Porous Alumina Ceramics Obtained by Particles Self-Assembly Combing Freeze Drying Method
Abstract
1. Introduction
2. Theoretical Background and Calculation
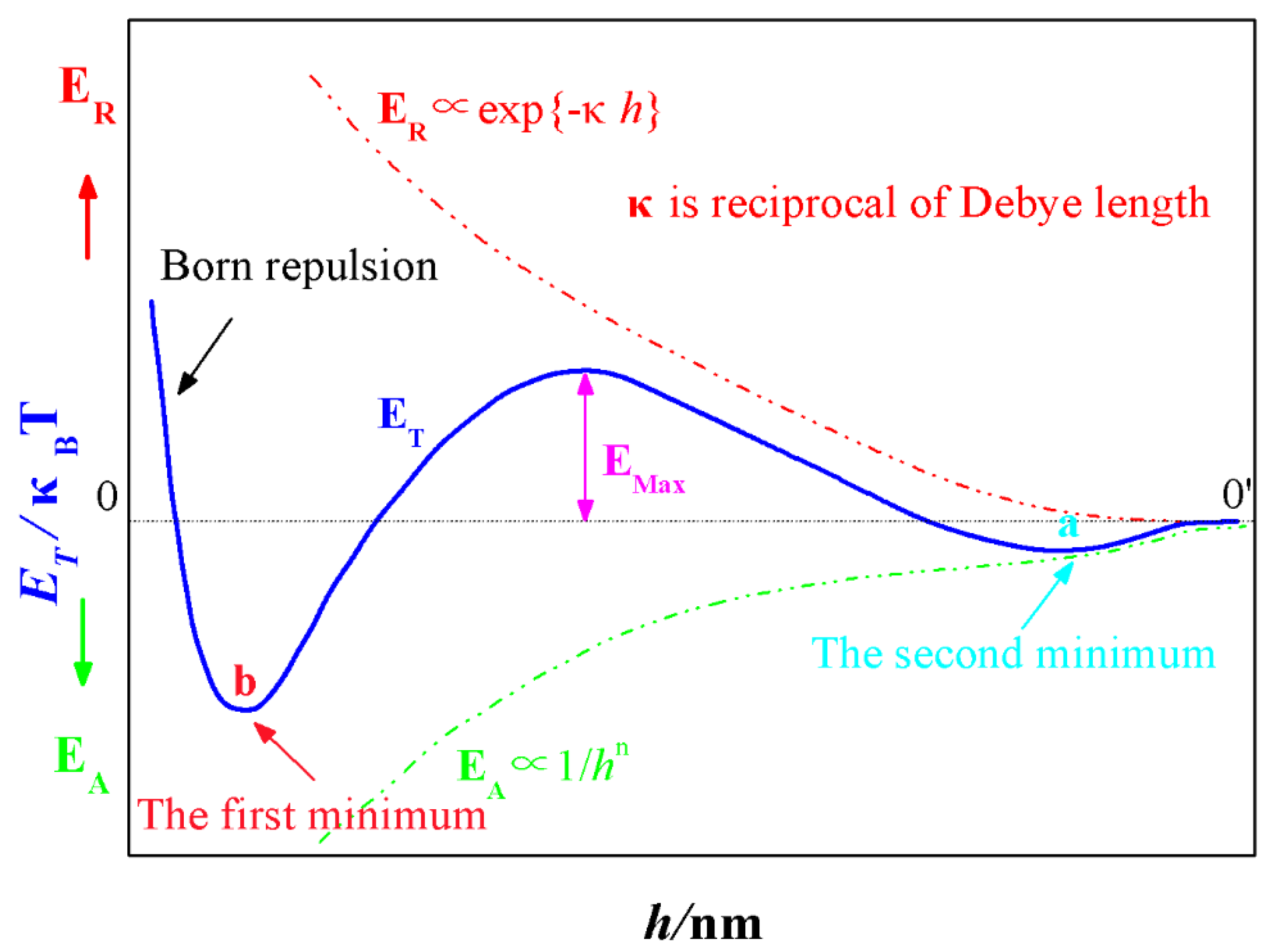
2.1. The Classical Derjaguin-Landau-Verwey-Overbeek (DLVO) Theory
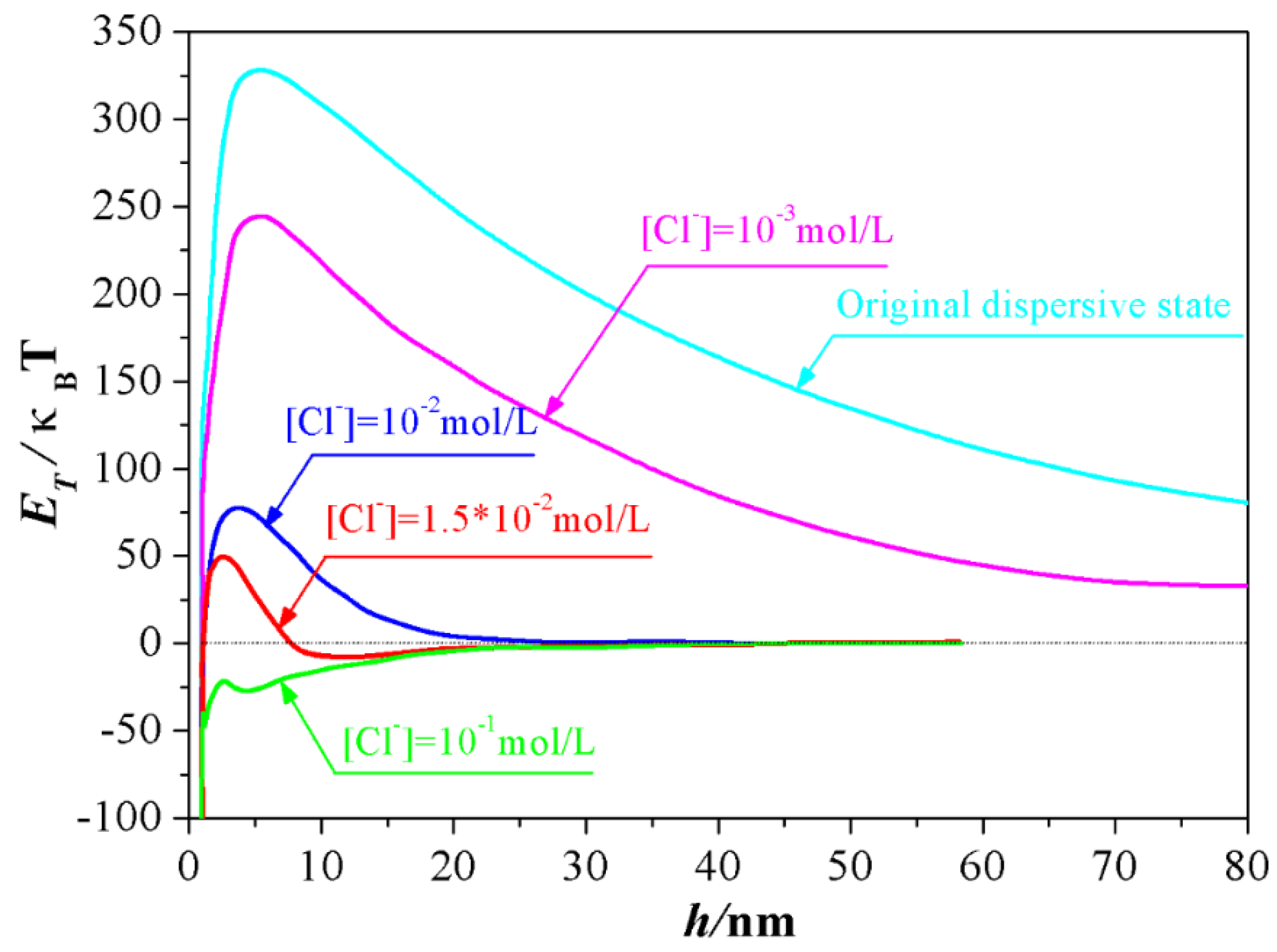
2.2. Influence of Salt Concentration on DLVO Interaction Energy
- (1)
- Van der Waals Interaction
- (2)
- Electrostatic Interaction
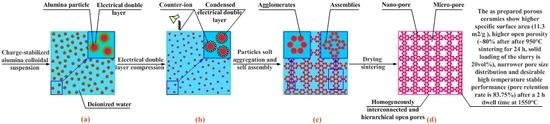
3. Experimental
3.1. Materials
3.2. Fabrication Procedure
3.3. Tests and Characterization Methods
4. Results and Discussion
4.1. Experimental Conditions Required for the Formation of the Second Minimum a
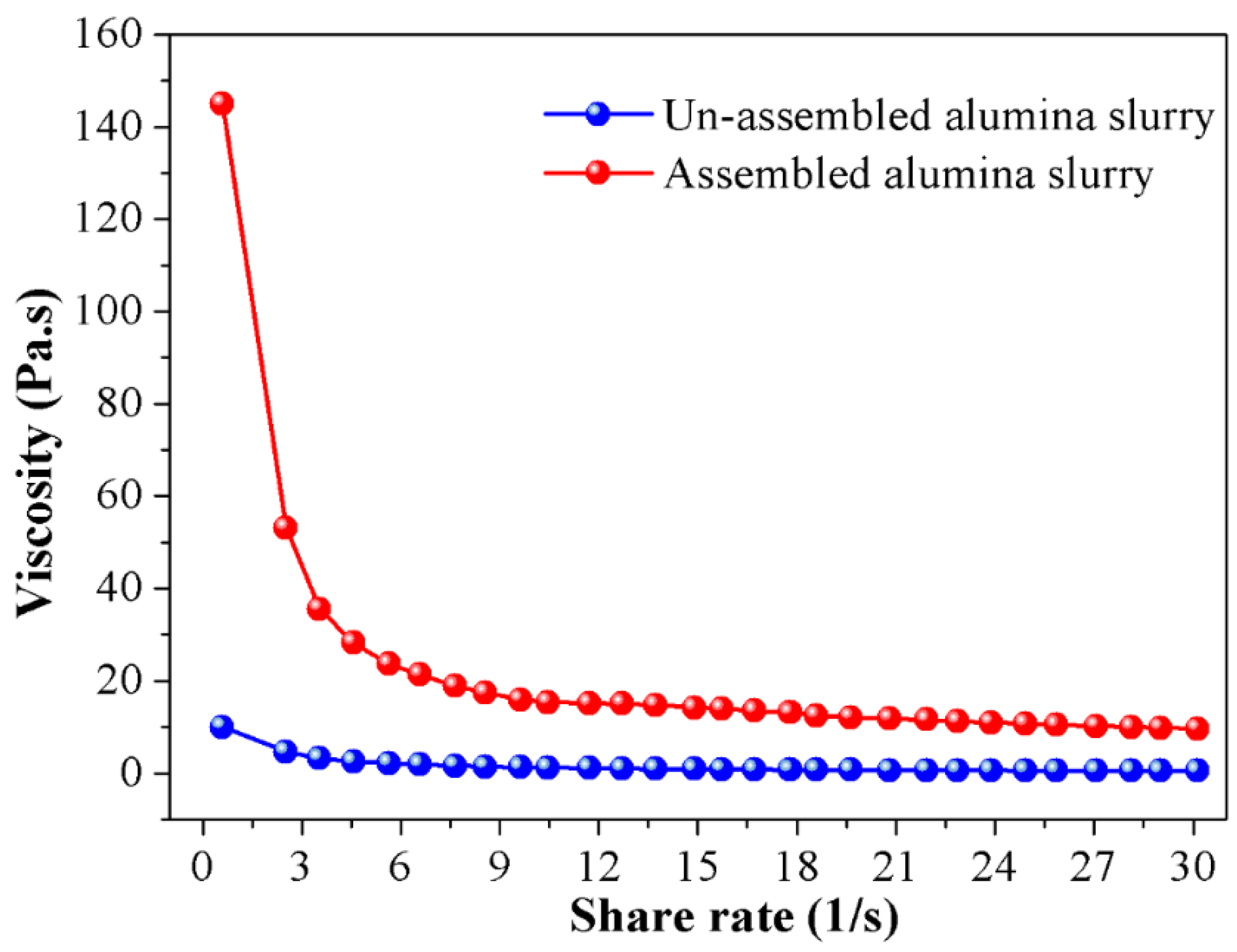
4.2. Effect of Particle Self-Assembly on Rheological Property of the Slurry
4.3. Effect of Particle Self-Assembly on Micro Structure Characteristics of the Porous Ceramics
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Han, M.; Yin, X.; Cheng, L.; Ren, S.; Li, Z. Effect of core-shell microspheres as pore-forming agent on the properties of porous alumina ceramics. Mater. Des. 2017, 113, 384–390. [Google Scholar] [CrossRef]
- Yang, J.F.; Ohji, T.; Kanzaki, S.; Diaz, A.; Hampshire, S. Microstructure and mechanical properties of silicon nitride ceramics with controlled porosity. J. Am. Ceram. Soc. 2002, 85, 1512–1516. [Google Scholar] [CrossRef]
- Lee, H.P.; Lin, D.J.; Yeh, M.L. Phenolic modified ceramic coating on biodegradable Mg alloy: The improved corrosion resistance and osteoblast-like cell activity. Materials 2017, 10, 696. [Google Scholar] [CrossRef]
- Faheemuddin, P.; Mirza, A.B.; Tahar, L. Processing of porous alumina substrate for multilayered ceramic filter. Desalin. Water Treat. 2011, 35, 33–38. [Google Scholar]
- Shimizu, T.; Matsuura, K.; Furue, H.; Matsuzak, K. Thermal conductivity of high porosity alumina refractory bricks made by a slurry gelation and foaming method. J. Eur. Ceram. Soc. 2013, 33, 3429–3435. [Google Scholar] [CrossRef]
- Montero, M.; Molina, T.; Szafran, M.; Moreno, R.; Nieto, M.I. Alumina porous nanomaterials obtained by colloidal processing using D-fructose as dispersant and porosity promoter. Ceram. Int. 2012, 38, 2779–2784. [Google Scholar] [CrossRef]
- Li, X.; Wu, P.; Zhu, D. Properties of porous alumina ceramics prepared by technique combining cold-drying and sintering. Int. J. Refract. Met. Hard Mater. 2013, 41, 437–441. [Google Scholar] [CrossRef]
- Ma, N.; Du, L.J.; Liu, W.T. Synthesis of honeycomb-like structured porous Si3N4 ceramics with exceptionally high number of cells per square inch. Mater. Lett. 2016, 175, 152–156. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, D.; Meggs, C. Porous Al2O3-ZrO2 composites fabricated by an ice template method. Scr. Mater. 2010, 62, 466–468. [Google Scholar] [CrossRef]
- Prabhakaran, K.; Sooraj, R.; Melkeri, A. A new direct coagulation casting process for alumina slurries prepared using poly(acrylate) dispersant. Ceram Int. 2009, 35, 979–985. [Google Scholar] [CrossRef]
- Omatete, O.O.; Janney, M.A.; Strelow, R.A. Gel-casting-a new ceramic forming process. Am. Ceram. Soc. Bull. 1991, 70, 1641–1649. [Google Scholar]
- Yang, J.L.; Yu, J.L.; Huang, Y. Recent developments in gel-casting of ceramics. J. Eur. Ceram. Soc. 2011, 31, 2569–2591. [Google Scholar] [CrossRef]
- Deville, S.; Saiz, E.; Nalla, R.K. Freezing as a path to build complex composites. Science 2006, 311, 515–518. [Google Scholar] [CrossRef]
- Naleway, S.E.; Yu, C.F.; Porter, M.M. Bioinspired composites from free drying with clathrate hydrates. Mater. Des. 2015, 71, 62–67. [Google Scholar] [CrossRef]
- Han, J.C.; Hong, C.Q.; Zhang, X.H. Highly porous ZrO2 ceramics fabricated by a camphene-based freeze-casting route: Microstructure and properties. J. Eur. Ceram. Soc. 2010, 30, 53–60. [Google Scholar] [CrossRef]
- Zhao, J.Z.; Li, Y.; Wu, Y.; Lv, S.S.; Lu, K. Microstructure of TiO2 porous ceramics by free drying of nanoparticle suspensions. Ceram. Int. 2017, 43, 14593–14598. [Google Scholar] [CrossRef]
- Deville, S.; Saiz, E.; Tomsia, A.P. Ice-templated porous alumina structures. Acta Mater. 2007, 55, 1965–1974. [Google Scholar] [CrossRef]
- Ye, F.; Zhang, J.; Liu, L.; Zhan, H. Effect of solid content on pore structure and mechanical properties of porous silicon nitride ceramics produced by free drying. Mater. Sci. Eng. A 2011, 528, 1421–1424. [Google Scholar] [CrossRef]
- Monmaturapoj, N.; Soodsawang, W.; Thepsuwan, W. Porous hydroxyapatite scaffolds produced by the combination of the gel-casting and freeze drying techniques. J. Porous Mater. 2012, 19, 441–447. [Google Scholar] [CrossRef]
- Furlana, K.P.; Pasquarellia, R.M.; Krekelerb, T.; Ritter, M.; Zierold, R.; Nielsch, K.; Schneider, G.A.; Janssen, R. Highly porous α-Al2O3 ceramics obtained by sintering atomic layer deposited inverse opals. Ceram. Int. 2017, 43, 11260–11264. [Google Scholar] [CrossRef]
- Derjaguin, B.V.; Landau, L.D. Theory of the stability of strongly charged lyophobic sols and of the adhesion of strongly charged particles in solution of electrolytes. Acta Physicochim URSS. 1993, 14, 30–59. [Google Scholar] [CrossRef]
- Derjaguin, B.V.; Churaev, N.V.; Muller, V.M. The Derjaguin-Landau-Verwey-Overbeek (DLVO) Theory of Stability of Lyophobic Colloids, Surface Forces; Springer: Boston, MA, USA, 1987. [Google Scholar]
- Yu, Z.; Zhang, J.; Zhang, C.; Xin, X.; Li, H. The coupling effects of soil organic matter and particle interaction forces on soil aggregate stability. Soil Tillage Res. 2017, 174, 251–260. [Google Scholar] [CrossRef]
- Horinek, D. DLVO Theory. In Encyclopedia of Applied Electrochemistry; Kreysa, G., Ota, K., Savinell, R.F., Eds.; Springer: New York, NY, USA, 2014. [Google Scholar]
- Petsev, D.N.; Denkov, N.D.; Kralchevsky, P.A. DLVO and NON-DLVO surface forces and interactions in colloidal dispersions. J. Dispers. Sci. Technol. 1997, 18, 647–659. [Google Scholar] [CrossRef]
- Fornes, J.A. Secondary minimum analysisin the DLVO-theory. Colloid Polym. Sci. 1985, 263, 1004–1007. [Google Scholar] [CrossRef]
- Butler, J.A.V. Theory of the stability of lyophobic colloids. Nature 1948, 162, 315–316. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Hu, Y.H.; Wang, Y.H. Effect of metallic ions on dispersibility of fine diaspora. Trans. Nonferr. Met. Soc. China 2011, 21, 1166–1171. [Google Scholar] [CrossRef]
- Nosrati, A.; Addai-Mensah, J.; Skinner, W. Muscovite clay mineral particle interactions in aqueous media. Powder Technol. 2012, 219, 228–238. [Google Scholar] [CrossRef]
- Yu, Y.X.; Ma, L.; Xu, H.; Sun, X.; Zhang, Z.; Ye, G. DLVO theoretical analyses between montmorillonite and fine coal under different pH and divalent cations. Powder Technol. 2018, 330, 147–151. [Google Scholar] [CrossRef]
- Hao, H.Q.; Li, L.; Yuan, Z.; Liu, J. PH-controlled dispersion of micro-fine siderite from hematite and quartz. Powder Technol. 2018, 339, 710–716. [Google Scholar] [CrossRef]
- Volkova, A.V.; Ermakova, L.E.; Golikova, E.V. Peculiarities of coagulation of the pseudohydrophilic colloids:Aggregate stability of the positively charged-Al2O3 hydrosol in NaCl solutions. Colloid Surf. A-Physicochem. Eng. Asp. 2017, 516, 129–138. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Fang, X.; Somasundaran, P.; Attinger, D. Self-assembly of colloidal particles from evaporating droplets: Role of DLVO interactions and proposition of a phase diagram. Langmuir 2010, 26, 7833–7842. [Google Scholar] [CrossRef]
- Behrens, S.H.; Christl, D.I.; Emmerzael, R.; Schurtenberger, P.; Borkovec, M. Charging and aggregation properties of carboxyl latex particles: Experiments versus DLVO theory. Langmuir 2000, 16, 2566–2575. [Google Scholar] [CrossRef]
- Oats, W.J.; Ozdemir, O.; Nguyen, A.V. Effect of mechanical and chemical clay removals by hydrocyclone and dispersants on coal flotation. Miner. Eng. 2010, 23, 413–419. [Google Scholar] [CrossRef]
- Yoon, R.; Mao, L. Application of extended DLVO theory, IV: Derivation of flotation rateequation from first principles. J. Colloid Interface Sci. 1996, 181, 613–626. [Google Scholar] [CrossRef]
- Bandini, P.; Prestidge, C.; Ralston, J. Colloidal iron oxide slime coatings and galena particle flotation. Miner. Eng. 2001, 14, 487–497. [Google Scholar] [CrossRef]
- Gui, X.; Xing, Y.; Rong, G.; Cao, Y.; Liu, J. Interaction forces between coal and kaolinite particles measured by atomic force microscopy. Powder Technol. 2016, 301, 349–355. [Google Scholar] [CrossRef]
- Xing, Y.; Gui, X.; Cao, Y. Effect of calcium ion on coal flotation in the presence of kaolinite clay. Energy Fuel 2016, 30, 1517–1523. [Google Scholar] [CrossRef]
- Wang, F.; Gu, H.; Yin, J.W. Porous Si3N4 ceramics fabricated through a modified incomplete gel-casting and freeze-drying method. Ceram. Int. 2017, 43, 14678–14682. [Google Scholar] [CrossRef]
- Kristen, L.S.; Dunand, D.C. Free drying—A review of processing, microstructure and properties via the open data repository, FreezeCasting.net. Prog. Mater. Sci. 2018, 94, 243–305. [Google Scholar]
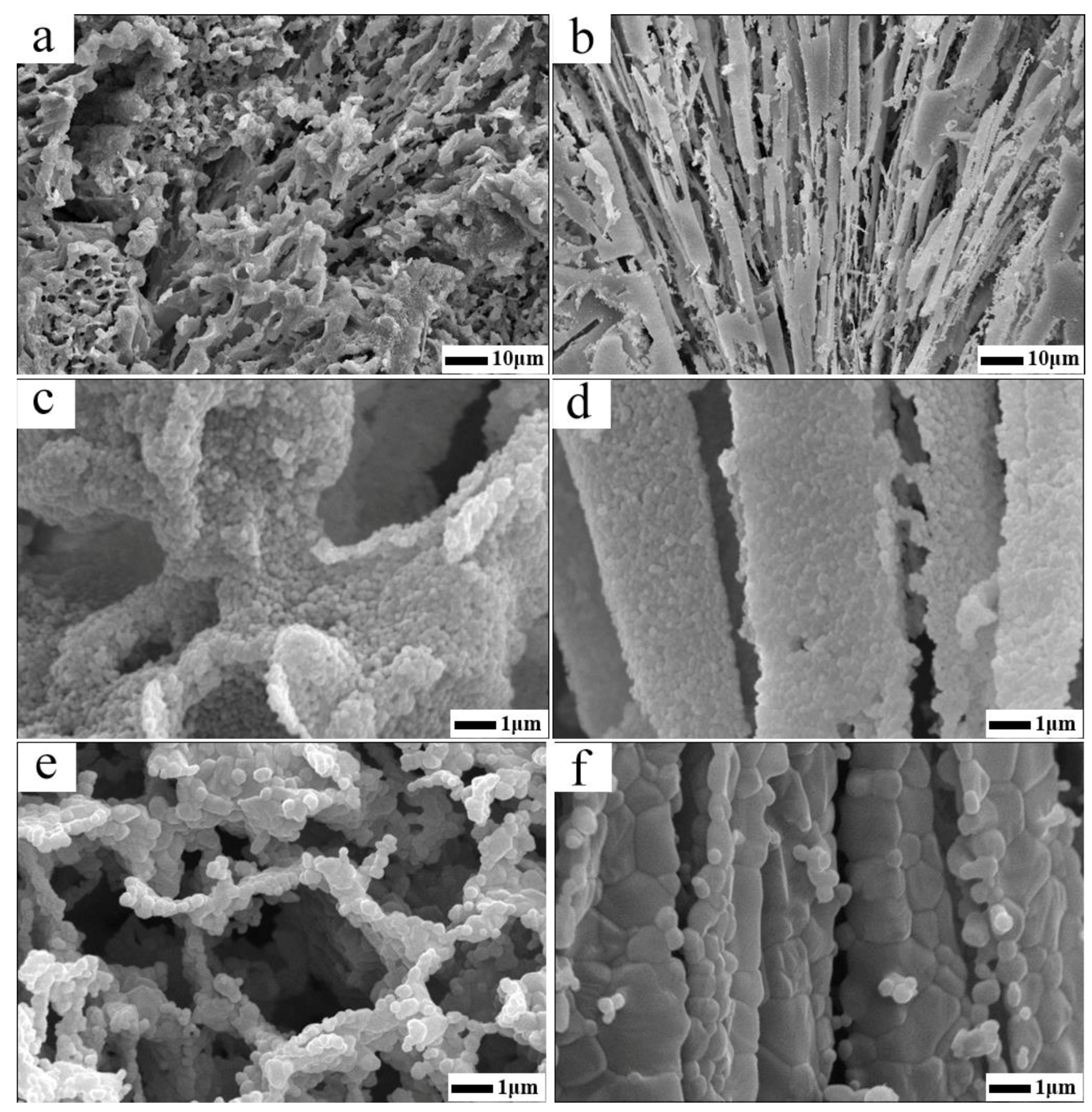
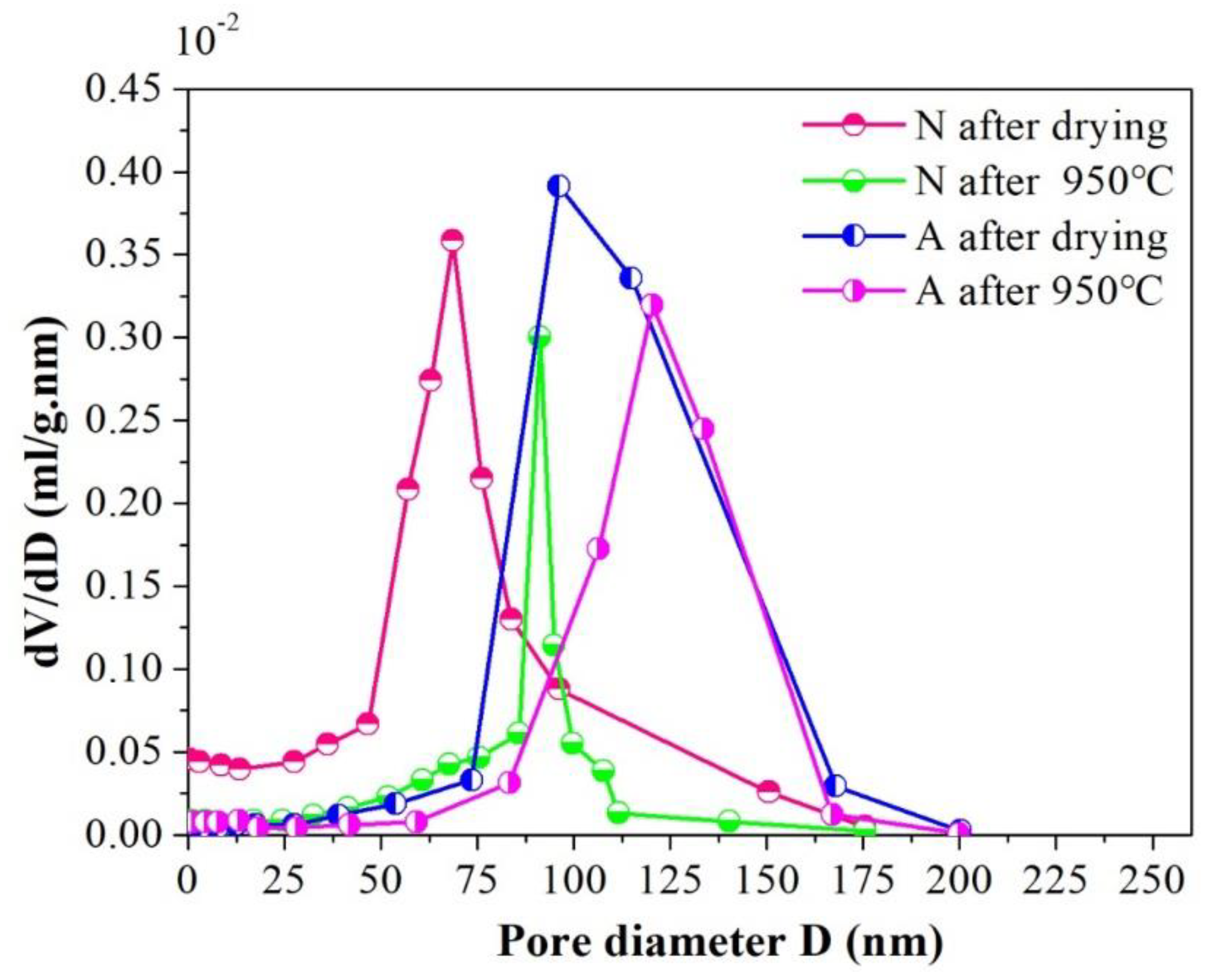

| Sample | Pore Characteristics | |||
|---|---|---|---|---|
| Specific Surface Area (m2/g) | Porosity (%) | Porosity Retention Rate (%) | Volume Density (g/cm3) | |
| N-0 °C | 6.8 | 79.04 | 98.8 | 0.83 |
| N-950 °C | 2.9 | 72.96 | 91.2 | 1.07 |
| N-1550 °C | 0.02 | 39.97 | 49.96 | 2.37 |
| A-0 °C | 11.3 | 80 | 100 | 0.72 |
| A-950 °C | 7.2 | 79.19 | 98.99 | 0.78 |
| A-1550 °C | 1.71 | 67.01 | 83.75 | 1.37 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, S.; Feng, B.; Tang, X.; Zhang, Y. Porous Alumina Ceramics Obtained by Particles Self-Assembly Combing Freeze Drying Method. Materials 2019, 12, 897. https://doi.org/10.3390/ma12060897
Hu S, Feng B, Tang X, Zhang Y. Porous Alumina Ceramics Obtained by Particles Self-Assembly Combing Freeze Drying Method. Materials. 2019; 12(6):897. https://doi.org/10.3390/ma12060897
Chicago/Turabian StyleHu, Shujuan, Bo Feng, Xiaoxia Tang, and Yue Zhang. 2019. "Porous Alumina Ceramics Obtained by Particles Self-Assembly Combing Freeze Drying Method" Materials 12, no. 6: 897. https://doi.org/10.3390/ma12060897
APA StyleHu, S., Feng, B., Tang, X., & Zhang, Y. (2019). Porous Alumina Ceramics Obtained by Particles Self-Assembly Combing Freeze Drying Method. Materials, 12(6), 897. https://doi.org/10.3390/ma12060897