Development of a Novel Biosensor Based on Tyrosinase/Platinum Nanoparticles/Chitosan/Graphene Nanostructured Layer with Applicability in Bioanalysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Materials
2.3. Development of Platinum Nanoparticles/Chitosan/Graphene-Carbon Screen-Printed Electrode (PtNP/Chit/GPH-CSPE)
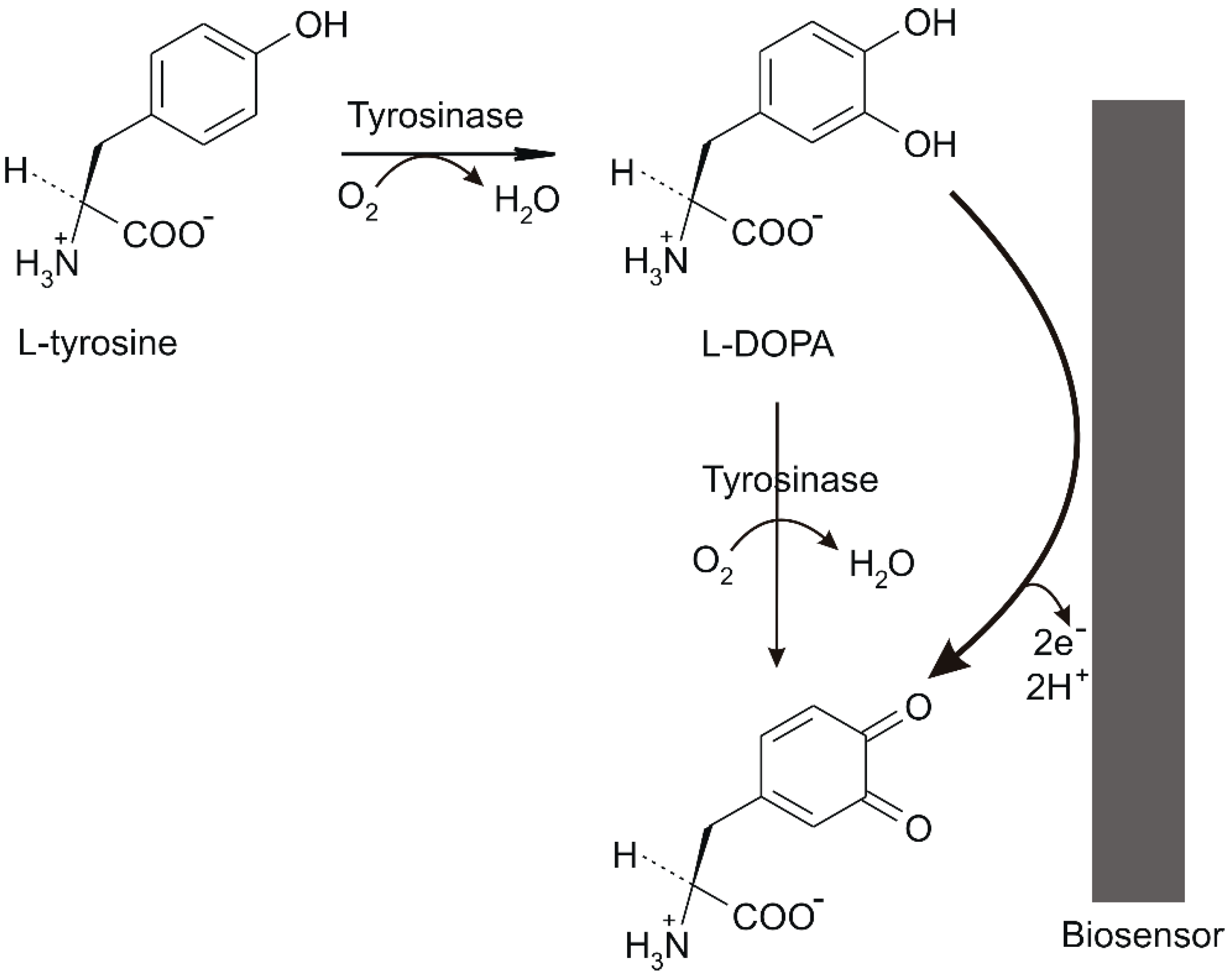
2.4. Development of Tyrosinase (Ty)/PtNP/GPH/Chit-CSPE
2.5. Apparatus
2.6. Biological and Pharmaceutical Samples
3. Results
3.1. Characterization of Nanostructured Biomaterial
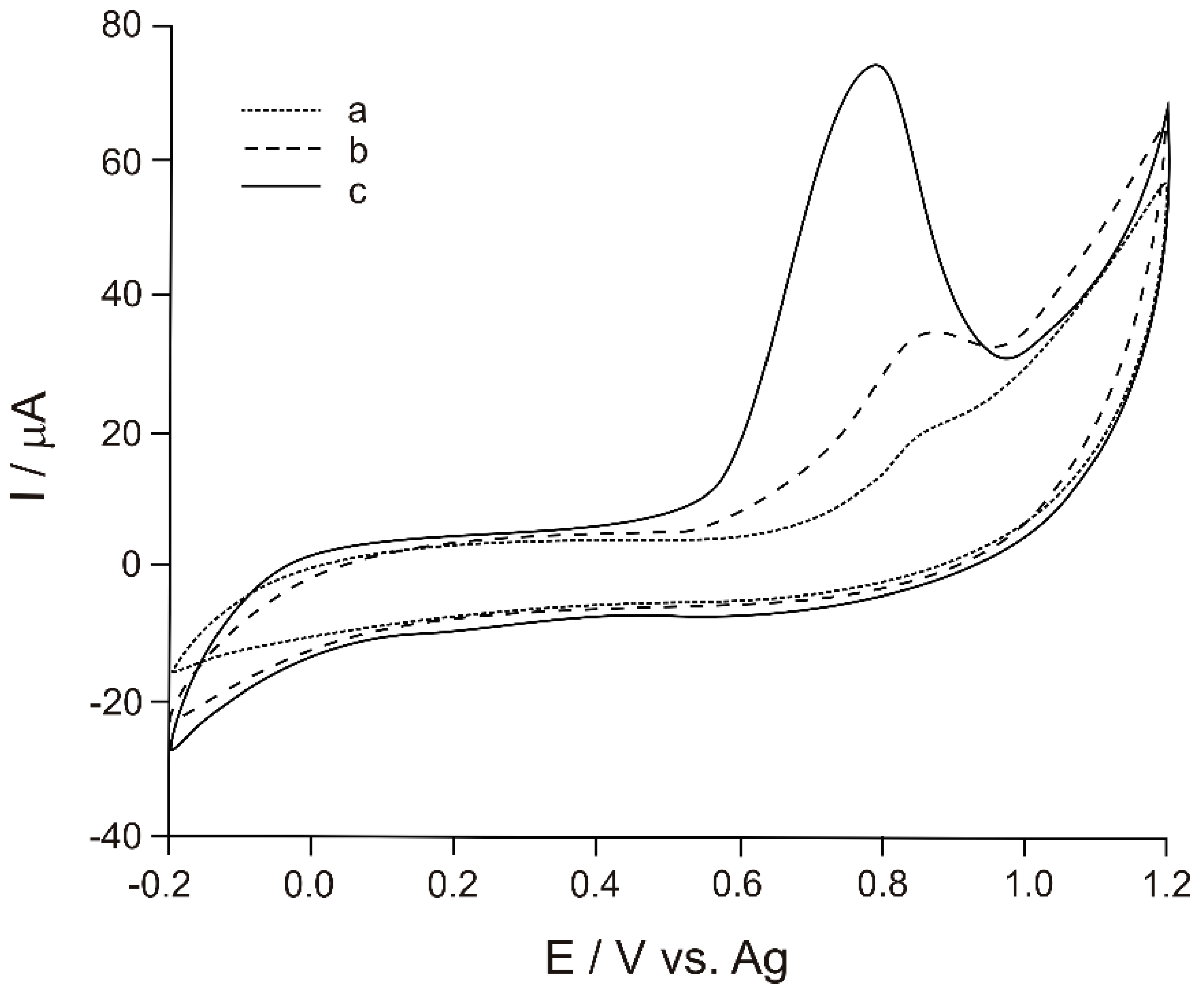
3.2. Exploratory Studies for the Detection of Tyr by Biosensor
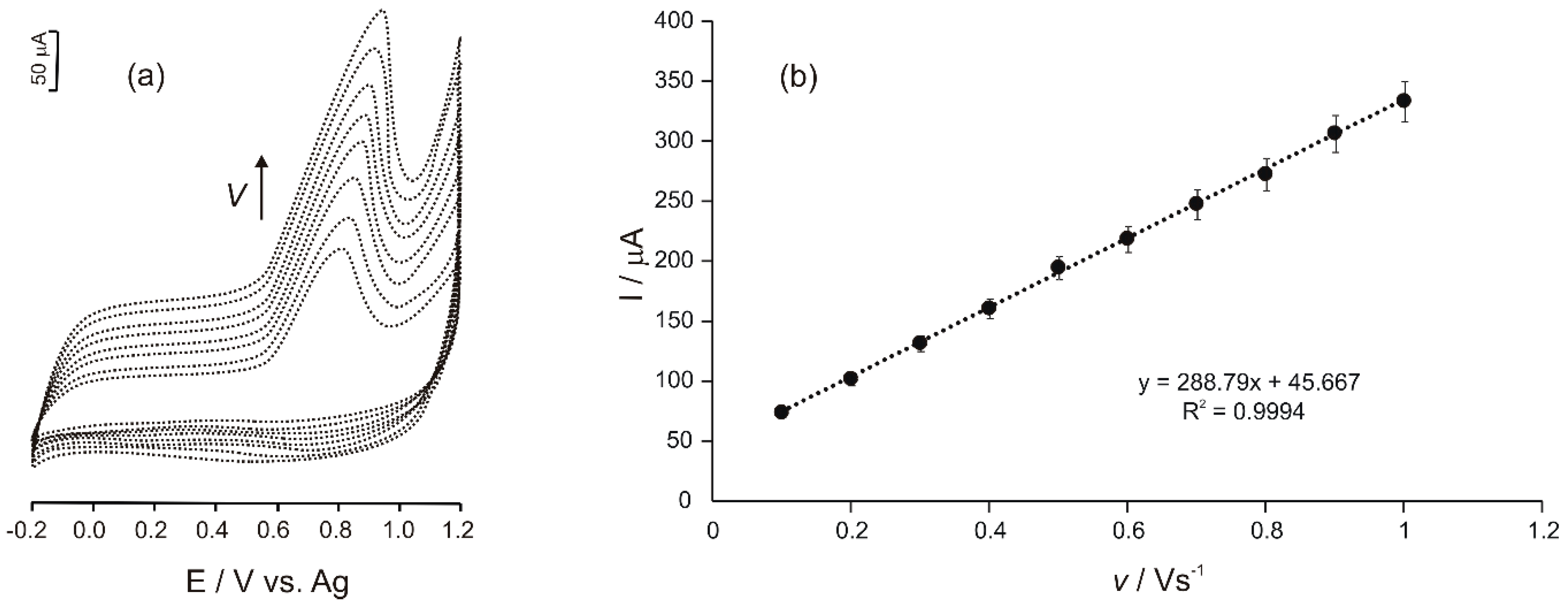
3.3. Influence of the Scan Rate in the Biosensor Response
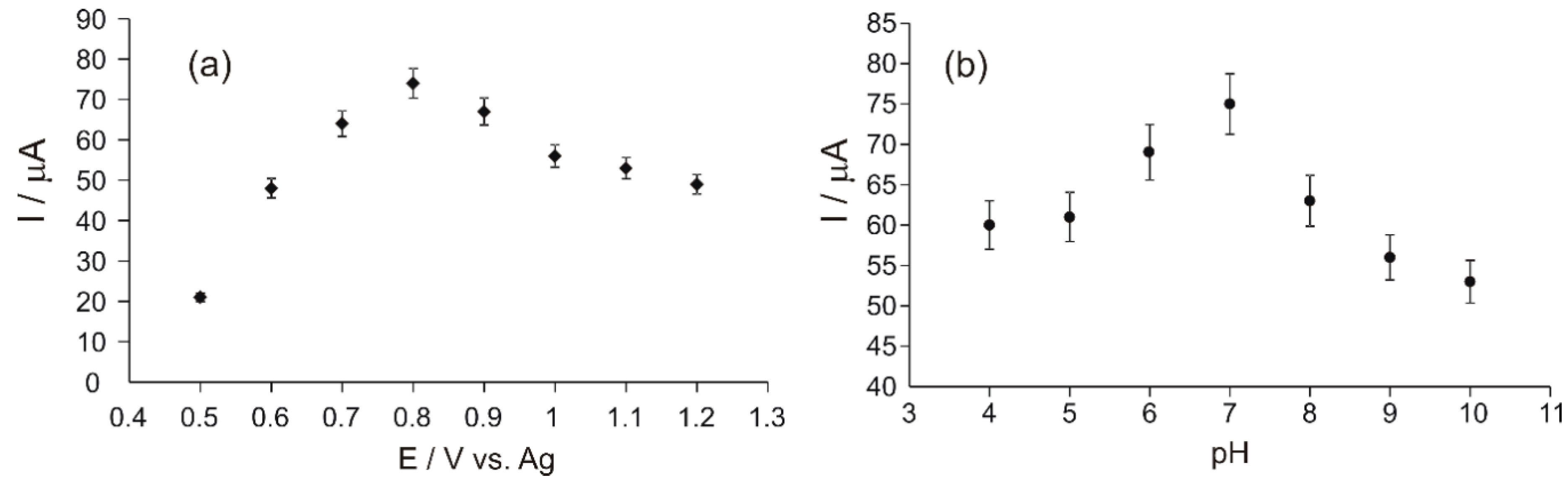
3.4. Studies for the Optimization of the Experimental Parameters
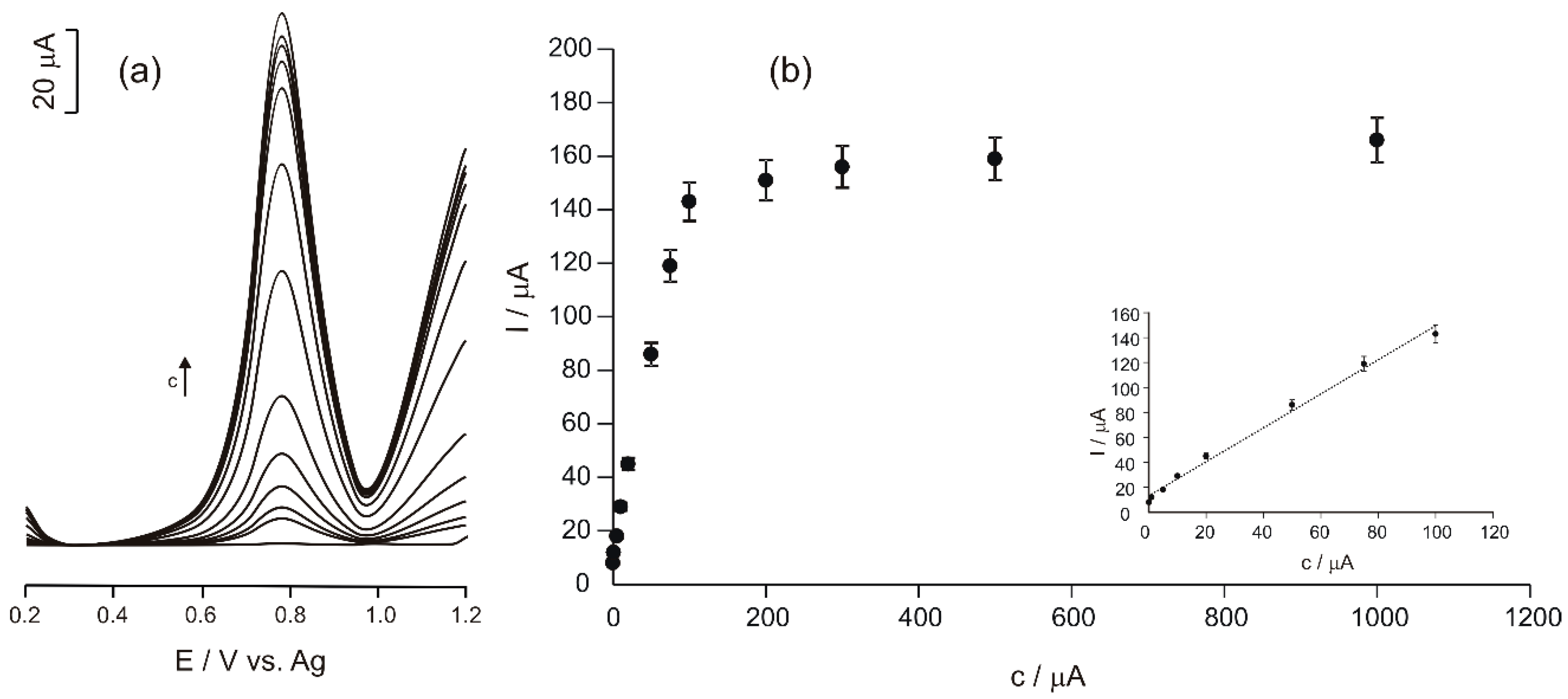
3.5. Analytical Performance Characteristics of the Ty/PtNP/GPH/Chit-CSPE Biosensor
3.6. A Comparative Approach to the (Bio)Sensors Reported in the Literature
3.7. Interference Study
3.8. Repeatability and Stability of the Ty/PtNP/GPH/Chit-CSPE Biosensor
3.9. Use of the Biosensor in Bioassay and Validation of the Quantitative Determination Method
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- El Harrad, L.; Bourais, I.; Mohammadi, H.; Amine, A. Recent Advances in Electrochemical Biosensors Based on Enzyme Inhibition for Clinical and Pharmaceutical Applications. Sensors 2018, 18, 164. [Google Scholar] [CrossRef]
- Hoffer, L.; Sher, K.; Saboohi, F.; Bernier, P.; MacNamara, E.; Rinzler, D. N-Acetyl-l-Tyrosine as a Tyrosine Source in Adult Parenteral Nutrition. J. Parenter. Enter. Nutr. 2003, 27, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Moizé, V.; Pi-Sunyer, X.; Vidal, J.; Miner, P.; Boirie, Y.; Laferrère, B. Effect on Nitrogen Balance, Thermogenesis, Body Composition, Satiety, and Circulating Branched Chain Amino Acid Levels up to One Year after Surgery: Protocol of a Randomized Controlled Trial on Dietary Protein During Surgical Weight Loss. JMIR Res. Protoc. 2016, 5, e220. [Google Scholar] [CrossRef]
- Voet, D.; Voet, J.G.; Pratt, C.W. Fundamentals of Biochemistry: Life at the Molecular Level, 5th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Fernstrom, J.D.; Fernstrom, M.H. Tyrosine, Phenylalanine, and Catecholamine Synthesis and Function in the Brain. J. Nutr. 2007, 137, 1539S–1547S. [Google Scholar] [CrossRef] [PubMed]
- Scriver, C.R.; Beaudet, A.L.; Sly, W.S.; Valle, D. “The Metabolic & Molecular Bases of Inherited Disease, Edition 7: Hyperphenylalaninemia: Phenylalanine Hydroxylase Deficiency”, The Metabolic and Molecular Bases of Inherited Disease, Chapter 77, Hyperphenylalaninemias: Phenylalanine Hydroxylase Deficiency; McGraw-Hill: New York, NY, USA, 2001; pp. 1667–1724. [Google Scholar]
- Fernandes, J. (Ed.) Inborn Metabolic Diseases: Diagnosis and Treatment, 4th ed.; Springer: Heidelberg, Germany, 2006. [Google Scholar] [CrossRef]
- Rahimi-Mohseni, M.; Raoof, J.B.; Ojani, R.; Aghajanzadeh, T.A.; Bagheri Hashkavayi, A. Development of a New Paper Based Nano-Biosensor Using the Co-Catalytic Effect of Tyrosinase from Banana Peel Tissue (Musa Cavendish) and Functionalized Silica Nanoparticles for Voltammetric Determination of l-Tyrosine. Int. J. Biol. Macromol. 2018, 113, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Hazra, C.; Samanta, T.; Mahalingam, V. A Resonance Energy Transfer Approach for the Selective Detection of Aromatic Amino Acids. J. Mater. Chem. C 2014, 2, 10157–10163. [Google Scholar] [CrossRef]
- Li, S.; Xing, M.; Wang, H.; Zhang, L.; Zhong, Y.; Chen, L. Determination of Tryptophan and Tyrosine by Chemiluminescence Based on a Luminol–N-Bromosuccinimide–ZnS Quantum Dots System. RSC Adv. 2015, 5, 59286–59291. [Google Scholar] [CrossRef]
- Dailey, C.A.; Garnier, N.; Rubakhin, S.S.; Sweedler, J.V. Automated Method for Analysis of Tryptophan and Tyrosine Metabolites Using Capillary Electrophoresis with Native Fluorescence Detection. Anal. Bioanal. Chem. 2013, 405, 2451–2459. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, B.; Yang, Y.; Wan, Y. A Simple and Reliable Ultra-High Performance Liquid Chromatography Coupled with Tandem Mass Spectrometry Method for Simultaneous Quantification of Tyrosine and Its Metabolites in Human Urine. J. Liquid Chromatogr. Relat. Technol. 2019, 1–7. [Google Scholar] [CrossRef]
- Varmira, K.; Mohammadi, G.; Mahmoudi, M.; Khodarahmi, R.; Rashidi, K.; Hedayati, M.; Goicoechea, H.C.; Jalalvand, A.R. Fabrication of a Novel Enzymatic Electrochemical Biosensor for Determination of Tyrosine in Some Food Samples. Talanta 2018, 183, 1–10. [Google Scholar] [CrossRef]
- Labib, M.; Sargent, E.H.; Kelley, S.O. Electrochemical Methods for the Analysis of Clinically Relevant Biomolecules. Chem. Rev. 2016, 116, 9001–9090. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Wang, Y. The Application of Chemically Modified Electrodes in Analytical Chemistry. Electroanalysis 1989, 1, 99–106. [Google Scholar] [CrossRef]
- Sánchez-Ferrer, Á.; Neptuno Rodríguez-López, J.; García-Cánovas, F.; García-Carmona, F. Tyrosinase: A Comprehensive Review of Its Mechanism. Biochim. Biophys. Acta (BBA) Protein Struct. Mol. Enzymol. 1995, 1247, 1–11. [Google Scholar] [CrossRef]
- Mohan, V.B.; Lau, K.; Hui, D.; Bhattacharyya, D. Graphene-Based Materials and Their Composites: A Review on Production, Applications and Product Limitations. Compos. Part B Eng. 2018, 142, 200–220. [Google Scholar] [CrossRef]
- Nag, A.; Mitra, A.; Mukhopadhyay, S.C. Graphene and Its Sensor-Based Applications: A Review. Sens. Actuators A Phys. 2018, 270, 177–194. [Google Scholar] [CrossRef]
- Apetrei, I.; Apetrei, C. Amperometric Biosensor Based on Diamine Oxidase/Platinum Nanoparticles/Graphene/Chitosan Modified Screen-Printed Carbon Electrode for Histamine Detection. Sensors 2016, 16, 422. [Google Scholar] [CrossRef] [PubMed]
- Minch, R.; Es-Souni, M. A Versatile Approach to Processing of High Active Area Pillar Coral- and Sponge-like Pt-Nanostructures. Application to Electrocatalysis. J. Mater. Chem. 2011, 21, 4182. [Google Scholar] [CrossRef]
- Muthusankar, E.; Ragupathy, D. Chitosan Based Nanocomposite Biosensors: A Recent Review. Sens. Lett. 2018, 16, 81–91. [Google Scholar] [CrossRef]
- Carralero, V.; Mena, M.L.; Gonzalez-Cortés, A.; Yáñez-Sedeño, P.; Pingarrón, J.M. Development of a High Analytical Performance-Tyrosinase Biosensor Based on a Composite Graphite–Teflon Electrode Modified with Gold Nanoparticles. Biosens. Bioelectron. 2006, 22, 730–736. [Google Scholar] [CrossRef]
- Yang, L.; Xiong, H.; Zhang, X.; Wang, S. A Novel Tyrosinase Biosensor Based on Chitosan-Carbon-Coated Nickel Nanocomposite Film. Bioelectrochemistry 2012, 84, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhai, F.; Hasebe, Y.; Jia, H.; Zhang, Z. A Highly Sensitive Electrochemical Biosensor for Phenol Derivatives Using a Graphene Oxide-Modified Tyrosinase Electrode. Bioelectrochemistry 2018, 122, 174–182. [Google Scholar] [CrossRef]
- Singh, S.; Jain, D.V.S.; Meena, V.K. Organic-Inorganic Hybrid Matrix for Electrochemical Biosensing of Tyrosine. Mater. Res. Bull. 2017, 94, 520–527. [Google Scholar] [CrossRef]
- Apetrei, I.M.; Apetrei, C. The Biocomposite Screen-Printed Biosensor Based on Immobilization of Tyrosinase onto the Carboxyl Functionalised Carbon Nanotube for Assaying Tyramine in Fish Products. J. Food Eng. 2015, 149, 1–8. [Google Scholar] [CrossRef]
- Eremia, S.A.V.; Vasilescu, I.; Radoi, A.; Litescu, S.-C.; Radu, G.-L. Disposable Biosensor Based on Platinum Nanoparticles-Reduced Graphene Oxide-Laccase Biocomposite for the Determination of Total Polyphenolic Content. Talanta 2013, 110, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, K.U.; Ali, A.S.; Ali, S.A.; Naaz, I. Microbial Tyrosinases: Promising Enzymes for Pharmaceutical, Food Bioprocessing, and Environmental Industry. Biochem. Res. Int. 2014, 2014, 854687. [Google Scholar] [CrossRef]
- Ozkan, S.A.; Kauffmann, J.-M.; Zuman, P.; Oliveira Brett, A.M.; Brett, C.; Uslu, B.; Hubert, P.; Rozet, E.; Parsajoo, C.; Patris, S.; et al. (Eds.) Electroanalysis in Biomedical and Pharmaceutical Sciences: Voltammetry, Amperometry, Biosensors, Applications; Monographs in Electrochemistry; Springer: Heidelberg, Germany, 2015. [Google Scholar]
- Klingler, R.J.; Kochi, J.K. Electron-Transfer Kinetics from Cyclic Voltammetry. Quantitative Description of Electrochemical Reversibility. J. Phys. Chem. 1981, 85, 1731–1741. [Google Scholar] [CrossRef]
- German, N.; Ramanavicius, A.; Ramanaviciene, A. Electrochemical Deposition of Gold Nanoparticles on Graphite Rod for Glucose Biosensing. Sens. Actuators B Chem. 2014, 203, 25–34. [Google Scholar] [CrossRef]
- Behpour, M.; Masoum, S.; Meshki, M. Study and Electrochemical Determination of Tyrosine at Graphene Nanosheets Composite Film Modified Glassy Carbon Electrode. J. Nanostruct. 2013, 3, 243–251. [Google Scholar] [CrossRef]
- O’Hare, D. Biosensors and Sensor Systems. In Body Sensor Networks; Yang, G.-Z., Ed.; Springer: London, UK, 2014; pp. 55–115. [Google Scholar] [CrossRef]
- Apetrei, C.; Alessio, P.; Constantino, C.J.L.; de Saja, J.A.; Rodriguez-Mendez, M.L.; Pavinatto, F.J.; Fernandes, E.G.R.; Zucolotto, V.; Oliveira, O.N. Biomimetic Biosensor Based on Lipidic Layers Containing Tyrosinase and Lutetium Bisphthalocyanine for the Detection of Antioxidants. Biosens. Bioelectron. 2011, 26, 2513–2519. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; Wiley: New York, NY, USA, 2001. [Google Scholar]
- He, Y.; Yang, X.; Han, Q.; Zheng, J. The Investigation of Electrochemistry Behaviors of Tyrosinase Based on Directly-Electrodeposited Grapheneon Choline-Gold Nanoparticles. Molecules 2017, 22, 1047. [Google Scholar] [CrossRef] [PubMed]
- Apetrei, I.M.; Apetrei, C. Amperometric tyrosinase based biosensors for serotonin detection. Rom. Biotechnol. Lett. 2013, 18, 8253–8262. [Google Scholar]
- Babaei, A.; Mirzakhani, S.; Khalilzadeh, B. A sensitive simultaneous determination of epinephrine and tyrosine using an iron (III) doped zeolite-modified carbon paste electrode. J. Braz. Chem. Soc. 2009, 20, 1862–1869. [Google Scholar] [CrossRef]
- Xu, J. Preparation of Multiwall Carbon Nanotubes Film Modified Electrode and Its Application to Simultaneous Determination of Oxidizable Amino Acids in Ion Chromatography. Talanta 2003, 60, 1123–1130. [Google Scholar] [CrossRef]
- Kanchana, P.; Lavanya, N.; Sekar, C. Development of Amperometric L-Tyrosine Sensor Based on Fe-Doped Hydroxyapatite Nanoparticles. Mater. Sci. Eng. C 2014, 35, 85–91. [Google Scholar] [CrossRef]
- Li, Y.J. Optical Determination of L-Tyrosine Based on Eggshell Membrane Immobilized Tyrosinase. J. AOAC Int. 2010, 93, 1912–1915. [Google Scholar] [PubMed]
- Schmid, F.-X. Biological Macromolecules: UV-Visible Spectrophotometry. Encyclopedia of Life Sciences; Macmillan Publishers Ltd.: New York, NY, USA; Nature Publishing Group: New York, NY, USA, 2001; pp. 1–4. Available online: http://www.life.illinois.edu/biochem/455/Lab%20exercises/2Photometry/spectrophotometry.pdf (accessed on 5 March 2018).

| (Bio)Sensor | Detection Principle | Linear Range (M) | LOD (M) | Reference |
|---|---|---|---|---|
| Zeolite, CPE | DPV | 1.2 × 10−6–90 × 10−6 | 3.2 × 10−7 | [38] |
| MWCNT, GCE | HDV | 9 × 10−7–3.5 × 10−4 | 3.5 × 10−7 | [39] |
| B.P.Tyr/M/SN-MPTS/SPE | DPV | 5 × 10−8–6 × 10−4 | 2 × 10−8 | [8] |
| Fe-HA/Ty | Amperometry | 1 × 10−7–1.1 × 10−5 | 2.45 × 10−7 | [40] |
| EM/Ty | Optical | 5 × 10−6–2 × 10−4 | 1 × 10−6 | [41] |
| Ty/Chit/PtNP/GPH-CSPE | SWV | 1 × 10−7–1 × 10−4 | 4.75 × 10−8 | This work |
| Interfering Chemical Species | c/M | [Tyr]/M | RE (%) | Recovery (%) |
|---|---|---|---|---|
| Na+ | 0.1 | 4.98 × 10−5 | −0.4 | 99.6 |
| K+ | 0.1 | 5.04 × 10−5 | 0.8 | 100.8 |
| Mg2+ | 0.1 | 4.95 × 10−5 | −1 | 99 |
| Ca2+ | 0.1 | 5.14 × 10−5 | 2.8 | 102.8 |
| L-Lysine | 0.01 | 5.15 × 10−5 | 3 | 103 |
| L-Asparagine | 0.01 | 5.22 × 10−5 | 4.4 | 104.4 |
| Glycine | 0.01 | 4.9 × 10−5 | −2 | 98 |
| L-Phenylalanine | 0.01 | 4.94 × 10−5 | −1.2 | 98.8 |
| L-Ascorbic acid | 0.001 | 5.45 × 10−5 | 9 | 109 |
| Uric acid | 0.001 | 5.44 × 10−5 | 8.8 | 108.8 |
| d-Glucose | 0.001 | 4.92 × 10−5 | −1.6 | 98.4 |
| L-Glutathione | 0.001 | 5.25 × 10−5 | 5 | 105 |
| Sample | Method | [Tyr]/M Detected | [Tyr]/M Added | [Tyr]/M Found | Recovery (%) |
|---|---|---|---|---|---|
| L-Tyrosine (Solgar) | A | 20 × 10−6 | 20 × 10−6 | 40.1 × 10−6 | 100.5 |
| 500 mg/capsule | B | 18 × 10−6 | 20 × 10−6 | 38.2 × 10−6 | 101.0 |
| L-Tyrosine (Solaray) 500 mg/capsule | A | 30 × 10−6 | 20 × 10−6 | 49.9 × 10−6 | 99.5 |
| B | 32 × 10−6 | 20 × 10−6 | 51.9 × 10−6 | 99.5 | |
| L-Tyrosine (Organika) 500 mg/capsule | A | 25 × 10−6 | 20 × 10−6 | 45.1 × 10−6 | 100.5 |
| B | 24 × 10−6 | 20 × 10−6 | 44.05 × 10−5 | 100.3 | |
| HBPS 1 | A | 54 × 10−6 | 50 × 10−6 | 104.2 × 10−6 | 100.4 |
| B | 52 × 10−6 | 50 × 10−6 | 101.8 × 10·6 | 99.6 | |
| HBPS 2 | A | 78 × 10−6 | 50 × 10−6 | 127.9 × 10−6 | 99.8 |
| B | 81 × 10−6 | 50 × 10−6 | 131.5 × 10−6 | 101.0 | |
| HBPS 3 | A | 95 × 10−6 | 50 × 10−6 | 145 × 10−6 | 100.0 |
| B | 93 × 10−6 | 50 × 10−6 | 142.8 × 10−6 | 99.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apetrei, I.M.; Apetrei, C. Development of a Novel Biosensor Based on Tyrosinase/Platinum Nanoparticles/Chitosan/Graphene Nanostructured Layer with Applicability in Bioanalysis. Materials 2019, 12, 1009. https://doi.org/10.3390/ma12071009
Apetrei IM, Apetrei C. Development of a Novel Biosensor Based on Tyrosinase/Platinum Nanoparticles/Chitosan/Graphene Nanostructured Layer with Applicability in Bioanalysis. Materials. 2019; 12(7):1009. https://doi.org/10.3390/ma12071009
Chicago/Turabian StyleApetrei, Irina Mirela, and Constantin Apetrei. 2019. "Development of a Novel Biosensor Based on Tyrosinase/Platinum Nanoparticles/Chitosan/Graphene Nanostructured Layer with Applicability in Bioanalysis" Materials 12, no. 7: 1009. https://doi.org/10.3390/ma12071009
APA StyleApetrei, I. M., & Apetrei, C. (2019). Development of a Novel Biosensor Based on Tyrosinase/Platinum Nanoparticles/Chitosan/Graphene Nanostructured Layer with Applicability in Bioanalysis. Materials, 12(7), 1009. https://doi.org/10.3390/ma12071009






