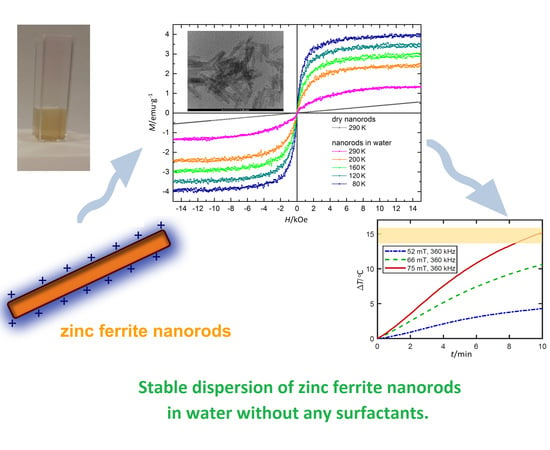
One-Step Synthesis of Long Term Stable Superparamagnetic Colloid of Zinc Ferrite Nanorods in Water
Abstract
1. Introduction
1.1. Ferrite Nanoparticles Applications
1.2. Synthesis Methods
1.3. Applications of Colloidal Dispersions
1.4. Stability of Colloidal Dispersion
- (1)
- (2)
1.5. Present Research
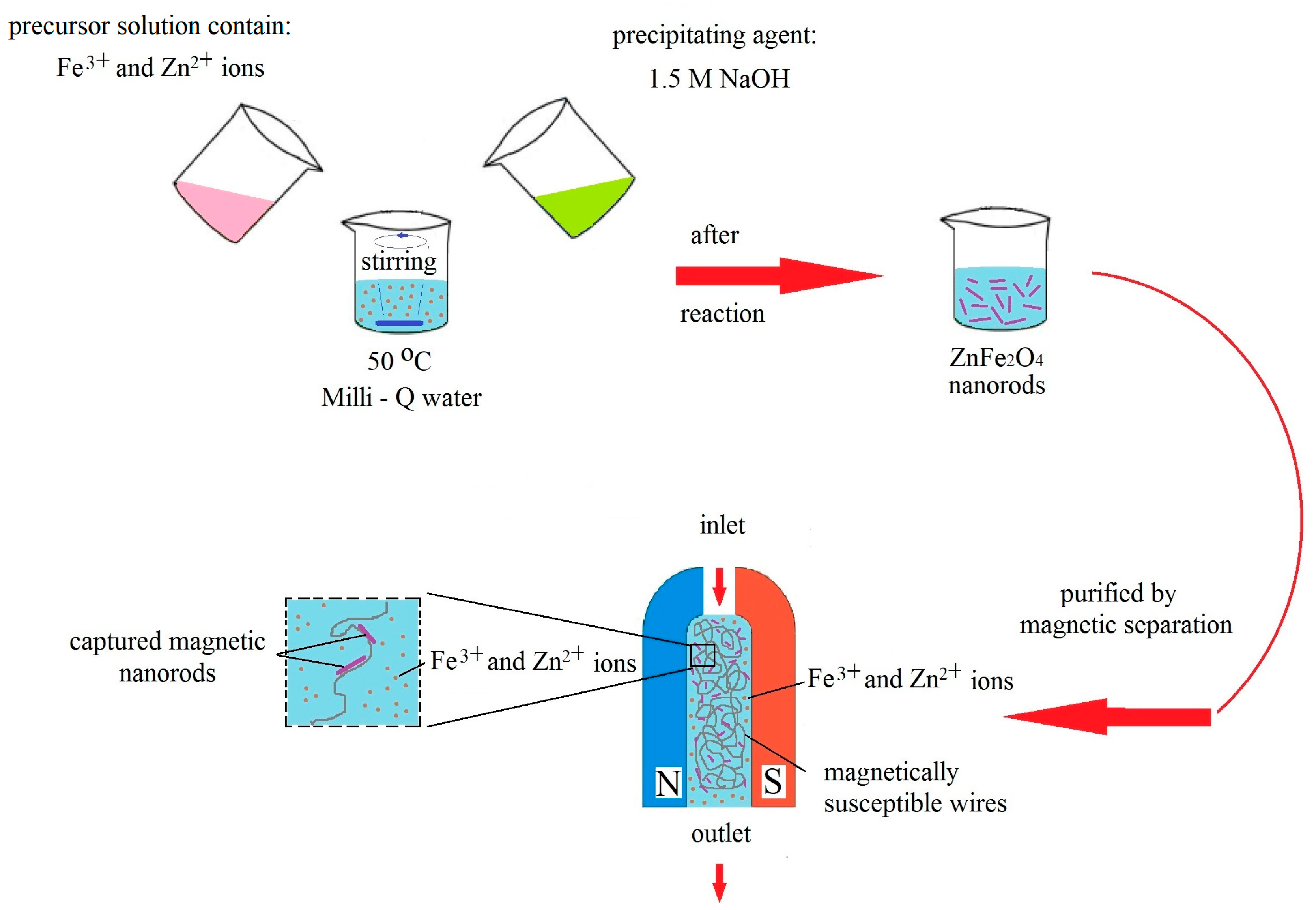
2. Materials and Methods
2.1. Materials
Synthesis of ZnFe2O4 Nanorods in Water Solution
2.2. Methods
2.2.1. X-ray Powder Diffraction (XRD)
2.2.2. Transmission Electron Microscopy (TEM)
2.2.3. Small Angle X-ray Scattering (SAXS)
2.2.4. Mössbauer Spectroscopy
2.2.5. Near Edge X-ray Absorption Structure (XANES)
2.2.6. Dynamic Light Scattering (DLS) and Zeta Potential Measurement
2.2.7. Vibrating Sample Magnetometry (VSM)
2.2.8. Magnetic Hyperthermia
3. Results and Discussion
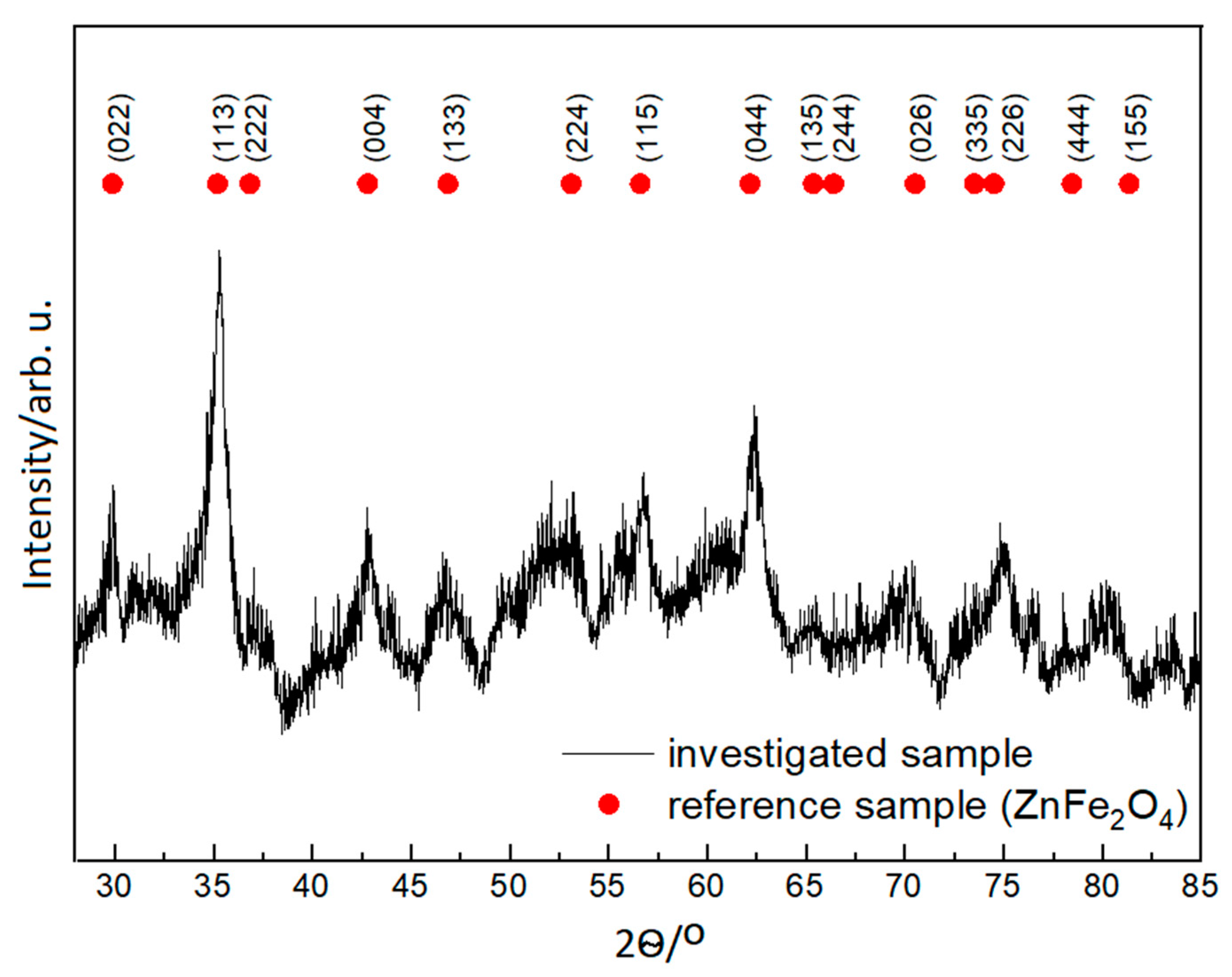
3.1. X-ray Powder Diffraction and Transmission Electron Microscopy
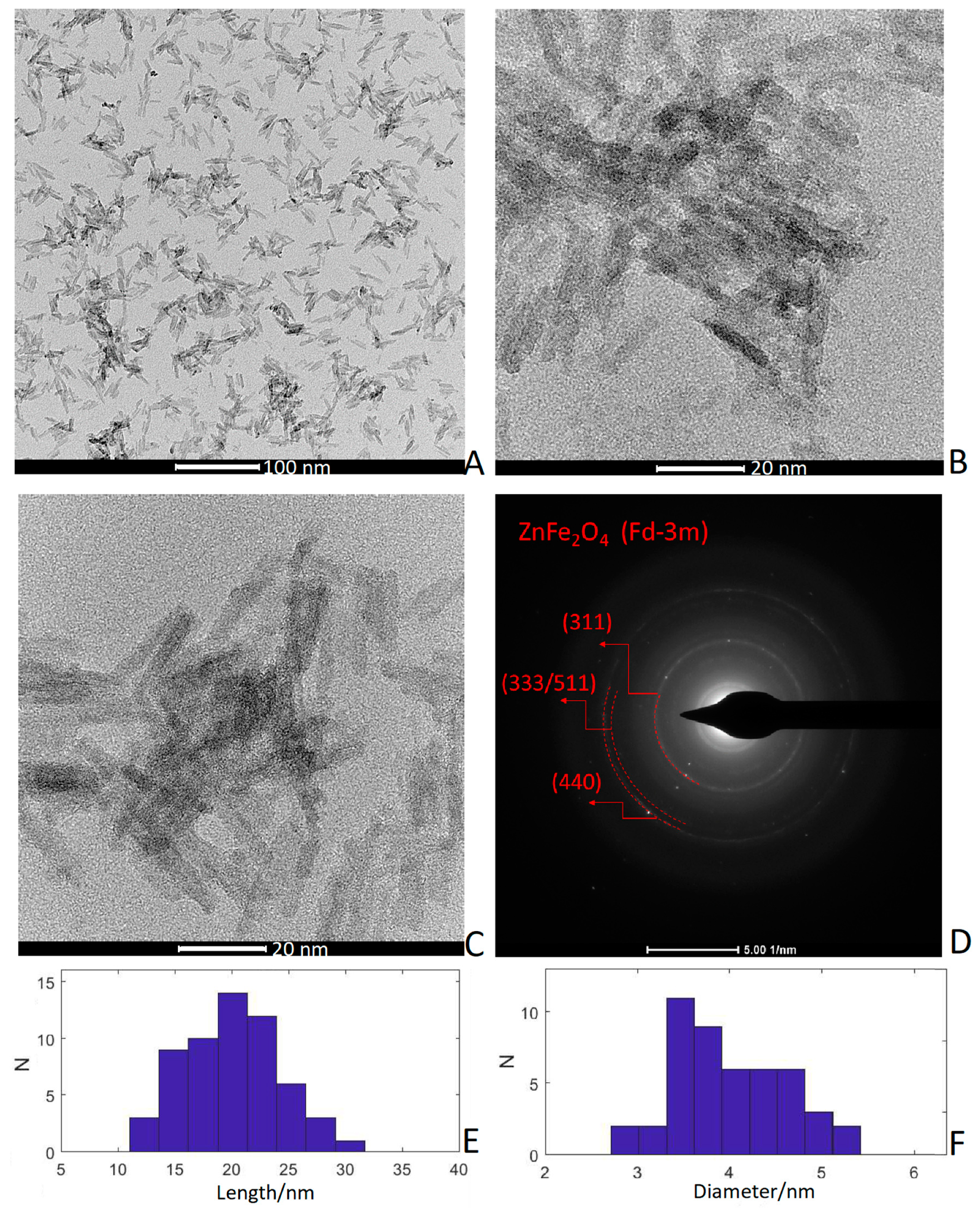
3.2. TEM Images and Selected-Area Electron Diffraction (SAED)
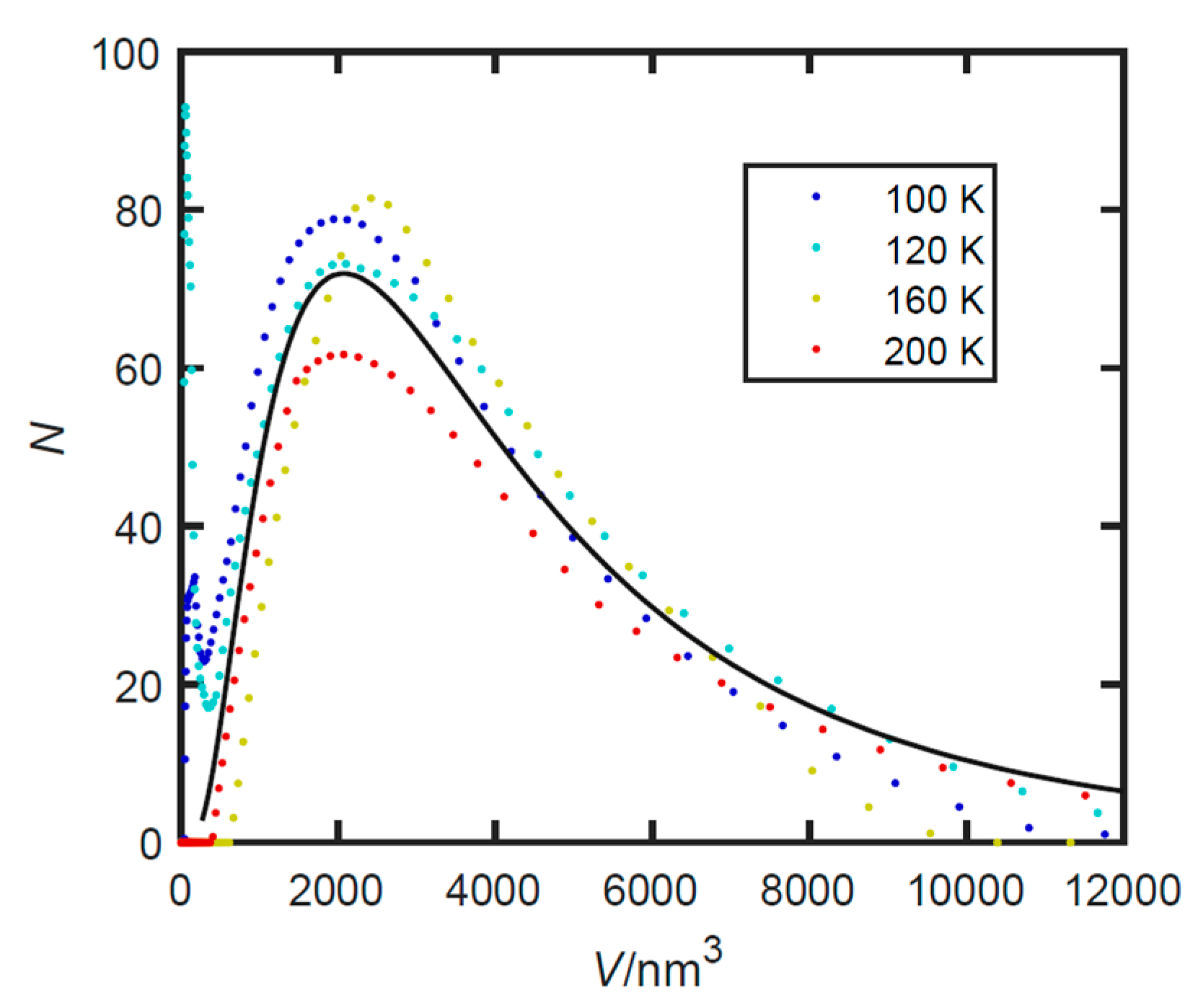
3.3. Small Angle X-ray Scattering
3.4. Zeta Potential (ζ) and Dynamic Light Scattering
3.5. Mössbauer Spectroscopy
3.6. X-ray Absorption Spectroscopy
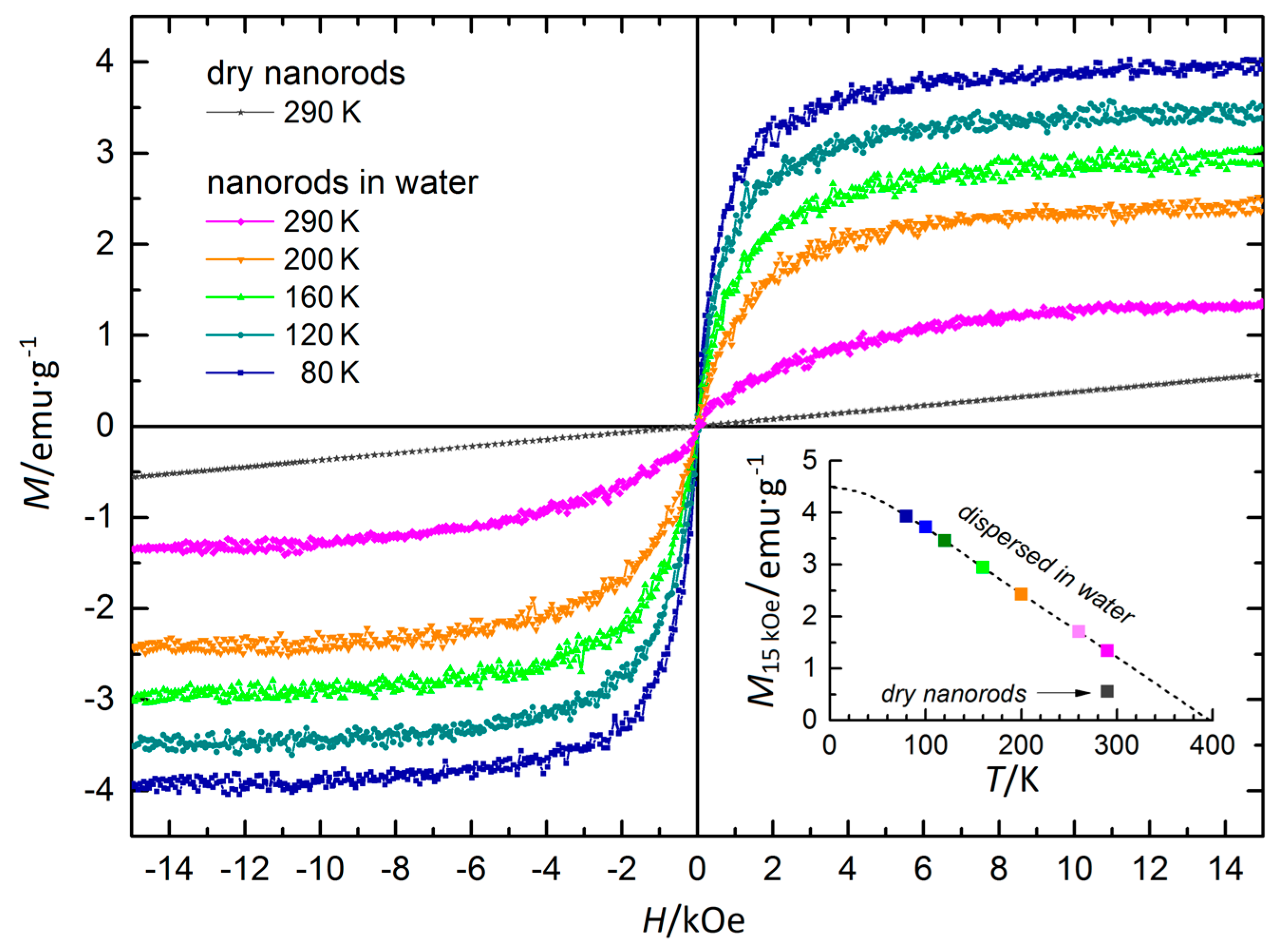
3.7. Magnetic Properties
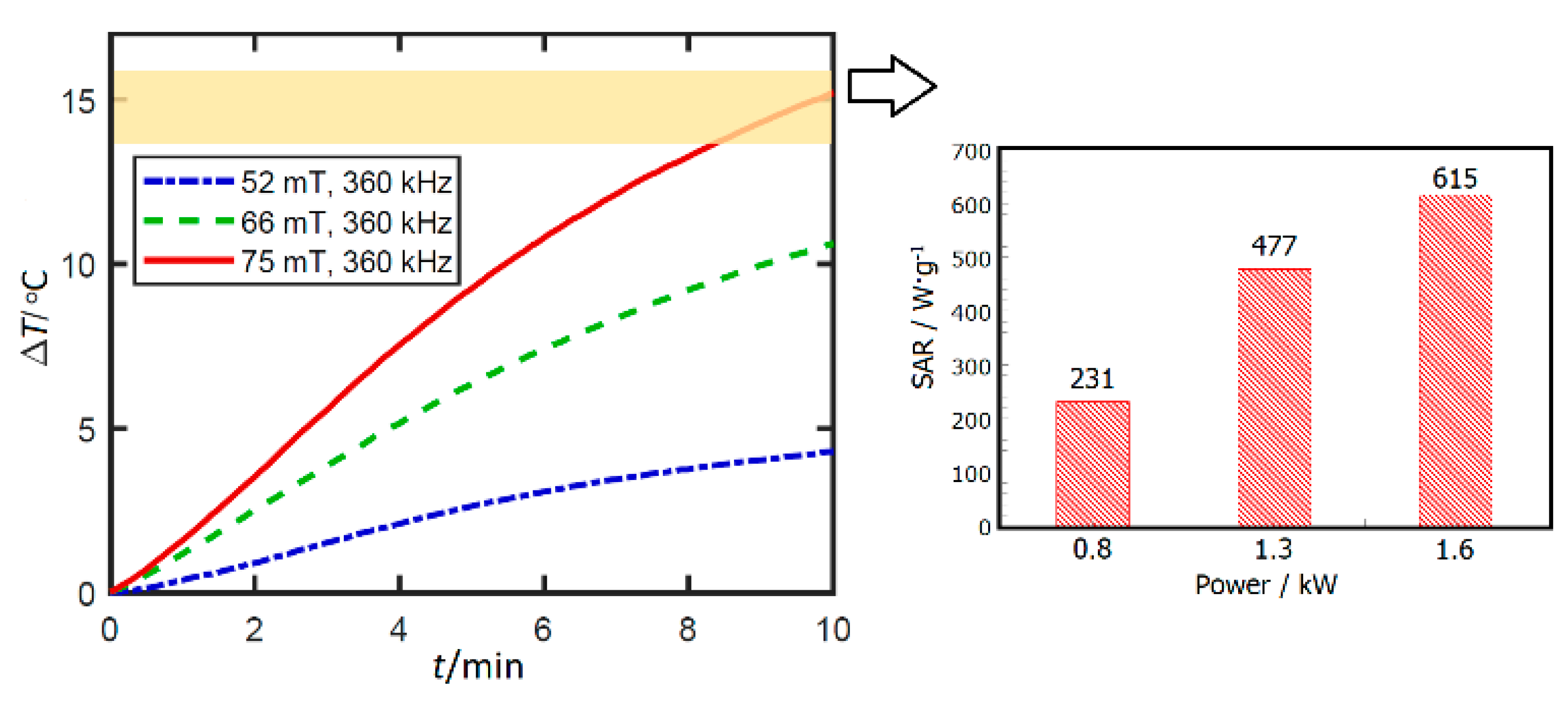
3.8. Potential Application of ZnFe2O4 Nanorods Dispersed in Water in Magnetic Hyperthermia Therapy
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xu, Y.; Qin, Y.; Palchoudhury, S.; Bao, Y. Water-soluble iron oxide nanoparticles with high stability and selective surface functionality. Langmuir 2011, 27, 8990–8997. [Google Scholar] [CrossRef]
- Singh, A.; Singh, A.; Singh, S.; Tandon, P.; Yadav, B.C.; Yadav, R.R. Synthesis, characterization and performance of zinc ferrite nanorods for room temperature sensing applications. J. Alloys Compd. 2015, 618, 475–483. [Google Scholar] [CrossRef]
- Gavilán, H.; Kowalski, A.; Heinke, D.; Sugunan, A.; Sommertune, J.; Varón, M.; Bogart, L.K.; Posth, O.; Zeng, L.; González-Alonso, D.; et al. Colloidal flower-shaped iron oxide nanoparticles: Synthesis strategies and coatings. Part. Part. Syst. Charact. 2017, 34, 1–12. [Google Scholar] [CrossRef]
- Ullrich, S.; Scheeler, S.P.; Pacholski, C.; Spatz, J.P.; Kudera, S. Formation of large 2D arrays of shape-controlled colloidal nanoparticles at variable interparticle distances. Part. Part. Syst. Charact. 2013, 30, 102–108. [Google Scholar] [CrossRef]
- Upadhyay, C.; Verma, H.C.; Sathe, V.; Pimpale, A.V. Effect of size and synthesis route on the magnetic properties of chemically prepared nanosize ZnFe2O4. J. Magn. Magn. Mater. 2007, 312, 271–279. [Google Scholar] [CrossRef]
- Jia, Z.; Ren, D.; Liang, Y.; Zhu, R. A new strategy for the preparation of porous zinc ferrite nanorods with subsequently light-driven photocatalytic activity. Mater. Lett. 2011, 65, 3116–3119. [Google Scholar] [CrossRef]
- Kim, J.H.; Jang, Y.J.; Kim, J.H.; Jang, J.-W.; Choi, S.H.; Lee, J.S. Defective ZnFe2O4 nanorods with oxygen vacancy for photoelectrochemical water splitting. Nanoscale 2015, 7, 19144–19151. [Google Scholar] [CrossRef]
- Dolcet, P.; Diodati, S.; Zorzi, F.; Voepel, P.; Seitz, C.; Smarsly, B.M.; Mascotto, S.; Nestola, F.; Gross, S. Very fast crystallisation of MFe2O4 spinel ferrites (M = Co, Mn, Ni, Zn) under low temperature hydrothermal conditions: A time-resolved structural investigation. Green Chem. 2018, 20, 2257–2268. [Google Scholar] [CrossRef]
- Kharisov, B.I.; Dias, H.V.R.; Kharissova, O.V. Mini-review: Ferrite nanoparticles in the catalysis. Arab. J. Chem. 2014. [Google Scholar] [CrossRef]
- Rezlescu, N.; Rezlescu, E.; Sachelarie, L.; Popa, P.D.; Doroftei, C. Structural and catalytic properties of mesoporous nanocrystalline mixed oxides containing magnesium. Catal. Commun. 2014, 46, 51–56. [Google Scholar] [CrossRef]
- Kmita, A.; Żukrowski, J.; Hodor, K.; Smogór, H.; Sikora, M. Zinc ferrite nanoparticles as perspective functional materials for applications in casting technologies. Metalurgija 2017, 56, 29–32. [Google Scholar]
- Qin, M.; Shuai, Q.; Wu, G.; Zheng, B.; Wang, Z.; Wu, H. Zinc ferrite composite material with controllable morphology and its applications. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2017, 224, 125–138. [Google Scholar] [CrossRef]
- Kmita, A.; Pribulova, A.; Holtzer, M.; Futas, P.; Roczniak, A. Use of specific properties of zinc ferrite in innovative technologies. Arch. Metall. Mater. 2016, 61. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, X.; Yang, Y.; Xiao, W.; Li, Z.; Xue, D.; Li, F.; Ding, J. Synthesis of nonstoichiometric zinc ferrite nanoparticles with extraordinary room temperature magnetism and their diverse applications. J. Mater. Chem. C 2013, 1, 2875–2885. [Google Scholar] [CrossRef]
- Liu, F.; Li, X.; Zhao, Q.; Hou, Y.; Quan, X.; Chen, G. Structural and photovoltaic properties of highly ordered ZnFe2O4 nanotube arrays fabricated by a facile sol-gel template method. Acta Mater. 2009, 57, 2684–2690. [Google Scholar] [CrossRef]
- Li, Q.; Bo, C.; Wang, W. Preparation and magnetic properties of ZnFe2O4 nanofibers by coprecipitation-air oxidation method. Mater. Chem. Phys. 2010, 124, 891–893. [Google Scholar] [CrossRef]
- Zhao, J.; Mi, L.; Hou, H.; Shi, X.; Fan, Y. The preparation of zinc ferrite nanorods by using single ferrocenyl complex as precursor. Mater. Lett. 2007, 61, 4196–4198. [Google Scholar] [CrossRef]
- Philip, J.; Laskar, J.M. Optical properties and applications of ferrofluids—A review. J. Nanofluids 2012, 1, 3–20. [Google Scholar] [CrossRef]
- Katz, E.; Willner, I. Integrated nanoparticle-biomolecule hybrid systems: Synthesis, properties, and applications. Angew. Chemie Int. Ed. 2004, 43, 6042–6108. [Google Scholar] [CrossRef] [PubMed]
- Sawant, V.J.; Bamane, S.R.; Shejwal, R.V.; Patil, S.B. Comparison of drug delivery potentials of surface functionalized cobalt and zinc ferrite nanohybrids for curcumin in to MCF-7 breast cancer cells. J. Magn. Magn. Mater. 2016, 417, 222–229. [Google Scholar] [CrossRef]
- Ghayour, H.; Abdellahi, M.; Ozada, N.; Jabbrzare, S.; Khandan, A. Hyperthermia application of zinc doped nickel ferrite nanoparticles. J. Phys. Chem. Solids 2017, 111, 464–472. [Google Scholar] [CrossRef]
- Verde, E.L.; Landi, G.T.; Carrião, M.S.; Drummond, A.L.; Gomes, J.A.; Vieira, E.D.; Sousa, M.H.; Bakuzis, A.F. Field dependent transition to the non-linear regime in magnetic hyperthermia experiments: Comparison between maghemite, copper, zinc, nickel and cobalt ferrite nanoparticles of similar sizes. AIP Adv. 2012, 2, 032120. [Google Scholar] [CrossRef]
- Mathew, D.S.; Juang, R.S. An overview of the structure and magnetism of spinel ferrite nanoparticles and their synthesis in microemulsions. Chem. Eng. J. 2007, 129, 51–65. [Google Scholar] [CrossRef]
- Kant Sharma, R.; Ghose, R. Synthesis and characterization of nanocrystalline zinc ferrite spinel powders by homogeneous precipitation method. Ceram. Int. 2015, 41, 14684–14691. [Google Scholar] [CrossRef]
- Raeisi Shahraki, R.; Ebrahimi, M.; Seyyed Ebrahimi, S.A.; Masoudpanah, S.M. Structural characterization and magnetic properties of superparamagnetic zinc ferrite nanoparticles synthesized by the coprecipitation method. J. Magn. Magn. Mater. 2012, 324, 3762–3765. [Google Scholar] [CrossRef]
- Yao, C.; Zeng, Q.; Goya, G.F.; Torres, T.; Liu, J.; Wu, H.; Ge, M.; Zeng, Y.; Wang, Y.; Jiang, J.Z. ZnFe2O4 nanocrystals: Synthesis and magnetic properties. J. Phys. Chem. C 2007, 111, 12274–12278. [Google Scholar] [CrossRef]
- Ghasemi, A.; Mousavinia, M. Structural and magnetic evaluation of substituted NiZnFe2O4 particles synthesized by conventional sol-gel method. Ceram. Int. 2014, 40, 2825–2834. [Google Scholar] [CrossRef]
- Fan, G.; Gu, Z.; Yang, L.; Li, F. Nanocrystalline zinc ferrite photocatalysts formed using the colloid mill and hydrothermal technique. Chem. Eng. J. 2009, 155, 534–541. [Google Scholar] [CrossRef]
- Yan, W.; Jiang, W.; Zhang, Q.; Li, Y.; Wang, H. Structure and magnetic properties of nickel-zinc ferrite microspheres synthesized by solvothermal method. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2010, 171, 144–148. [Google Scholar] [CrossRef]
- Kotsikau, D.; Ivanovskaya, M.; Pankov, V.; Fedotova, Y. Structure and magnetic properties of manganese-zinc-ferrites prepared by spray pyrolysis method. Solid State Sci. 2015, 39, 69–73. [Google Scholar] [CrossRef]
- Lee, H.; Jung, J.C.; Kim, H.; Chung, Y.M.; Kim, T.J.; Lee, S.J.; Oh, S.H.; Kim, Y.S.; Song, I.K. Effect of pH in the preparation of ZnFe2O4 for oxidative dehydrogenation of n-butene to 1,3-butadiene: Correlation between catalytic performance and surface acidity of ZnFe2O4. Catal. Commun. 2008, 9, 1137–1142. [Google Scholar] [CrossRef]
- Vékás, L.; Bica, D.; Avdeev, M.V. Magnetic nanoparticles and concentrated magnetic nanofluids: Synthesis, properties and some applications. China Particuology 2007, 5, 43–49. [Google Scholar] [CrossRef]
- Bañobre-López, M.; Bran, C.; Rodríguez-Abreu, C.; Gallo, J.; Vázquez, M.; Rivas, J. A colloidally stable water dispersion of Ni nanowires as an efficient: T2-MRI contrast agent. J. Mater. Chem. B 2017, 5, 3338–3347. [Google Scholar] [CrossRef]
- Kharisov, B.I.; Dias, H.V.R.; Kharissova, O.V.; Vázquez, A.; Peña, Y.; Gómez, I. Solubilization, dispersion and stabilization of magnetic nanoparticles in water and non-Aqueous solvents: Recent trends. RSC Adv. 2014, 4, 45354–45381. [Google Scholar] [CrossRef]
- Din, F.U.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef]
- Milanovic, M.; Stijepovic, I.; Pavlovic, V.; Srdic, V. Functionalization of zinc ferrite nanoparticles: Influence of modification procedure on colloidal stability. Process. Appl. Ceram. 2016, 10, 287–293. [Google Scholar] [CrossRef]
- Szpak, A.; Kania, G.; Skórka, T.; Tokarz, W.; Zapotoczny, S.; Nowakowska, M. Stable aqueous dispersion of superparamagnetic iron oxide nanoparticles protected by charged chitosan derivatives. J. Nanoparticle Res. 2013, 15, 1372. [Google Scholar] [CrossRef]
- Gittins, D.I.; Caruso, F. Spontaneous phase transfer of nanoparticulate metals from organic to aqueous media. Angew. Chem. Int. Ed. Engl. 2001, 40, 3001–3004. [Google Scholar] [CrossRef]
- Laurent, S.; Roch, A.; Robic, C.; Forge, D.; Vander Elst, L.; Muller, R.N.; Port, M. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2009, 110, 2574. [Google Scholar] [CrossRef]
- Glatzel, P.; Sikora, M.; Smolentsev, G.; Fernández-García, M. Hard X-ray photon-in photon-out spectroscopy. Catal. Today 2009, 145, 294–299. [Google Scholar] [CrossRef]
- Koziej, D. Revealing complexity of nanoparticle synthesis in solution by in situ hard X-ray spectroscopy - today and beyond. Chem. Mater. 2016, 28, 2478–2490. [Google Scholar] [CrossRef]
- Thunemann, A.F.; Kegel, J.; Polte, J.; Emmering, F. Superparamagnetic maghemite nanorods: Analysis by coupling field-flow fractional and small-angle X-ray scattering. Anal. Chem. 2008, 80, 5905–5911. [Google Scholar] [CrossRef]
- Gopinath, S.; Philip, J. Prepeartion of metal oxide nanoparticles of different sizes and morphologies, their characterization using small angle X-ray scattering and study of thermal properties. Mater. Chem. Phys. 2014, 145, 213–221. [Google Scholar] [CrossRef]
- Szczerba, W.; Costo, R.; Veintemillas-Verdaguer, S.; Del Puerto Morales, M.; Thünemann, A.F. SAXS analysis of single-and multi-core iron oxide magnetic nanoparticles. J. Appl. Crystallogr. 2017, 50, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Rebbouh, L.; Hermann, R.P.; Grandjean, F.; Hyeon, T.; An, K.; Amato, A.; Long, G.J. Fe57 Mössbauer spectral and muon spin relaxation study of the magnetodynamics of monodispersed γ-Fe2O3 nanoparticles. Phys. Rev. B Condens. Matter Mater. Phys. 2007, 76, 1–12. [Google Scholar] [CrossRef]
- Szczerba, W.; Zukrowski, J.; Przybylski, M.; Sikora, M.; Safonova, O.; Shmeliov, A.; Nicolosi, V.; Schneider, M.; Granath, T.; Oppmann, M.; et al. Pushing up the magnetisation values for iron oxide nanoparticles via zinc doping: X-ray studies on the particle’s sub-nano structure of different synthesis routes. Phys. Chem. Chem. Phys. 2016, 18, 25221–25229. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Guti Errez, V.; Jim Enez-Villacorta, F.; Bonville, P.; Torralvo-Fern, M.J.; Aez-Puche, R. X-ray absorption spectroscopy and Mossbauer spectroscopy studies of superparamagnetic ZnFe2O4 nanoparticles. J. Phys. Chem. C 2011, 115, 1627–1634. [Google Scholar] [CrossRef]
- Bullita, S.; Casu, A.; Casula, M.F.; Concas, G.; Congiu, F.; Corrias, A.; Falqui, A.; Loche, D.; Marras, C. ZnFe2O4 nanoparticles dispersed in a highly porous silica aerogel matrix: A magnetic study. Phys. Chem. Chem. Phys. 2014, 16, 4843. [Google Scholar] [CrossRef] [PubMed]
- Řezníček, R.; Chlan, V.; Štěpánková, H.; Novák, P.; Zukrowski, J.; Kozłowski, A.; Kakol, Z.; Tarnawski, Z.; Honig, J.M. Understanding the Mössbauer spectrum of magnetite below the Verwey transition: Ab initio calculations, simulation, and experiment. Phys. Rev. B 2017, 96. [Google Scholar] [CrossRef]
- Berry, F.J.; Skinner, S.; Thomas, M.F. Mössbauer spectroscopic examination of a single crystal of Fe3O4. J. Phys. Condens. Matter 1998, 10, 215–220. [Google Scholar] [CrossRef]
- Bruce, D.W. Local Structural Characterisation; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013. [Google Scholar]
- Wilke, M.; Farges, F.; Petit, P.E.; Brown, G.E.; Martin, F. Oxidation state and coordination of Fe in minerals: An Fe K-XANES spectroscopic study. Am. Mineral. 2001, 86, 714–730. [Google Scholar] [CrossRef]
- Signorini, L.; Pasquini, L.; Savini, L.; Carboni, R.; Boscherini, F.; Bonetti, E.; Giglia, A.; Pedio, M.; Mahne, N.; Mahne, N.; et al. Size-dependent oxidation in iron/iron oxide core-shell nanoparticles. Phys. Rev. B Condens. Matter Mater. Phys. 2003, 68, 1–8. [Google Scholar] [CrossRef]
- Joly, Y.; Lorenzo, J.E.; Nazarenko, E.; Hodeau, J.-L.; Mannix, D.; Marin, C. Low-temperature structure of magnetite studied using resonant x-ray scattering. Phys. Rev. B 2008, 78, 134110. [Google Scholar] [CrossRef]
- Rijssel, J.V.; Kuipers, B.W.M.; Erné, B.H. Non-regularized inversion method from light scattering applied to ferro fluid magnetization curves for magnetic size distribution analysis. J. Magn. Magn. Mater. 2014, 353, 110–115. [Google Scholar] [CrossRef]
- Spirou, S.; Basini, M.; Lascialfari, A.; Sangregorio, C.; Innocenti, C. Magnetic hyperthermia and radiation therapy: Radiobiological principles and current practice. Nanomaterials 2018, 8, 40. [Google Scholar] [CrossRef]
- Hanini, A.; Lartigue, L.; Gavard, J.; Kacem, K.; Wilhelm, C.; Gazeau, F.; Chau, F.; Ammar, S. Zinc substituted ferrite nanoparticles with Zn0.9Fe2.1O4 formula used as heating agents for in vitro hyperthermia assay on glioma cells. J. Magn. Magn. Mater. 2016, 416, 315–320. [Google Scholar] [CrossRef]
- Carrey, J.; Mehdaoui, B.; Respaud, M. Simple models for dynamic hysteresis loop calculations of magnetic single-domain nanoparticles: Application to magnetic hyperthermia optimization. J. Appl. Phys. 2011, 109, 083921. [Google Scholar] [CrossRef]
- Vallejo-Fernandez, G.; Whear, O.; Roca, A.G.; Hussain, S.; Timmis, J.; Patel, V.; O’Grady, K. Mechanisms of hyperthermia in magnetic nanoparticles. J. Phys. D Appl. Phys. 2013, 46, 312001. [Google Scholar] [CrossRef]
- Ota, S.; Kitaguchi, R.; Takeda, R.; Yamada, T.; Takemura, Y. Rotation of magnetization derived from Brownian relaxation in magnetic fluids of different viscosity evaluated by dynamic hysteresis measurements over a wide frequency range. Nanomaterials 2016, 6, 170. [Google Scholar] [CrossRef]
- Brown, W.F., Jr. Thermal fluctuations of a single-domain particle. Phys. Rev. 1963, 130, 1677. [Google Scholar] [CrossRef]
- Giustini, A.J.; Petryk, A.A.; Cassim, S.M.; Tate, J.A.; Baker, I.; Hoopes, P.J. Magnetic nanoparticle hyperthermia in cancer treatment. Nano Life 2010, 1, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Hankiewicz, J.H.; Celinski, Z.; Stupic, K.F.; Anderson, N.R.; Camley, R.E. Ferromagnetic particles as magnetic resonance imaging temperature sensors. Nat. Commun. 2016, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
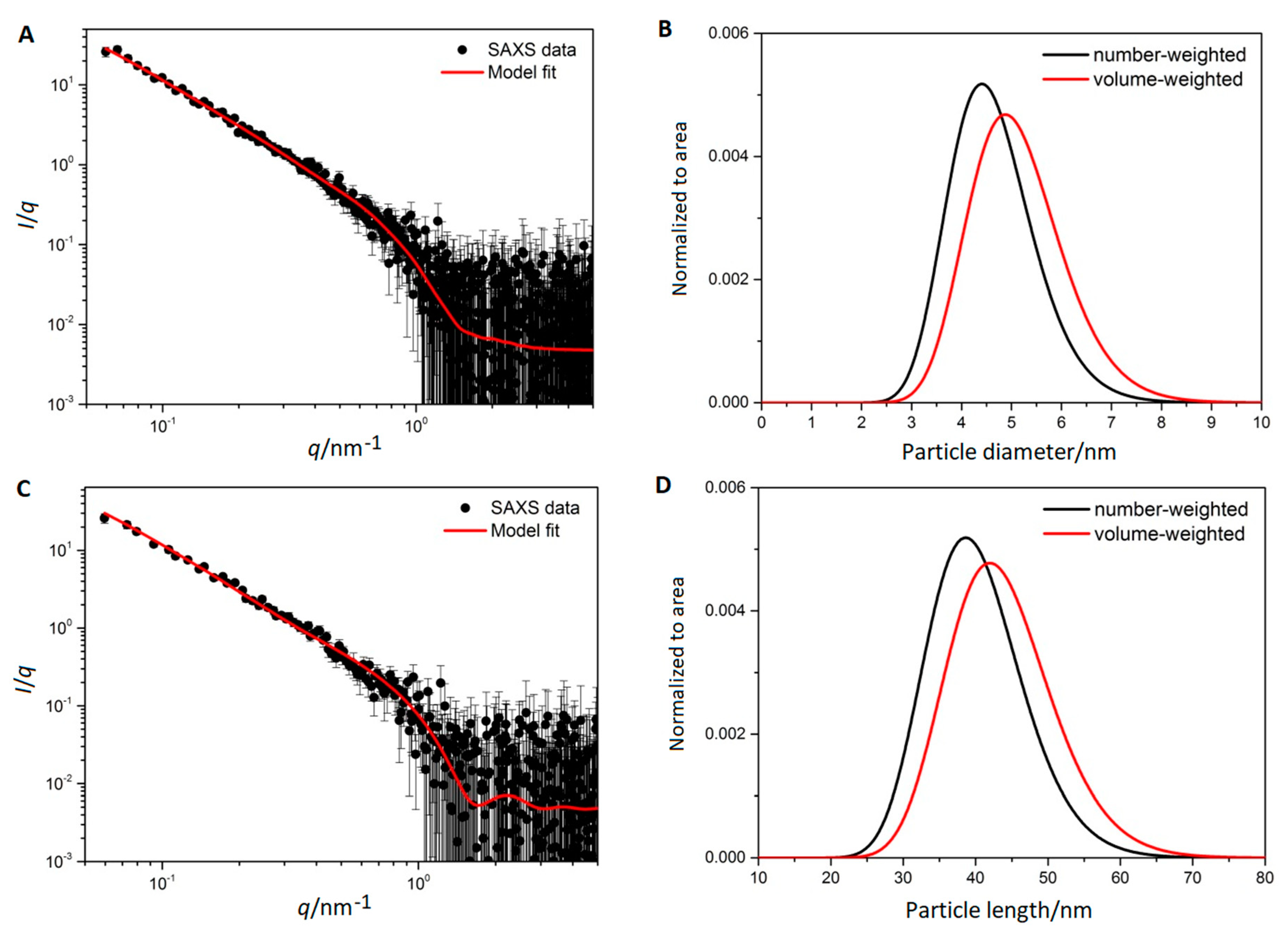
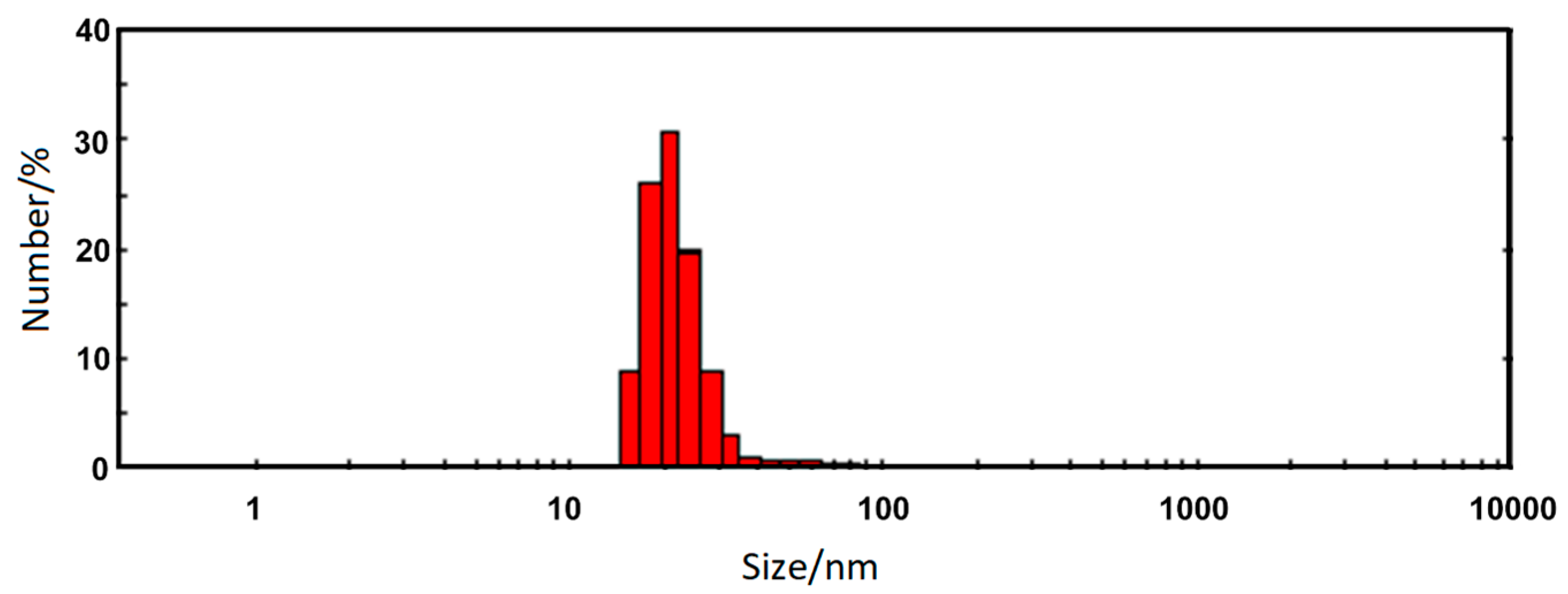
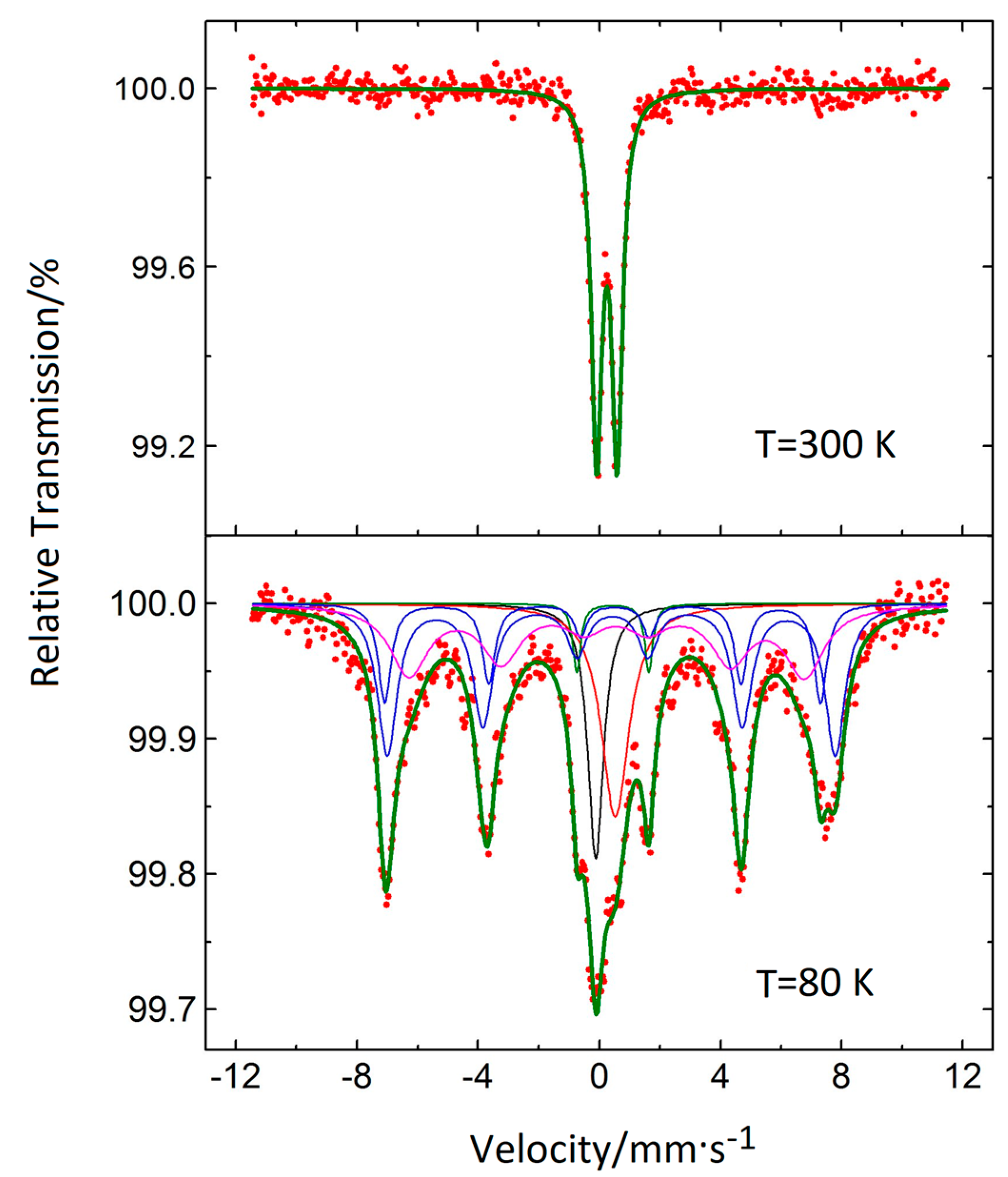
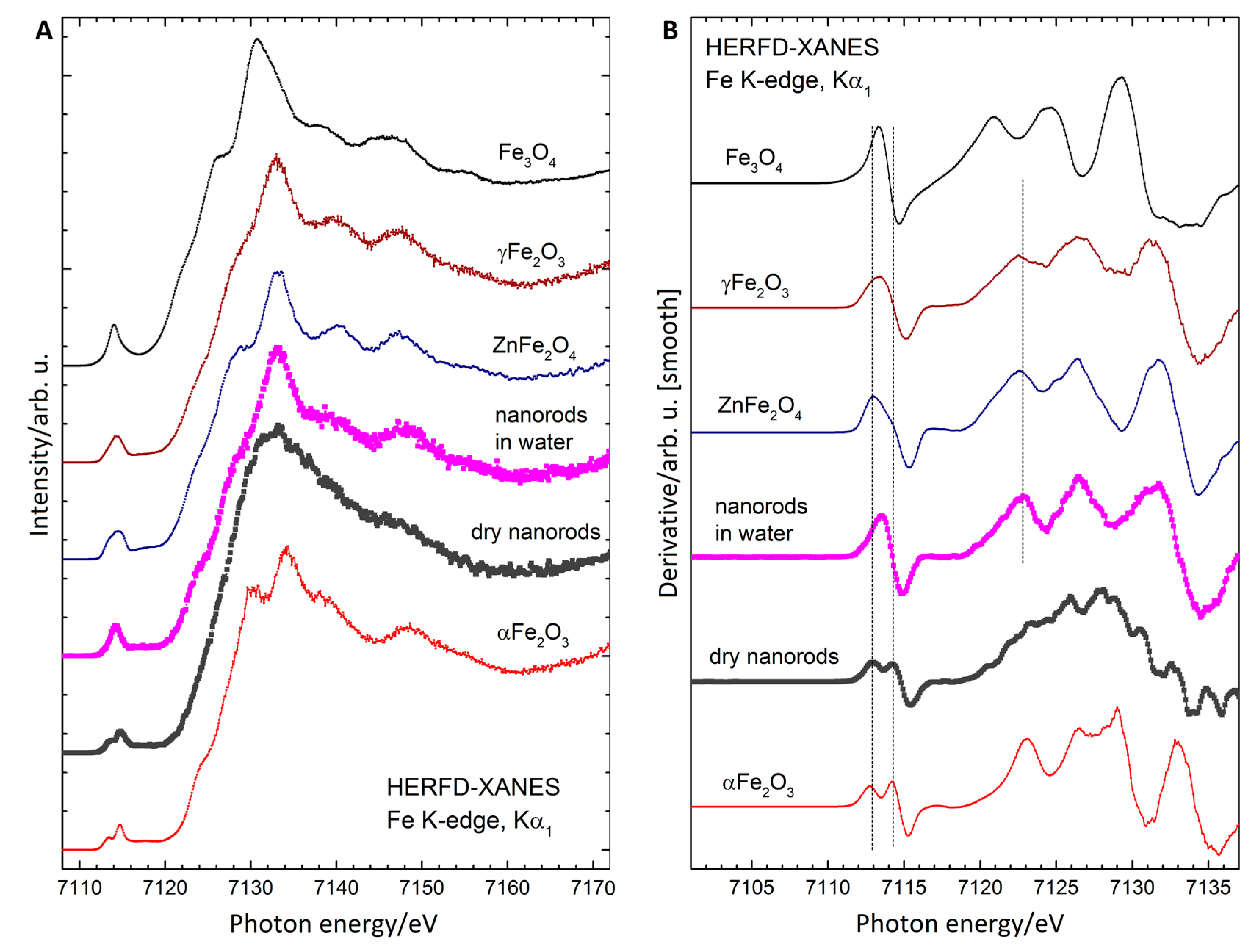
| Sample | dmax/nm | PdI | ζ/mV |
|---|---|---|---|
| Nanorods ZnFe2O4 | 22.5 ± 7.9 | 0.26 | +47.7 ± 2.4 |
| Component No. | C % | IS/mm·s−1 | <IS>/mm·s−1 | H/kGs | <H>/kGs | QS/mm·s−1 | Γ/2/mm·s−1 |
|---|---|---|---|---|---|---|---|
| 1 | 10.3 | −0.1170 | - | - | - | - | 0.312 |
| 2 | 15.4 | 0.5256 | - | - | 0.558 | ||
| 3 | 2.8 | 0.4424 | - | 1.1894 | 0.157 | ||
| 4 | 30.6 | 0.415 | 0.390 | 458.3 | 435.3 | −0.022 | 0.394 |
| 5 | 13.1 | 0.310 | 445.8 | −0.202 | 0.256 | ||
| 6 | 27.8 | 0.399 | 404.9 | −0.152 | 0.771 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kmita, A.; Lachowicz, D.; Żukrowski, J.; Gajewska, M.; Szczerba, W.; Kuciakowski, J.; Zapotoczny, S.; Sikora, M. One-Step Synthesis of Long Term Stable Superparamagnetic Colloid of Zinc Ferrite Nanorods in Water. Materials 2019, 12, 1048. https://doi.org/10.3390/ma12071048
Kmita A, Lachowicz D, Żukrowski J, Gajewska M, Szczerba W, Kuciakowski J, Zapotoczny S, Sikora M. One-Step Synthesis of Long Term Stable Superparamagnetic Colloid of Zinc Ferrite Nanorods in Water. Materials. 2019; 12(7):1048. https://doi.org/10.3390/ma12071048
Chicago/Turabian StyleKmita, Angelika, Dorota Lachowicz, Jan Żukrowski, Marta Gajewska, Wojciech Szczerba, Juliusz Kuciakowski, Szczepan Zapotoczny, and Marcin Sikora. 2019. "One-Step Synthesis of Long Term Stable Superparamagnetic Colloid of Zinc Ferrite Nanorods in Water" Materials 12, no. 7: 1048. https://doi.org/10.3390/ma12071048
APA StyleKmita, A., Lachowicz, D., Żukrowski, J., Gajewska, M., Szczerba, W., Kuciakowski, J., Zapotoczny, S., & Sikora, M. (2019). One-Step Synthesis of Long Term Stable Superparamagnetic Colloid of Zinc Ferrite Nanorods in Water. Materials, 12(7), 1048. https://doi.org/10.3390/ma12071048