
6] and construction and demolition (C&D) waste [
7,
8,
9,
10].
As an environmentally friendly material, geopolymer, has been found to be an ideal substitute for ordinary Portland cement (OPC) [
11,
12,
13]. Geopolymer, first described by Davidovits [
14], is an inorganic polymer material with a three-dimensional network structure, which can be prepared by the alkali-activated polymerization of silica tetrahedron and alumina tetrahedron from industrial waste [
15]. Fly ash [
12,
16,
17], GGBS [
18,
19], calcined clays [
20,
21], and other by-products [
22,
23] from recycled waste, as geopolymers, have been investigated by different researchers. It is well accepted in these studies that geopolymer concrete is able to provide a better mechanical performance, durability, and environmental protection than ordinary concrete.
In addition, transforming C&D waste into aggregates in concrete production is also considered a promising recycling technology, promoting the reuse of solid waste and consequently reducing not only environmental pollution, but also the consumption of raw materials [
24,
25]. This may be one of the significant efforts required for achieving sustainable construction. However, because recycled aggregates are characterized by a high porosity, high water absorption, and low strength, concrete containing a high content of recycled aggregates exhibits an inferior performance, compared to ordinary concrete [
26,
27]. Therefore, extensive efforts have been devoted to increasing the performance of recycled aggregate concretes (RC) [
9,
28,
29]. Among the reported approaches, using geopolymer to replace OPC is believed to be one of the most promising engineering technologies to improve the mechanical properties of RC [
9]. This is because, in addition to the defects of recycled aggregates themselves, most of the damage caused to concrete can be traced back to chemical and mechanical defects in the cement structure [
30], which can be modified by the outstanding chemical and physical properties of geopolymer. Previous studies [
9,
17,
31,
32,
33,
34] focused on the mechanical or microstructural characteristics of GRAC. It was found that the combination of geopolymer and recycled aggregate concrete (GRAC) not only provides a significant enhancement of the internal matrix of RC, but also reduces environmental impact, absolutely promoting the development of recycled construction materials. However, it should be mentioned that very limited information is available on the durability aspects of GRAC, especially concerning its sulfate resistance.
In fact, sulfate attack is one of the main durability problems for concrete structures, especially concrete in environments with sulfate ions [
35]. The ingress of sulfate ions contributes to the formation of ettringite and cracks when a critical stress, generated by the expansion force of ettringite, is reached. Thus, sulfate attack could cause spalling, cracking, and decreased strength of concrete structures. The sulfate resistance was also reported to be worse in RC, compared to ordinary concrete (NC), when a high content of recycled aggregates was used [
36,
37,
38,
39]. Therefore, how to prevent or reduce the damage of sulfate attack to concrete structures and improve the sulfate corrosion resistance of recycled concrete has aroused widespread concern [
40,
41]. A few studies have shown that geopolymer can provide a better sulfate resistance to ordinary concrete than OPC [
42,
43]. Fernandez–Jimenez et al. [
42] soaked the fly ash-based geopolymer in Na
2SO
4 solution for one year and found that the compressive strength of the specimens increased continuously. Hardjito et al. [
43] also reported similar experimental results. It is noted that no substantial work has been performed on the sulfate resistance of alkali-activated GRAC.
2. Research Significance
The demand for reducing the detrimental impact of C&D waste on the environment and addressing the shortage of natural resources places a burden on the government. Additionally, as the main binder in conventional concrete, Portland cement has been reported as the second most consumed product on Earth, second only to water, and its production is expected to increase in the coming years. To reduce the need for cement as well as the disposal of C&D wastes, using C&D waste and geopolymer together, as a green and eligible alternative to conventional concrete, is a promising technology. Numerous studies have reported on the performance of geopolymer concrete. However, the majority of these studies focused on natural aggregate concrete, with a limited number on recycled concrete. Additionally, these previous studies focused on the independent influence of fly ash on the mechanical properties of geopolymer concrete [
44], with limited information on the coupling effects of GGBS and fly ash on the performance of geopolymer concrete, especially recycled concrete. In fact, some researchers have found that single fly ash-based geopolymer concrete may exhibit a low early strength, which has been recognized as an apparent disadvantage [
45,
46]. In order to improve the mechanical properties of concrete with single fly ash-based geopolymer, the incorporation of GGBS into it was reported to be an effective method [
47,
48]. Therefore, it is necessary to develop a green concrete that incorporates recycled aggregates and GGBS and fly ash-based geopolymer.
In order to reduce the consumption of natural resources [
49,
50,
51], recycle the waste materials [
52,
53,
54,
55,
56], and effectively apply this new type of concrete in infrastructure exposed to soils, groundwater, rivers, seawater, and industrial wastes containing high concentrations of sulfate ions, it is essential to study its sulfate resistance. To the best of the authors’ knowledge, no data are available in the literature on the sulfate resistance of recycled aggregate concrete with GGBS and fly ash-based geopolymer. The object of this work is to develop this environment-friendly GRAC, with an improved sulfate resistance. In this study, recycled aggregates from construction and demolition (C&D) waste are used as coarse aggregates, and GGBS and fly ash-based geopolymer is used to totally replace cement in concrete. A series of specimens were prepared by considering different amounts of GGBS and sulfate exposure durations. They were then exposed to a sulfate environment and tested under uniaxial compression. The coupling effects of the GGBS and fly ash-based geopolymer and the recycled coarse aggregates are examined in terms of the sulfate resistance of GRAC. The mass loss, crack propagation, and residual compressive strength are analyzed. The optimum content of GGBS in GRAC, based on the experimental results, is also discussed. Finally, XRD and SEM tests are conducted to reveal the attack mechanisms of the sulfate ions and hydration mechanisms of GRAC.
3. Experiment Set-Up
3.1. Materials
In this study, recycled coarse aggregates, produced by crushed waste concrete, were used. The recycled coarse aggregates were obtained from demolished buildings. The original concrete was characterized by a compressive strength of 20–30 MPa. To improve the purity and quality of recycled coarse aggregates, a high-pressure (5 MPa) water jet was used to remove the mud in recycled aggregates. Wood and glass impurities were removed manually. Then, they were dried by sunshine before use. The ingredient proportions in the recycled coarse aggregates are presented in
Table 1, which met the requirements of class III of recycled aggregates, according to the Chinese standard GB/T 25177-2010 [
57]. For comparison, natural coarse aggregates, made of granite, were used in the control specimen (ordinary concrete). Both natural coarse aggregates and recycled coarse aggregates have a continuous grain size distribution of between 5 and 20 mm. The main properties of coarse aggregates are summarized in
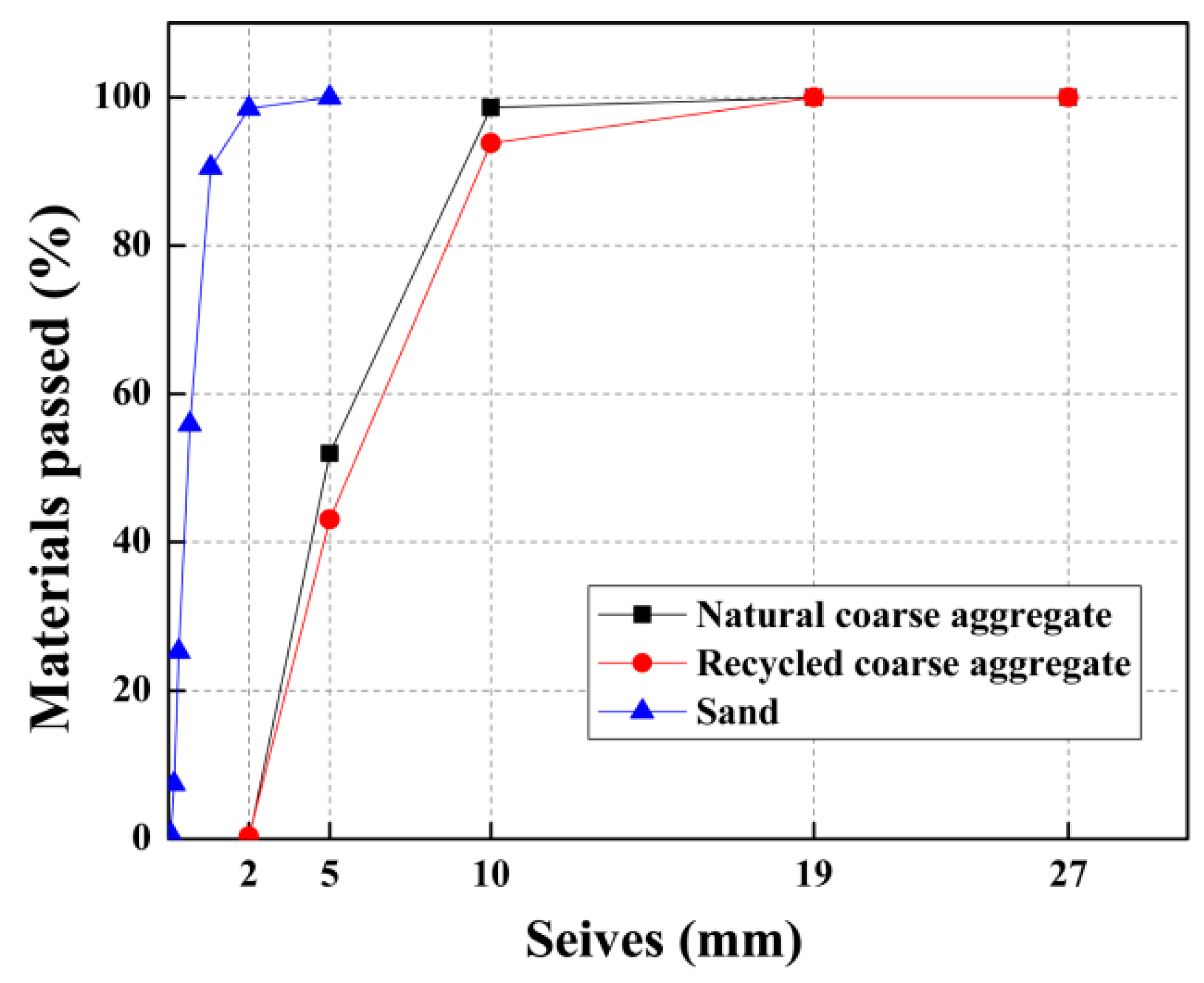
Table 2. River sand was extracted as fine aggregates, with a fineness modulus of 2.52, a water absorption rate of 0.8%, and a specific gravity of 2.69. The size grading of all aggregates is presented in
Figure 1.
Ordinary Portland cement (OPC), with a strength of 42.5 MPa, complying with the Chinese standard GB175-2007 [
58], was used in the control specimen. The properties and chemical compositions of the OPC, fly ash, and GGBS were shown in
Table 3.
The alkaline activator used in this study was a mixture of NaOH and Na
2SiO
3 solutions. The properties of NaOH and Na
2SiO
3 solutions are listed in
Table 4 and
Table 5, respectively. The solid NaOH used for NaOH solution had a purity of 99%. Following the findings in the previous literature [
43,
59,
60,
61], the mass ratio of NaOH to Na
2SiO
3 solution was 1:2.5.
To improve the setting time of GRAC, a retarding water reducer (RWR), consisting mainly of calcium sucrose, was used in this study. The properties of the RWR were reported in
Table 6.
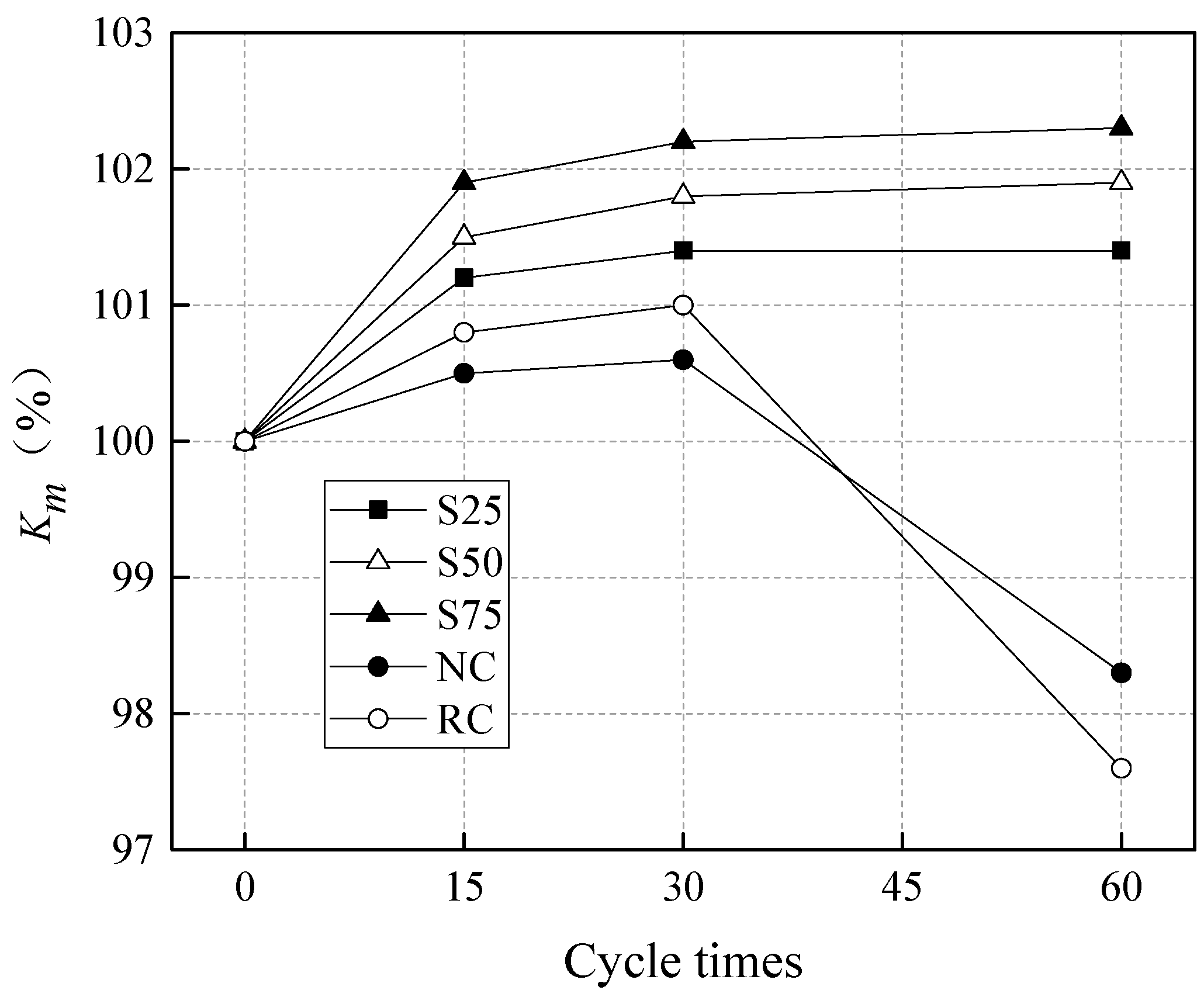
In total, 20 groups of test specimens were considered in this study. There were 5 mixes (see
Table 7), which were exposed to 4 different cycles of sulfate attack (see
Table 8). Each group included 3 replicas of test specimens, with dimensions of 100 mm × 100 mm × 100 mm. In all mixes, the water-binder ratio (W/B) was kept constant and equal to 0.5. The specimens NC and RC in
Table 7 were OPC-based concrete with natural aggregates and recycled aggregates, respectively. In geopolymer concretes, 3 levels of GGBS content were considered. The RWR solution in all mixes was fixed at 1.5%, according to the flowability. The slump test results show that the combination of GGBS and fly ash can provide acceptable workability for the geopolymer concrete with recycled coarse aggregates. The consistency of GRAC is significantly influenced by GGBS content. An increase of GGBS content can reduce the slump value of GRAC. A detailed discussion of this can be found in our previous study [
48]. In terms of the curing condition, the geopolymer specimens, S25, S50, and S75, were demolded after 24 h and cured under steam and a high temperature of 80 °C for another 24 h, before being put under a standard curing condition (20 °C and RH > 95%). The specimens, NC and RC, were cured under the standard curing condition, until testing.
3.2. Test Procedures
The test of sulfate attack in this study was conducted according to the Chinese standard GBT 50082 [
62]. At the age of 26 days, the specimens were oven-dried (80 ± 5 °C) for 24 h, and then, after they were cooled down to room temperature, the weight of each specimen was measured (
m1). Subsequently, the specimens were put into a wetting–drying chamber, containing sulfate solution with 5% Na
2SO
4. During the wetting stage, the level of the solution was at least 20 mm higher than the top surface of the specimens. Each wetting stage lasted for 15 h. During the drying stage, the specimens were air-dried for 30 min and then dried at 80 °C for 6 h. They were then cooled down to room temperature for 2 h. Each wetting–drying cycle lasted for around 24 h. The sulfate solution was replaced weekly in order to maintain the same sulfate content and pH level. This is because the migration of alkali from the concretes to the solution could increase the pH of the sulfate solution noticeably [
63]. After the targeted cycles of attack, the specimens were oven-dried (80 ± 5 °C) for 24 h and cooled down to room temperature. The weight was then measured (
m2), and the compressive strength was tested.
To investigate the residual compressive behavior, after exposure to sulfate attack, all specimens were tested under axial compression loading at room temperature. The compression test was conducted by the Italian MATEST material testing machine (Matest, Treviolo, Bergamo, Italy), with a capacity of 4000 kN. The axial load, controlled by the stress, was applied at a speed of 0.5 MPa/s.
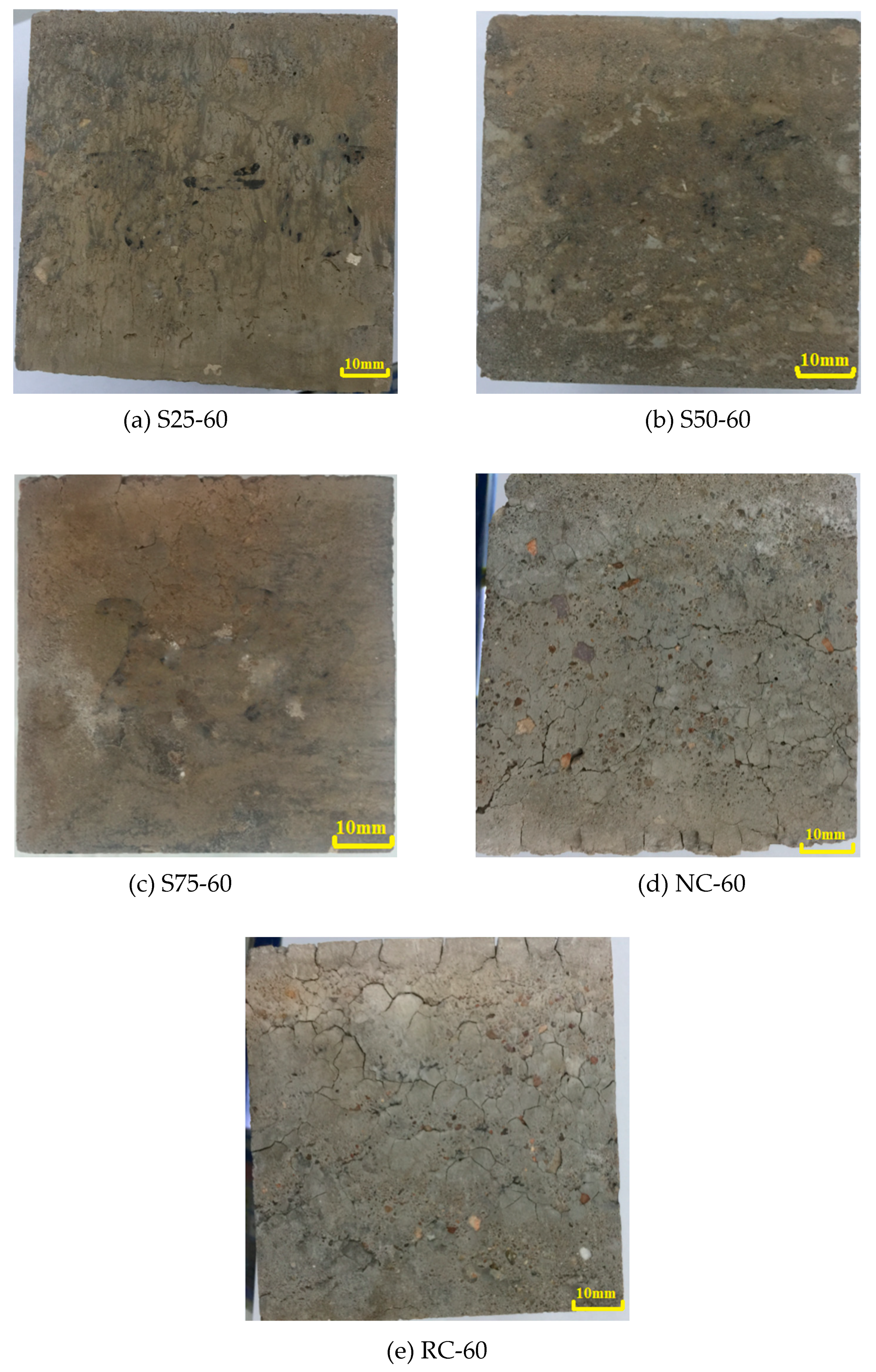
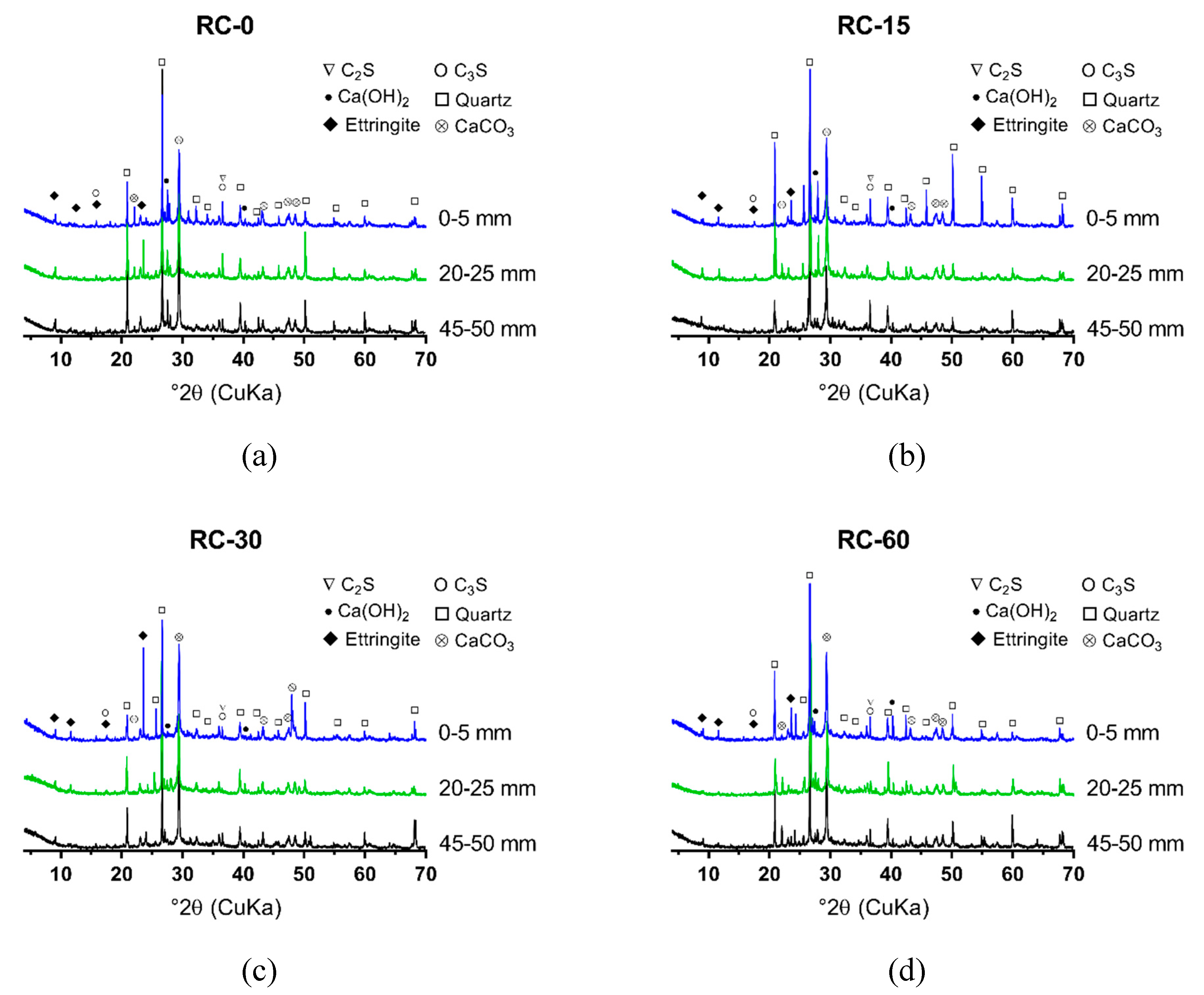
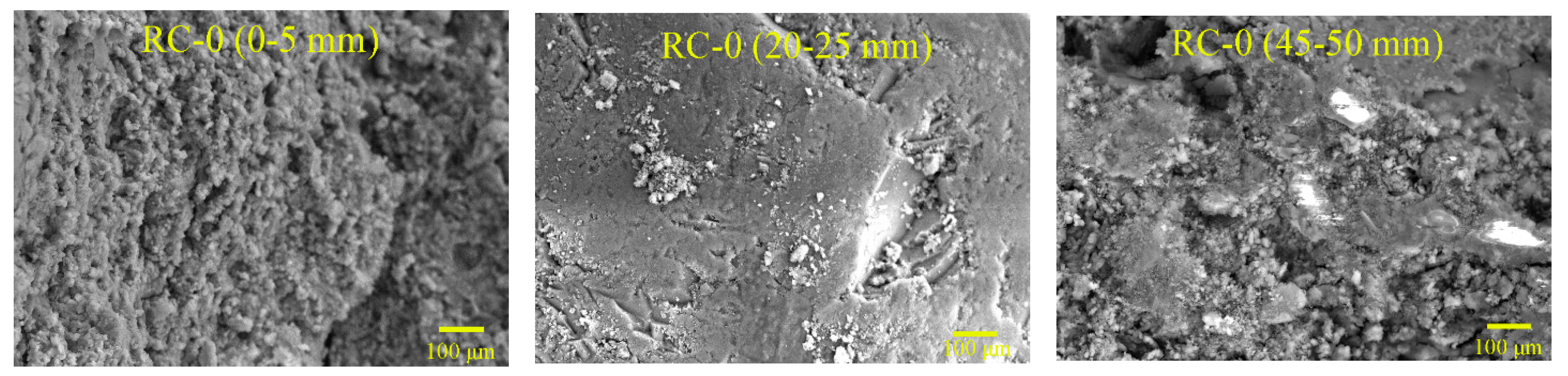
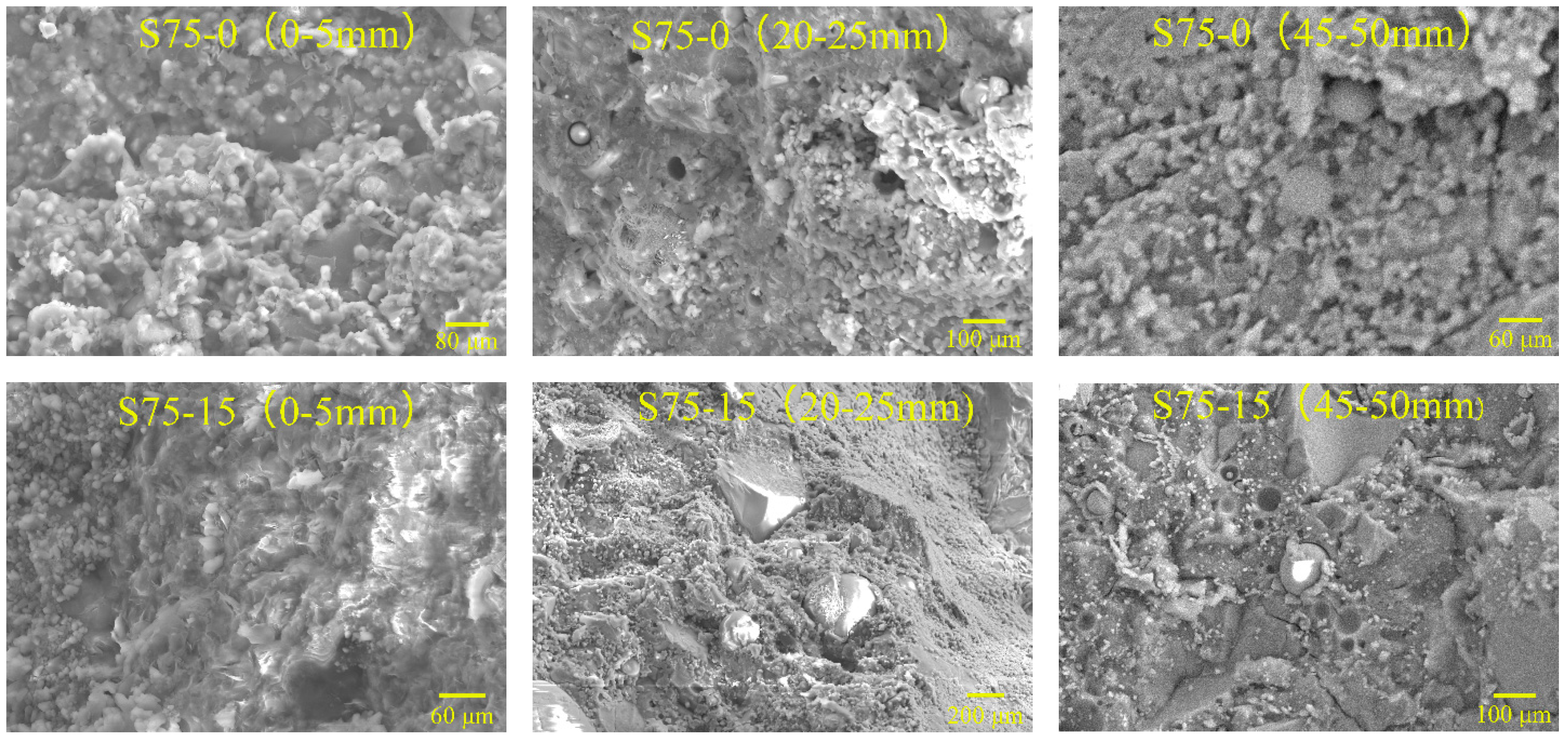
To examine the hydration mechanism and sulfate resistance of GRAC, X-ray diffraction (XRD), and Scanning Electron Microscopy (SEM) tests were conducted for the samples, from different depths (0–5 mm, 20–25 mm, and 45–50 mm) below the surface of specimens. Copper was used as the anode material. For each measurement, 1.5 g of the powder sample, passed through a sieve with an aperture of 48 µm, was used for XRD measurement with a Bruker D8 ADVANCE X-Ray Powder Diffractometer (Bruker, Karlsruhe, Baden-Wü rttemberg, Germay). The scanning rate was 4°/min and from 4° to 80° (2θ) for a continuous measurement.
As for SEM observations, an electron microscopy, LYRA 3 XMU (Tescan, Brno, Southern Moravia, Czech Republic), made by TESCAN was used in this study. The magnification could be adjusted from 1 to 1000 thousand times. The SEM samples were prepared by extraction from the crack surface of specimens, with a size of less than 10 mm × 10 mm × 10 mm. They were carefully classified and stored in sealed plastic bags, before SEM observation. Additionally, the spray gold coating was implemented to make the concrete samples electronically conductive, which enabled their microstructures and micro scale damage to be observed in SEM.