Reduction Temperature-Dependent Nanoscale Morphological Transformation and Electrical Conductivity of Silicate Glass Microchannel Plate
Abstract
1. Introduction
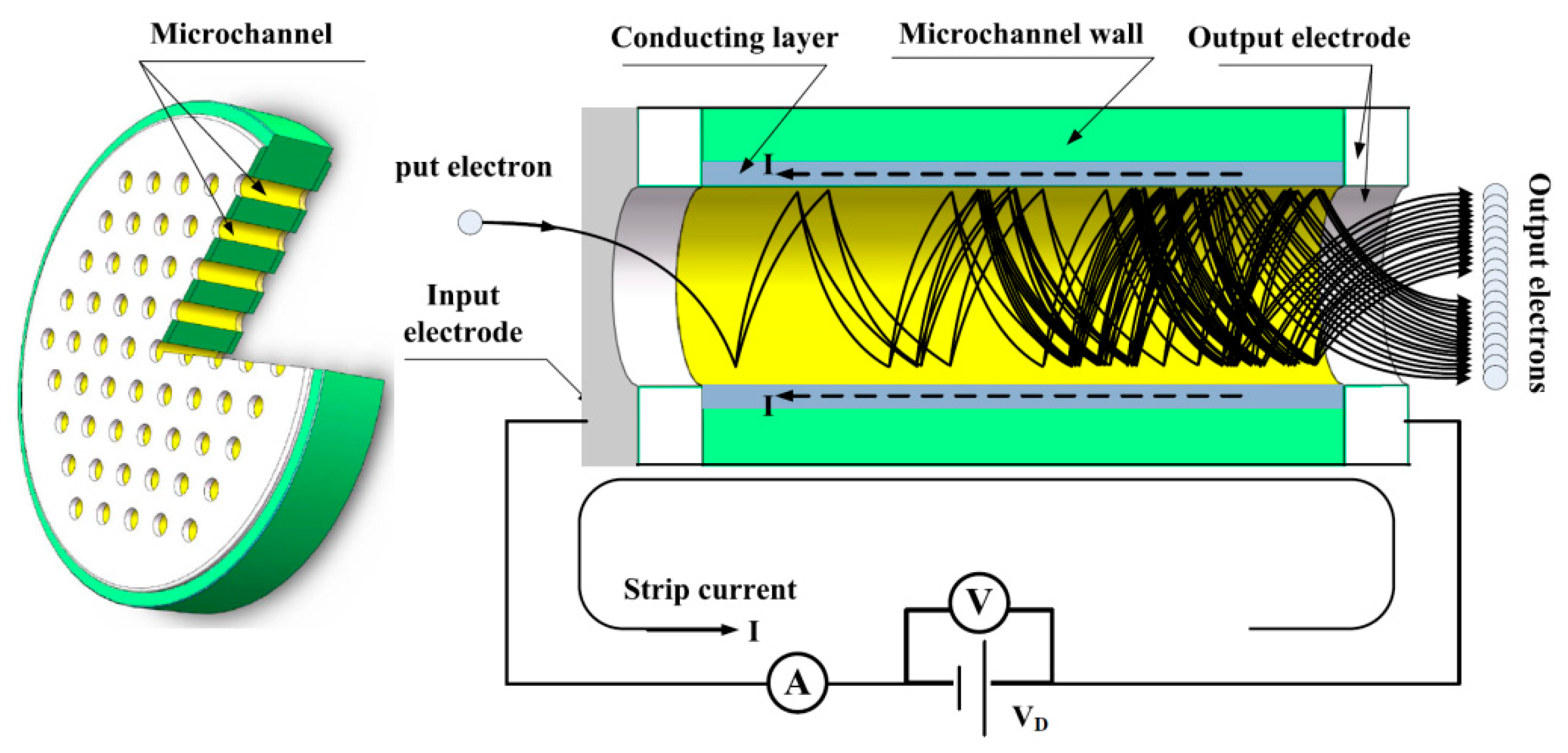
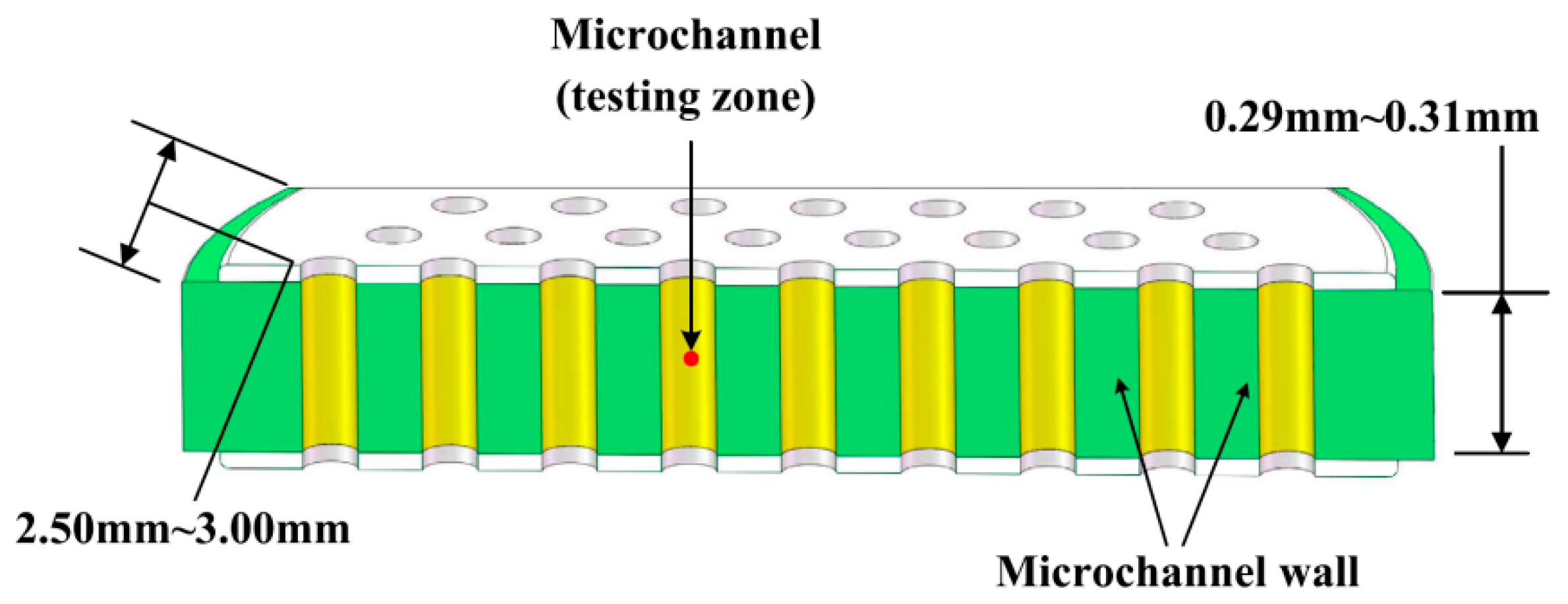
2. Materials and Methods
3. Results and Discussion
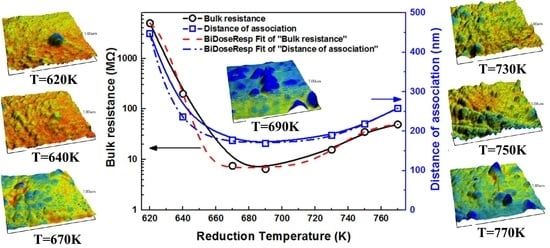
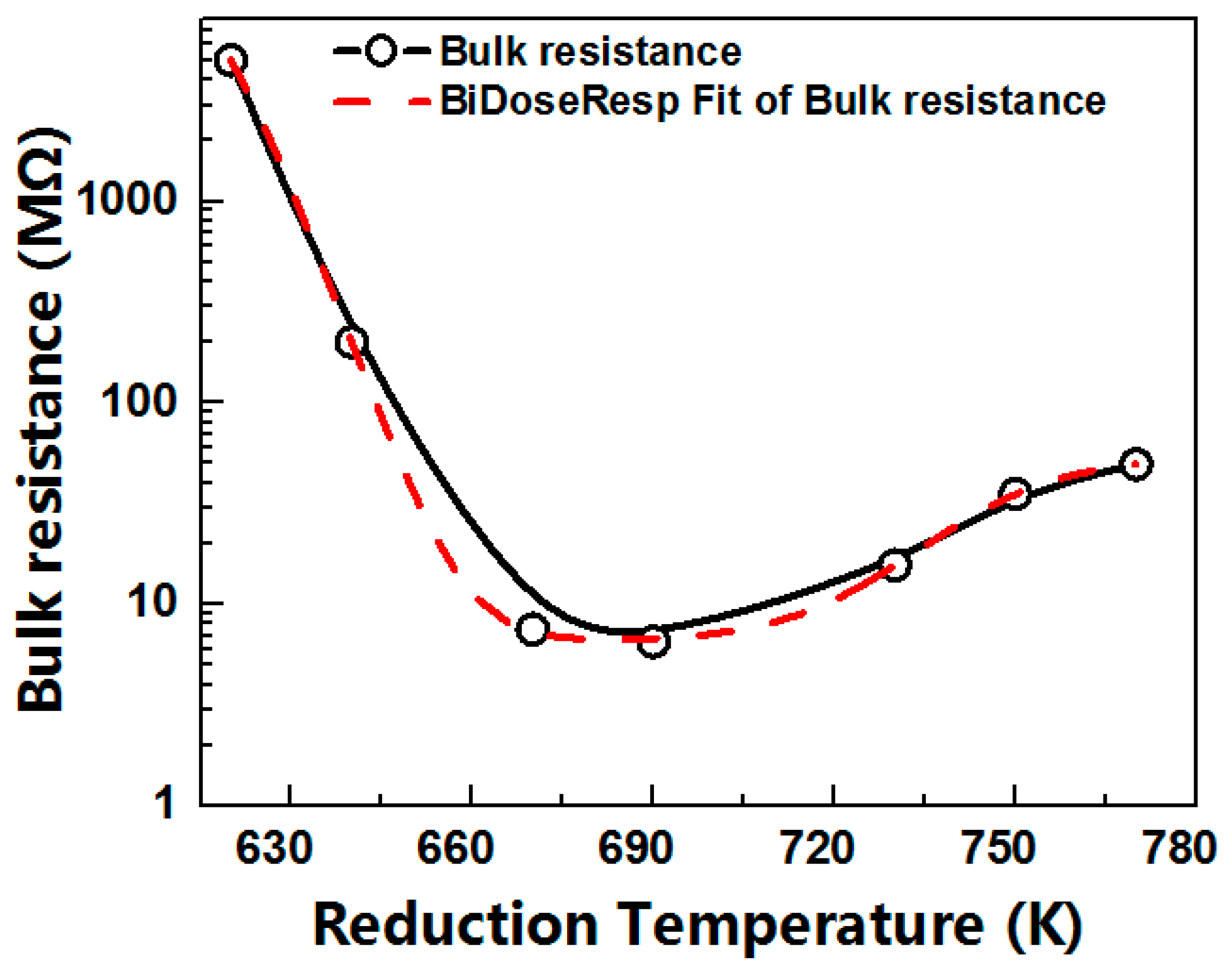
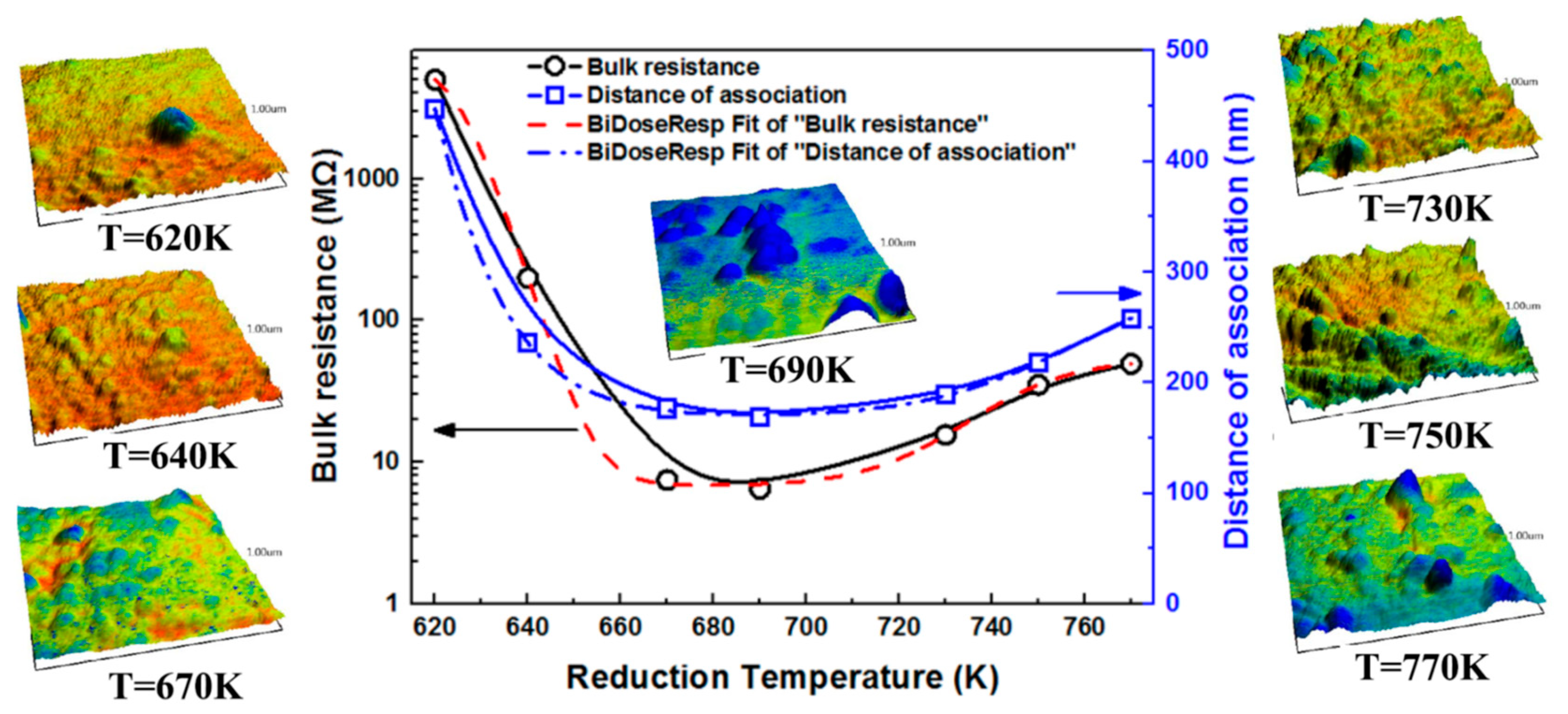
3.1. Bulk Resistances at Different Reduction Temperatures
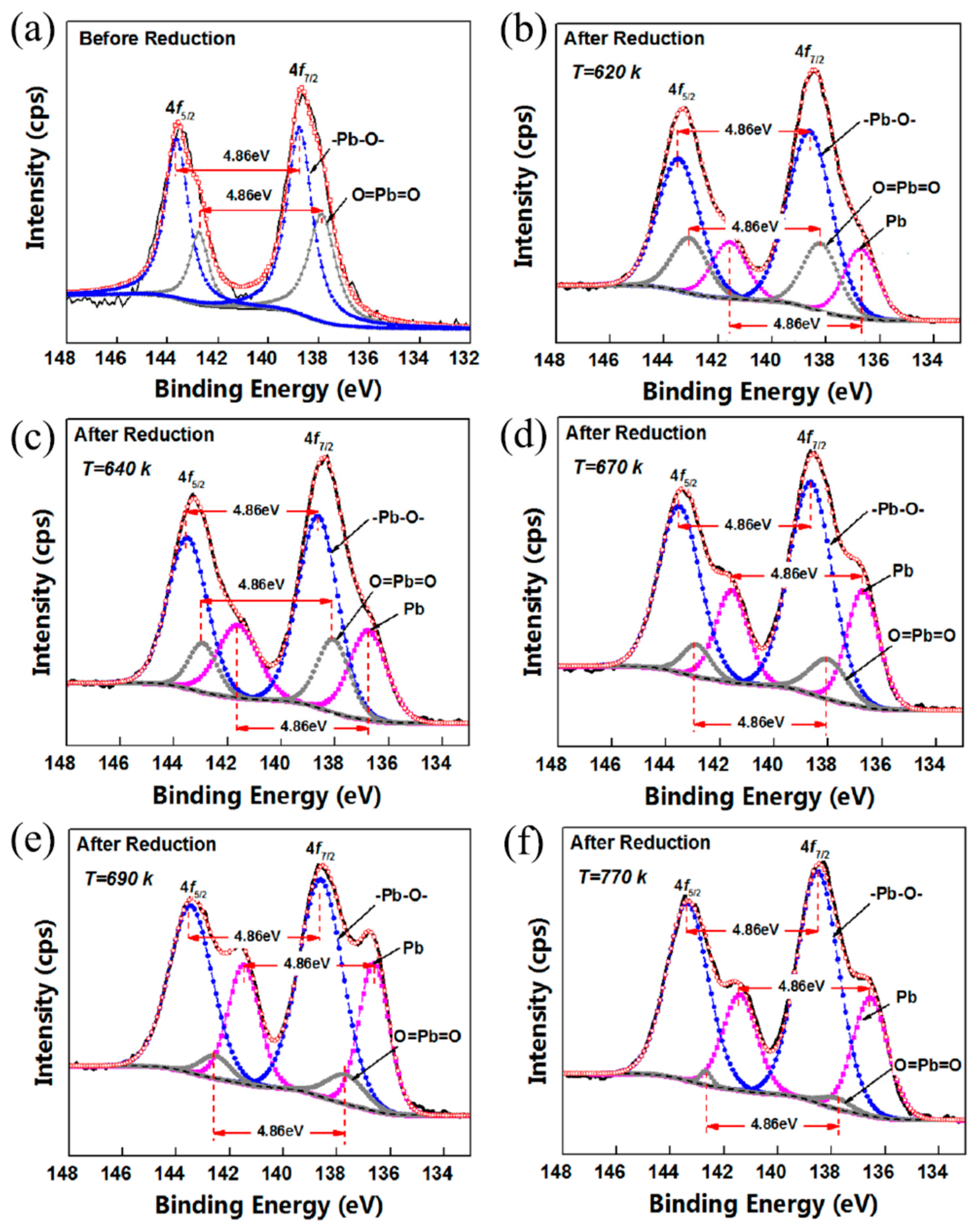
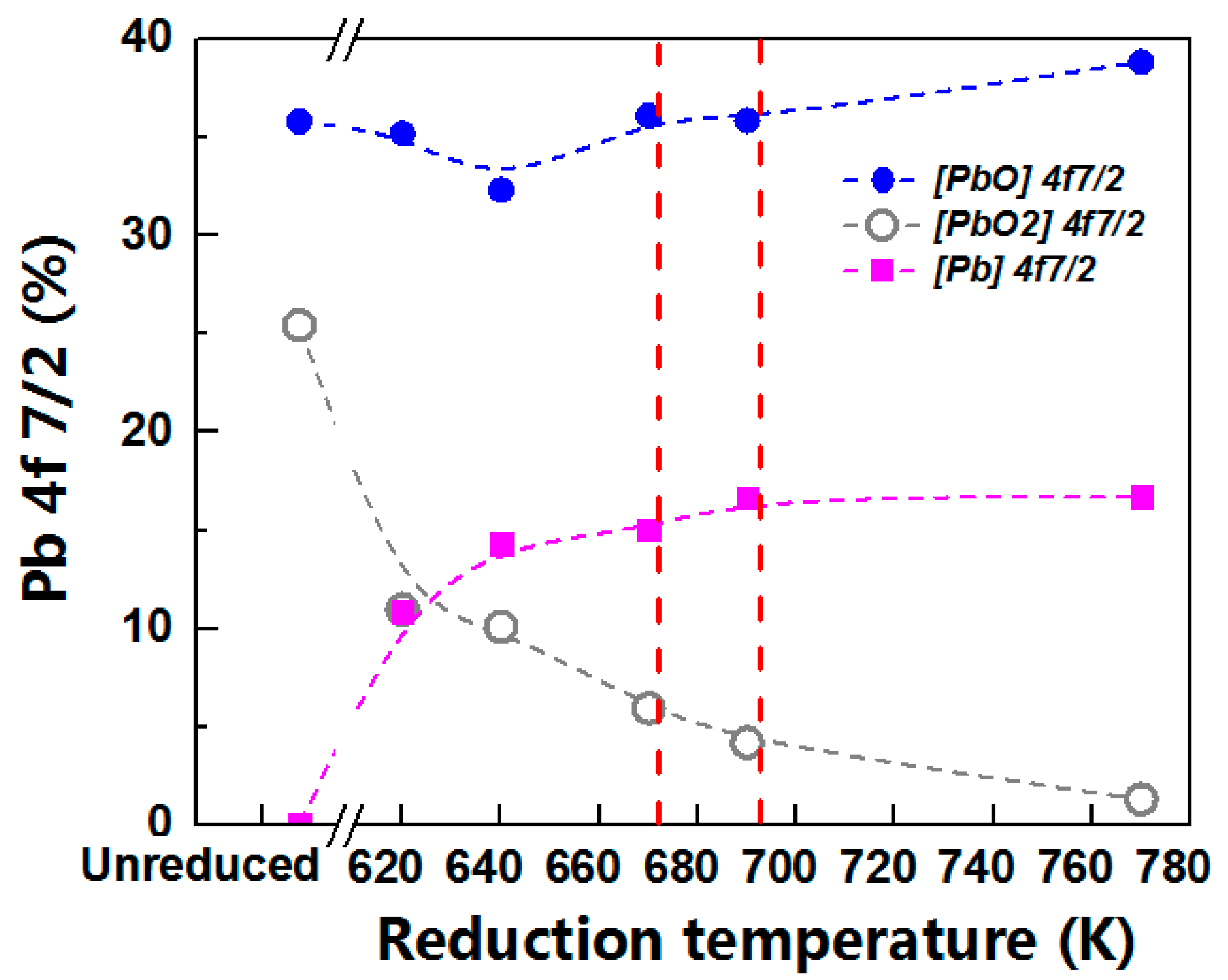
3.2. XPS Spectra of Pb
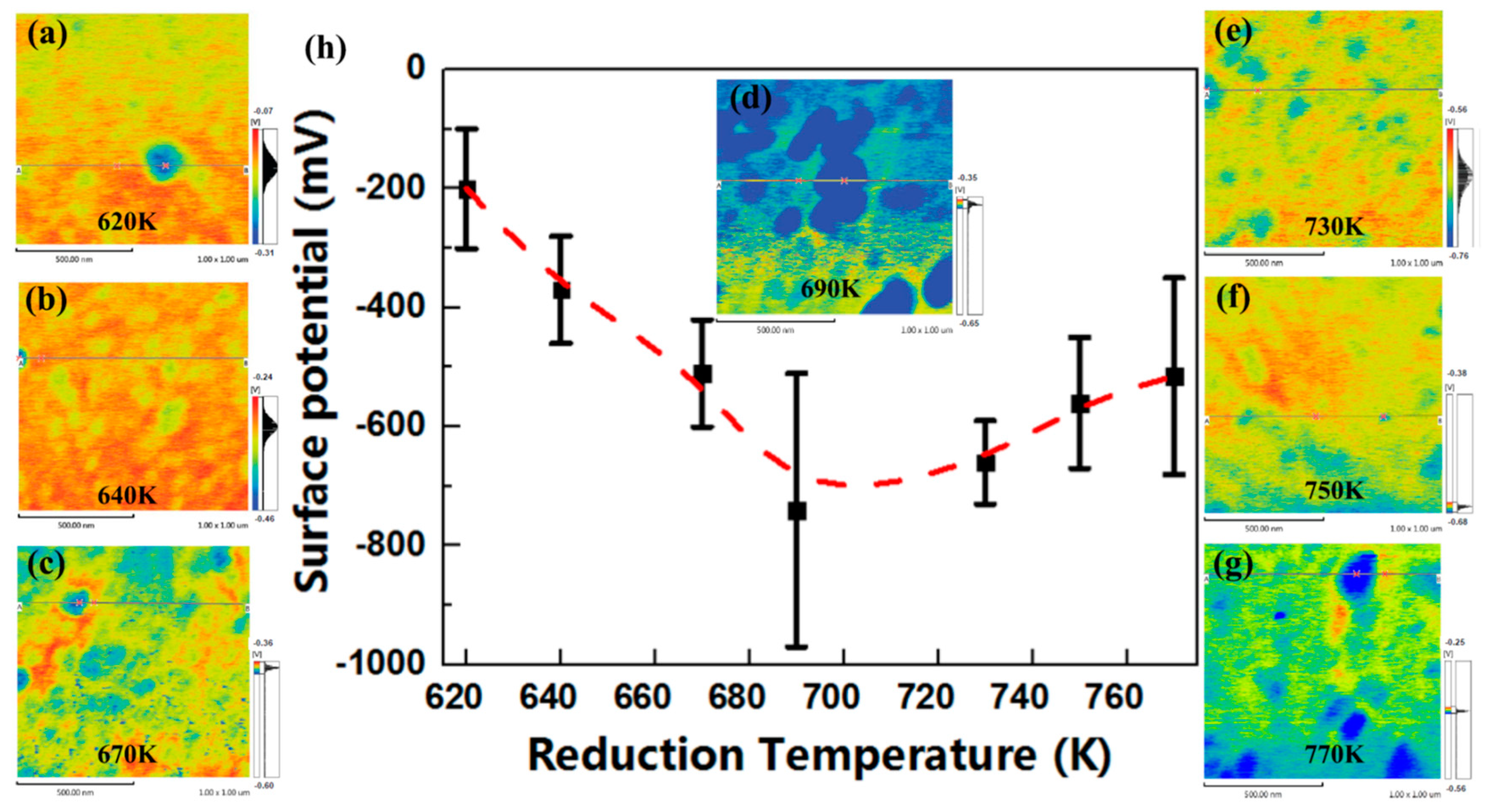
3.3. Morphology Evolution of Pb Aggregates
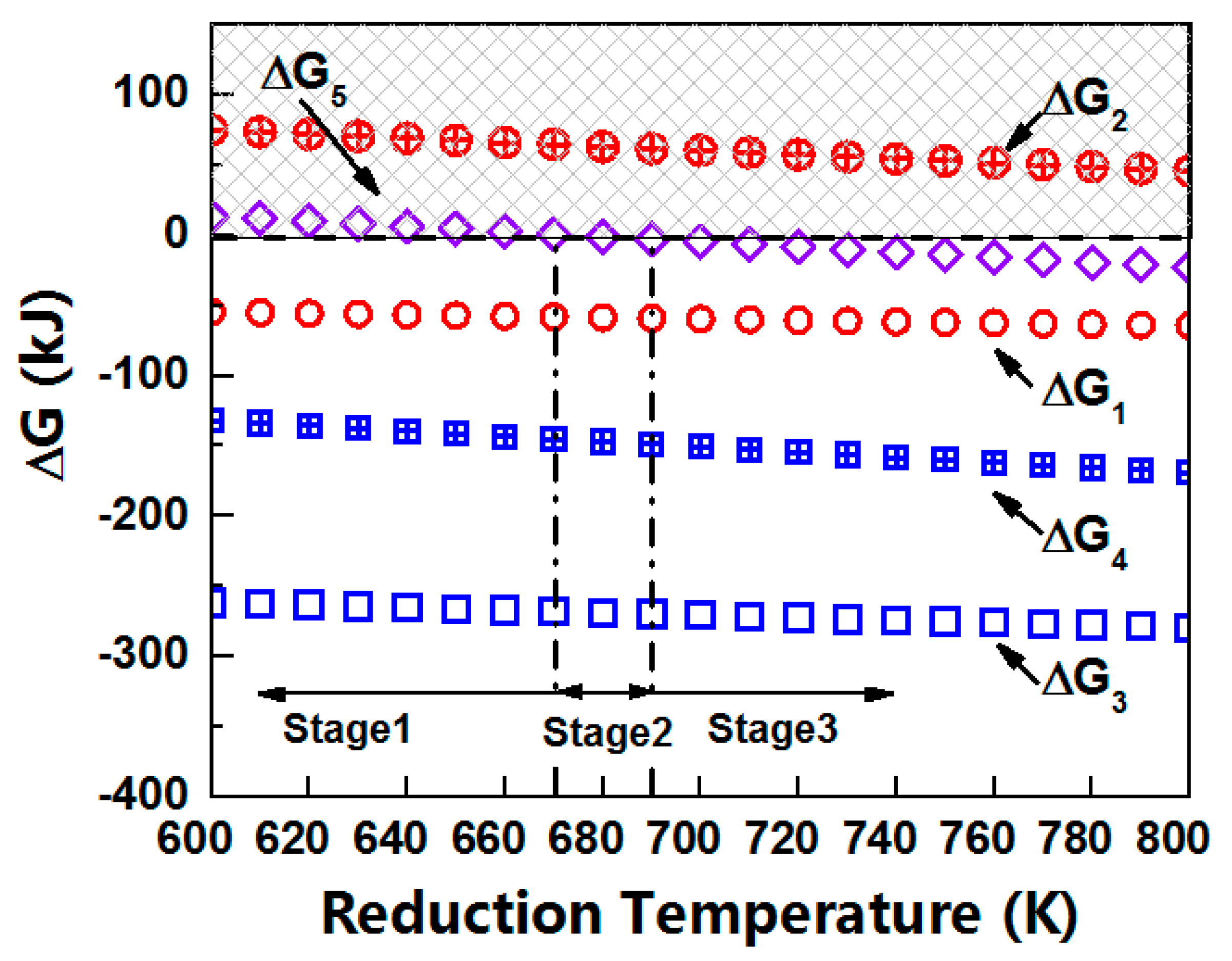
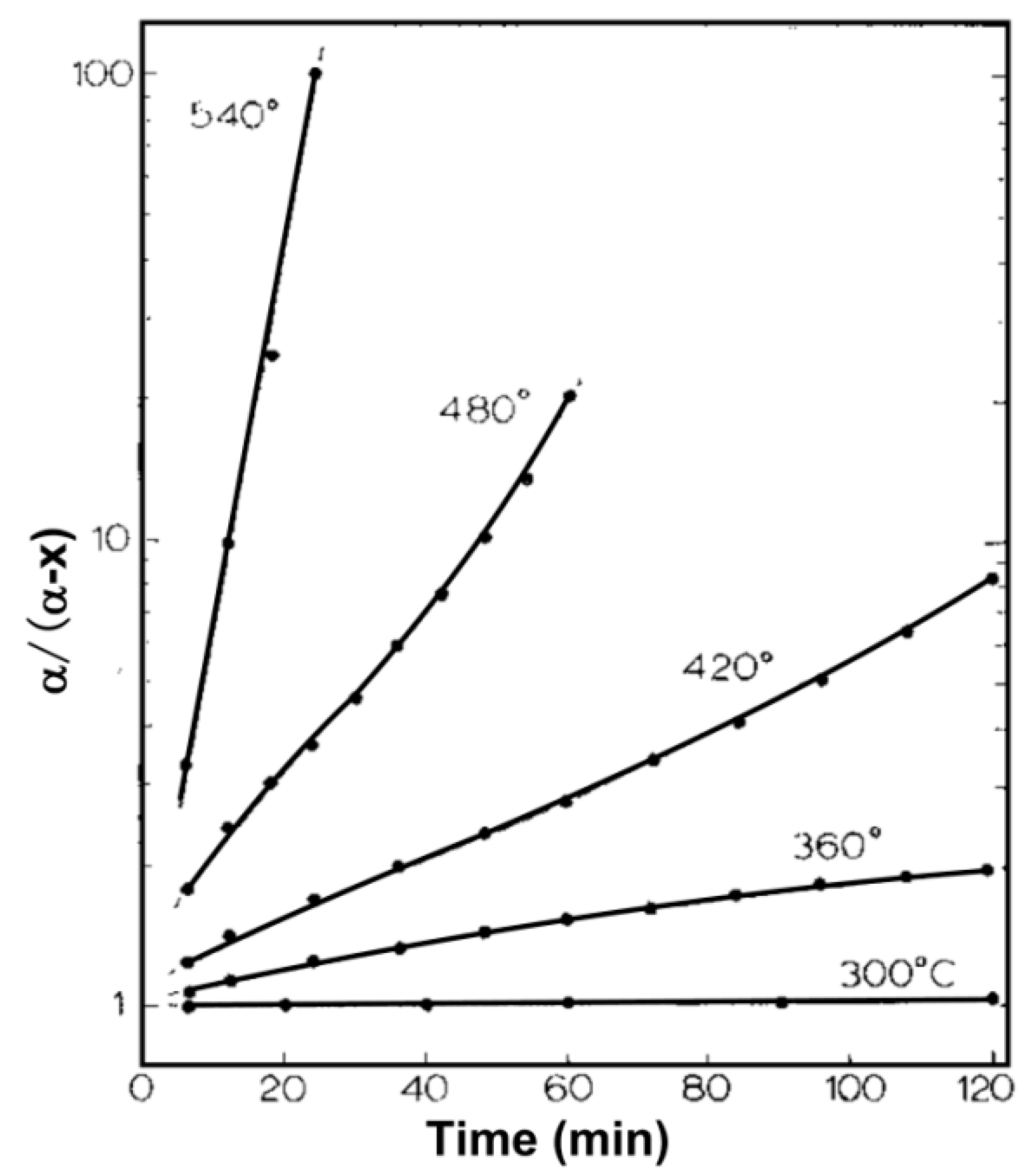
3.4. Thermodynamic and Kinetic of the Reducing Pb
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hans, A.; Schmidt, P.; Ozga, C.; Hartmann, G.; Holzapfel, X.; Ehresmann, A.; Knie, A. Extreme Ultraviolet to Visible Dispersed Single Photon Detection for Highly Sensitive Sensing of Fundamental Processes in Diverse Samples. Materials 2018, 11, 869. [Google Scholar] [CrossRef] [PubMed]
- Pertot, Y.; Schmidt, C.; Matthews, M.; Chauvet, A.; Huppert, M.; Svoboda, V.; von Conta, A.; Tehlar, A.; Baykusheva, D.; Wolf, J.P.; et al. Time-resolved x-ray absorption spectroscopy with a water window high-harmonic source. Science 2017, 355, 264–267. [Google Scholar] [CrossRef]
- Booth, D.; Rittenhouse, S.T.; Yang, J.; Sadeghpour, H.R.; Shaffer, J.P. Production of trilobite Rydberg molecule dimers with kilo-Debye permanent electric dipole moments. Science 2015, 348, 99–102. [Google Scholar] [CrossRef]
- Priedhorsky, W.; Bloch, J.J. Optical detection of rapidly moving objects in space. Appl. Opt. 2005, 44, 423–433. [Google Scholar] [CrossRef]
- Wiggins, B.B.; Vadas, J.; Alexander, A.; Hudan, S. Achieving high spatial resolution using a microchannel plate detector with an economic and scalable approach. Nucl. Instrum. Methods Phys. Res. A 2017, 872, 144–149. [Google Scholar] [CrossRef]
- Lehmann, A.; Böhm, M.; Britting, A.; Eyrich, W.; Pfaffinger, M.; Uhlig, F.; Belias, A.; Dzhygadlo, R.; Gerhardt, A.; Götzen, K.; et al. Recent Developments with Microchannel-Plate PMTs. Nucl. Instrum. Methods Phys. Res. A 2017, 876, 42–47. [Google Scholar] [CrossRef]
- Barnyakov, A.Y.; Barnyakov, M.Y.; Prisekin, V.G.; Karpov, S.V.; Katcin, A.A. Test of microchannel plates in magnetic fields up to 4.5 T. Nucl. Instrum. Methods Phys. Res. A 2017, 845, 588–590. [Google Scholar] [CrossRef]
- Mazuritskiy, M.I.; Dabagov, S.B.; Lerer, A.M.; Dziedzic-Kocurek, K.; Sokolov, A.; Coreno, M.; Turchini, S.; D’Elia, A.; Sacchi, M.; Marcelli, A. Transmission diffractive patterns of large microchannel plates at soft X-ray energies. Nucl. Instrum. Methods Phys. Res. B 2017, 402, 282–286. [Google Scholar] [CrossRef]
- Bruce, N.L.; Bruce, N.L.; Maureen, W.; Maureen, W. Dynamic range optimization in microchannel plates. Proc. SPIE 1990, 1243, 132–139. [Google Scholar]
- Ertley, C.; Siegmund, O.; Hull, J.; Tremsin, A.; O’Mahony, A.; Craven, C.; Minot, M. Microchannel Plate Imaging Detectors for High Dynamic Range Applications. IEEE Trans. Nucl. Sci. 2017, 64, 1774–1780. [Google Scholar] [CrossRef]
- Wiza, J.L. Microchannel plate detectors. Nucl. Instrum. Methods 1979, 162, 587–601. [Google Scholar] [CrossRef]
- Huang, Y.; Gu, Z.; Zhang, Y.; Liu, H.; Li, G. Nano-scale morphology on micro-channel plate lead silicate glass surface. J. Chin. Ceram. Soc. 2012, 40, 994–999. [Google Scholar]
- Leskovar, B. Microchannel plates. Phys. Today 1977, 30, 42–49. [Google Scholar] [CrossRef]
- Simeonova, J.M.; Kourtev, J.S.; Dimitriev, Y.B. Surface compositional studies of heat reduced lead silicate glass. J. Non-Cryst. Solids 1983, 57, 177–187. [Google Scholar] [CrossRef]
- Czajka, R.; Trzebiatowski, K.; Polewsk, W.; Kościelsk, B.; Kaszczyszyn, S.; Susla, B. AFM investigation of bismuth doped silicate glasses. Vacuum 1997, 48, 213–216. [Google Scholar] [CrossRef]
- Cai, H.; Li, F.; Xu, Y.; Bo, T.; Zhou, D.; Lian, J.; Li, Q.; Cao, Z.; Xu, T.; Wang, C.; et al. Effect of nano-scale morphology on micro-channel wall surface and electrical characterization in lead silicate glass micro-channel plate. Proc. SPIE 2017, 10460, 104601G. [Google Scholar]
- Huang, Y.; Zhang, Y.; Liu, H.; Gu, Z. XPS study on microporous surface composition of microchannel plates. Proc. SPIE 2011, 8194, 81941Q. [Google Scholar]
- Grévin, B.; Schwartz, P.O.; Biniek, L.; Brinkmann, M.; Leclerc, N.; Zaborova, E.; Méry, S. High-resolution noncontact AFM and Kelvin probe force microscopy investigations of self-assembled photovoltaic donor–acceptor dyads. Beilstein J. Nanotechnol. 2016, 7, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Hua, Z.; Zhu, S.; An, B.; Iijima, T.; Gu, C.; Zheng, J. The finding of hydrogen trapping at phase boundary in austenitic stainless steel by scanning Kelvin probe force microscopy. Scr. Mater. 2019, 162, 219–222. [Google Scholar] [CrossRef]
- Yivlialin, R.; Pace, G.; Bussetti, G.; Caironi, M.; Duò, L. Contact potential and scanning Kelvin force microscopy measurements on sulphate-anion intercalated graphite. Electrochim. Acta 2018, 267, 20–23. [Google Scholar] [CrossRef]
- Zemek, J.; Jiricek, P.; Houdkova, J.; Jurek, K.; Gedeon, O. Lead-silicate glass surface sputtered by an argon cluster ion beam investigated by XPS. J. Non-Cryst. Solids 2017, 469, 1–6. [Google Scholar] [CrossRef]
- Moulder, J.F.; Chastain, J.; King, R.C. (Eds.) Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer Corp.: Chanhassen, MN, USA, 1995. [Google Scholar]
- Sascha, S.; Thilo, G. (Eds.) Kelvin Probe Force Microscopy: Measuring and Compensating Electrostatic Forces; Springer International Publishing AG: Berlin, Germany, 2018. [Google Scholar]
- Haertling, G.H.; Cook, R.L. Kinetics of the reduction of lead compounds and glasses. J. Am. Ceram. 1965, 48, 35–40. [Google Scholar] [CrossRef]
- Bates, C.W.; Helmer, J.; Wiechert, N. Induced electron emission spectroscopy of reduced lead silicate glass. Solid State Commun. 1972, 10, 847–851. [Google Scholar] [CrossRef]

| Pb3O4 | K2O | Na2O | BaO | MgO | Al2O3 | SiO2 | |
|---|---|---|---|---|---|---|---|
| CML865 | 13.6 | 6.1 | 2.0 | 1.2 | 1.7 | 1.1 | Bal. |
| No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Reduction Temperature/K | 620 | 640 | 670 | 690 | 730 | 750 | 770 |
| Reduction Time/h | 180 | ||||||
| Hydrogen Flow/mL·min−1 | 10 | ||||||
| Hydrogen Pressure/105 Pa | 1.1 | ||||||
| Pb0 | –Pb–O– | O=Pb=O | ||||||
|---|---|---|---|---|---|---|---|---|
| 4f 7/2 | 4f 5/2 | 4f 7/2 | 4f 5/2 | 4f 7/2 | 4f 5/2 | |||
| Before reduction | B.E (eV) | - | - | 138.8 | 143.6 | 138.2 | 142.7 | |
| Area% | - | - | 35.8 | 31.1 | 22.4 | 10.6 | ||
| FWHM (eV) | - | - | 1.19 | 1.11 | 1.29 | 0.91 | ||
| After reduction | T = 620 K | B.E (eV) | 136.7 | 141.6 | 138.6 | 143.5 | 138.2 | 143.1 |
| Area% | 10.9 | 9.0 | 35.2 | 25.1 | 11.0 | 8.8 | ||
| FWHM (eV) | 1.50 | 1.52 | 1.92 | 1.84 | 1.55 | 1.55 | ||
| T = 640 K | B.E (eV) | 136.8 | 141.6 | 138.6 | 143.5 | 138.1 | 142.9 | |
| Area% | 14.3 | 12.6 | 32.3 | 24.9 | 10.1 | 5.8 | ||
| FWHM (eV) | 1.65 | 1.80 | 1.75 | 1.72 | 1.42 | 1.23 | ||
| T = 670 K | B.E (eV) | 136.7 | 141.6 | 138.6 | 143.5 | 138.0 | 142.9 | |
| Area% | 15.1 | 11.8 | 36.1 | 27.5 | 5.9 | 3.6 | ||
| FWHM (eV) | 1.41 | 1.41 | 1.87 | 1.82 | 1.63 | 1.22 | ||
| T = 690 K | B.E (eV) | 136.6 | 141.5 | 138.6 | 143.4 | 137.7 | 142.5 | |
| Area% | 16.7 | 14.0 | 35.9 | 26.7 | 4.2 | 2.6 | ||
| FWHM (eV) | 1.32 | 1.38 | 1.94 | 1.89 | 1.67 | 1.33 | ||
| T = 770 K | B.E (eV) | 136.6 | 141.1 | 138.5 | 143.4 | 137.8 | 142.7 | |
| Area% | 16.7 | 13.2 | 38.9 | 29.4 | 1.3 | 0.6 | ||
| FWHM (eV) | 1.54 | 1.51 | 1.84 | 1.82 | 1.28 | 0.51 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, H.; Sun, Y.; Zhang, X.; Zhang, L.; Liu, H.; Li, Q.; Bo, T.; Zhou, D.; Wang, C.; Lian, J. Reduction Temperature-Dependent Nanoscale Morphological Transformation and Electrical Conductivity of Silicate Glass Microchannel Plate. Materials 2019, 12, 1183. https://doi.org/10.3390/ma12071183
Cai H, Sun Y, Zhang X, Zhang L, Liu H, Li Q, Bo T, Zhou D, Wang C, Lian J. Reduction Temperature-Dependent Nanoscale Morphological Transformation and Electrical Conductivity of Silicate Glass Microchannel Plate. Materials. 2019; 12(7):1183. https://doi.org/10.3390/ma12071183
Chicago/Turabian StyleCai, Hua, Yong Sun, Xian Zhang, Lei Zhang, Hui Liu, Qing Li, Tiezhu Bo, Dongzhan Zhou, Chen Wang, and Jiao Lian. 2019. "Reduction Temperature-Dependent Nanoscale Morphological Transformation and Electrical Conductivity of Silicate Glass Microchannel Plate" Materials 12, no. 7: 1183. https://doi.org/10.3390/ma12071183
APA StyleCai, H., Sun, Y., Zhang, X., Zhang, L., Liu, H., Li, Q., Bo, T., Zhou, D., Wang, C., & Lian, J. (2019). Reduction Temperature-Dependent Nanoscale Morphological Transformation and Electrical Conductivity of Silicate Glass Microchannel Plate. Materials, 12(7), 1183. https://doi.org/10.3390/ma12071183