The Influence of Si/Al Ratios on Adsorption and Desorption Characterizations of Pd/Beta Served as Cold-Start Catalysts
Abstract
:1. Introduction
2. Experimentation
2.1. Catalyst Preparation
2.2. Catalyst Characterization
2.3. Catalyst Evaluation
3. Result
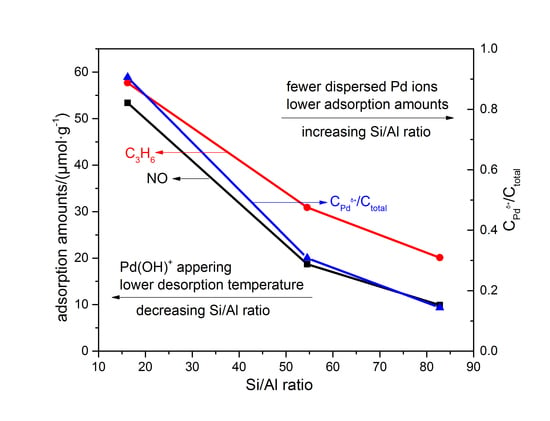
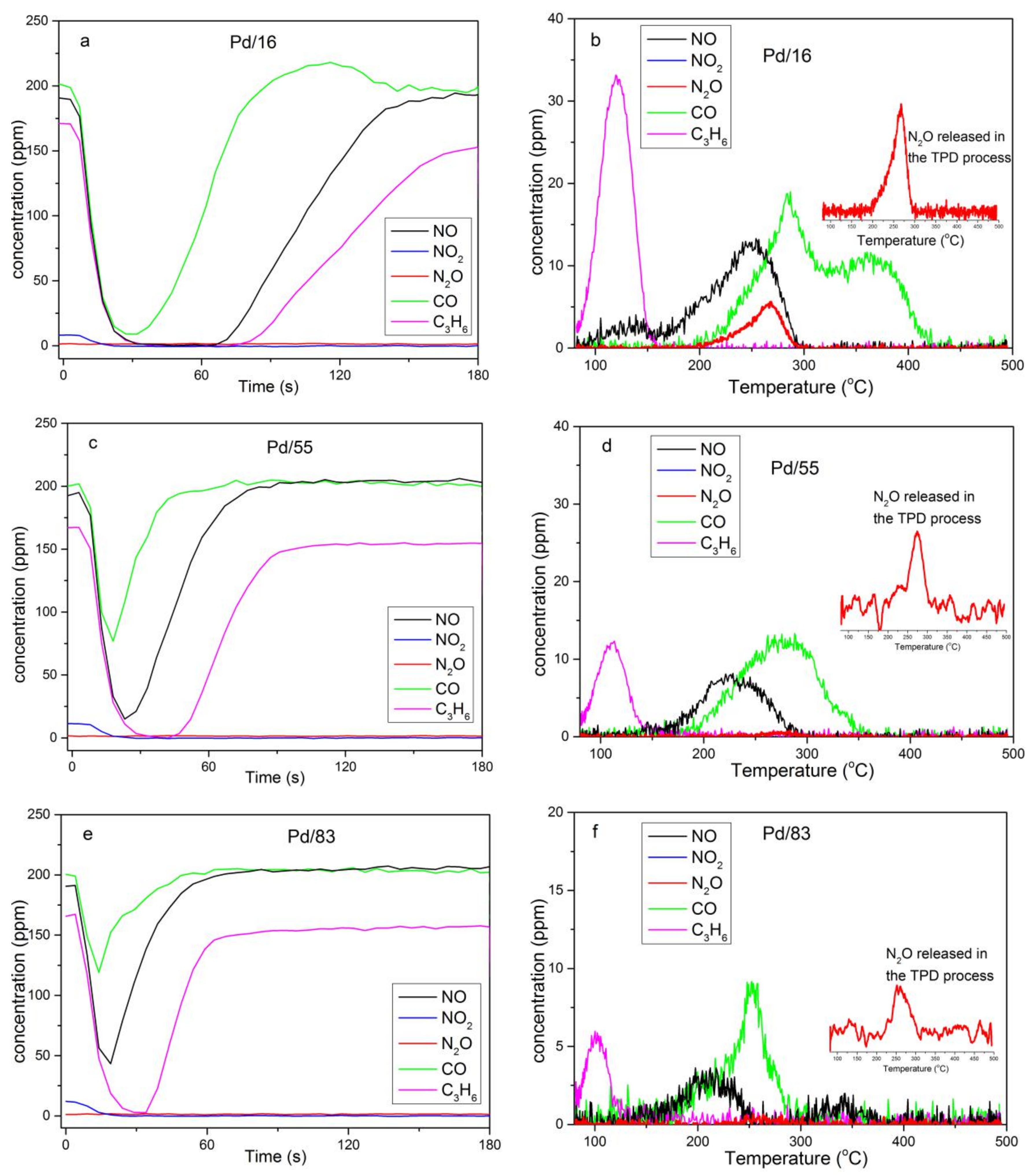
3.1. Catalyst Evaluation
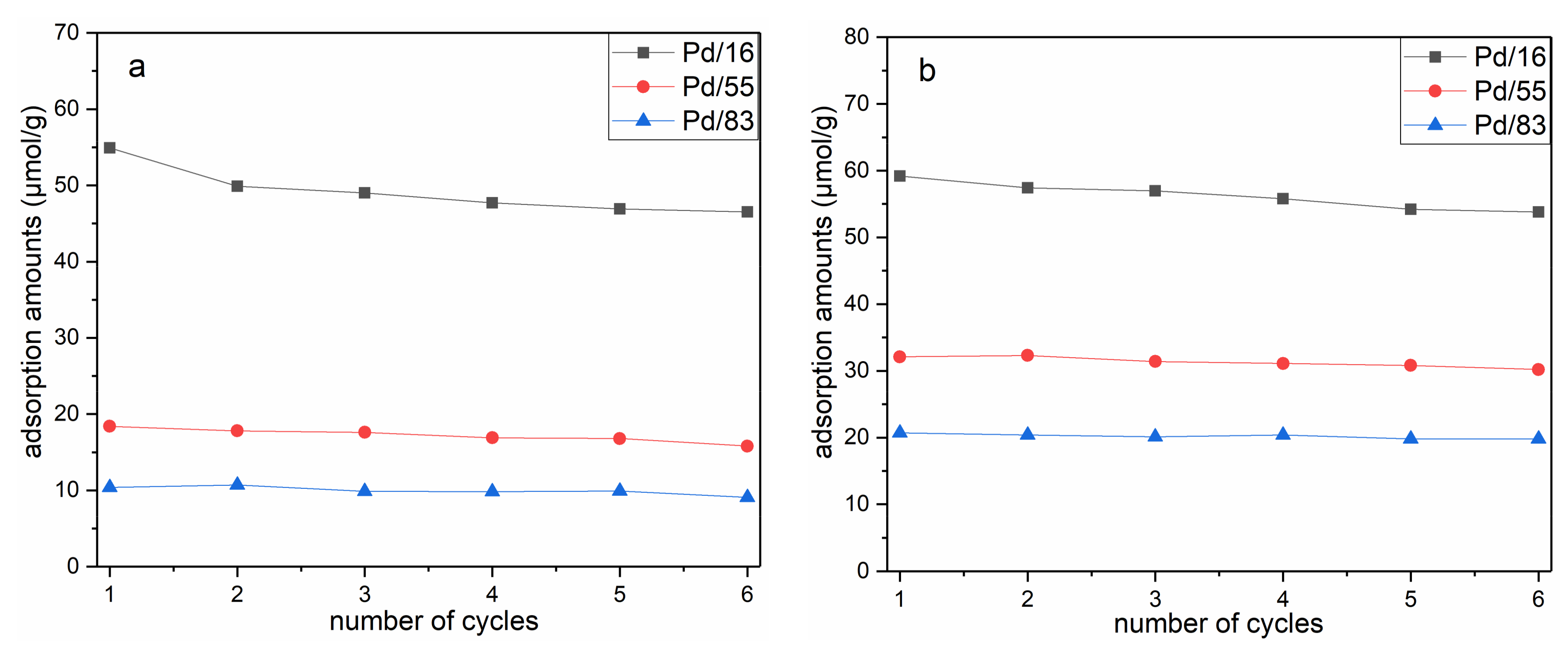
3.2. Adsorption–Desorption Cycling
3.3. Catalyst Characterization
3.3.1. Textural Properties
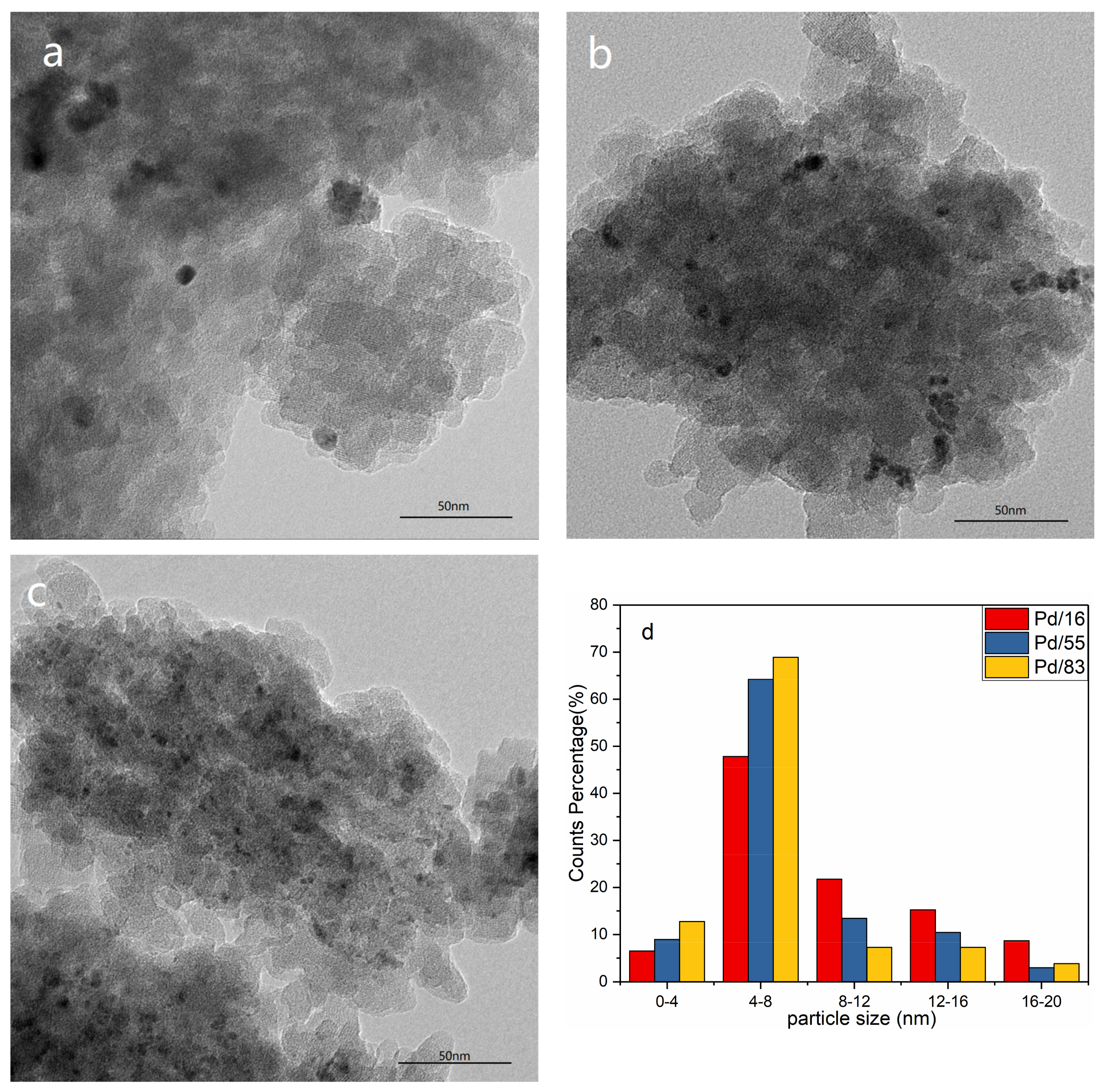
3.3.2. HRTEM
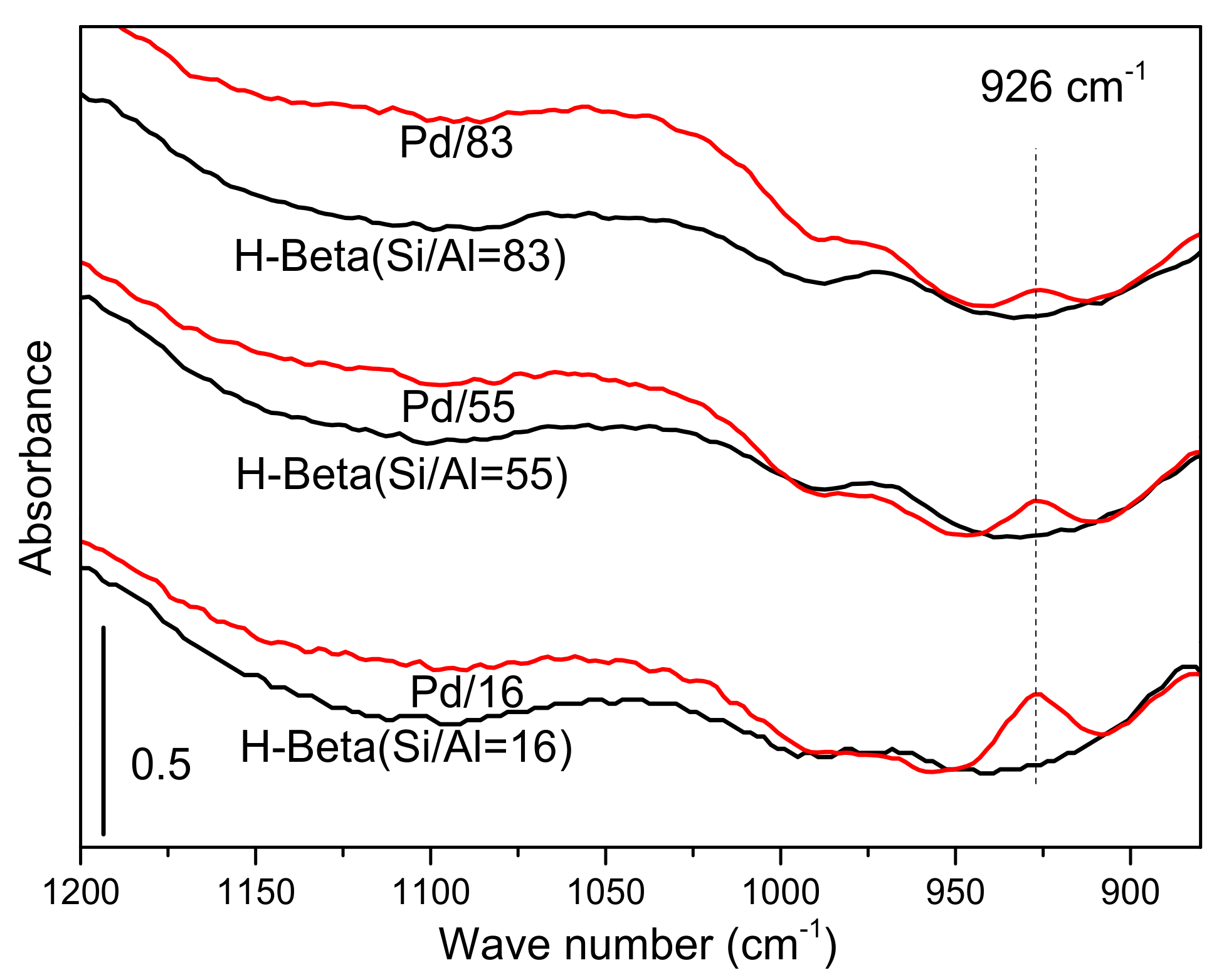
3.3.3. Ex-Situ FT-IR
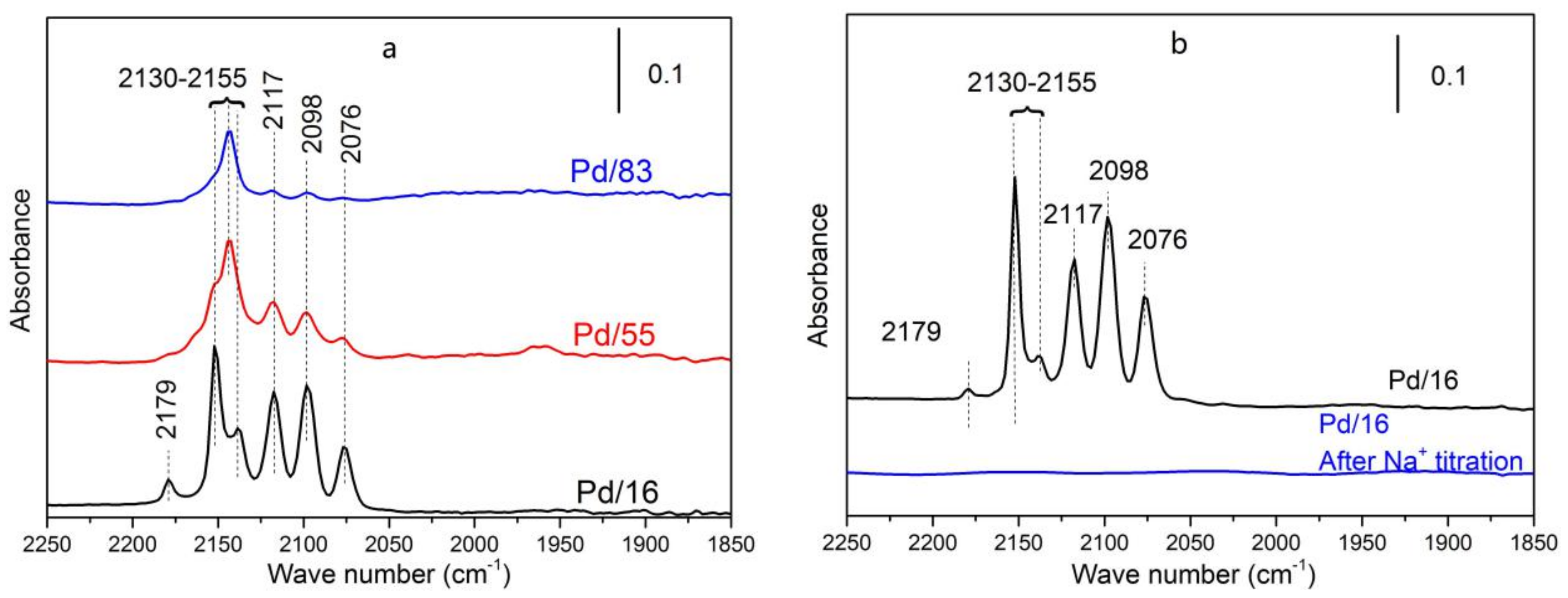
3.3.4. In Situ FT-IR of CO Adsorption
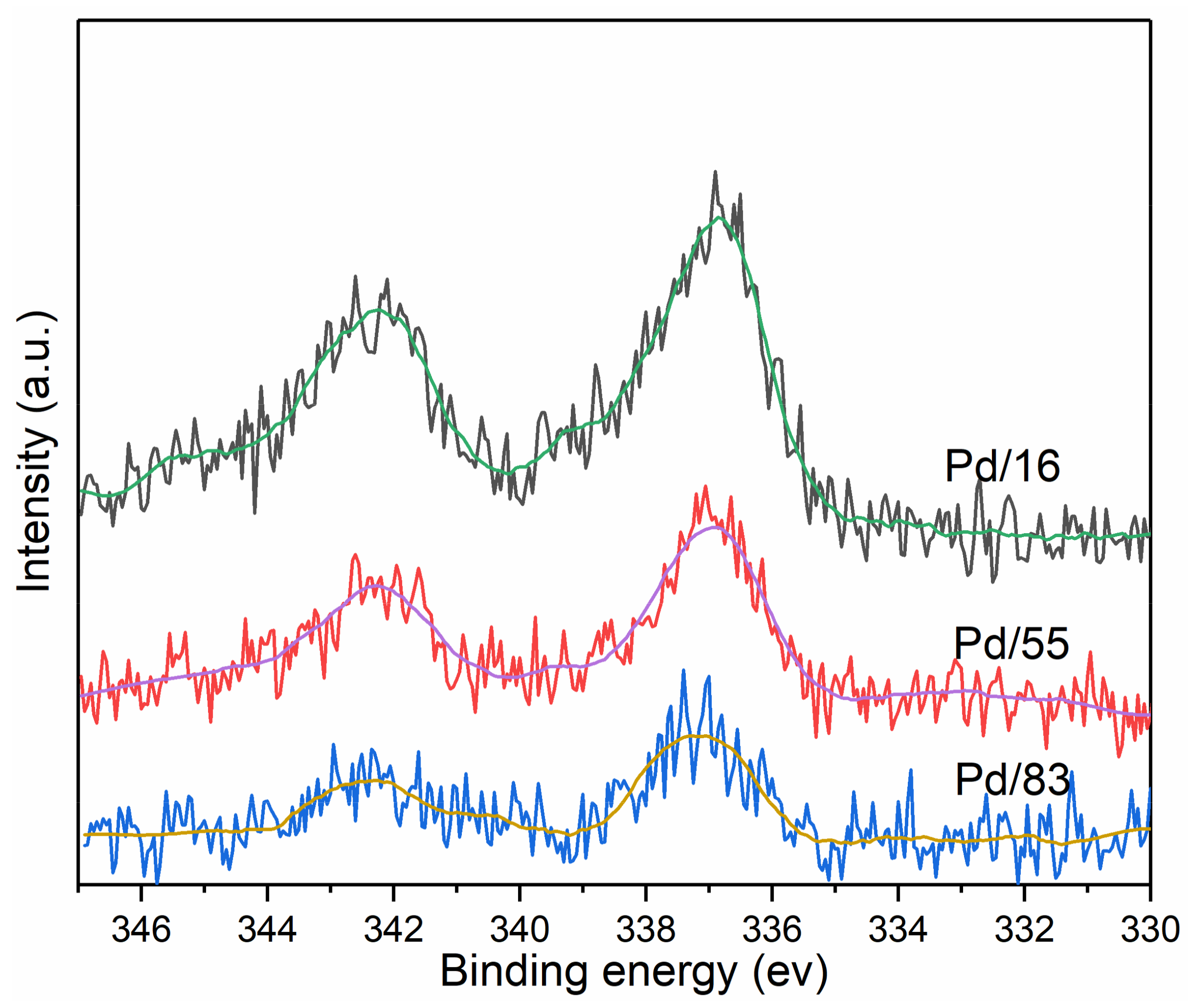
3.3.5. XPS
3.3.6. In Situ FT-IR of NH3 Adsorption
3.3.7. Na+ Titration
4. Discussion
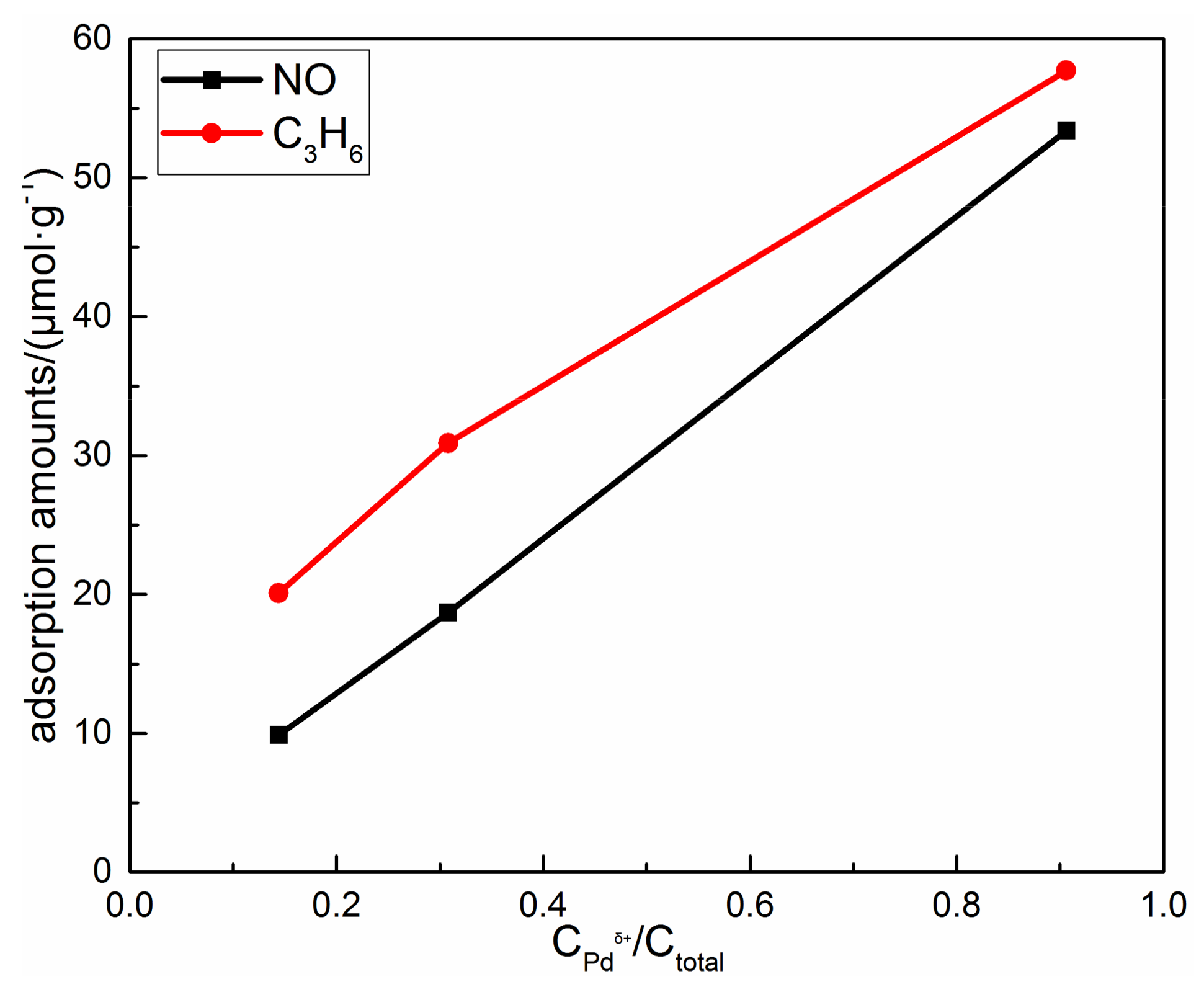
4.1. The Relationship of Pd Species and the NO Desorption Peak Centered at 130 °C of Pd/16
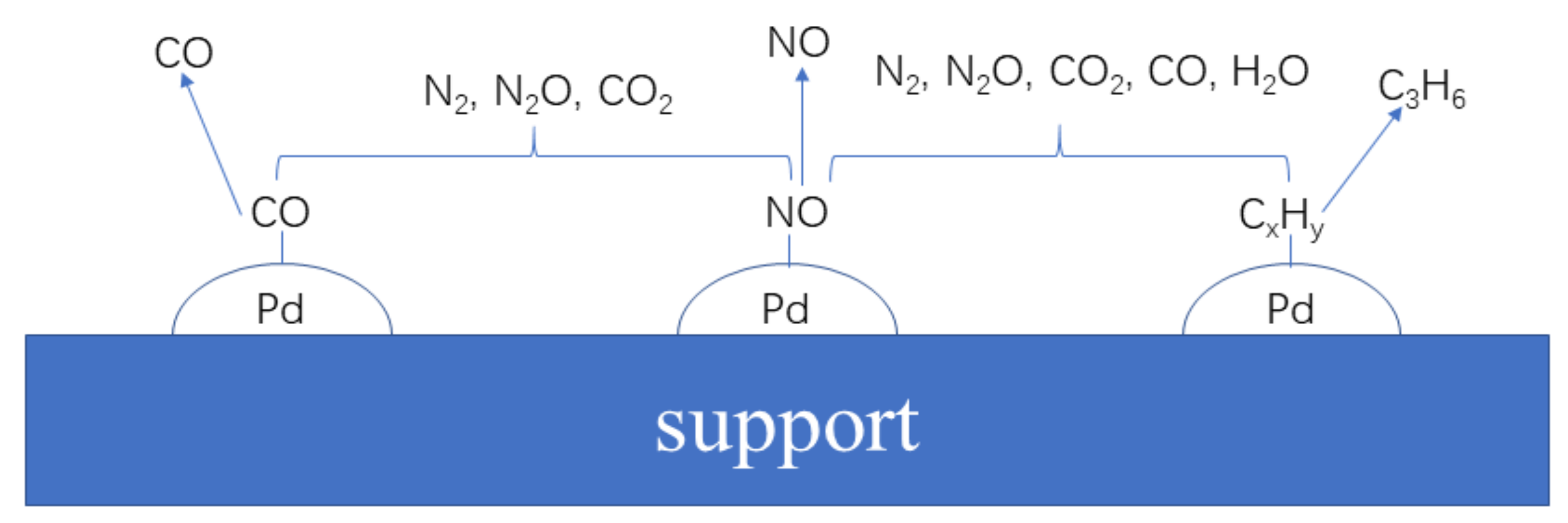
4.2. Conversion of NO and C3H6 in the Desorption Stage
4.3. Influence of Si/Al Ratios on Cold-Start Catalyst Performance of Pd/Beta
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Johnson, T.V. Vehicular emissions in review. SAE Int. J. Engines 2012, 5, 216–234. [Google Scholar] [CrossRef]
- Johnson, T.V. Review of vehicular emissions trends. SAE Int. J. Engines 2015, 8, 1152–1167. [Google Scholar] [CrossRef]
- Golden, S.; Nazarpoor, Z.; Launois, M.; Liu, R.F.; Maram, P. Development of Non-Copper Advanced Spinel Mixed Metal Oxides for Zero-Precious Metal and Ultra-Low Precious Metal Next-Generation TWC; Technical Paper; SAE: Warrendale, PA, USA, 2016. [Google Scholar] [CrossRef]
- Johnson, T.; Joshi, A. Review of Vehicle Engine Efficiency and Emissions; Technical Paper; SAE: Warrendale, PA, USA, 2018. [Google Scholar] [CrossRef]
- Kim, D.H.; Mudiyanselage, K.; Szanyi, J.; Kwak, J.H.; Zhu, H.Y.; Peden, C. Effect of K loadings on nitrate formation /decomposition and on NOx storage performance of K-based NOx storage-reduction catalysts. Appl. Catal. B Environ. 2013, 142, 472–478. [Google Scholar] [CrossRef]
- Ma, L.; Cheng, Y.S.; Cavataio, G.; McCabe, R.W.; Fu, L.X.; Li, J.H. In situ DRIFTS and temperature-programmed technology study on NH3-SCR of NOx over Cu-SSZ-13 and Cu-SAPO-34 catalysts. Appl. Catal. B Environ. 2014, 156, 428–437. [Google Scholar] [CrossRef]
- Chen, H.Y.; Collier, J.E.; Liu, D. Low temperature NO storage of zeolite supported Pd for low temperature diesel engine emission control. Catal. Lett. 2016, 146, 1706–1711. [Google Scholar] [CrossRef]
- Zheng, Y.; Kovarik, L.; Engelhard, M.H.; Wang, Y.L.; Wang, Y.; Gao, F.; Szanyi, J. Low-Temperature Pd/Zeolite Passive NOx Adsorbers: Structure, Performance, and Adsorption Chemistry. J. Phys. Chem. C 2017, 121, 15793–15803. [Google Scholar] [CrossRef]
- Gu, Y.; Epling, W.S. Passive NOx adsorber: An overview of catalyst performance and reaction chemistry. Appl. Catal. A Gen. 2019, 570, 1–14. [Google Scholar] [CrossRef]
- Moliner, M.; Corma, A. From metal-supported oxides to well-defined metal site zeolites: The next generation of passive NOx adsorbers for low-temperature control of emissions from diesel engines. React. Chem. Eng. 2019, 4, 223–234. [Google Scholar] [CrossRef]
- Lucas, A.; Ramos, M.J.; Dorado, F.; Sanchez, P.; Valerde, J.L. Influence of the Si/Al ratio in the hydroisomerization of n-octane over platinum and palladium beta zeolite-based catalysts with or without binder. Appl. Catal. A Gen. 2005, 289, 205–213. [Google Scholar] [CrossRef]
- Descorme, C.; Gélin, P.; Lécuyer, C.; Primet, M. Palladium-exchanged MFI-type zeolites in the catalytic reduction of nitrogen monoxide by methane. Influence of the Si/Al ratio on the activity and the hydrothermal stability. Appl. Catal. B Environ. 1997, 13, 185–195. [Google Scholar] [CrossRef]
- Ohtsuka, H.; Tabata, T. Influence of Si/Al ratio on the activity and durability of Pd-ZSM-5 catalysts for nitrogen oxide reduction by methane. Appl. Catal. B Environ. 2000, 26, 275–284. [Google Scholar] [CrossRef]
- Lee, J.; Ryou, Y.S.; Cho, S.J.; Lee, H.; Kim, C.H.; Kim, D.H. Investigation of the active sites and optimum Pd/Al of Pd/ZSM–5 passive NO adsorbers for the cold-start application: Evidence of isolated-Pd species obtained after a high-temperature thermal treatment. Appl. Catal. B Environ. 2018, 226, 71–82. [Google Scholar] [CrossRef]
- Mihai, O.; Trandafilović, L.; Wentworth, T.; Torres, F.F.; Olsson, L. The Effect of Si/Al Ratio for Pd/BEA and Pd/SSZ-13 Used as Passive NOx Adsorbers. Top. Catal. 2018, 61, 2007–2020. [Google Scholar] [CrossRef]
- Koyano, G.; Yokoyama, S.; Misono, M. States of Pd in Pd/H–ZSM-5 and Pd/Na–ZSM-5 catalysts and catalytic activity for the reduction of NO by CH4 in the presence of O2. Appl. Catal. A Gen. 1999, 188, 301–312. [Google Scholar] [CrossRef]
- Lobree, L.J.; Aylor, A.W.; Reimer, J.A.; Bell, A.T. NO Reduction by CH4 in the Presence of O2 over Pd-H-ZSM-5. J. Catal. 1999, 181, 189–204. [Google Scholar] [CrossRef]
- Adelman, B.J.; Sachtler, W.M.H. The effect of zeolitic protons on NOx reduction over Pd/ZSM-5 catalysts. Appl. Catal. B Environ. 1997, 14, 1–11. [Google Scholar] [CrossRef]
- Kikuchi, E.; Ogura, M. Palladium species in Pd/H-ZSM-5 zeolite catalysts for CH4-SCR. Res. Chem. Intermed. 2000, 26, 55–60. [Google Scholar] [CrossRef]
- Choi, K.H.; Shokouhimehr, M.; Sung, Y.E. Heterogeneous Suzuki cross-coupling reaction catalyzed by magnetically recyclable nanocatalyst. Bull. Korean Chem. Soc. 2013, 34, 1477–1480. [Google Scholar] [CrossRef]
- Aejung, K.; Mahdi, R.S.; Shahrouz, A.; Mohammadreza, S. Palladium nanocatalysts confined in mesoporous Silica for heterogeneous reduction of nitroaromatics. Energy Environ. Focus 2015, 4, 18–23. [Google Scholar]
- Penner, S.; Wang, D.; Jenewein, B.; Gabasch, H.; Klotzer, B.; Knop-Gericke, A.; Schlögl, R.; Hayek, K. Growth and decomposition of aligned and ordered PdO nanoparticles. J. Chem. Phys. 2006, 125, 094703. [Google Scholar] [CrossRef]
- Sobalík, Z.; Dědeček, J.; Ikonnikov, I.; Wichterlova, B. State and coordination of metal ions in high silica zeolites Incorporation, development and rearrangement during preparation and catalysis. Microporous Mesoporous Mater. 1998, 21, 525–532. [Google Scholar] [CrossRef]
- Palazov, A.; Chang, C.C.; Kokes, R.J. The infrared spectrum of carbon monoxide on reduced and oxidized palladium. J. Catal. 1975, 36, 338–350. [Google Scholar] [CrossRef]
- Aylor, A.W.; Lobree, L.J.; Reimer, J.A.; Bell, A.T. Investigations of the Dispersion of Pd in H-ZSM-5. J. Catal. 1997, 172, 453–462. [Google Scholar] [CrossRef]
- Okumura, K.; Amano, J.; Yasunobu, N.; Niwa, M. X-ray absorption fine structure study of the formation of the highly dispersed PdO over ZSM-5 and the structural change of Pd induced by adsorption of NO. J. Phys. Chem. B 2000, 104, 1050–1057. [Google Scholar] [CrossRef]
- Ryou, Y.S.; Lee, J.; Cho, S.J.; Lee, H.; Kim, C.H.; Kim, D.H. Activation of Pd/SSZ-13 catalyst by hydrothermal aging treatment in passive NO adsorption performance at low temperature for cold start application. Appl. Catal. B Environ. 2017, 212, 140–149. [Google Scholar] [CrossRef]
- Jin, R.B.; Liu, Y.; Wu, Z.B.; Wang, H.Q.; Gu, T.T. Low-temperature selective catalytic reduction of NO with NH3 over MnCe oxides supported on TiO2 and Al2O3: A comparative study. Chemosphere 2010, 78, 1160–1166. [Google Scholar] [CrossRef]
- Si, Z.C.; Weng, D.; Wu, X.D.; Jiang, Y. Roles of Lewis and Brønsted acid sites in NO reduction with ammonia on CeO2-ZrO2-NiO-SO42− catalyst. J. Rare Earths 2010, 28, 727–731. [Google Scholar] [CrossRef]
- Qi, G.; Yang, R.T. Ultra-active Fe/ZSM-5 catalyst for selective catalytic reduction of nitric oxide with ammonia. Appl. Catal. B Environ. 2005, 60, 13–22. [Google Scholar] [CrossRef]
- Gao, F.; Washton, N.M.; Wang, Y.; Kollar, M.; Szanyi, J.; Peden, C.H. Effects of Si/Al ratio on Cu/SSZ-13 NH3-SCR catalysts: Implications for the active Cu species and the roles of Brønsted acidity. J. Catal. 2015, 331, 25–38. [Google Scholar] [CrossRef]
- Wang, L.; Li, W.; Schmieg, S.J.; Weng, D. Role of Brønsted acidity in NH3 selective catalytic reduction reaction on Cu/SAPO-34 catalysts. J. Catal. 2015, 324, 98–106. [Google Scholar] [CrossRef]
- Ogura, M.; Hayashi, M.; Kage, S.; Matsukata, M.; Kikuchi, E. Determination of active palladium species in ZSM-5 zeolite for selective reduction of nitric oxide with methane. Appl. Catal. B Environ. 1999, 23, 247–257. [Google Scholar] [CrossRef]
- Wang, J.G.; Liu, C.J.; Fang, Z.P.; Liu, Y.; Han, Z.Q. DFT study of structural and electronic properties of PdO/HZSM-5. J. Phys. Chem. B 2004, 108, 1653–1659. [Google Scholar] [CrossRef]
- Okumura, K.; Niwa, M. Regulation of the dispersion of PdO through the interaction with acid sites of zeolite studied by extended X-ray absorption fine structure. J. Phys. Chem. B 2000, 104, 9670–9675. [Google Scholar] [CrossRef]
- Martınez-Arias, A.; Fernández-Garcıa, M.; Iglesias-Juez, A.; Hungria, A.B.; Anderson, J.A.; Conesa, J.C.; Soria, J. New Pd/CexZr1−xO2/Al2O3 three-way catalysts prepared by microemulsion: Part 2. In situ analysis of CO oxidation and NO reduction under stoichiometric CO+NO+O2. Appl. Catal. B Environ. 2001, 31, 51–60. [Google Scholar] [CrossRef]
- Ferrer, V.; Finol, D.; Solano, R.; Moronta, A.; Ramos, M.J. Reduction of NO by CO using Pd–CeTb and Pd–CeZr catalysts supported on SiO2 and La2O3−Al2O3. J. Environ. Sci. 2015, 27, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Radev, L.; Khristova, M.; Mehandjiev, D.; Samuneva, B. Sol-gel Ag+Pd/SiO2 as a catalyst for reduction of NO with CO. Catal. Lett. 2006, 112, 181–186. [Google Scholar] [CrossRef]
- Gao, F.; Kwak, J.H.; Szanyi, J.; Peden, C.H.F. Current understanding of Cu-exchanged chabazite molecular sieves for use as commercial diesel engine DeNOx catalysts. Top. Catal. 2013, 56, 1441–1459. [Google Scholar] [CrossRef]
- Postole, G.; Caldararu, M.; Bonnetot, B.; Auroux, A. Influence of the support surface chemistry on the catalytic performances of PdO/BN catalysts. J. Phys. Chem. C 2008, 112, 11385–11393. [Google Scholar] [CrossRef]
- Theis, J.R.; McCabe, R.W. The effects of high temperature lean exposure on the subsequent HC conversion of automotive catalysts. Catal. Today 2012, 184, 262–270. [Google Scholar] [CrossRef]
- Rice, M.J.; Chakraborty, A.K.; Bell, A.T. Al next nearest neighbor, ring occupation, and proximity statistics in ZSM-5. J. Catal. 1999, 186, 222–227. [Google Scholar] [CrossRef]
- Westermann, A.; Azambre, B.; Finqueneisel, G.; Costa, P.D. Evolution of unburnt hydrocarbons under “cold-start” conditions from adsorption/desorption to conversion: On the screening of zeolitic materials. Appl. Catal. B Environ. 2014, 158, 48–59. [Google Scholar] [CrossRef]
- Azambre, B.; Westermann, A.; Finqueneisel, G.; Can, F.; Comparot, J.D. Adsorption and desorption of a model hydrocarbon mixture over HY Zeolite under dry and wet conditions. J. Phys. Chem. C 2014, 119, 315–331. [Google Scholar] [CrossRef]
- Westermann, A.; Azambre, B.; Chebbi, M.; Koch, A. Modification of Y Faujasite zeolites for the trapping and elimination of a propene-toluene-decane mixture in the context of cold-start. Microporous Mesoporous Mater. 2016, 230, 76–88. [Google Scholar] [CrossRef]
- Westermann, A.; Azambre, B. Impact of the Zeolite Structure and Acidity on the Adsorption of Unburnt Hydrocarbons Relevant to Cold Start Conditions. J. Phys. Chem. C 2016, 120, 25903–25914. [Google Scholar] [CrossRef]

| Sample | Si/Al Ratio | Pd Loading (wt.%) |
|---|---|---|
| Pd/16 | 16.20 | 0.96 |
| Pd/55 | 54.52 | 1.04 |
| Pd/83 | 82.81 | 0.97 |
| Sample | Adsorption Amount (μmol/gcat) a | Desorption Amount (μmol/gcat) | ||||
|---|---|---|---|---|---|---|
| NO | C3H6 | CO | NO | C3H6 | CO | |
| Pd/16 | 53.4 | 57.7 | 28.6 | 20.1 | 23.2 | 33.1 |
| Pd/55 | 18.7 | 30.9 | 7.9 | 6.6 | 10.7 | 29.9 |
| Pd/83 | 9.9 | 21.1 | 5.4 | 1.5 | 5.7 | 9.1 |
| Sample | SBET (m2/g) | Vmicro (cm3/g) |
|---|---|---|
| H-Beta/16 | 682.8 | 0.181 |
| H-Beta/55 | 667.5 | 0.176 |
| H-Beta/83 | 625.4 | 0.163 |
| Pd/16 | 586.5 | 0.158 |
| Pd/55 | 571.3 | 0.153 |
| Pd/83 | 540.1 | 0.141 |
| Sample | ||
|---|---|---|
| Pd/16 | 0.87 | 0.09 |
| Pd/55 | 0.32 | 0.72 |
| Pd/83 | 0.14 | 0.83 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, M.; Wang, J.; Wang, J.; Shen, M. The Influence of Si/Al Ratios on Adsorption and Desorption Characterizations of Pd/Beta Served as Cold-Start Catalysts. Materials 2019, 12, 1045. https://doi.org/10.3390/ma12071045
Jiang M, Wang J, Wang J, Shen M. The Influence of Si/Al Ratios on Adsorption and Desorption Characterizations of Pd/Beta Served as Cold-Start Catalysts. Materials. 2019; 12(7):1045. https://doi.org/10.3390/ma12071045
Chicago/Turabian StyleJiang, Ming, Jun Wang, Jianqiang Wang, and Meiqing Shen. 2019. "The Influence of Si/Al Ratios on Adsorption and Desorption Characterizations of Pd/Beta Served as Cold-Start Catalysts" Materials 12, no. 7: 1045. https://doi.org/10.3390/ma12071045
APA StyleJiang, M., Wang, J., Wang, J., & Shen, M. (2019). The Influence of Si/Al Ratios on Adsorption and Desorption Characterizations of Pd/Beta Served as Cold-Start Catalysts. Materials, 12(7), 1045. https://doi.org/10.3390/ma12071045