Combustion Synthesis of Non-Precious CuO-CeO2 Nanocrystalline Catalysts with Enhanced Catalytic Activity for Methane Oxidation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Solution Combustion Synthesis of CeO2 and the CuO-CeO2 Catalysts
2.2.2. Characterization
2.2.3. Methane Oxidation Catalysis Measurements
3. Results
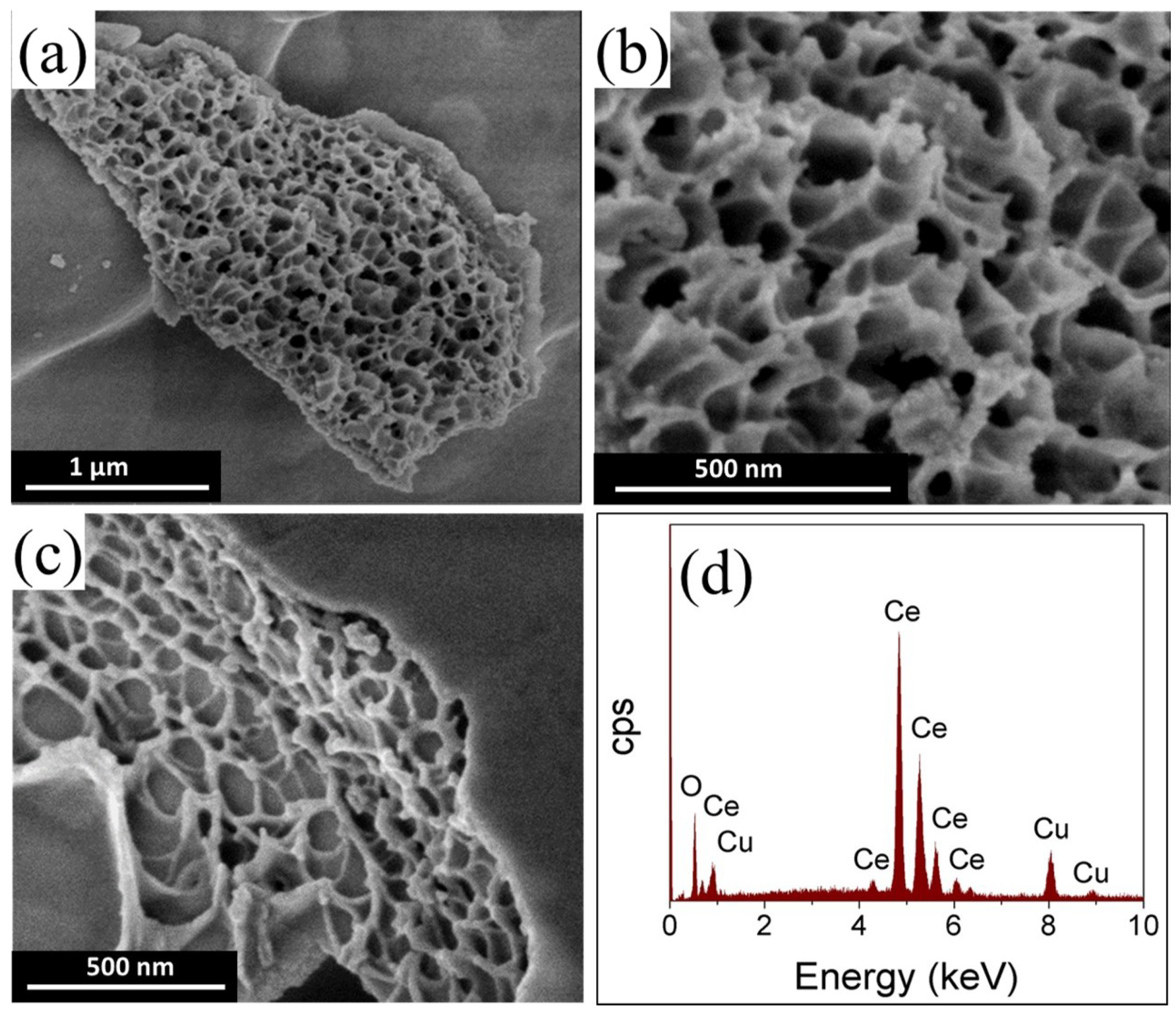
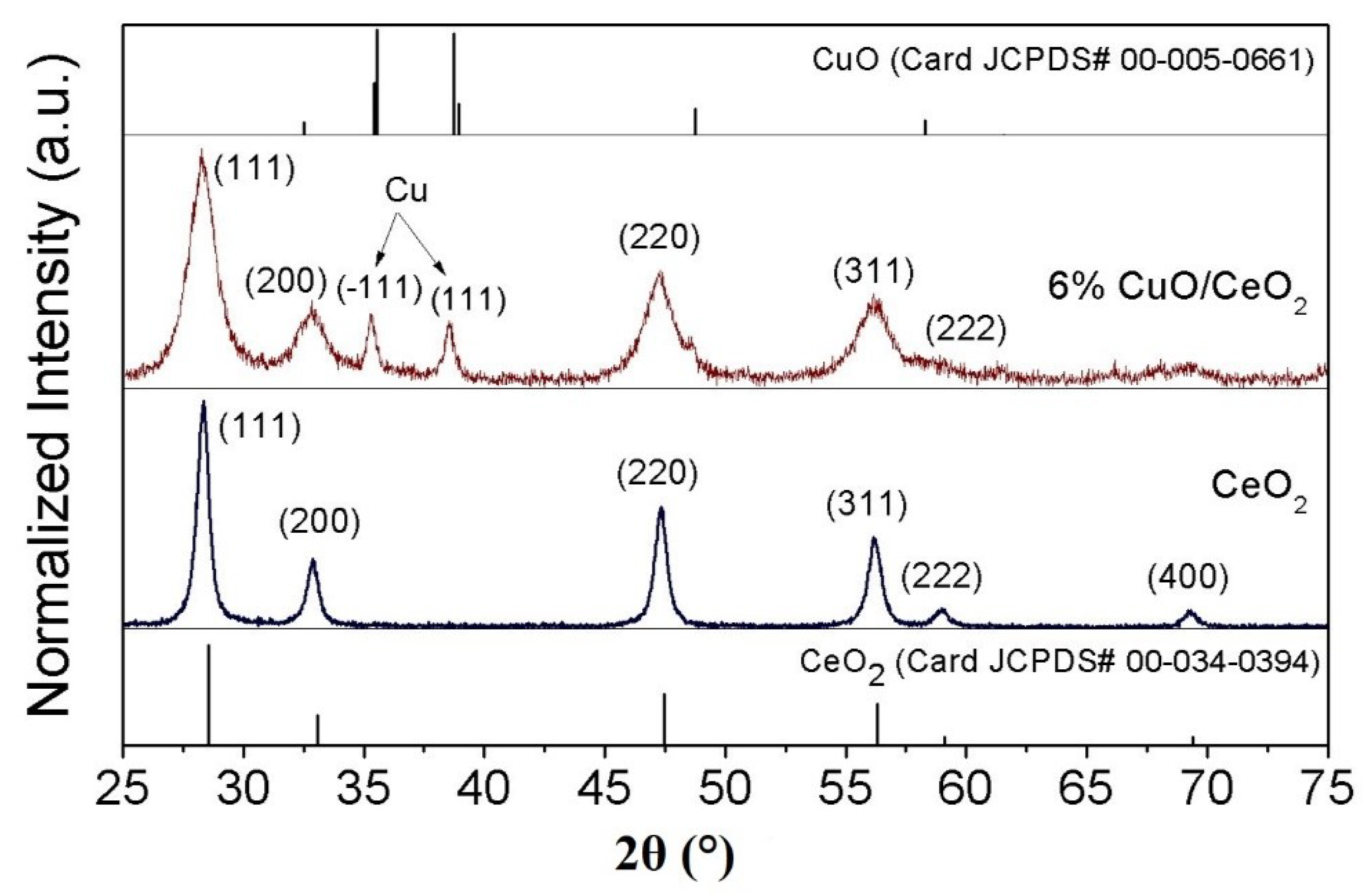
3.1. Morphological and Crystal Structure of the Catalysts
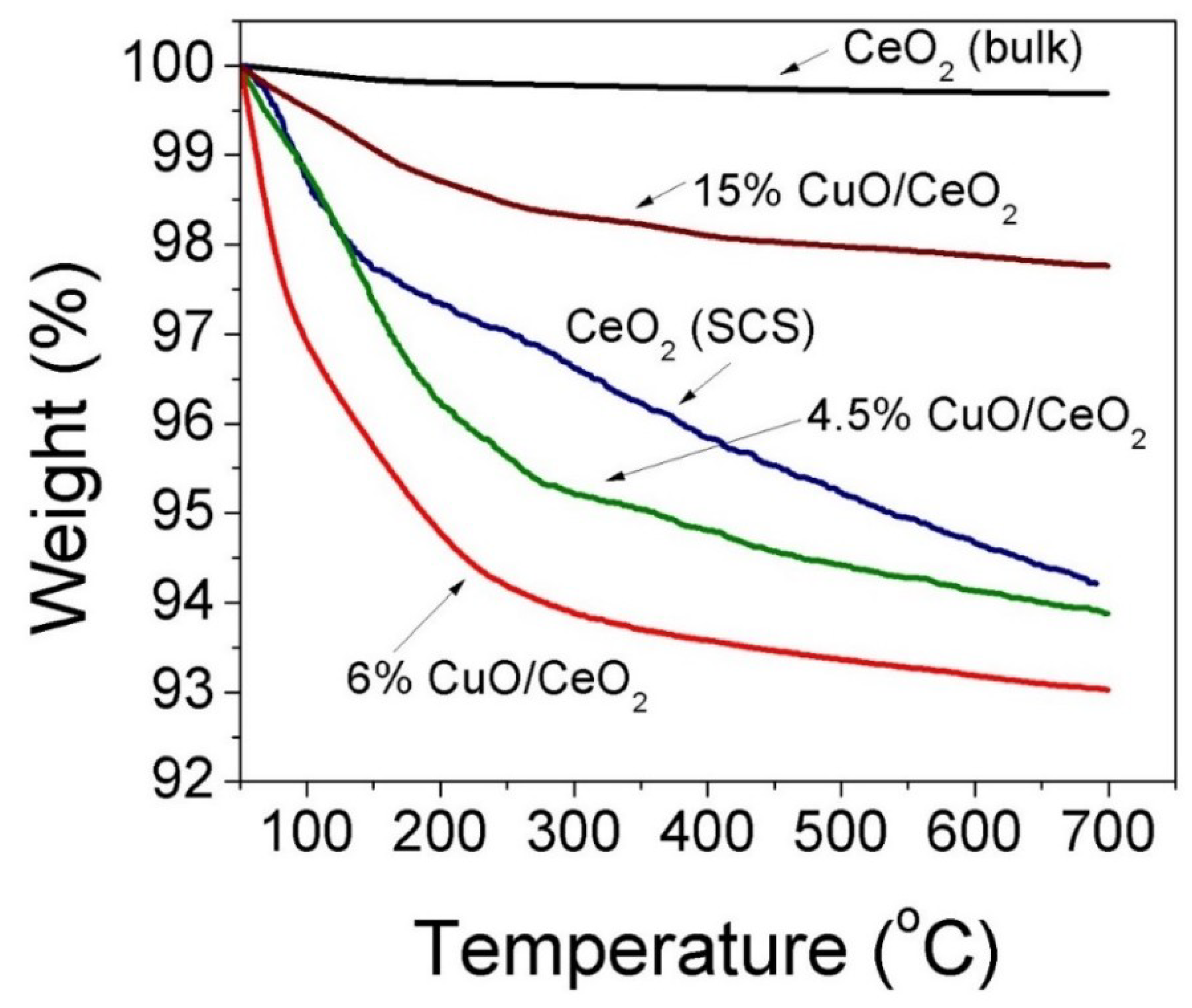
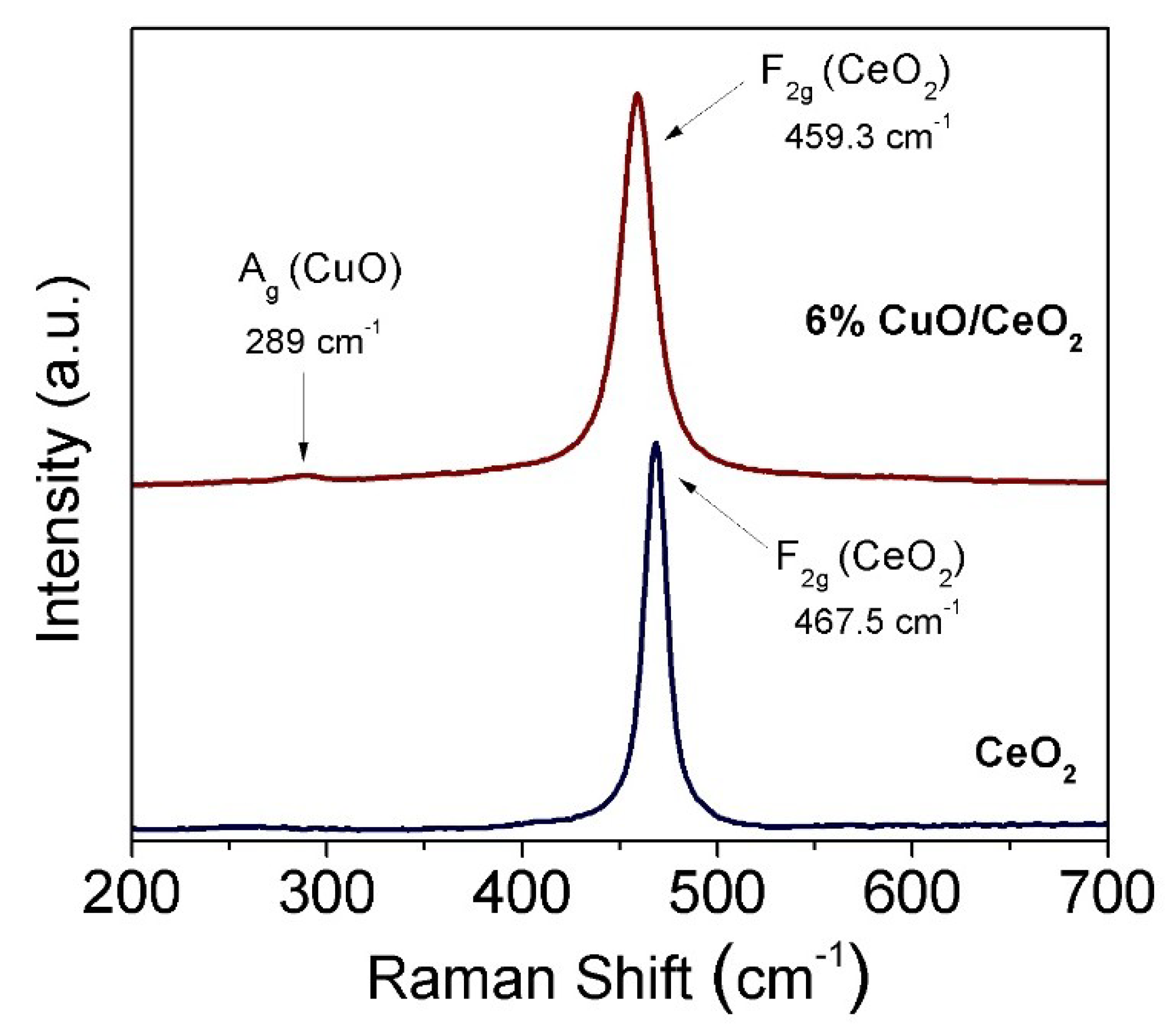
3.2. Thermal and Electronic Properties (TGA and Raman)
3.3. Surface Chemical Analysis (XPS)
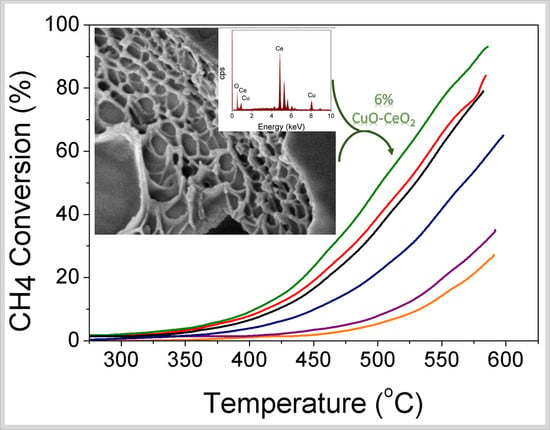
3.4. Catalytic CH4 Oxidation Study
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Maunula, T.; Kallinen, K.; Kinnunen, N.; Keenan, M.; Wolff, T. Methane Abatement and Catalyst Durability in Heterogeneous Lean-Rich and Dual-Fuel Conditions. Top. Catal. 2019. [Google Scholar] [CrossRef]
- Petrov, A.W.; Ferri, D.; Krumeich, F.; Nachtegaal, M.; van Bokhoven, J.A.; Kröcher, O. Stable complete methane oxidation over palladium based zeolite catalysts. Nat. Commun. 2018, 9, 2545. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Lou, Y.; Cai, Y.; Zhao, Z.; Wang, L.; Zhan, W.; Guo, Y.; Guo, Y. The relationship between the chemical state of Pd species and the catalytic activity for methane combustion on Pd/CeO2. Catal. Sci. Technol. 2018, 8, 2567–2577. [Google Scholar] [CrossRef]
- Qi, W.; Ran, J.; Zhang, Z.; Niu, J.; Zhang, P.; Fu, L.; Hu, B.; Li, Q. Methane combustion reactivity during the metal→metallic oxide transformation of Pd-Pt catalysts: Effect of oxygen pressure. Appl. Surf. Sci. 2018, 435, 776–785. [Google Scholar] [CrossRef]
- Habibi, A.H.; Hayes, R.E.; Semagina, N. Evaluation of hydrothermal stability of encapsulated PdPt@SiO2 catalyst for lean CH4 combustion. Appl. Catal. A Gen. 2018, 556, 129–136. [Google Scholar] [CrossRef]
- Specchia, S.; Conti, F.; Specchia, V. Kinetic Studies on Pd/CexZr1−xO2 Catalyst for Methane Combustion. Ind. Eng. Chem. Res. 2010, 49, 11101–11111. [Google Scholar] [CrossRef]
- Wang, D.; Gong, J.; Luo, J.; Li, J.; Kamasamudram, K.; Currier, N.; Yezerets, A. Distinct reaction pathways of methane oxidation on different oxidation states over Pd-based three-way catalyst (TWC). Appl. Catal. A Gen. 2019, 572, 44–50. [Google Scholar] [CrossRef]
- Dai, Y.; Kumar, V.P.; Zhu, C.; Wang, H.; Smith, K.J.; Wolf, M.O.; MacLachlan, M.J. Bowtie-Shaped NiCo2O4 Catalysts for Low-Temperature Methane Combustion. Adv. Funct. Mater. 2019, 29, 1807519. [Google Scholar] [CrossRef]
- Munoz, F.F.; Cabezas, M.D.; Acuna, L.M.; Leyva, A.G.; Baker, R.T.; Fuentes, R.O. Structural Properties and Reduction Behavior of Novel Nanostructured Pd/Gadolinia-Doped Ceria Catalysts with Tubular Morphology. J. Phys. Chem. C 2011, 115, 8744–8752. [Google Scholar] [CrossRef]
- Kockrick, E.; Borchardt, L.; Schrage, C.; Gaudillere, C.; Ziegler, C.; Freudenberg, T.; Farrusseng, D.; Eychmüller, A.; Kaskel, S. CeO2/Pt Catalyst Nanoparticle Containing Carbide-Derived Carbon Composites by a New In situ Functionalization Strategy. Chem. Mater. 2011, 23, 57–66. [Google Scholar] [CrossRef]
- Schwartz, W.R.; Pfefferle, L.D. Combustion of Methane over Palladium-Based Catalysts: Support Interactions. J. Phys. Chem. C 2012, 116, 8571–8578. [Google Scholar] [CrossRef]
- Cui, W.; Li, S.; Wang, D.; Deng, Y.; Chen, Y. High reactivity and sintering resistance of CH4 oxidation over modified Pd/Al2O3. Catal. Commun. 2019, 119, 86–90. [Google Scholar] [CrossRef]
- Guo, X.; Brault, P.; Zhi, G.; Caillard, A.l.; Guoqiang, J.; Coutanceau, C.; Baranton, S.; Guo, X. Synergistic Combination of Plasma Sputtered Pd–Au Bimetallic Nanoparticles for Catalytic Methane Combustion. J. Phys. Chem. C 2011, 115, 11240–11246. [Google Scholar] [CrossRef]
- Yang, J.; Guo, Y. Nanostructured perovskite oxides as promising substitutes of noble metals catalysts for catalytic combustion of methane. Chin. Chem. Lett. 2018, 29, 252–260. [Google Scholar] [CrossRef]
- Stanchovska, S.G.; Guergova, D.N.; Ivanov, G.M.; Stoyanova, R.K.; Zhecheva, E.N.; Naydenov, A.I. Supported Palladium Containing Perovskite Catalysts for Methane Combustion. Bulg. Chem. Commun. 2018, 50, 61–65. [Google Scholar]
- Wang, W.-W.; Du, P.-P.; Zou, S.-H.; He, H.-Y.; Wang, R.-X.; Jin, Z.; Shi, S.; Huang, Y.-Y.; Si, R.; Song, Q.-S.; et al. Highly Dispersed Copper Oxide Clusters as Active Species in Copper-Ceria Catalyst for Preferential Oxidation of Carbon Monoxide. Acs Catal. 2015, 5, 2088–2099. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, Y.; Wang, Z.; Liu, W.; Wang, L. Catalytic oxidation of CO over mesoporous copper-doped ceria catalysts via a facile CTAB-assisted synthesis. Rsc Adv. 2018, 8, 14888–14897. [Google Scholar] [CrossRef]
- Zabilskiy, M.; Djinović, P.; Tchernychova, E.; Tkachenko, O.P.; Kustov, L.M.; Pintar, A. Nanoshaped CuO/CeO2 Materials: Effect of the Exposed Ceria Surfaces on Catalytic Activity in N2O Decomposition Reaction. Acs Catal. 2015, 5, 5357–5365. [Google Scholar] [CrossRef]
- Polychronopoulou, K.; Jaoudé, M.A. Nano-architectural advancement of CeO2-driven catalysis via electrospinning. Surf. Coat. Technol. 2018, 350, 245–280. [Google Scholar] [CrossRef]
- Singhania, A.; Bhaskarwar, A.N. Effect of rare earth (RE–La, Pr, Nd) metal-doped ceria nanoparticles on catalytic hydrogen iodide decomposition for hydrogen production. Int. J. Hydrog. Energy 2018, 43, 4818–4825. [Google Scholar] [CrossRef]
- Di Sarli, V.; Landi, G.; Lisi, L.; Di Benedetto, A. Ceria-coated diesel particulate filters for continuous regeneration. Aiche J. 2017, 63, 3442–3449. [Google Scholar] [CrossRef]
- Polychronopoulou, K.; Zedan, A.F.; AlKetbi, M.; Stephen, S.; Ather, M.; Katsiotis, M.S.; Arvanitidis, J.; Christofilos, D.; Isakovic, A.F.; AlHassan, S. Tailoring the efficiency of an active catalyst for CO abatement through oxidation reaction: The case study of samarium-doped ceria. J. Environ. Chem. Eng. 2018, 6, 266–280. [Google Scholar] [CrossRef]
- Zhou, L.; Li, X.; Yao, Z.; Chen, Z.; Hong, M.; Zhu, R.; Liang, Y.; Zhao, J. Transition-Metal Doped Ceria Microspheres with Nanoporous Structures for CO Oxidation. Sci. Rep. 2016, 6, 23900. [Google Scholar] [CrossRef] [PubMed]
- Khalil, K.M.S.; Elkabee, L.A.; Murphy, B. Preparation and characterization of thermally stable porous ceria aggregates formed via a sol–gel process of ultrasonically dispersed cerium(IV) isopropoxide. Microporous Mesoporous Mater. 2005, 78, 83–89. [Google Scholar] [CrossRef]
- Shang, H.; Zhang, X.; Xu, J.; Han, Y. Effects of preparation methods on the activity of CuO/CeO2 catalysts for CO oxidation. Front. Chem. Sci. Eng. 2017, 11, 603–612. [Google Scholar] [CrossRef]
- AlKetbi, M.; Polychronopoulou, K.; Zedan, A.F.; Sebastián, V.; Baker, M.A.; AlKhoori, A.; Jaoude, M.A.; Alnuaimi, O.; Hinder, S.S.; Tharalekshmy, A.; et al. Tuning the activity of Cu-containing rare earth oxide catalysts for CO oxidation reaction: Cooling while heating paradigm in microwave-assisted synthesis. Mater. Res. Bull. 2018, 108, 142–150. [Google Scholar] [CrossRef]
- Konsolakis, M. The role of Copper–Ceria interactions in catalysis science: Recent theoretical and experimental advances. Appl. Catal. B Environ. 2016, 198, 49–66. [Google Scholar] [CrossRef]
- Sun, S.; Mao, D.; Yu, J.; Yang, Z.; Lu, G.; Ma, Z. Low-temperature CO oxidation on CuO/CeO2 catalysts: The significant effect of copper precursor and calcination temperature. Catal. Sci. Technol. 2015, 5, 3166–3181. [Google Scholar] [CrossRef]
- Lin, L.; Yao, S.; Liu, Z.; Zhang, F.; Li, N.; Vovchok, D.; Martínez-Arias, A.; Castañeda, R.; Lin, J.; Senanayake, S.D.; et al. In Situ Characterization of Cu/CeO2 Nanocatalysts for CO2 Hydrogenation: Morphological Effects of Nanostructured Ceria on the Catalytic Activity. J. Phys. Chem. C 2018, 122, 12934–12943. [Google Scholar] [CrossRef]
- Konsolakis, M.; Carabineiro, S.A.C.; Papista, E.; Marnellos, G.E.; Tavares, P.B.; Moreira, J.A.; Romaguera-Barcelay, Y.; Figueiredo, J.L. Effect of preparation method on the solid state properties and the deN2O performance of CuO–CeO2 oxides. Catal. Sci. Technol. 2015, 5, 3714–3727. [Google Scholar] [CrossRef]
- Chen, C.; Zhan, Y.; Zhou, J.; Li, D.; Zhang, Y.; Lin, X.; Jiang, L.; Zheng, Q. Cu/CeO2 Catalyst for Water-Gas Shift Reaction: Effect of CeO2 Pretreatment. ChemPhysChem 2018, 19, 1448–1455. [Google Scholar] [CrossRef] [PubMed]
- Barbato, P.S.; Colussi, S.; Di Benedetto, A.; Landi, G.; Lisi, L.; Llorca, J.; Trovarelli, A. Origin of High Activity and Selectivity of CuO/CeO2 Catalysts Prepared by Solution Combustion Synthesis in CO-PROX Reaction. J. Phys. Chem. C 2016, 120, 13039–13048. [Google Scholar] [CrossRef]
- Alammar, T.; Noei, H.; Wang, Y.; Grünert, W.; Mudring, A.-V. Ionic Liquid-Assisted Sonochemical Preparation of CeO2 Nanoparticles for CO Oxidation. Acs Sustain. Chem. Eng. 2015, 3, 42–54. [Google Scholar] [CrossRef]
- Arango-Díaz, A.; Moretti, E.; Talon, A.; Storaro, L.; Lenarda, M.; Núñez, P.; Marrero-Jerez, J.; Jiménez-Jiménez, J.; Jiménez-López, A.; Rodríguez-Castellón, E. Preferential CO oxidation (CO-PROX) catalyzed by CuO supported on nanocrystalline CeO2 prepared by a freeze-drying method. Appl. Catal. A Gen. 2014, 477, 54–63. [Google Scholar] [CrossRef]
- Li, Y.; Cai, Y.; Xing, X.; Chen, N.; Deng, D.; Wang, Y. Catalytic activity for CO oxidation of Cu–CeO2 composite nanoparticles synthesized by a hydrothermal method. Anal. Methods 2015, 7, 3238–3245. [Google Scholar] [CrossRef]
- Zagaynov, I.V.; Kutsev, S.V.; Shelekhov, E.V.; Naumkin, A.V. CuO–CeO2 composites: Synthesis from mixed sols. Colloids Surf. A Physicochem. Eng. Asp. 2014, 444, 159–164. [Google Scholar] [CrossRef]
- Kang, W.; Ozgur, D.O.; Varma, A. Solution Combustion Synthesis of High Surface Area CeO2 Nanopowders for Catalytic Applications: Reaction Mechanism and Properties. Acs Appl. Nano Mater. 2018, 1, 675–685. [Google Scholar] [CrossRef]
- Abdelsayed, V.; Aljarash, A.; El-Shall, M.S.; Al Othman, Z.A.; Alghamdi, A.H. Microwave Synthesis of Bimetallic Nanoalloys and CO Oxidation on Ceria-Supported Nanoalloys. Chem. Mater. 2009, 21, 2825–2834. [Google Scholar] [CrossRef]
- Zedan, A.F.; Allam, N.K.; AlQaradawi, S.Y. A Study of Low-Temperature CO Oxidation over Mesoporous CuO-TiO2 Nanotube Catalysts. Catalysts 2017, 7, 129. [Google Scholar] [CrossRef]
- Zedan, A.F.; Mohamed, A.T.; El-Shall, M.S.; AlQaradawi, S.Y.; AlJaber, A.S. Tailoring the reducibility and catalytic activity of CuO nanoparticles for low temperature CO oxidation. Rsc Adv. 2018, 8, 19499–19511. [Google Scholar] [CrossRef]
- Zedan, A.F.; Polychronopoulou, K.; Asif, A.; AlQaradawi, S.Y.; AlJaber, A.S. Cu-Ce-O catalyst revisited for exceptional activity at low temperature CO oxidation reaction. Surf. Coat. Technol. 2018, 354, 313–323. [Google Scholar] [CrossRef]
- Konar, S.; Kalita, H.; Puvvada, N.; Tantubay, S.; Mahto, M.K.; Biswas, S.; Pathak, A. Shape-dependent catalytic activity of CuO nanostructures. J. Catal. 2016, 336, 11–22. [Google Scholar] [CrossRef]
- Polychronopoulou, K.; Zedan, A.F.; Katsiotis, M.S.; Baker, M.A.; AlKhoori, A.A.; AlQaradawi, S.Y.; Hinder, S.J.; AlHassan, S. Rapid microwave assisted sol-gel synthesis of CeO2 and CexSm1−xO2 nanoparticle catalysts for CO oxidation. Mol. Catal. 2017, 428, 41–55. [Google Scholar] [CrossRef]
- Shi, S.; Hossu, M.; Hall, R.; Chen, W. Solution combustion synthesis, photoluminescence and X-ray luminescence of Eu-doped nanoceria CeO2:Eu. J. Mater. Chem. 2012, 22, 23461–23467. [Google Scholar] [CrossRef]
- Jadhav, L.D.; Patil, S.P.; Chavan, A.U.; Jamale, A.P.; Puri, V.R. Solution combustion synthesis of Cu nanoparticles: A role of oxidant-to-fuel ratio. IET Micro Nano Lett. 2011, 6, 812–815. [Google Scholar] [CrossRef]
- Varma, A.; Mukasyan, A.S.; Rogachev, A.S.; Manukyan, K.V. Solution Combustion Synthesis of Nanoscale Materials. Chem. Rev. 2016, 116, 14493–14586. [Google Scholar] [CrossRef] [PubMed]
- Cwele, T.; Mahadevaiah, N.; Singh, S.; Friedrich, H.B.; Yadav, A.K.; Jha, S.N.; Bhattacharyya, D.; Sahoo, N.K. CO oxidation activity enhancement of Ce0.95Cu0.05O2−δ induced by Pd co-substitution. Catal. Sci. Technol. 2016, 6, 8104–8116. [Google Scholar] [CrossRef]
- Khan, M.A.M.; Khan, W.; Ahamed, M.; Alhazaa, A.N. Microstructural properties and enhanced photocatalytic performance of Zn doped CeO2 nanocrystals. Sci. Rep. 2017, 7, 12560. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, I.; Kumar, P.P.; Annapoorna, I.; Sumliner, J.M.; Ramakrishna, M.; Hebalkar, N.Y.; Padmanabham, G.; Sundararajan, G. Preparation and characterization of Cu-doped TiO2 materials for electrochemical, photoelectrochemical, and photocatalytic applications. Appl. Surf. Sci. 2014, 293, 229–247. [Google Scholar] [CrossRef]
- Taniguchi, T.; Watanabe, T.; Sugiyama, N.; Subramani, A.K.; Wagata, H.; Matsushita, N.; Yoshimura, M. Identifying Defects in Ceria-Based Nanocrystals by UV Resonance Raman Spectroscopy. J. Phys. Chem. C 2009, 113, 19789–19793. [Google Scholar] [CrossRef]
- Sudarsanam, P.; Hillary, B.; Amin, M.H.; Rockstroh, N.; Bentrup, U.; Brückner, A.; Bhargava, S.K. Heterostructured Copper–Ceria and Iron–Ceria Nanorods: Role of Morphology, Redox, and Acid Properties in Catalytic Diesel Soot Combustion. Langmuir 2018, 34, 2663–2673. [Google Scholar] [CrossRef] [PubMed]
- Moumen, A.; Hartiti, B.; Thevenin, P.; Siadat, M. Synthesis and characterization of CuO thin films grown by chemical spray pyrolysis. Opt. Quantum Electron. 2017, 49, 70. [Google Scholar] [CrossRef]
- Patsalas, P.; Logothetidis, S.; Sygellou, L.; Kennou, S. Structure-dependent electronic properties of nanocrystalline cerium oxide films. Phys. Rev. B 2003, 68, 035104. [Google Scholar] [CrossRef]
- Zhong, L.-S.; Hu, J.-S.; Cao, A.-M.; Liu, Q.; Song, W.-G.; Wan, L.-J. 3D Flowerlike Ceria Micro/Nanocomposite Structure and Its Application for Water Treatment and CO Removal. Chem. Mater. 2007, 19, 1648–1655. [Google Scholar] [CrossRef]
- Sutradhar, N.; Sinhamahapatra, A.; Pahari, S.; Jayachandran, M.; Subramanian, B.; Bajaj, H.C.; Panda, A.B. Facile Low-Temperature Synthesis of Ceria and Samarium-Doped Ceria Nanoparticles and Catalytic Allylic Oxidation of Cyclohexene. J. Phys. Chem. C 2011, 115, 7628–7637. [Google Scholar] [CrossRef]
- Ong, H.R.; Rahman Khan, M.M.; Ramli, R.; Du, Y.; Xi, S.; Yunus, R.M. Facile synthesis of copper nanoparticles in glycerol at room temperature: Formation mechanism. RSC Adv. 2015, 5, 24544–24549. [Google Scholar] [CrossRef]
- Subalakshmi, P.; Ganesan, M.; Sivashanmugam, A. Synthesis of 3D architecture CuO micro balls and nano hexagons and its electrochemical capacitive behavior. Mater. Des. 2017, 119, 104–112. [Google Scholar] [CrossRef]
- Ola, O.; Mercedes Maroto-Valer, M. Copper based TiO2 honeycomb monoliths for CO2 photoreduction. Catal. Sci. Technol. 2014, 4, 1631–1637. [Google Scholar] [CrossRef]
- Khader, M.M.; Al-Marri, J.M.; Ali, S.; Abdelmoneim, G.A. Active and Stable Methane Oxidation Nano-Catalyst with Highly-Ionized Palladium Species Prepared by Solution Combustion Synthesis. Catalysts 2018, 8, 66. [Google Scholar] [CrossRef]
- Chrzan, M.; Chlebda, D.; Jodłowski, P.; Salomon, E.; Kołodziej, A.; Gancarczyk, A.; Sitarz, M.; Łojewska, J. Towards Methane Combustion Mechanism on Metal Oxides Supported Catalysts: Ceria Supported Palladium Catalysts. Top. Catal. 2019. [Google Scholar] [CrossRef]

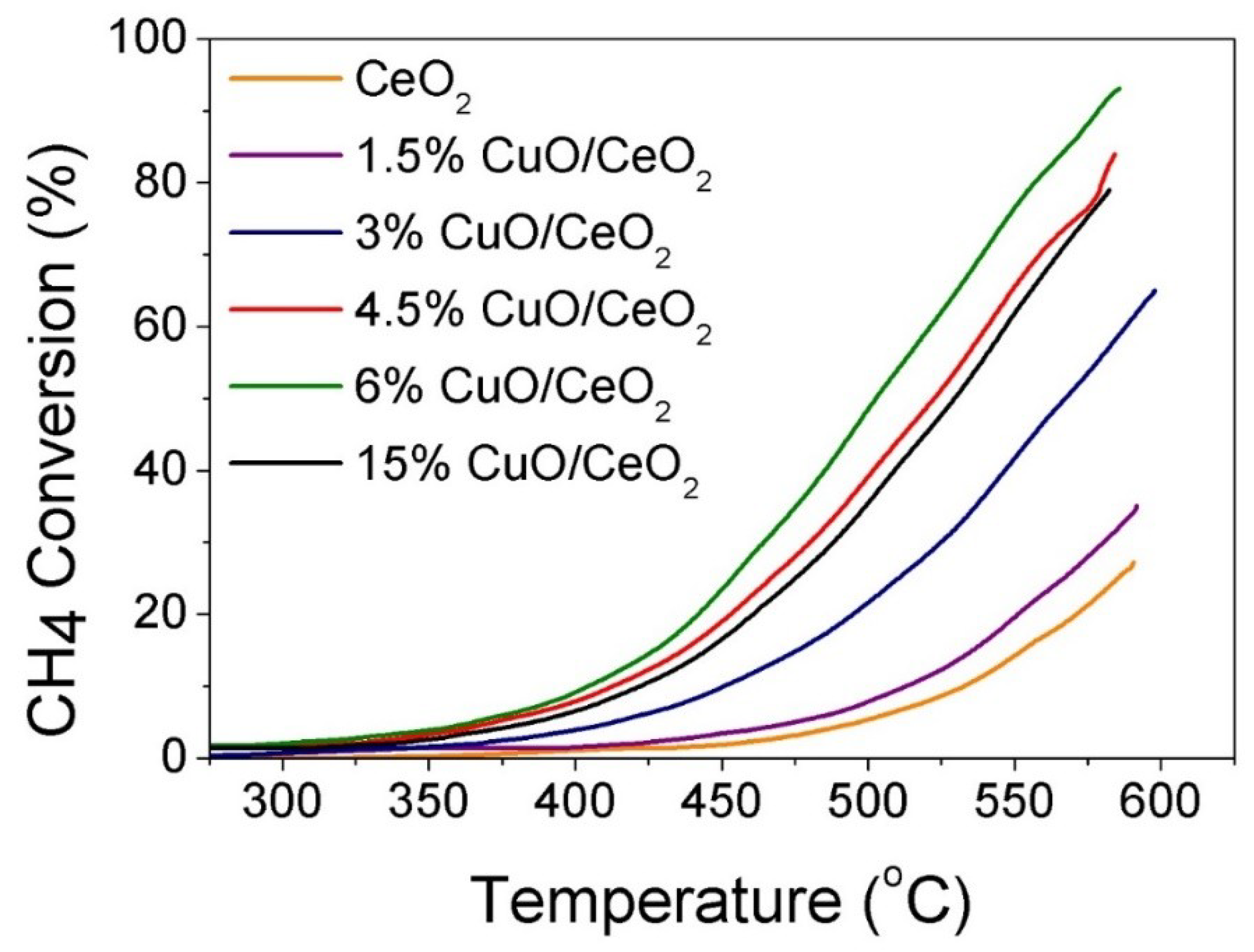
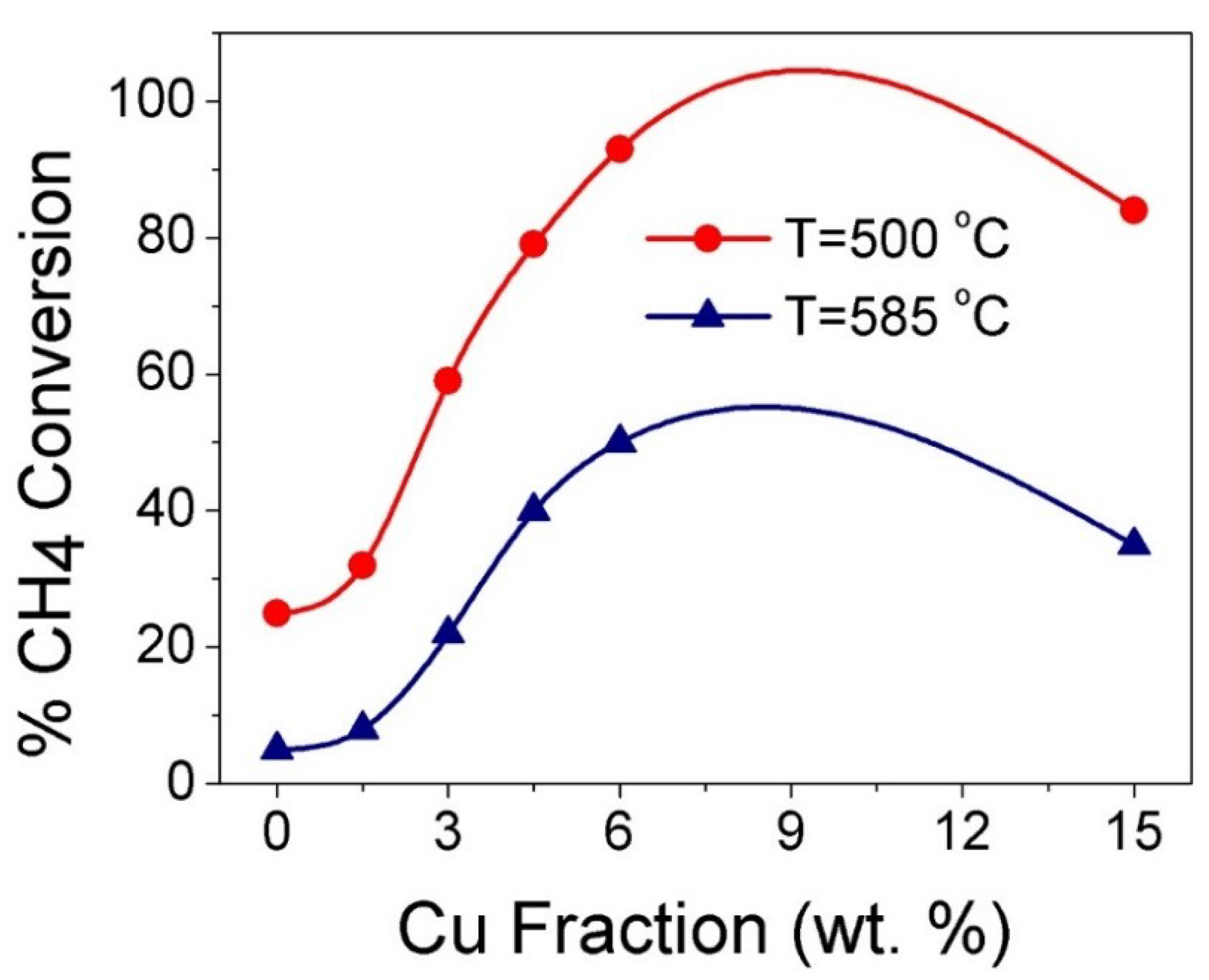
| Catalyst | T25 (°C) | T50 (°C) | T80 (°C) | % CH4 Conversion (T = 500 °C) | % CH4 Conversion (T = 585 °C) |
|---|---|---|---|---|---|
| CeO2 | 585 | - | - | 5 | 25 |
| 1.5% CuO-CeO2 | 565 | - | - | 8 | 32 |
| 3% CuO-CeO2 | 510 | 567 | - | 22 | 59 |
| 4.5% CuO-CeO2 | 465 | 522 | 580 | 40 | 84 |
| 6% CuO-CeO2 | 453 | 502 | 556 | 50 | 93 |
| 15% CuO-CeO2 | 475 | 530 | - | 35 | 79 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zedan, A.F.; AlJaber, A.S. Combustion Synthesis of Non-Precious CuO-CeO2 Nanocrystalline Catalysts with Enhanced Catalytic Activity for Methane Oxidation. Materials 2019, 12, 878. https://doi.org/10.3390/ma12060878
Zedan AF, AlJaber AS. Combustion Synthesis of Non-Precious CuO-CeO2 Nanocrystalline Catalysts with Enhanced Catalytic Activity for Methane Oxidation. Materials. 2019; 12(6):878. https://doi.org/10.3390/ma12060878
Chicago/Turabian StyleZedan, Abdallah F., and Amina S. AlJaber. 2019. "Combustion Synthesis of Non-Precious CuO-CeO2 Nanocrystalline Catalysts with Enhanced Catalytic Activity for Methane Oxidation" Materials 12, no. 6: 878. https://doi.org/10.3390/ma12060878
APA StyleZedan, A. F., & AlJaber, A. S. (2019). Combustion Synthesis of Non-Precious CuO-CeO2 Nanocrystalline Catalysts with Enhanced Catalytic Activity for Methane Oxidation. Materials, 12(6), 878. https://doi.org/10.3390/ma12060878