The Effect of Elevated Curing Temperatures on High Ye’elimite Calcium Sulfoaluminate Cement Mortars
Abstract
:1. Introduction
2. Experimental Methods
2.1. Material Properties and Preparation
2.2. Experimental Procedures
3. Experimental Results
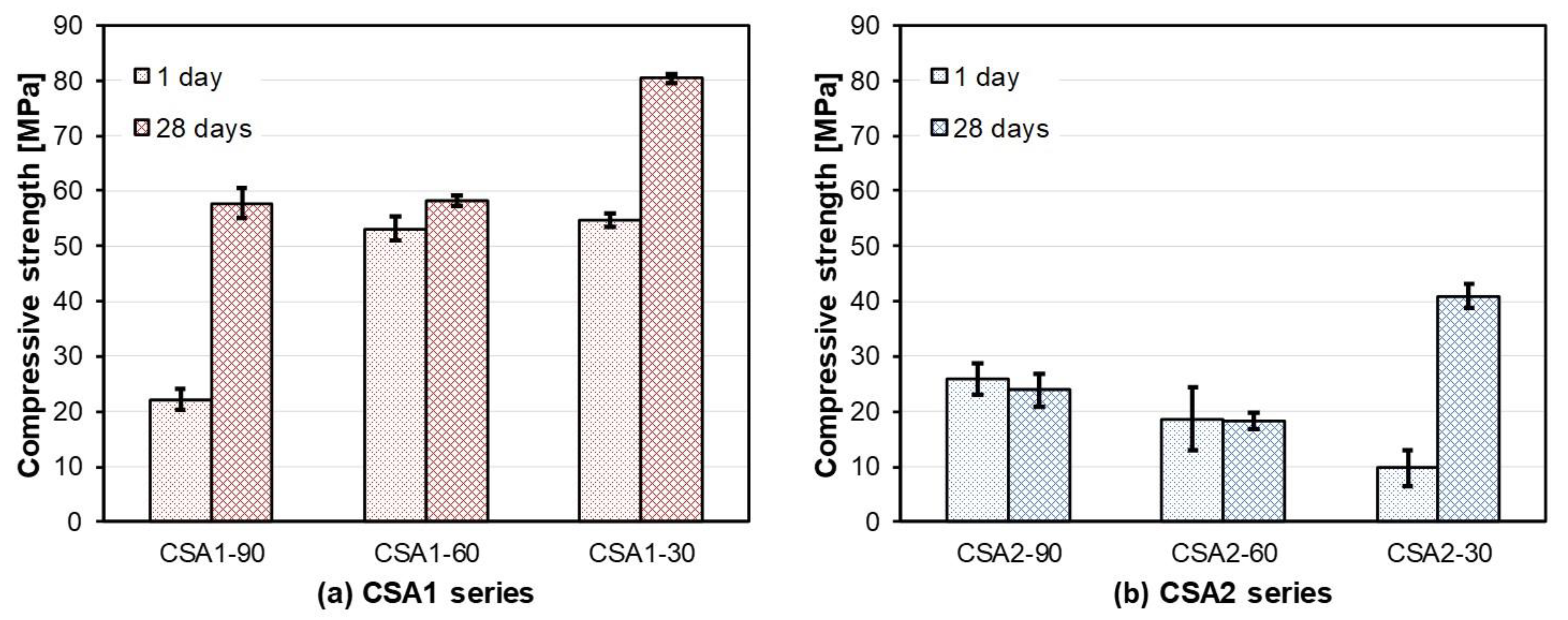
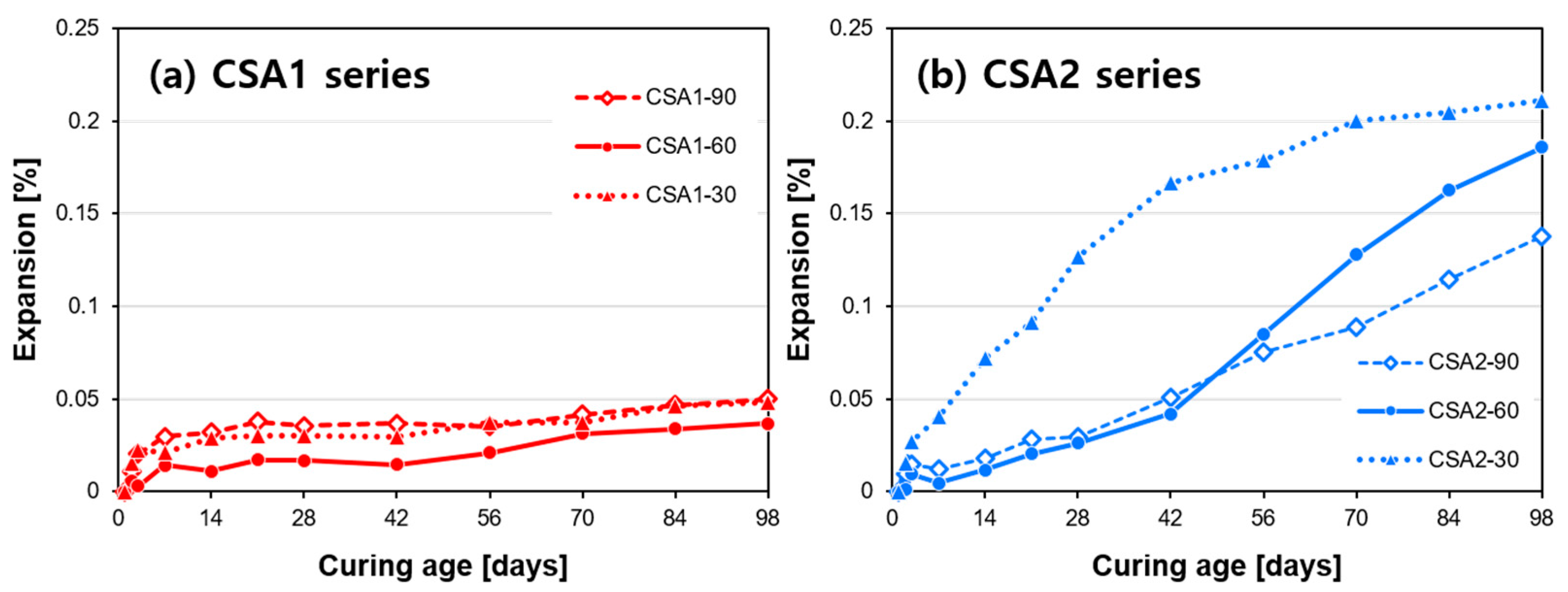
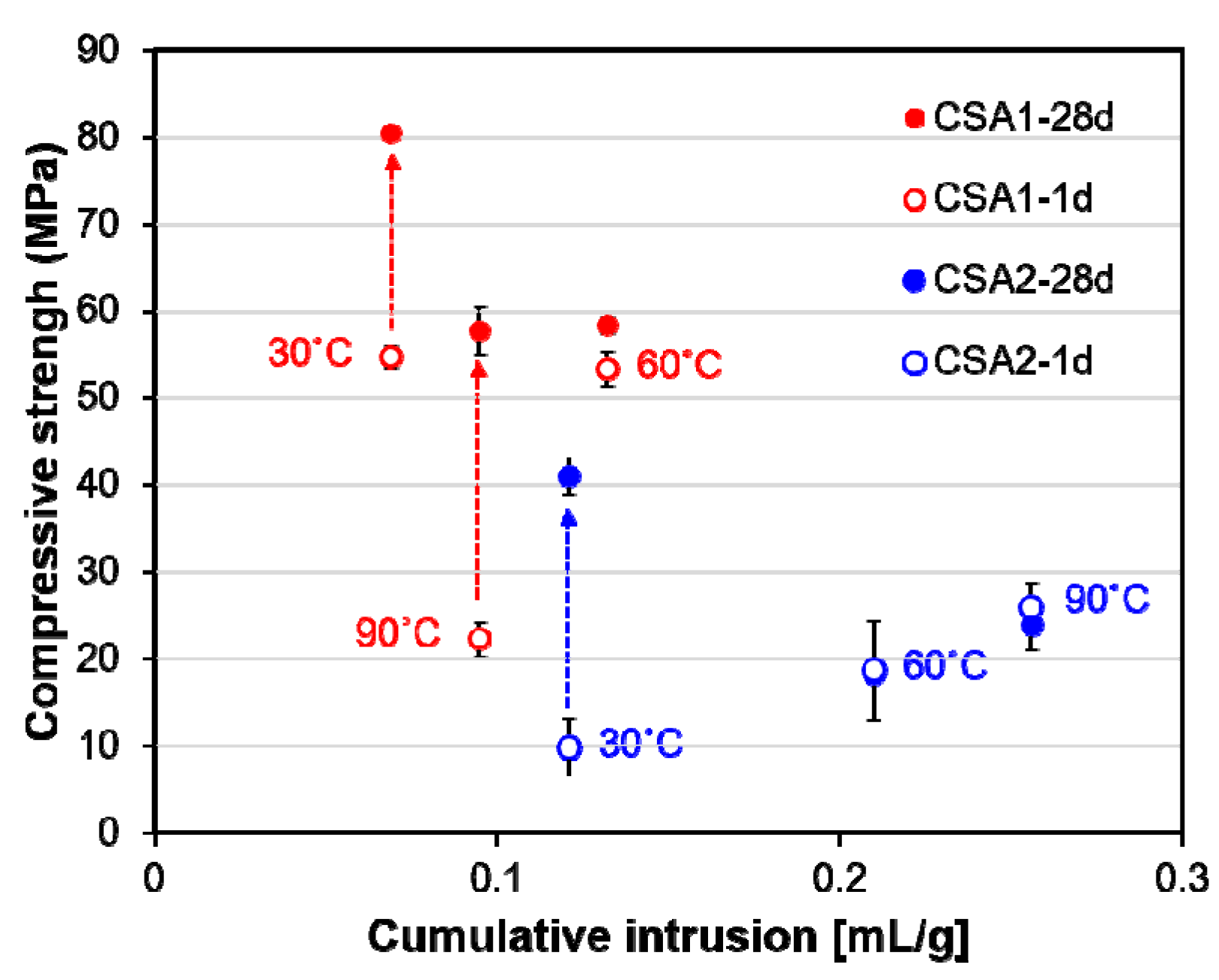
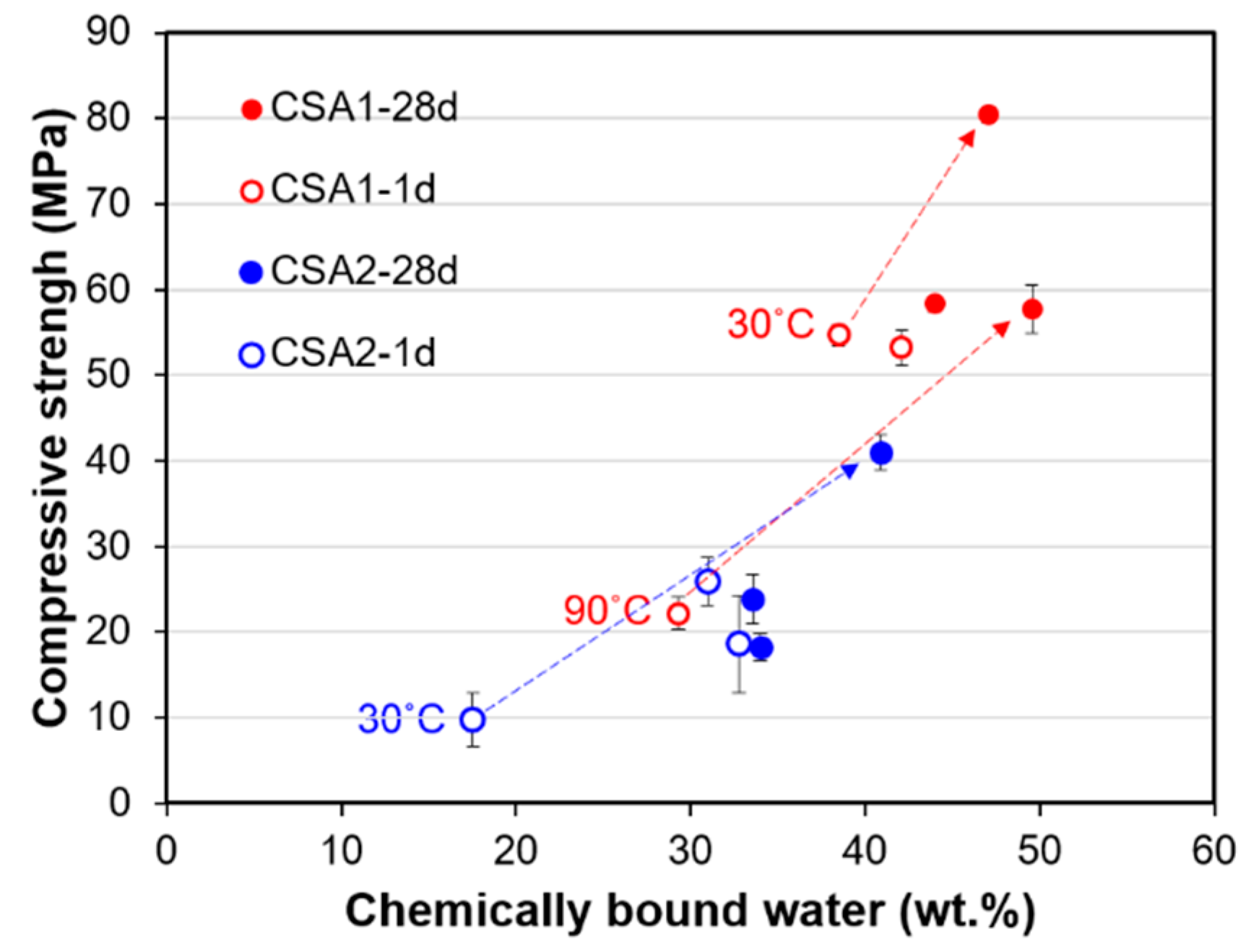
3.1. Compressive Strength and Dimensional Stability
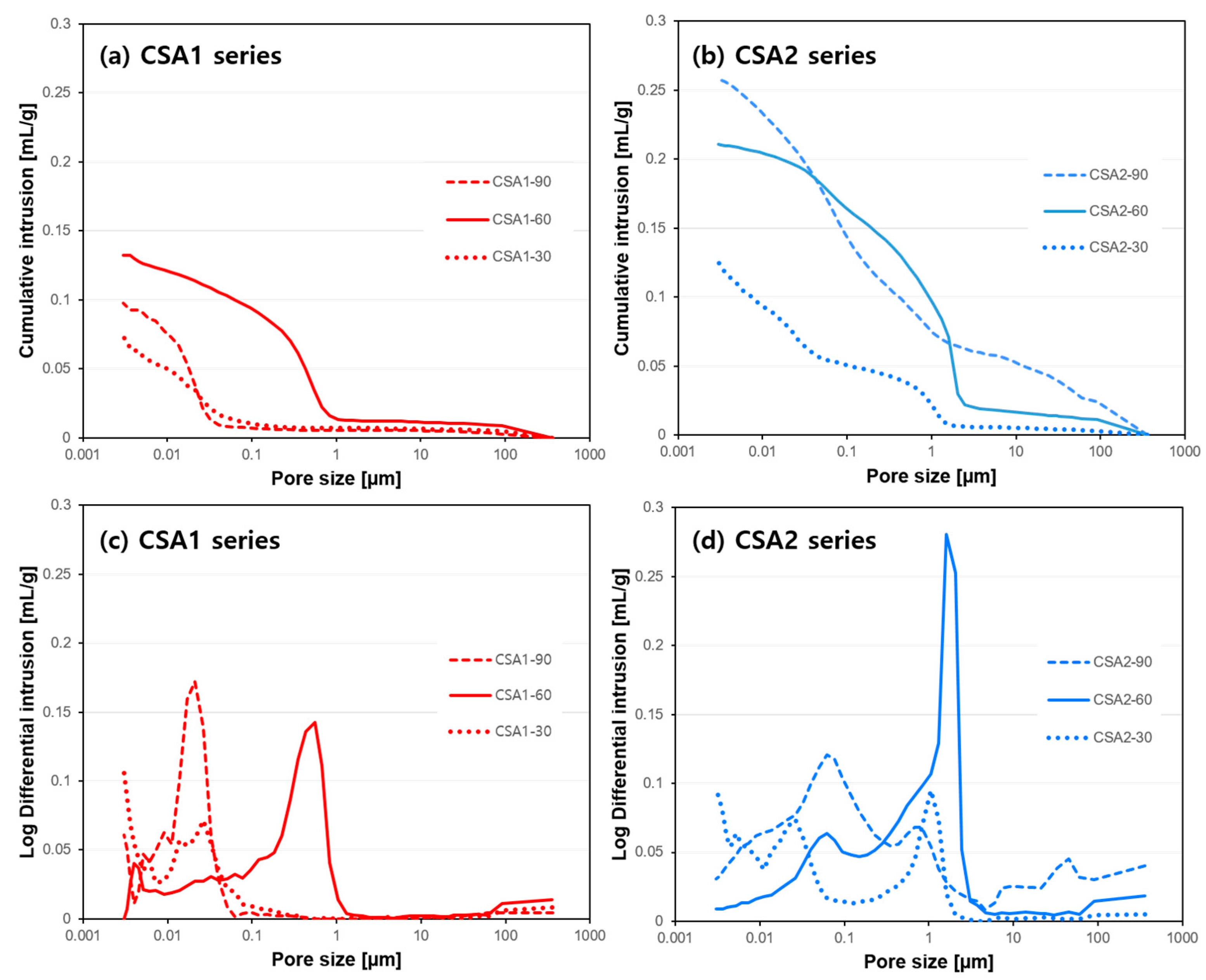
3.2. Pore Structure
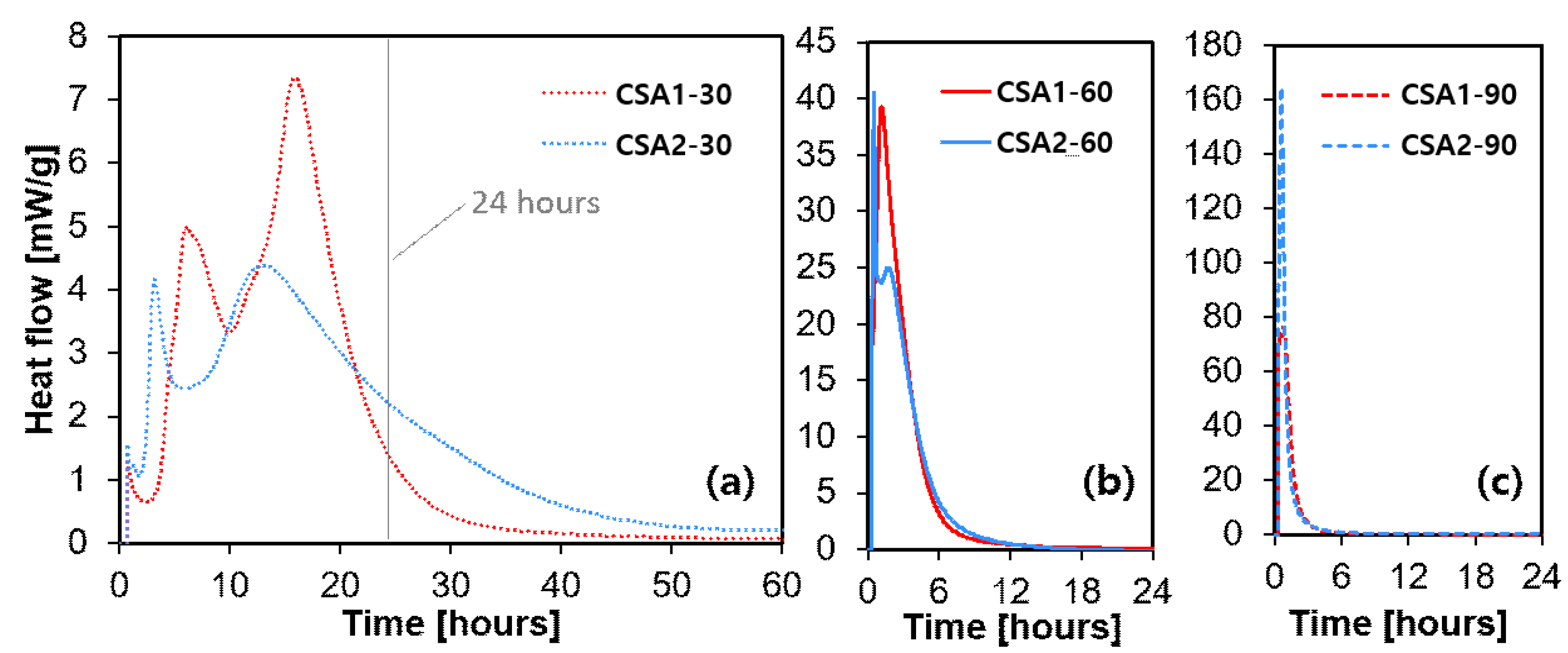
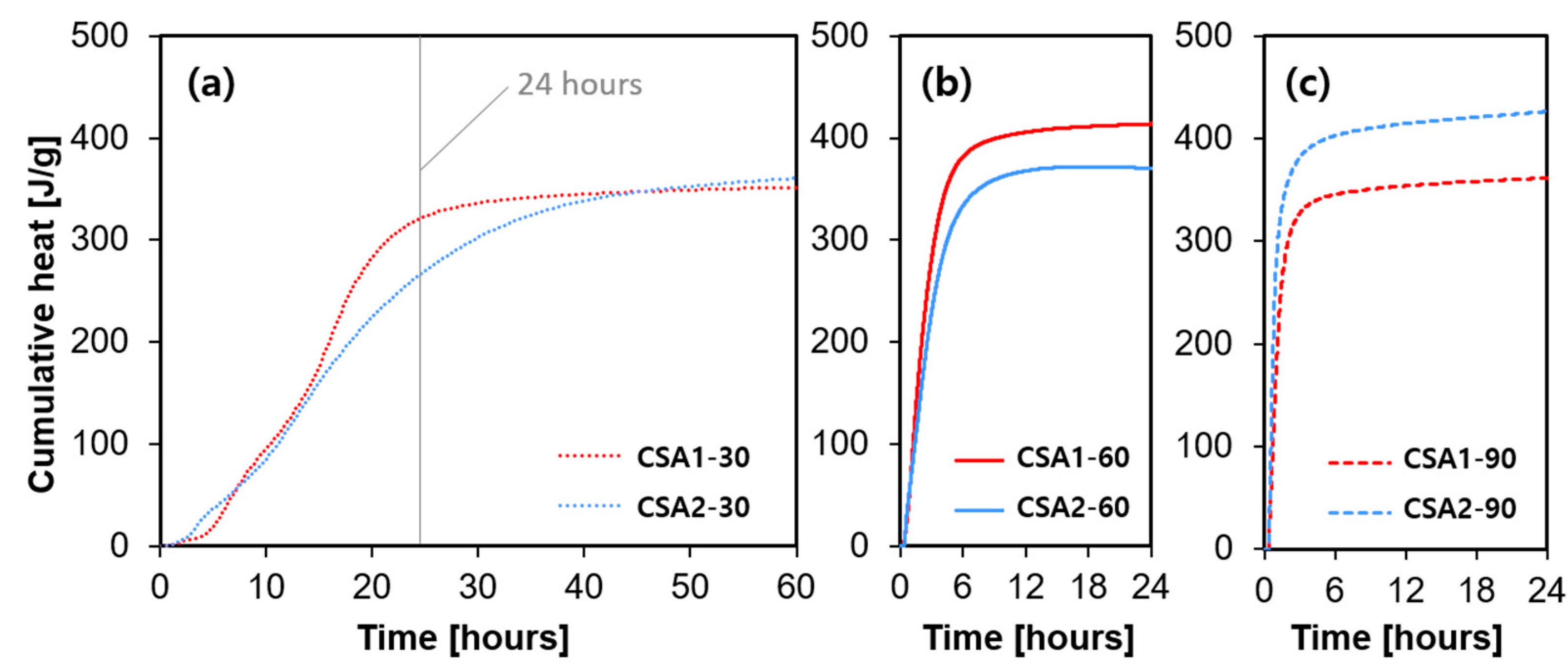
3.3. Heat of Hydration
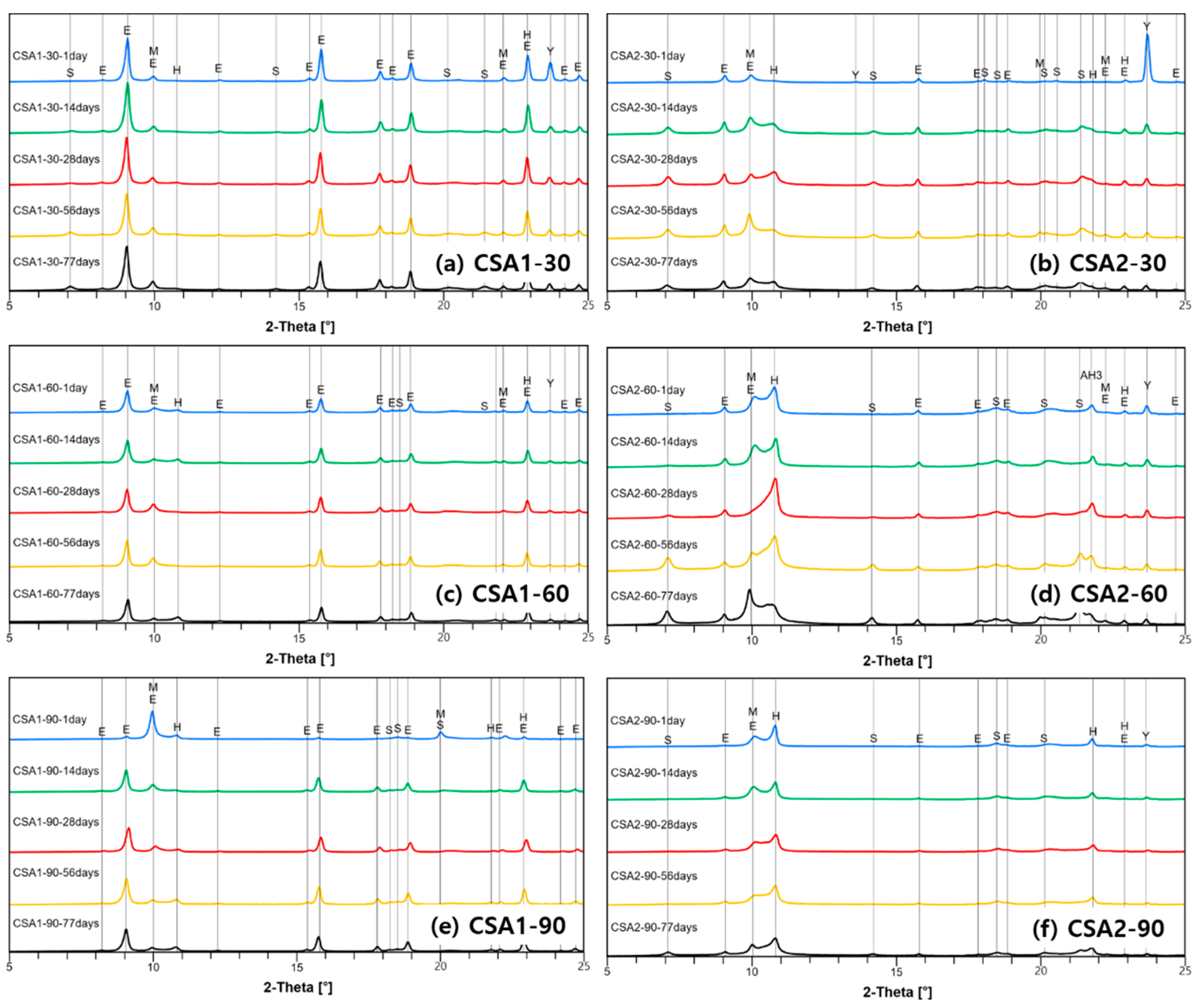
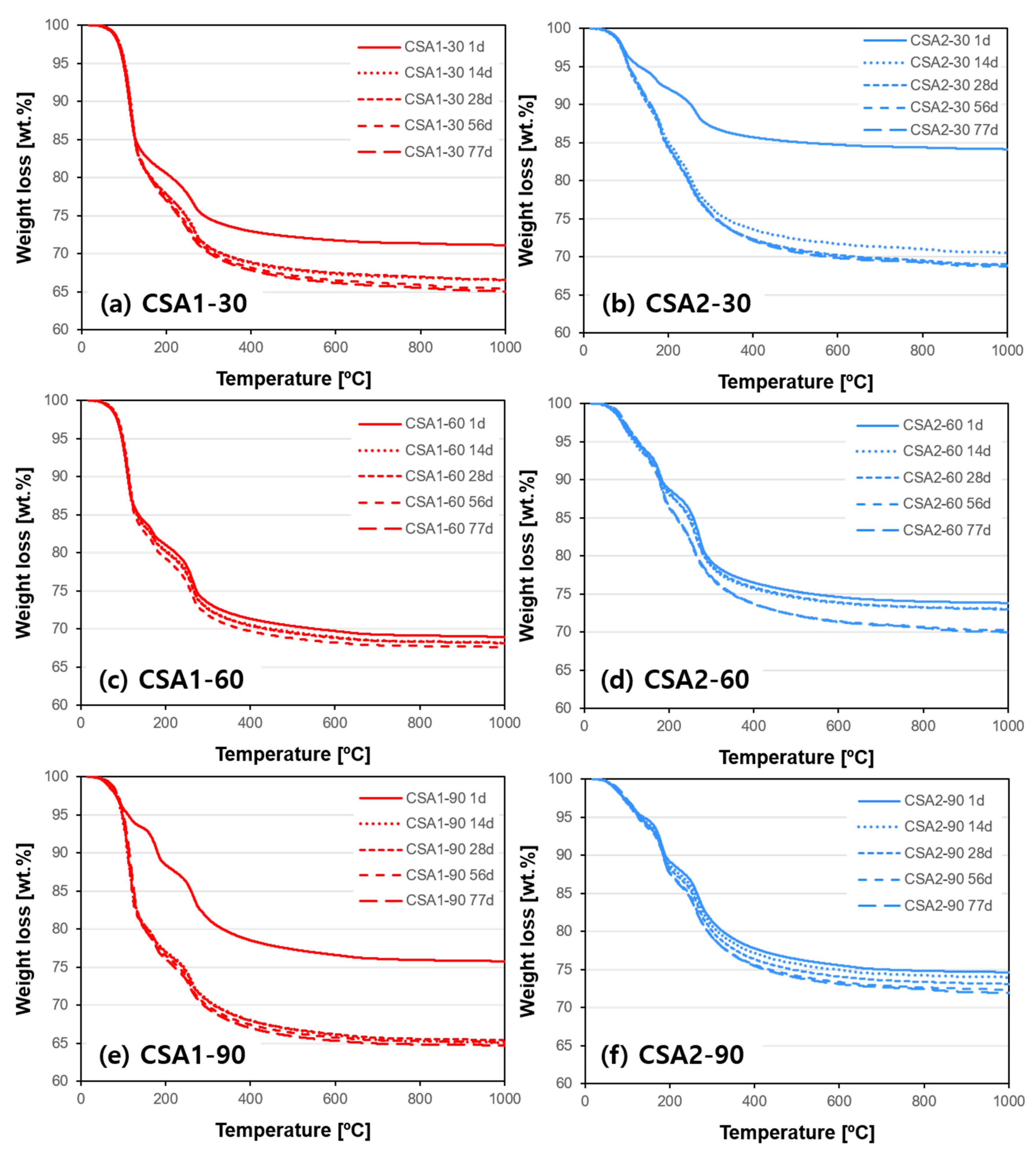
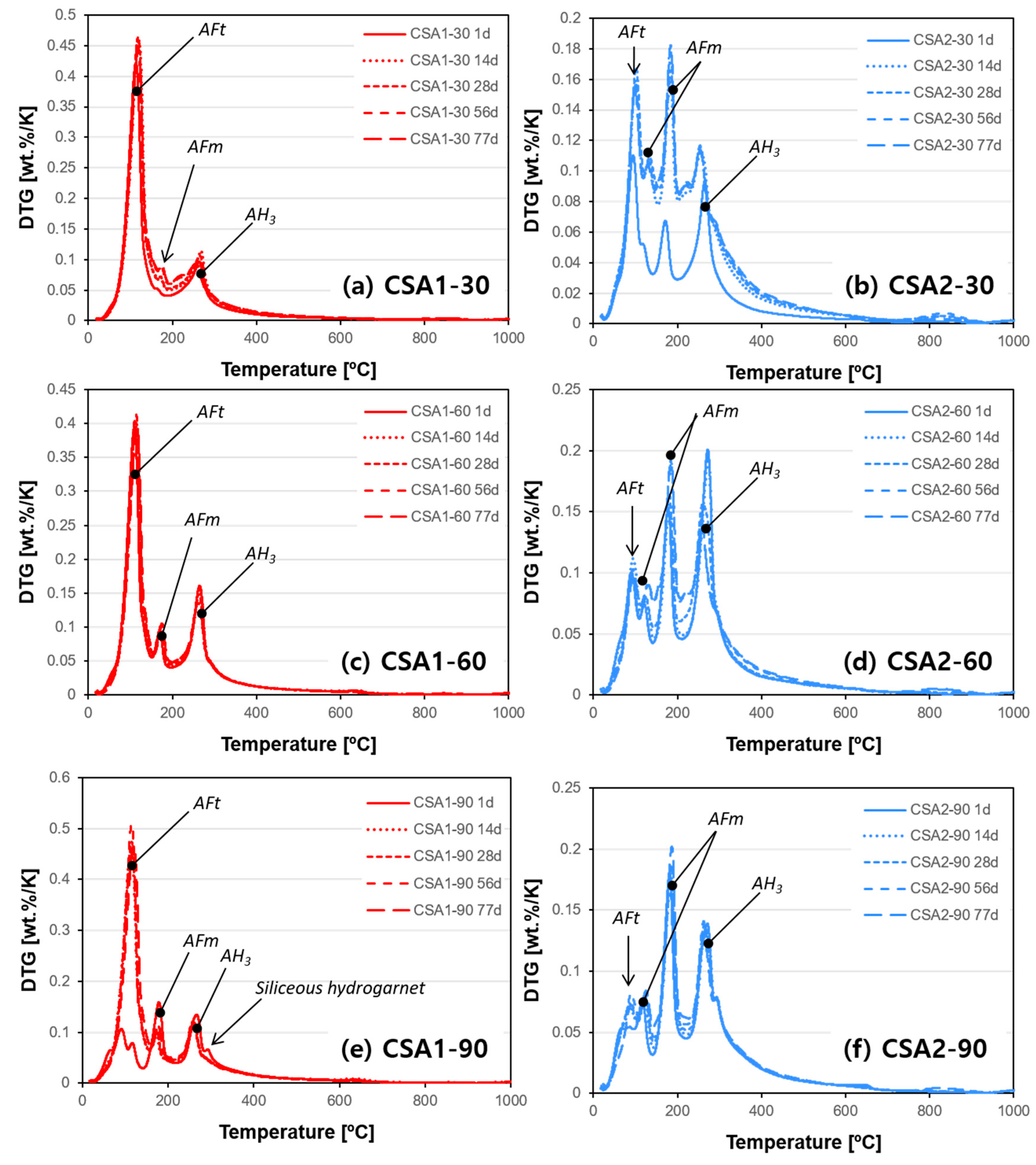
3.4. X-ray Diffraction and Thermogravimetric Analysis
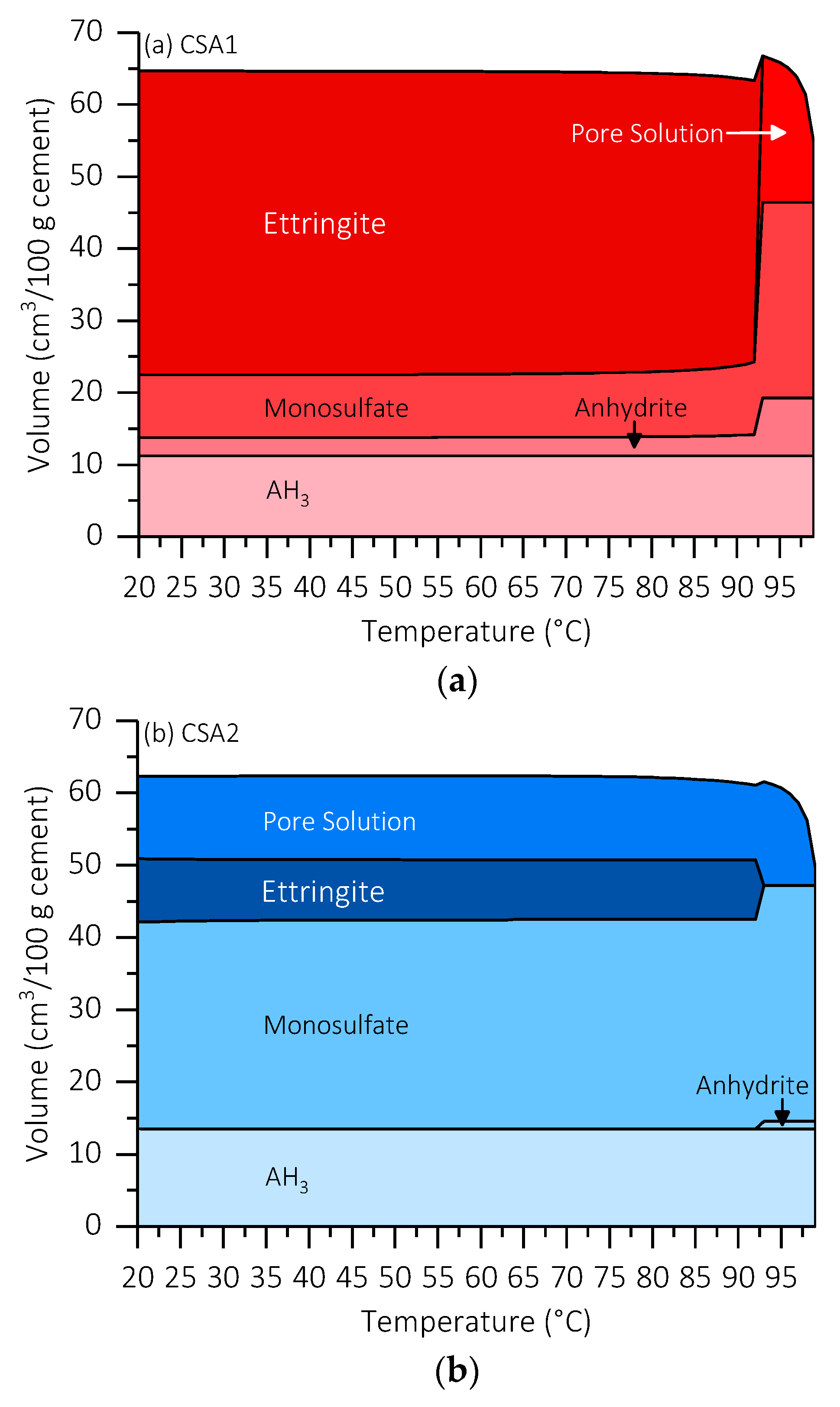
3.5. Thermodynamic Modeling
4. Discussion
4.1. Hydration Characteristics at 30 °C Curing Temperature
4.2. Decomposition and Re-Formation of Ettringite
4.3. Relations of Material Properties with Hydration Characteristics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alexander, K. Calcium Aluminosulfate and Expansive Cements Containing Same. US3155526A, 3 November 1964. [Google Scholar]
- Al Horr, Y.; Elhoweris, A.; Elsarrag, E. The development of a novel process for the production of calcium sulfoaluminate. Int. J. Sustain. Built Environ. 2017, 6, 734–741. [Google Scholar] [CrossRef]
- Chen, I.A.; Hargis, C.W.; Juenger, M.C. Understanding expansion in calcium sulfoaluminate–belite cements. Cem. Concr. Res. 2012, 42, 51–60. [Google Scholar] [CrossRef]
- Jeong, Y.; Hargis, C.; Chun, S.; Moon, J. Effect of calcium carbonate fineness on calcium sulfoaluminate-belite cement. Materials 2017, 10, 900. [Google Scholar] [CrossRef]
- Jeong, Y.; Hargis, C.W.; Chun, S.-C.; Moon, J. The effect of water and gypsum content on strätlingite formation in calcium sulfoaluminate-belite cement pastes. Constr. Build. Mater. 2018, 166, 712–722. [Google Scholar] [CrossRef]
- Schneider, M.; Romer, M.; Tschudin, M.; Bolio, H. Sustainable cement production—Present and future. Cem. Concr. Res. 2011, 41, 642–650. [Google Scholar] [CrossRef]
- Glasser, F.P.; Zhang, L. High-performance cement matrices based on calcium sulfoaluminate–belite compositions. Cem. Concr. Res. 2001, 31, 1881–1886. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, M.; Zhang, Y. Preparation and properties of self-pulverizing calcium sulfoaluminate cement. Constr. Build. Mater. 2012, 34, 107–113. [Google Scholar] [CrossRef]
- Juenger, M.; Winnefeld, F.; Provis, J.L.; Ideker, J. Advances in alternative cementitious binders. Cem. Concr. Res. 2011, 41, 1232–1243. [Google Scholar] [CrossRef]
- Winnefeld, F.; Lothenbach, B. Hydration of calcium sulfoaluminate cements—Experimental findings and thermodynamic modeling. Cem. Concr. Res. 2010, 40, 1239–1247. [Google Scholar] [CrossRef]
- Telesca, A.; Marroccoli, M.; Pace, M.; Tomasulo, M.; Valenti, G.; Monteiro, P. A hydration study of various calcium sulfoaluminate cements. Cem. Concr. Res. 2014, 53, 224–232. [Google Scholar] [CrossRef]
- Winnefeld, F.; Barlag, S. Calorimetric and thermogravimetric study on the influence of calcium sulfate on the hydration of ye’elimite. J. Therm. Anal. Calorim. 2009, 101, 949–957. [Google Scholar] [CrossRef]
- Zhang, L. Microstructure and Performance of Calcium Sulfoaluminate Cements. Ph.D. Thesis, University of Aberdeen, Aberdeen, UK, 28 June 2000. [Google Scholar]
- Beltagui, H.; Jen, G.; Whittaker, M.; Imbabi, M.S. The influence of variable gypsum and water content on the strength and hydration of a belite-calcium sulphoaluminate cement. J. Adv. Appl. Ceram. 2017, 116, 199–206. [Google Scholar] [CrossRef]
- Martin, L.H.; Winnefeld, F.; Müller, C.J.; Lothenbach, B. Contribution of limestone to the hydration of calcium sulfoaluminate cement. Cem. Concr. Res. 2015, 62, 204–211. [Google Scholar] [CrossRef]
- Pelletier-Chaignat, L.; Winnefeld, F.; Lothenbach, B.; Müller, C.J. Beneficial use of limestone filler with calcium sulphoaluminate cement. Constr. Build. Mater. 2012, 26, 619–627. [Google Scholar] [CrossRef]
- Martin, L.H.; Winnefeld, F.; Tschopp, E.; Müller, C.J.; Lothenbach, B. Influence of fly ash on the hydration of calcium sulfoaluminate cement. Cem. Concr. Res. 2017, 95, 152–163. [Google Scholar] [CrossRef]
- García-Maté, M.; De la Torre, A.G.; León-Reina, L.; Aranda, M.A.; Santacruz, I. Hydration studies of calcium sulfoaluminate cements blended with fly ash. Cem. Concr. Res. 2013, 54, 12–20. [Google Scholar] [CrossRef]
- Coppola, L.; Coffetti, D.; Crotti, E. Use of tartaric acid for the production of sustainable Portland-free CSA-based mortars. Constr. Build. Mater. 2018, 171, 243–249. [Google Scholar] [CrossRef]
- Coppola, L.; Coffetti, D.; Crotti, E.; Pastore, T. CSA-based Portland-free binders to manufacture sustainable concretes for jointless slabs on ground. Constr. Build. Mater. 2018, 187, 691–698. [Google Scholar] [CrossRef]
- Zhou, Q.; Glasser, F.P. Thermal stability and decomposition mechanisms of ettringite at <120 °C. Cem. Concr. Res. 2001, 31, 1333–1339. [Google Scholar] [CrossRef]
- Ghorab, H.; Kishar, E. Studies on the stability of the calcium sulfoaluminate hydrates. Part 1: Effect of temperature on the stability of ettringite in pure water. Cem. Concr. Res. 1985, 15, 93–99. [Google Scholar] [CrossRef]
- Kaufmann, J.; Winnefeld, F.; Lothenbach, B. Stability of ettringite in calcium sulfoaluminate based cement pastes at elevated temperatures. J. Adv. Cem. Res. 2016, 28, 251–261. [Google Scholar] [CrossRef]
- Chen, B.; Kuznik, F.; Horgnies, M.; Johannes, K.; Morin, V.; Gengembre, E. Physicochemical properties of ettringite/meta-ettringite for thermal energy storage. Solar Energy Mater. Solar Cells 2019, 193, 320–334. [Google Scholar] [CrossRef]
- Struble, L.J.; Brown, P.W. Heats of dehydration and specific heats of compounds found in concrete and their potential for thermal energy storage. J. Solar Energy Mater. 1986, 14, 1–12. [Google Scholar] [CrossRef]
- Ndiaye, K.; Cyr, M.; Ginestet, S. Durability and stability of an ettringite-based material for thermal energy storage at low temperature. Cem. Concr. Res. 2017, 99, 106–115. [Google Scholar] [CrossRef]
- Wang, P.; Li, N.; Xu, L. Hydration evolution and compressive strength of calcium sulphoaluminate cement constantly cured over the temperature range of 0 to 80 °C. Cem. Concr. Res. 2017, 100, 203–213. [Google Scholar] [CrossRef]
- Li, N.; Xu, L.; Wang, R.; Li, L.; Wang, P. Experimental study of calcium sulfoaluminate cement-based self-leveling compound exposed to various temperatures and moisture conditions: Hydration mechanism and mortar properties. Cem. Concr. Res. 2018, 108, 103–115. [Google Scholar] [CrossRef]
- Hwang, S.-D.; Khatib, R.; Lee, H.K.; Lee, S.-H.; Khayat, K.H. Optimization of steam-curing regime for high-strength, self-consolidating concrete for precast, prestressed concrete applications. PCI J. 2012, 57, 48–62. [Google Scholar] [CrossRef]
- Standard Practice for Mechanical Mixing of Hydraulic Cement Pastes and Mortars of Plastic Consistency; ASTM International: West Conshohocken, PA, USA, 2014.
- Standard test method for compressive strength of hydraulic cement mortars (using 2-in. or [50-mm] cube specimens). In Annual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 2013; Volume 4, pp. 1–9.
- Standard Practice for Use of Apparatus for the Determination of Length Change of Hardened Cement Paste, Mortar, and Concrete; ASTM International: West Conshohocken, PA, USA, 2004.
- Snellings, R.; Chwast, J.; Cizer, Ö.; De Belie, N.; Dhandapani, Y.; Durdzinski, P.; Elsen, J.; Haufe, J.; Hooton, D.; Patapy, C.; et al. Report of TC 238-SCM: hydration stoppage methods for phase assemblage studies of blended cements—Results of a round robin test. Mater. Struct. 2018, 51, 111. [Google Scholar] [CrossRef]
- Scrivener, K.; Snellings, R.; Lothenbach, B. A Practicl Guide to Microstructural Analysis of Cementitious Materials; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- X’Pert HighScore Plus, 3.0.5 ed.; PANalytical: Almelo, The Netherlands, 2012.
- Allmann, R.; Hinek, R. The introduction of structure types into the Inorganic Crystal Structure Database ICSD. Acta Crystallogr. Sect. A 2007, 63, 412–417. [Google Scholar] [CrossRef]
- Daškevič, A.; Merkys, A.; Le Bail, A.; Chateigner, D.; Lutterotti, L.; Quirós, M.; Serebryanaya, N.R.; Moeck, P.; Downs, R.T.; Gražulis, S. Crystallography Open Database (COD): An open-access collection of crystal structures and platform for world-wide collaboration. Nucleic Acids Res. 2011, 40, D420–D427. [Google Scholar]
- Kulik, D.A.; Wagner, T.; Dmytrieva, S.V.; Kosakowski, G.; Hingerl, F.F.; Chudnenko, K.V.; Berner, U.R. GEM-Selektor geochemical modeling package: Revised algorithm and GEMS3K numerical kernel for coupled simulation codes. J. Comput. Geosci. 2013, 17, 1–24. [Google Scholar] [CrossRef]
- Wagner, T.; Kulik, D.A.; Hingerl, F.F.; Dmytrieva, S.V. GEM-Selektor geochemical modeling package: TSolMod library and data interface for multicomponent phase models. Can. Mineral. 2012, 50, 1173–1195. [Google Scholar] [CrossRef]
- Hummel, W.; Berner, U.; Curti, E.; Pearson, F.J.; Thoenen, T. Nagra/PSI chemical thermodynamic data base 01/01. Radiochim. Acta 2002, 90, 805–813. [Google Scholar] [CrossRef]
- Lothenbach, B.; Matschei, T.; Möschner, G.; Glasser, F.P. Thermodynamic modeling of the effect of temperature on the hydration and porosity of Portland cement. Cem. Concr. Res. 2008, 38, 1–18. [Google Scholar] [CrossRef]
- Matschei, T.; Lothenbach, B.; Glasser, F.P. Thermodynamic properties of Portland cement hydrates in the system CaO–Al2O3–SiO2–CaSO4–CaCO3–H2O. Cem. Concr. Res. 2007, 37, 1379–1410. [Google Scholar] [CrossRef]
- Lothenbach, B.; Pelletier-Chaignat, L.; Winnefeld, F. Stability in the system CaO–Al2O3–H2O. Cem. Concr. Res. 2012, 42, 1621–1634. [Google Scholar] [CrossRef]
- Dilnesa, B.Z.; Lothenbach, B.; Renaudin, G.; Wichser, A.; Kulik, D. Synthesis and characterization of hydrogarnet Ca3 (AlxFe1− x) 2 (SiO4) y (OH) 4 (3− y). Cem. Concr. Res. 2014, 59, 96–111. [Google Scholar] [CrossRef]
- Cuesta, A.; De la Torre, A.G.; Losilla, E.R.; Santacruz, I.; Aranda, M.A. Pseudocubic crystal structure and phase transition in doped ye’elimite. Cryst. Growth Des. 2014, 14, 5158–5163. [Google Scholar] [CrossRef]
- Ossorio, M.; Van Driessche, A.; Pérez, P.; García-Ruiz, J. The gypsum–anhydrite paradox revisited. J. Chem. Geol. 2014, 386, 16–21. [Google Scholar] [CrossRef]
- Zhang, L.; Glasser, F. Hydration of calcium sulfoaluminate cement at less than 24 h. Adv. Cem. Res. 2002, 14, 141–156. [Google Scholar] [CrossRef]
- Glasser, F.P.; Zhang, L. Calculation of Chemical Water Demand for Hydration of Calcium Sulfoaluminate Cement. In Proceedings of the 4th International Symposium on Cement and Concrete, Shanghai, China, 26–29 October 1998. [Google Scholar]
- Pera, J.; Ambroise, J. New applications of calcium sulfoaluminate cement. Cem. Concr. Res. 2004, 34, 671–676. [Google Scholar] [CrossRef]
- Li, G.; Walenta, G.; Gartner, E. Formation and hydration of low-CO2 cements based on belite, calcium sulfoaluminate and calcium aluminoferrite. In Proceedings of the 12th ICCC, Montreal, QC, Canada, 8 July 2007; pp. 9–12. [Google Scholar]
- Hargis, C.W.; Telesca, A.; Monteiro, P.J. Calcium sulfoaluminate (Ye’elimite) hydration in the presence of gypsum, calcite, and vaterite. Cem. Concr. Res. 2014, 65, 15–20. [Google Scholar] [CrossRef]
- Hargis, C.W.; Lothenbach, B.; Müller, C.J.; Winnefeld, F. Further insights into calcium sulfoaluminate cement expansion. Adv. Cem. Res. 2018, 1–18. [Google Scholar] [CrossRef]
- Scherer, G.W. Crystallization in pores. Cem. Concr. Res. 1999, 29, 1347–1358. [Google Scholar] [CrossRef]
- Bizzozero, J.; Gosselin, C.; Scrivener, K.L. Expansion mechanisms in calcium aluminate and sulfoaluminate systems with calcium sulfate. Cem. Concr. Res. 2014, 56, 190–202. [Google Scholar] [CrossRef]
- Hall, C.; Barnes, P.; Billimore, A.D.; Jupe, A.C.; Turrillas, X. Thermal decomposition of ettringite Ca6[Al(OH)6]2(SO4)3·26H2O. J. Chem. Soc. 1996, 92, 2125–2129. [Google Scholar]
- Meller, N.; Kyritsis, K.; Hall, C. The hydrothermal decomposition of calcium monosulfoaluminate 14-hydrate to katoite hydrogarnet and β-anhydrite: An in-situ synchrotron X-ray diffraction study. J. Solid State Chem. 2009, 182, 2743–2747. [Google Scholar] [CrossRef]
- Šatava, V.; Vepřek, O. Thermal decomposition of ettringite under hydrothermal conditions. J. Am. Ceram. Soc. 1975, 58, 357–359. [Google Scholar] [CrossRef]

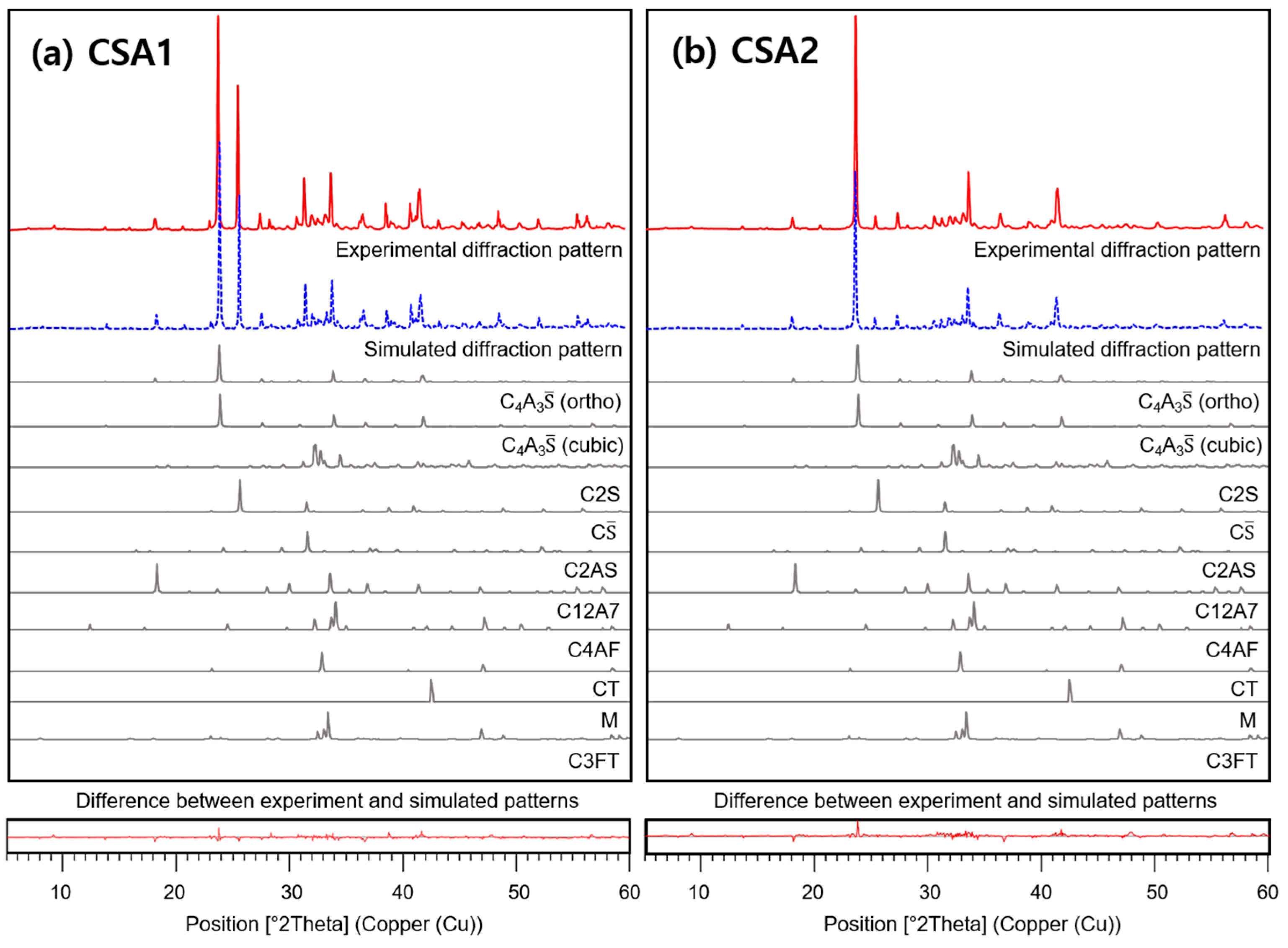
| XRF | QXRD | ||||
|---|---|---|---|---|---|
| Oxide | CSA1 (wt.%) | CSA2 (wt.%) | Phases | CSA1 (wt.%) | CSA2 (wt.%) |
| CaO | 41.3 | 41.3 | C4A3 (ortho) | 40.0 | 51.0 |
| Al2O3 | 28.1 | 34.5 | C4A3 (cubic) | 13.6 | 13.4 |
| SiO2 | 5.6 | 6.96 | β-C2S | 9.7 | 15.0 |
| SO3 | 18.0 | 9.46 | C | 23.8 | 3.2 |
| Fe2O3 | 1.6 | 2.0 | C2AS | 1.6 | 4.0 |
| TiO2 | 1.2 | 1.4 | C12A7 | 1.7 | 2.1 |
| MgO | 1.1 | 1.4 | C4AF | 0.9 | 1.0 |
| Br | 1.4 | 1.4 | CT | 1.1 | 1.4 |
| K2O | 0.2 | 0.2 | M | 0.6 | 0.7 |
| SrO | 0.1 | 0.1 | C3FT | 6.8 | 8.1 |
| ZrO2 | 0.1 | 0.1 | - | ||
| LOI † | 1.2 | 1.0 | |||
| Label | CSA Cement | Sand | Water | Curing Condition | |
|---|---|---|---|---|---|
| CSA1 | CSA2 | ||||
| CSA1-30 | 100 | - | 240 | 50 | Cured at 30 °C for all times |
| CSA2-30 | - | 100 | 240 | 50 | |
| CSA1-60 | 100 | - | 240 | 50 | Cured at 60 °C for 24 h then cured at 30 °C until testing |
| CSA2-60 | - | 100 | 240 | 50 | |
| CSA1-90 | 100 | - | 240 | 50 | Cured at 90 °C for 24 h then cured at 30 °C until testing |
| CSA2-90 | - | 100 | 240 | 50 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, Y.; Hargis, C.W.; Kang, H.; Chun, S.-C.; Moon, J. The Effect of Elevated Curing Temperatures on High Ye’elimite Calcium Sulfoaluminate Cement Mortars. Materials 2019, 12, 1072. https://doi.org/10.3390/ma12071072
Jeong Y, Hargis CW, Kang H, Chun S-C, Moon J. The Effect of Elevated Curing Temperatures on High Ye’elimite Calcium Sulfoaluminate Cement Mortars. Materials. 2019; 12(7):1072. https://doi.org/10.3390/ma12071072
Chicago/Turabian StyleJeong, Yeonung, Craig W. Hargis, Hyunuk Kang, Sung-Chul Chun, and Juhyuk Moon. 2019. "The Effect of Elevated Curing Temperatures on High Ye’elimite Calcium Sulfoaluminate Cement Mortars" Materials 12, no. 7: 1072. https://doi.org/10.3390/ma12071072
APA StyleJeong, Y., Hargis, C. W., Kang, H., Chun, S.-C., & Moon, J. (2019). The Effect of Elevated Curing Temperatures on High Ye’elimite Calcium Sulfoaluminate Cement Mortars. Materials, 12(7), 1072. https://doi.org/10.3390/ma12071072






