Metal Oxide Thin Films Prepared by Magnetron Sputtering Technology for Volatile Organic Compound Detection in the Microwave Frequency Range
Abstract
1. Introduction
2. Materials and Methods
2.1. Sputtering Technology
2.1.1. Copper Oxide
2.1.2. Titanium Dioxide
2.1.3. Tin Dioxide
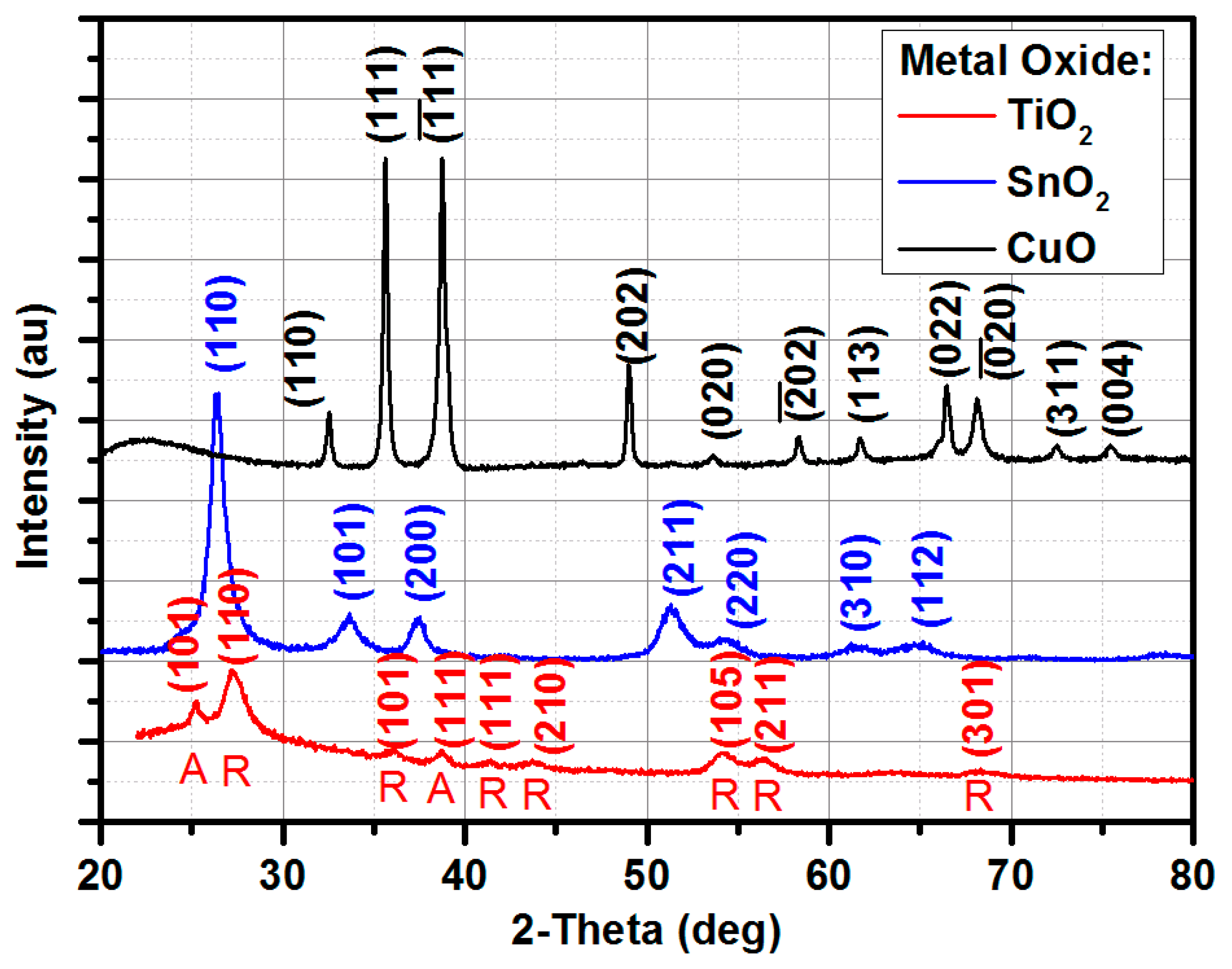
2.2. X-ray Diffraction
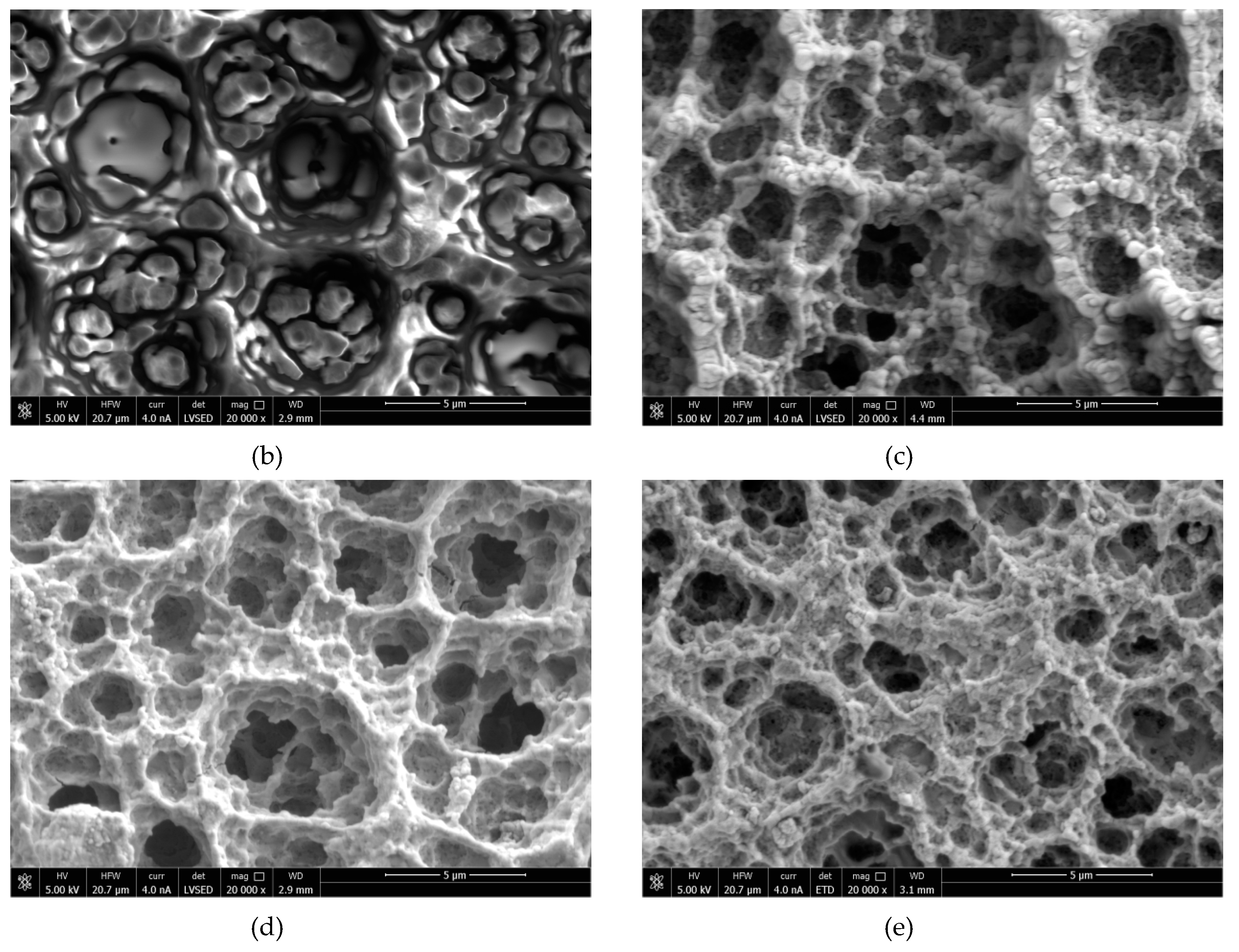
2.3. Scanning Electron Microscopy
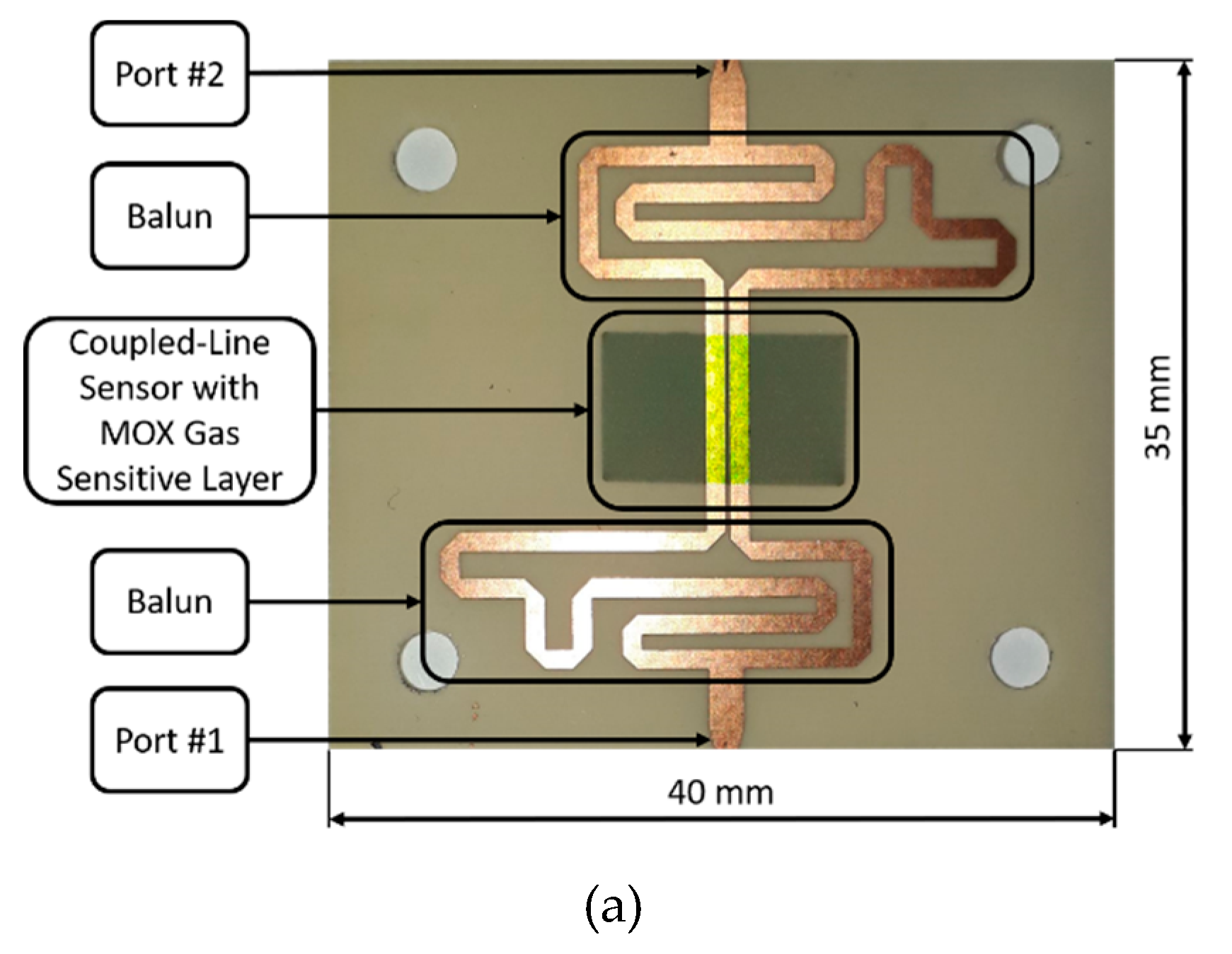
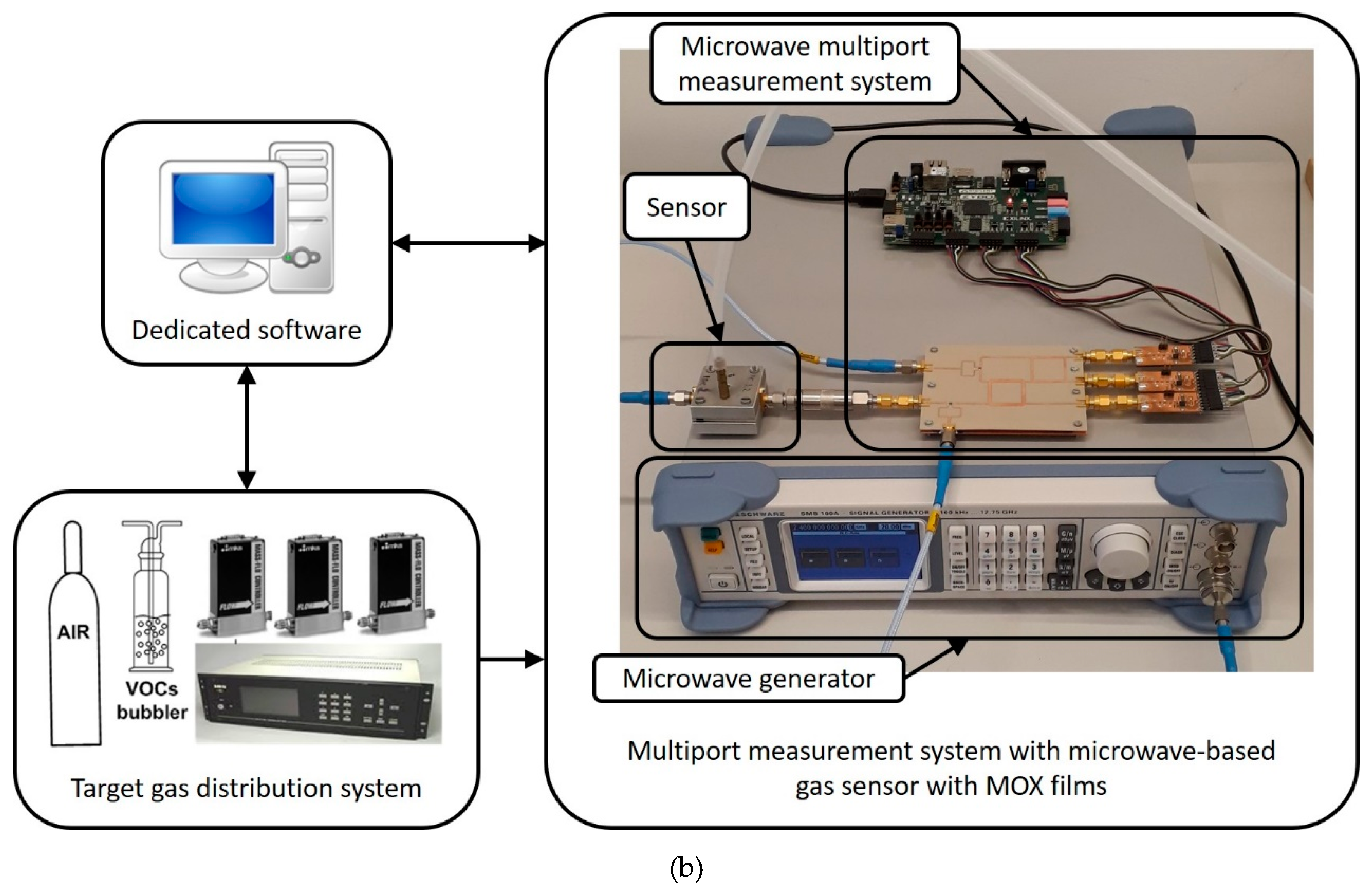
2.4. Gas-Sensing Measurements
3. Results and Discussion
3.1. XRD Results
3.2. SEM Results
3.3. Gas-Sensing Results
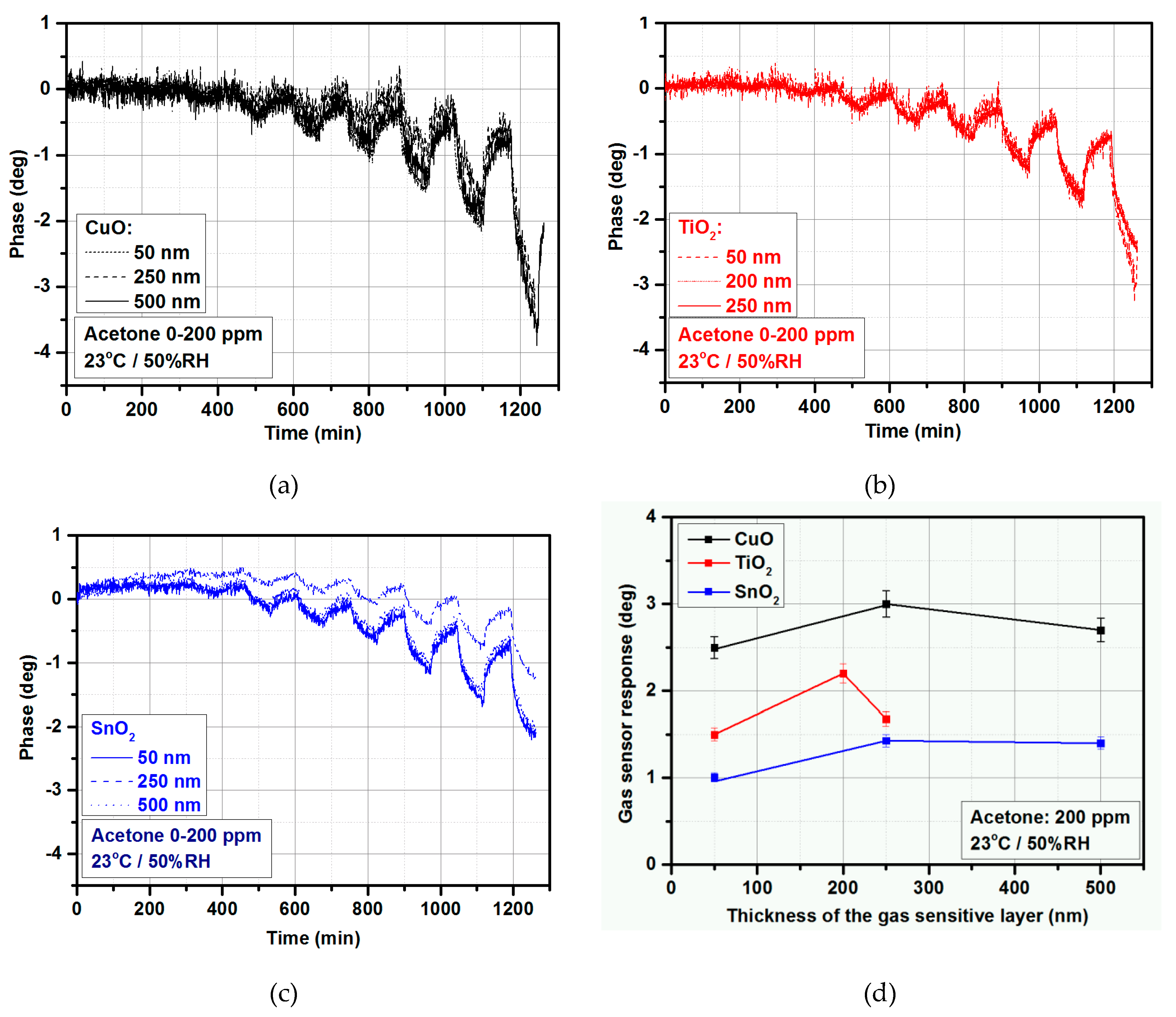
3.3.1. Thickness Selection
3.3.2. Acetone Detection
3.3.3. Ethanol/Methanol Detection
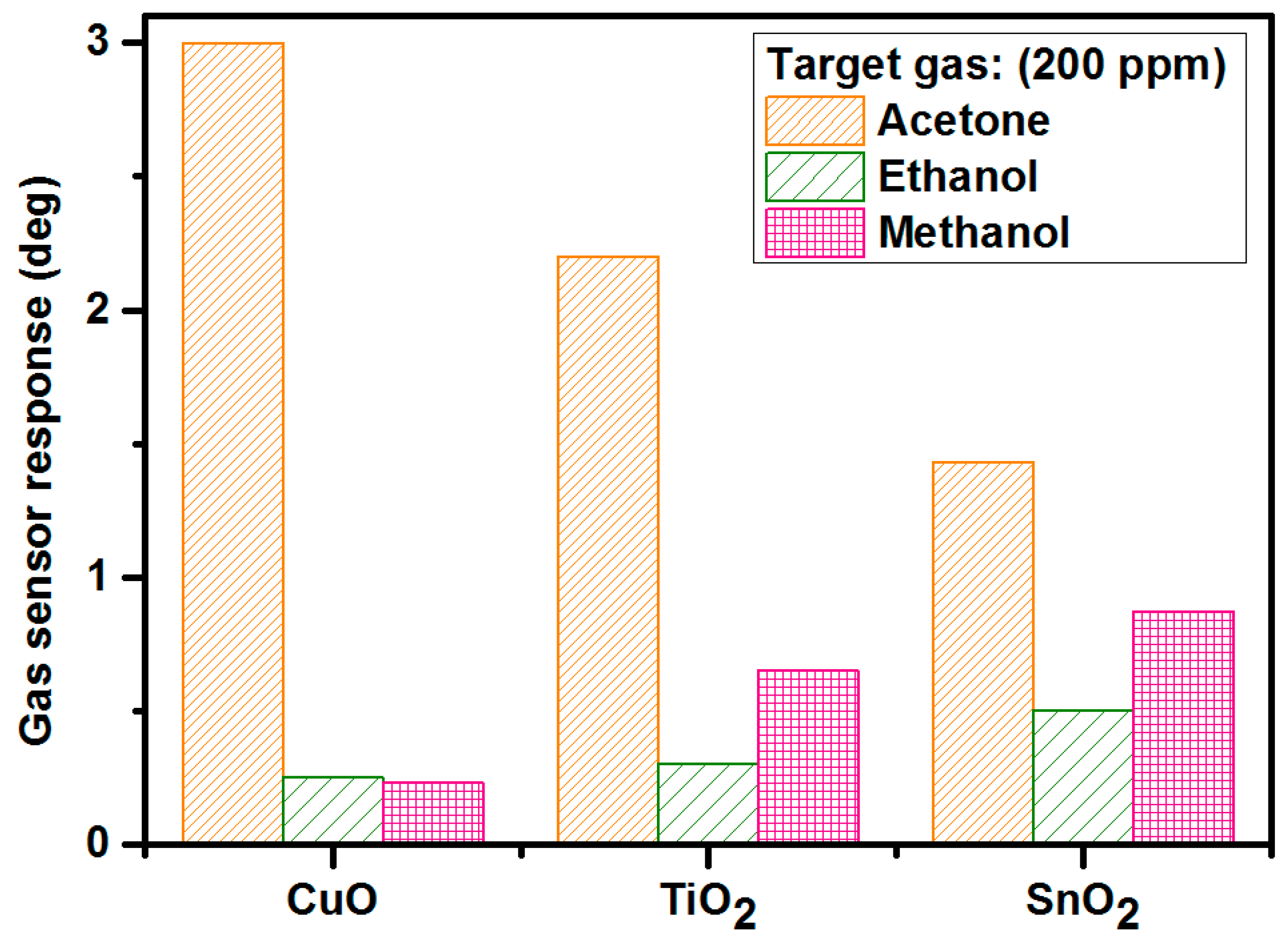
3.3.4. Selectivity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nafarizal, N. Precise Control of Metal Oxide Thin Films Deposition in Magnetron Sputtering Plasmas for High Performance Sensing Devices Fabrication. Procedia Chem. 2016, 20, 93–97. [Google Scholar] [CrossRef]
- Chuang, K.-T.; Abdullah, H.; Leu, S.-J.; Cheng, K.-B.; Kuo, D.-H.; Chen, H.-C.H.; Chien, J.-H.; Hu, W.-T. Metal oxide composite thin films made by magnetron sputtering for bactericidal application. J. Photochem. Photobiol. A Chem. 2017, 337, 151–164. [Google Scholar] [CrossRef]
- Tan, X.-Q.; Liu, J.-Y.; Niu, J.-R.; Liu, J.-Y.; Tian, J.-Y. Recent Progress in Magnetron Sputtering Technology Used on Fabrics. Materials 2018, 11, 1953. [Google Scholar] [CrossRef] [PubMed]
- Snyders, R.; Dauchot, J.-P.; Hecq, M. Synthesis of Metal Oxide Thin Films by Reactive Magnetron Sputtering in Ar/O2 Mixtures: An Experimental Study of the Chemical Mechanisms. Plasma Process. Polym. 2007, 4, 113–126. [Google Scholar] [CrossRef]
- Gil-Rostra, J.; Chaboy, J.; Yubero, F.; Vilajoana, A.; Gonzalez-Elipe, A.R. Colored and Transparent Oxide Thin Films Prepared by Magnetron Sputtering: The Glass Blower Approach. ACS Appl. Mater. Interfaces 2013, 5, 1967–1976. [Google Scholar] [CrossRef] [PubMed]
- Brudnik, A.; Czternastek, H.; Zakrzewska, K.; Jachimowski, M. Plasma-emission-controlled d.c. magnetron sputtering of TiO2-x thin films. Thin Solid Films 1991, 199, 45–58. [Google Scholar] [CrossRef]
- Kelly, P.J.; Arnell, R.D. Magnetron sputtering: a review of recent developments and applications. Vaccum 2000, 56, 159–172. [Google Scholar] [CrossRef]
- Safi, I. Recent aspects concerning DC reactive magnetron sputtering of thin films: A review. Surf. Coat. Technol. 2000, 127, 203–219. [Google Scholar] [CrossRef]
- Brauer, G.; Szyszka, B.; Vergohl, M.; Bandorf, R. Magnetron sputtering—Milestones of 30 years. Vacuum 2010, 84, 1354–1359. [Google Scholar] [CrossRef]
- Depla, D.; Mahieu, S. Reactive Sputter Deposition; Springer Series in Materials Science; Springer: Belin, Germany, 2008. [Google Scholar]
- Penning, F.M. Coating by Cathode Disintegration. U.S. Patent 2,146,025, 7 February 1939. [Google Scholar]
- Eranna, G. Metal Oxide Nanostructures as Gas Sensing Devices; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Shivya, P.; Prasad, A.K.; Sridharan, M. Magnetron sputtered nanostructured cadmium oxide films for ammonia sensing. J. Solid State Chem. 2014, 214, 24–29. [Google Scholar]
- Matsumiya, M.; Qiu, F.B.; Shin, W.; Izu, N.; Matsubara, I.; Murayama, N.; Kanzaki, S. Thermoelectric CO gas sensor using thin-film catalyst of Au and Co3O4. J. Electrochem. Soc. 2004, 151, H7–H10. [Google Scholar] [CrossRef]
- Rydosz, A. Amorphous and Nanocrystalline Magnetron Sputtered CuO Thin Films Deposited on Low Temperature Cofired Ceramics Substrates for Gas Sensor Applications. IEEE Sens. J. 2014, 14, 1600–1608. [Google Scholar] [CrossRef]
- Szkudlarek, A.; Kollbek, K.; Klejna, S.; Rydosz, A. Electronic sensitization of CuO thin films by Cr-doping for enhanced gas sensor response at low detection limit. Mater. Res. Express 2018, 5, 126406. [Google Scholar] [CrossRef]
- Ogita, M.; Higo, K.; Nakanishi, Y.; Hatanaka, Y. Ga2O3 thin film for oxygen sensor at high temperature. Appl. Surf. Sci. 2001, 175–176, 721–725. [Google Scholar] [CrossRef]
- Comini, E.; Cristalli, A.; Faglia, G.; Sberveglieri, G. Light enhanced gas sensing properties of indium oxide and tin dioxide sensors. Sens. Actuators B 2000, 65, 260–263. [Google Scholar] [CrossRef]
- Kollbek, K.; Szkudlarek, A.; Rydosz, A.; Łyson-Sypien, B.; Marzec, M.; Przybylski, M. Detection of Low-Level Acetone Using Semiconductor Gas Sensors Based on CuO/Fe2O3 Hetero-Junctions. 2018. Available online: http://ma.ecsdl.org/content/MA2018-01/25/1530.abstract?sid=ac83f4af-cbbe-470b-a596-d5f05f1e39a7 (accessed on 15 February 2019).
- Rydosz, A.; Ziabka, M.; Michon, D.; Kanak, J.; Maziarz, W.; Pisarkiewicz, T. Gas Sensing Characteristics of MoO3 Thin Films Prepared by Glancing Angle Magnetron Sputtering. Sens. Lett. 2017, 15, 1–8. [Google Scholar] [CrossRef]
- Turgut, E.; Coban, O.; Saritas, S.; Tuzemen, S.; Yildrim, M.; Gur, E. Oxygen partial pressure effects on the RF sputtered p-type NiO hydrogen gas sensors. Appl. Surf. Sci. 2018, 435, 880–885. [Google Scholar] [CrossRef]
- Chen, H.-I.; Hsiao, C.-Y.; Chen, W.-C.; Chang, C.-H.; Chou, T.-C.; Liu, I.P.; Lin, K.W.; Liu, W.-C. Characteristics of a Pt/NiO thin film-based ammonia gas sensor. Sens. Actuators B 2018, 256, 962–967. [Google Scholar] [CrossRef]
- Moon, H.G.; Jang, H.W.; Kim, J.-S.; Park, H.-H.; Yoon, S.-J. A route to high sensitivity and rapid response Nb2O5-based gas sensors: TiO2 doping, surface embossing, and voltage optimization. Sens. Actuators B 2011, 153, 37–43. [Google Scholar] [CrossRef]
- Sharma, A.; Tomar, M.; Gupta, V. A low temperature operated NO2 gas sensors based on TeO2/SnO2 p-n heterointerface. Sens. Actuators B 2013, 176, 875–883. [Google Scholar] [CrossRef]
- Siciliano, T.; Di Giulio, M.; Tepore, M.; Filippo, E.; Micocci, G.; Tepore, A. Room temperature NO2 sensing properties of reactively sputtered TeO2 thin films. Sens. Actuators B 2009, 137, 644–648. [Google Scholar] [CrossRef]
- Jeong, H.-S.; Park, M.-J.; Kwon, S.-H.; Joo, H.-J.; Song, S.-H.; Kwon, H.-I. Low temperature NO2 sensing properties of RF-sputtered SnO-SnO2 heterojunction thin-film with p-type semiconducting behavior. Ceram. Int. 2018, 44, 17283–17289. [Google Scholar] [CrossRef]
- Oros, C.; Horprathum, M.; Wisitsoraat, A.; Srichaiyaperk, T.; Samransuksamer, B.; Limwichean, S.; Eiamchai, P.; Phokharatkul, D.; Nuntawong, N.; Chananonnawathorn, C.; et al. Ultra-sensitive NO2 sensor based on vertically aligned SnO2 nanorods deposited by DC reactive magnetron sputtering with glancing angle deposition technique. Sens. Actuators B 2016, 223, 936–945. [Google Scholar] [CrossRef]
- Radecka, M.; Zakrzewska, K.; Rekas, M. SnO2-TiO2 solid solutions for gas sensors. Sens. Actuators B 1998, 47, 1–3. [Google Scholar] [CrossRef]
- Salaman, S.; Shihab, A.; Elttayef, A.H. Design and Construction of Nanostructure TiO2 Thin Film Gas Sensor Prepared by, R.F Magnetron Sputtering Technique. Energy Procedia 2019, 157, 283–289. [Google Scholar] [CrossRef]
- Rydosz, A.; Szkudlarek, A.; Ziabka, M.; Domanski, K.; Maziarz, W.; Pisarkiewicz, T. Performance of Si-Doped WO3 Thin Films for Acetone Sensing Prepared by Glancing Angle DC Magnetron Sputtering. IEEE Sens. J. 2016, 16, 1004–1012. [Google Scholar] [CrossRef]
- Jolly Bose, R.; Illyasukutty, N.; Tan, K.S.; Rawat, R.S.; Matham, M.V.; Kohler, H.; Pillai, V.P. Preparation and characterization of Pt loaded WO3 films suitable for gas sensing applications. Appl. Surf. Sci. 2018, 440, 320–330. [Google Scholar] [CrossRef]
- Liang, J.; Liu, J.; Li, W.; Hu, M. Preparation and room temperature methane sensing properties of platinum-decorated vanadium oxide films. Mater. Res. Bull. 2016, 84, 332–339. [Google Scholar] [CrossRef]
- Girija, K.G.; Somasundaram, K.; Topkar, A.; Vatsa, R.K. Highly selective H2S gas sensor based on Cu-doped ZnO nanocrystalline films deposited by RF magnetron sputtering of powder target. J. Alloys Compd. 2016, 684, 15–20. [Google Scholar] [CrossRef]
- Bhati, V.S.; Ranwa, S.; Fanetti, M.; Valant, M.; Kumar, M. Efficient hydrogen sensor based on Ni-doped ZnO nanostructures by RF sputtering. Sens. Actuators B 2018, 255, 558–597. [Google Scholar] [CrossRef]
- Bae, J.W.; Park, J.Y.; Hwang, S.W.; Yeom, G.Y.; Kim, K.D.; Cho, Y.A.; Jeon, J.S.; Choi, D. Characterization of Yittria-Stabilized Zirconia Thin Films Prepared by Radio Frequency Magnetron Sputtering for a Combustion Control Oxygen Sensor. J. Electrochem. Soc. 2000, 147, 2380–2384. [Google Scholar] [CrossRef]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal oxide gas sensors: sensitivity and influencing factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, P.; Acharyya, D.; Dutta, K. Resistive and Capacitive Measurement of Nano-Structured Gas Sensors. Environ. Nanotechnol. 2018, 21, 25–62. [Google Scholar]
- Rydosz, A.; Maziarz, W.; Pisarkiewicz, T. Formation a uniform temperature distribution in semiconductors resistance gas sensors in LTCC technology. Electr. Rev. 2011, 87, 249–252. [Google Scholar]
- Bartsch, H.; Stoepel, D.; Mueller, J.; Rydosz, A. Printed heater elements for smart sensor packages in LTCC. In Proceedings of the 2017 21st European Microelectronics and Packaging Conference (EMPC) & Exhibition, Warsaw, Poland, 10–13 September 2017. [Google Scholar] [CrossRef]
- Kita, J.; Schubert, F.; Retting, F.; Engelbrecht, A.; Gross, A.; Moos, R. Ceramic Alumina Substrates for High-temperature Gas Sensors—Implications for Applicability. Procedia Eng. 2017, 87, 1505–1508. [Google Scholar] [CrossRef]
- Kim, I.-D.; Rothschild, A. Nanostructured metal oxide gas sensors prepared by electrospinning. Polym. Adv. Technol. 2011, 22, 318–325. [Google Scholar] [CrossRef]
- Rockerby, D.; Serventi, A.M. CHAPTER 6—Nanostructured Metal Oxide Gas Sensors for Air-Quality Monitoring. In Enironanotechnology; Elsevier: Amsterdam, The Netherlands, 2010; pp. 99–136. [Google Scholar]
- Atanosova, G.; Dikovks, A.Og.; Dilova, T.; Georgieva, B.; Avdeev, G.V.; Stefanov, P.; Nedyalkov, N.N. Metal-oxide nanostructures produced by PLD in open air for gas sensor applications. Appl. Surf. Sci. 2019, 470, 861–869. [Google Scholar] [CrossRef]
- Zappa, D.; Galstyan, V.; Kaur, N.; Arachchige, H.M.M.M.; Sisman, O.; Comini, E. Metal oxide-based heterostructures for gas sensors—A review. Anal. Chim. Acta 2019, 1039, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, A.; Kim, S.S.; Kim, H.W. Resistance-based H2S gas sensors using metal oxide nanostructures: A review of recent advances. J. Hazard. Mater. 2018, 357, 314–331. [Google Scholar] [CrossRef] [PubMed]
- Gwizdz, P.; Radecka, M.; Zakrzewska, K. Array of Chromium Doped Nanostructured TiO2 Metal Oxide Gas Sensors. Procedia Eng. 2014, 87, 1059–1062. [Google Scholar] [CrossRef]
- Movlaee, K.; Periasamy, P.; Krishnakumar, T.; Ganali, M.R.; Leonardi, S.G.; Neri, G.; Chavali, M.; Siril, P.F.; Devarajan, V.P. Microwave-assisted synthesis and characterization of WOx nanostructures for gas sensor application. J. Alloys Compd. 2018, 762, 745–753. [Google Scholar] [CrossRef]
- Huotari, J.; Kekkonen, V.; Haapalainen, T.; Leidinger, M.; Sauerwald, T.; Puustinen, J.; Liimatainen, J.; Lappalainen, J. Pulsed laser deposition of metal oxide nanostructures for highly sensitive gas sensor applications. Sens. Actuators B 2016, 236, 978–987. [Google Scholar] [CrossRef]
- Singh, G.; Virpal; Singh, R.C. Highly sensitive gas sensor based on Er-doped SnO2 nanostructures and its temperature dependent selectivity towards hydrogen and ethanol. Sens. Actuators B 2019, 282, 373–383. [Google Scholar] [CrossRef]
- Rydosz, A.; Maciak, E.; Wincza, K.; Gruszczynski, S. Microwave-based sensors with phthalocyanine films for acetone, ethanol and methanol detection. Sens. Actuators B 2016, 237, 876–886. [Google Scholar] [CrossRef]
- Staszek, K.; Rydosz, A.; Maciak, E.; Wincza, K.; Gruszczynski, S. Six-port microwave system for volatile organic compounds detection. Sens. Actuators B 2017, 245, 882–894. [Google Scholar] [CrossRef]
- Rossignol, J.; Barochi, G.; de Fonseca, B.; Brunet, J.; Bouvet, M.; Pauly, A.; Markey, L. Microwave-based gas sensor with phthalocyanine film at room temperature. Sens. Actuators B 2013, 189, 213–216. [Google Scholar] [CrossRef]
- Jones, T.A.; Boot, B.; Thorpe, S.C. Fast response metal phthalocyanine-based gas sensors. Sens. Actuators 1989, 17, 467–474. [Google Scholar] [CrossRef]
- Jouhannaud, J.; Rossignol, J.; Stuerga, D. Développement d’un nouveau capteur de gaz basé sur la détectionà large bande micro-onde. C. R. Phys. 2007, 8, 456–461. [Google Scholar] [CrossRef]
- Bailly, G.; Harrabi, A.; Rossignol, J.; Stuerga, D.; Pribetich, P. Microwave gas sensing with a microstrip interDigital capacitor: Detection of NH3 with TiO2 nanoparticles. Sens. Actuators B 2016, 236, 554–564. [Google Scholar] [CrossRef]
- Rydosz, A.; Szkudlarek, A. Gas sensing Performance of M-Doped CuO-Based Thin Films Working at Different Temperatures upon Exposure to Propane. Sensors 2015, 15, 20069–20085. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewska, K.; Brudnik, A.; Radecka, M.; Posadowski, W. Reactively sputtered TiO2-x thin films with plasma-emission-controlled departure from stoichiometry. Thin Solid Films 1999, 343–344, 152–155. [Google Scholar] [CrossRef]
- Remler, R.F. The solvent properties of acetone. Ind. Eng. Chem. 1923, 7, 717–720. [Google Scholar] [CrossRef]
- Rydosz, A. Sensors for Enhanced Detection of Acetone as a Potential Tool for Noninvasive Diabetes Monitoring. Sensors 2018, 18, 2298. [Google Scholar] [CrossRef] [PubMed]
- Staszek, K.; Piekarz, I.; Sorocki, J.; Koryciak, S.; Wincza, K.; Gruszczyński, S. Low-cost microwave vector system for liquid properties monitoring. IEEE Trans. Ind. Electron. 2018, 65, 1665–1674. [Google Scholar] [CrossRef]
- Saasa, V.; Malwela, T.; Beukes, M.; Mokhotho, M.; Liu, C.-P.; Mwakikunga, B. Sensing Technologies for Detection of Acetone in Human Breath for Diabetes Diagnosis and Monitoring. Diagnostics 2018, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Rydosz, A. Micropreconcentrator in LTCC technology with mass spectrometry for the detection of acetone in healthy and type-1 diabetes mellitus patient breath. Metabolities 2014, 4, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Available online: http://www.hmdb.ca/metabolites/HMDB0004327 (accessed on 15 February 2018).
- Available online: http://www.hmdb.ca/metabolites/HMDB0001875 (accessed on 15 February 2018).



| Metal Oxide | Magnetron Sputtering Mode | Target Gases | Operating Temperature (°C) | References |
|---|---|---|---|---|
| CdO | DC | NH3 | 150 | [13] |
| Co3O4 | RF | CO | 200 | [14] |
| CuO | DC | NO2 | 200 | [15] |
| MF | C3H6O | 450 | [16] | |
| Ga2O3 | RF | O2 | 1000 | [17] |
| In2O3 | RF | CO/NO2 | 25 | [18] |
| Fe2O3 | RF | C3H6O | 375 | [19] |
| MoO3 | DC | H2S | 280 | [20] |
| NiO | RF | H2 | 200 | [21] |
| NH3 | 300 | [22] | ||
| Nb2O3 | RF | CO | 350 | [23] |
| TeO2 | RF | NO2 | 90 | [24] |
| 25 | [25] | |||
| SnO2 | RF | NO2 | 60 | [26] |
| DC | NO2 | 150 | [27] | |
| TiO2 | RF | H2 | 500 25 | [28] [29] |
| WO3 | DC RF | C3H6O CO | 450 200 | [30] [31] |
| V2O3 | DC | CH4 | 25 | [32] |
| ZnO | RF | H2S H2 | 250 75 | [33] [34] |
| ZrO2 | RF | O2 | 500 | [35] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rydosz, A.; Brudnik, A.; Staszek, K. Metal Oxide Thin Films Prepared by Magnetron Sputtering Technology for Volatile Organic Compound Detection in the Microwave Frequency Range. Materials 2019, 12, 877. https://doi.org/10.3390/ma12060877
Rydosz A, Brudnik A, Staszek K. Metal Oxide Thin Films Prepared by Magnetron Sputtering Technology for Volatile Organic Compound Detection in the Microwave Frequency Range. Materials. 2019; 12(6):877. https://doi.org/10.3390/ma12060877
Chicago/Turabian StyleRydosz, Artur, Andrzej Brudnik, and Kamil Staszek. 2019. "Metal Oxide Thin Films Prepared by Magnetron Sputtering Technology for Volatile Organic Compound Detection in the Microwave Frequency Range" Materials 12, no. 6: 877. https://doi.org/10.3390/ma12060877
APA StyleRydosz, A., Brudnik, A., & Staszek, K. (2019). Metal Oxide Thin Films Prepared by Magnetron Sputtering Technology for Volatile Organic Compound Detection in the Microwave Frequency Range. Materials, 12(6), 877. https://doi.org/10.3390/ma12060877






