Effects of Finish Line Design and Fatigue Cyclic Loading on Phase Transformation of Zirconia Dental Ceramics: A Qualitative Micro-Raman Spectroscopic Analysis
Abstract
:1. Introduction
2. Materials and Methods
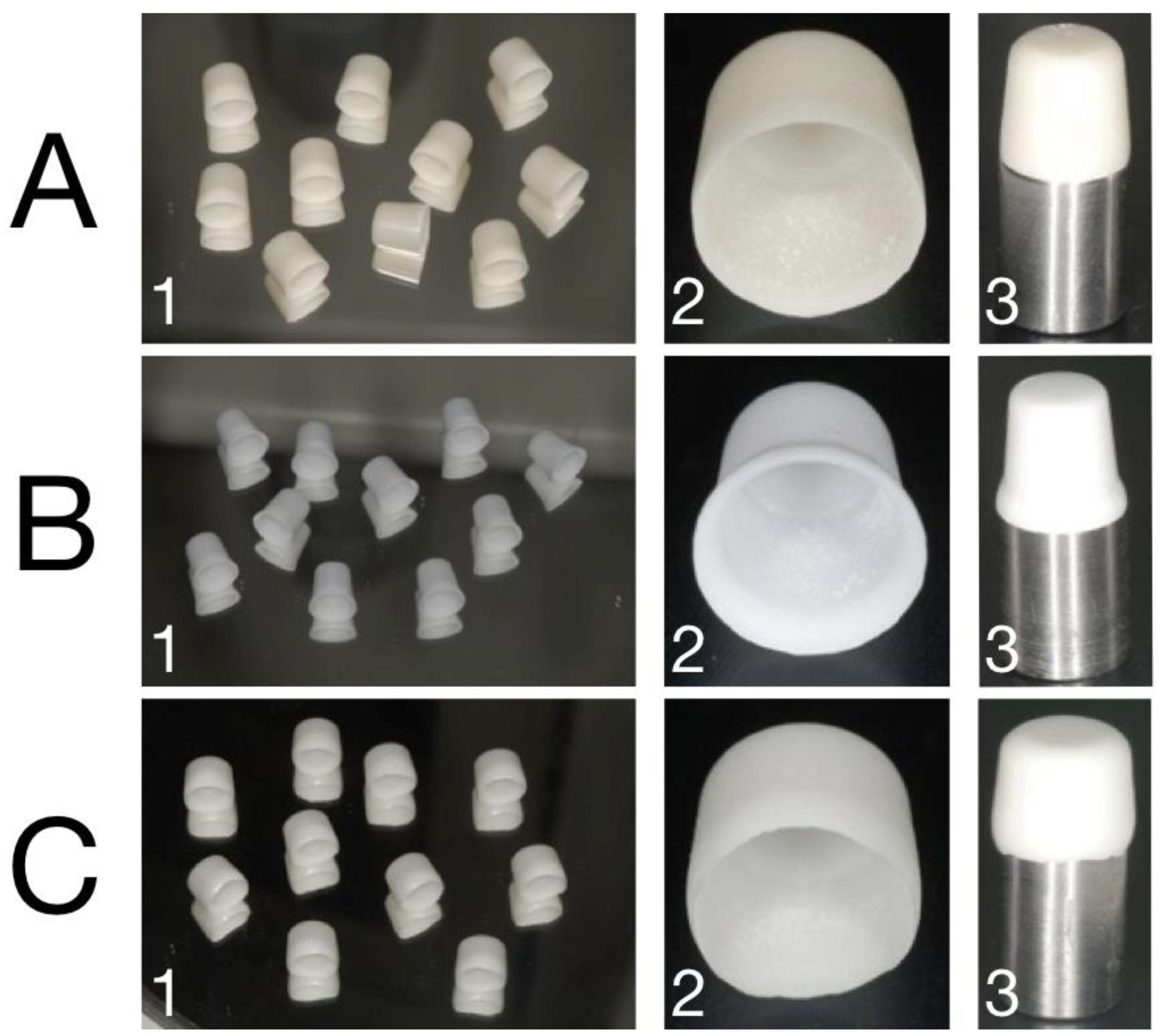
2.1. Sample Preparation
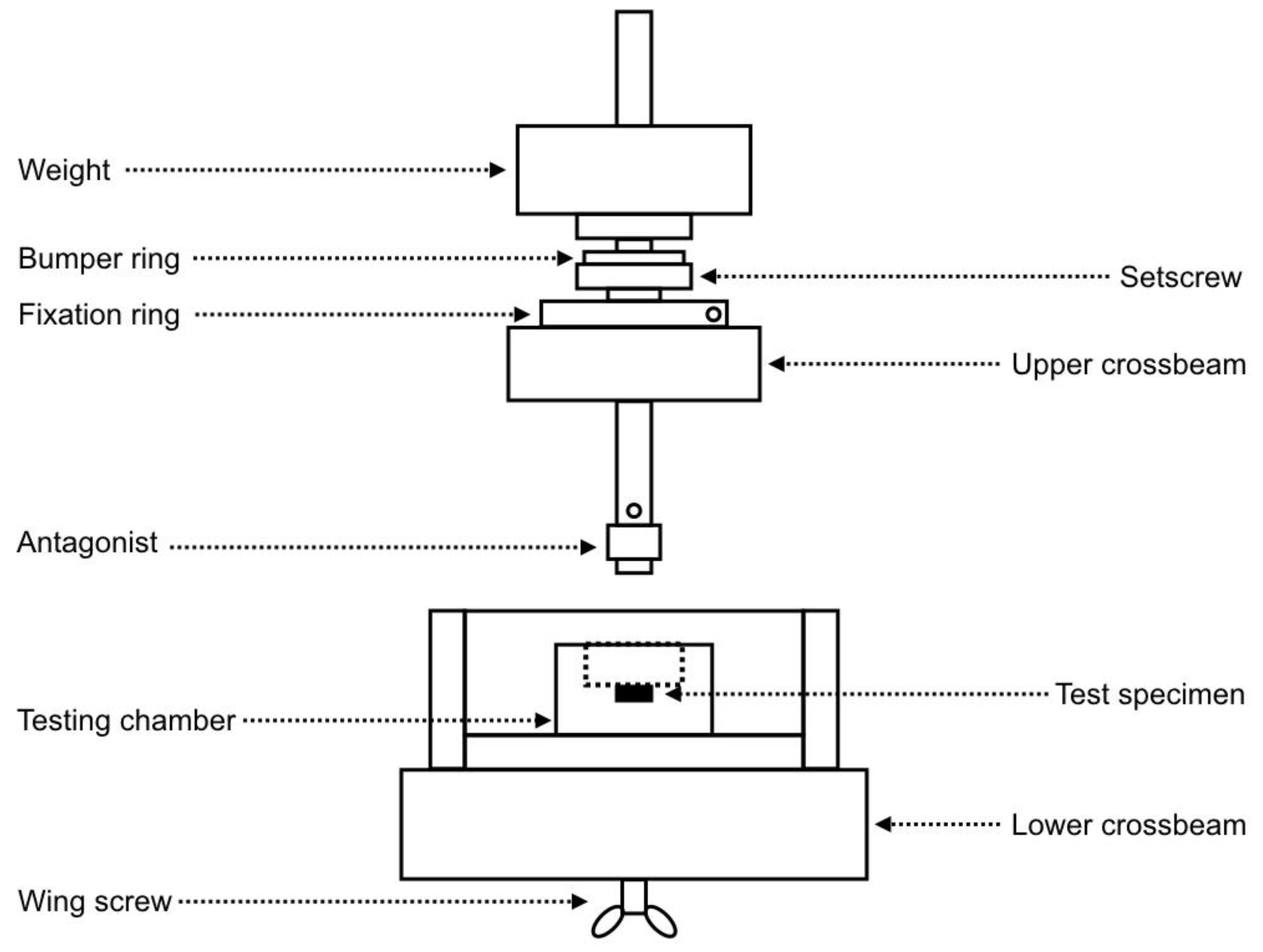
2.2. Chewing Simulation
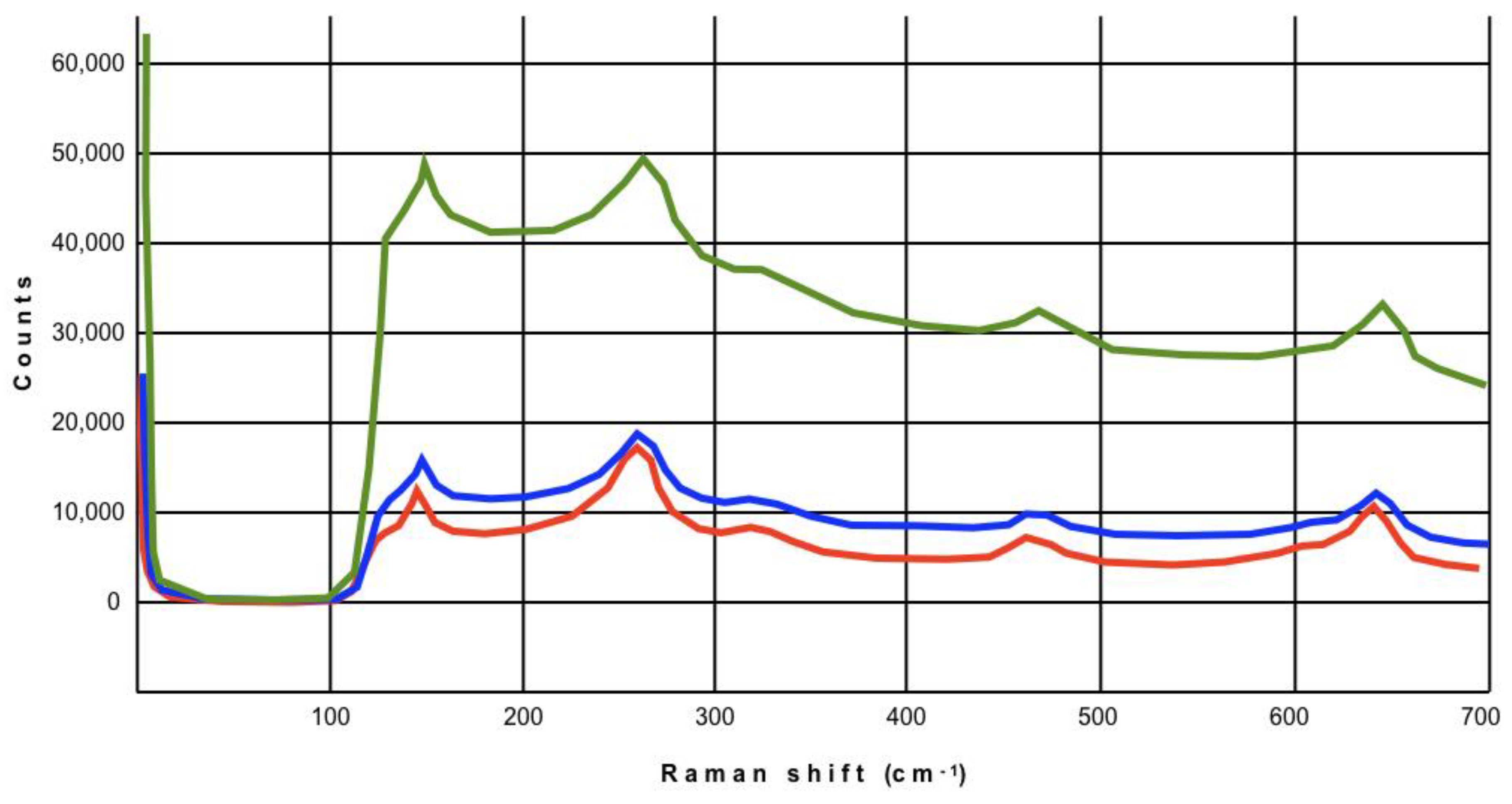
2.3. Micro-Raman Analysis
3. Results
4. Discussion
5. Conclusions
- irrespective of preparation geometry, the manufacturing processes needed to obtain different marginal finish lines (deep-chamfer, slight-chamfer, feather-edge) did not generate the phenomenon of “transformation toughening” either in the load application areas or at the margins of the restorations in the analyzed brands of zirconia;
- after wear-simulation of one year of chewing (fatigue cyclic load), monoclinic zirconia was not found either on the top or at the margins of the copings;
- manufacturing milling, even in thin thickness, did not cause any structural modification of zirconia ceramics “as received by manufacturers” both before and after chewing simulation;
- further laboratory studies and RCTs are needed to investigate if longer chewing time can produce negative effects on zirconia.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vigolo, P.; Mutinelli, S. Evaluation of zirconium-oxide-based ceramic single-unit posterior fixed dental prostheses (FDPs) generated with two CAD/CAM systems compared to porcelain-fused-to-metal single-unit posterior FDPs: A 5-year clinical prospective study. J. Prosthodont. 2012, 21, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Zarone, F.; Russo, S.; Sorrentino, R. From porcelain-fused-to-metal to zirconia: Clinical and experimental considerations. Dent. Mater. 2011, 27, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, R.; De Simone, G.; Tetè, S.; Russo, S.; Zarone, F. Five-year prospective clinical study of posterior three-unit zirconia-based fixed dental prostheses. Clin. Oral Investig. 2012, 16, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Iwai, T.; Komine, F.; Kobayashi, K.; Saito, A.; Matsumura, H. Influence of convergence angle and cement space on adaptation of zirconium dioxide ceramic copings. Acta Odontol. Scand. 2008, 66, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, Y.; Tsumita, M.; Kano, T.; Sakurai, S.; Fukushima, S. Clinical marginal and internal gaps of zirconia all-ceramic crowns. J. Prosthodont. Res. 2011, 55, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; Vichi, A.; Zarone, F. Zirconia abutments and restorations: From laboratory to clinical investigations. Dent. Mater. 2015, 31, e63–e76. [Google Scholar] [CrossRef] [PubMed]
- Fuzzi, M.; Tricarico, M.G.; Ferrari Cagidiaco, E.; Bonadeo, G.; Sorrentino, R.; Ferrari, M. Nanoleakage and internal adaptation of zirconia and lithium disilicate single crowns with knife edge preparation. J. Osseointegr. 2017, 9, 262–274. [Google Scholar]
- Sorrentino, R.; Triulzio, C.; Tricarico, M.G.; Bonadeo, G.; Gherlone, E.F.; Ferrari, M. In vitro analysis of the fracture resistance of CAD-CAM monolithic zirconia molar crowns with different occlusal thickness. J. Mech. Behav. Biomed. Mater. 2016, 61, 328–333. [Google Scholar] [CrossRef] [PubMed]
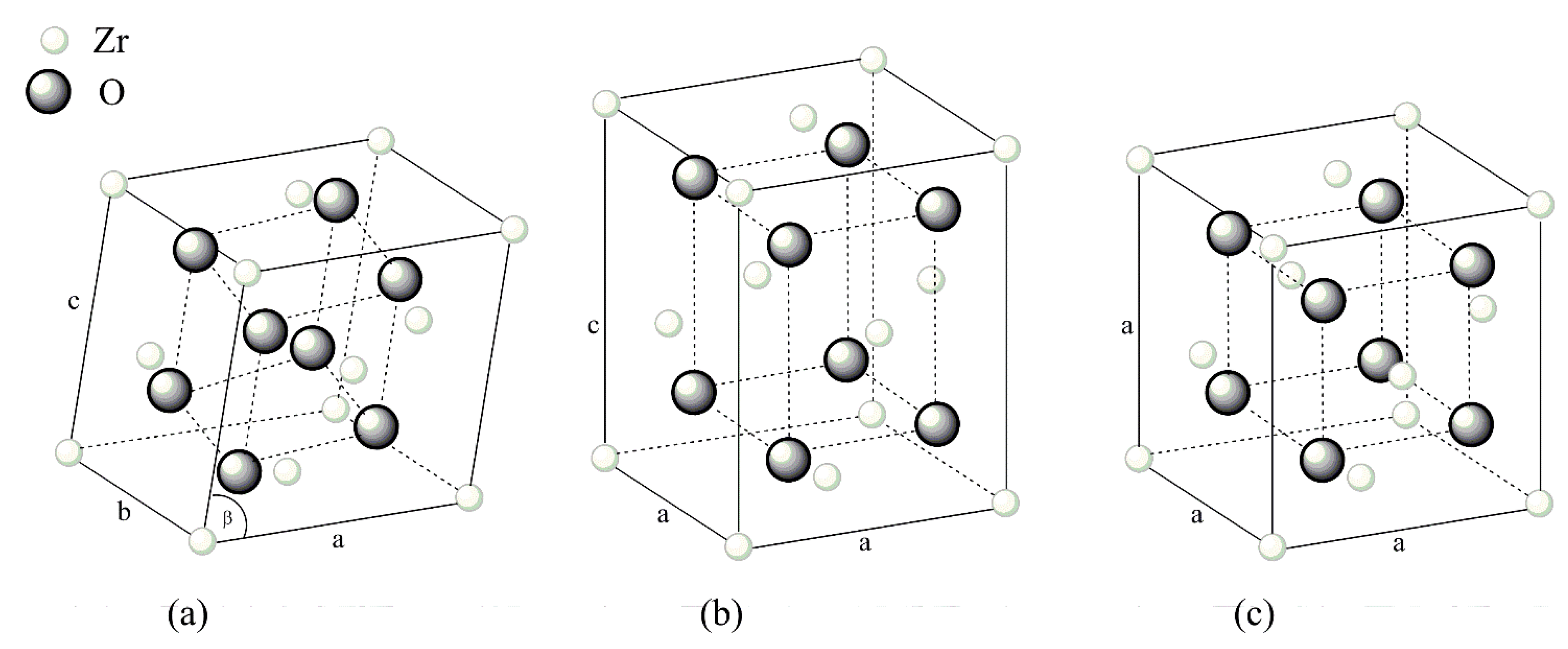
- Kisi, E.H.; Howard, C.J. Crystal structures of zirconia phases and their inter-relation. Key Eng. Mater. 1998, 153, 1–36. [Google Scholar] [CrossRef]
- Camposilvan, E.; Leone, R.; Gremillard, L.; Sorrentino, R.; Zarone, F.; Ferrari, M.; Chevalier, J. Aging resistance, mechanical properties and translucency of different yttria-stabilized zirconia ceramics for monolithic dental crown applications. Dent. Mater. 2018, 34, 879–890. [Google Scholar] [CrossRef]
- Subarrao, E.C. Zirconia: An overview. In Science and Technology of Zirconia; Heuer, A.H., Hobbs, L.W., Eds.; The American Ceramic Society: Columbus, OH, USA, 1981; pp. 1–24. [Google Scholar]
- Fabbri, G.; Fradeani, M.; Dellificorelli, G.; De Lorenzi, M.; Zarone, F.; Sorrentino, R. Clinical evaluation of the influence of connection type and restoration height on the reliability of zirconia abutments: A retrospective study on 965 abutments with a mean 6-year follow-up. Int. J. Periodontics Restor. Dent. 2017, 37, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Comlekoglu, M.; Dundar, M.; Ozcan, M.; Gungor, M.; Gokce, B.; Artunc, C. Influence of cervical finish line type on the marginal adaptation of zirconia ceramic crowns. Oper. Dent. 2009, 34, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Patroni, S.; Chiodera, G.; Caliceti, C.; Ferrari, P. CAD/CAM technology and zirconium oxide with feather-edge marginal preparation. Eur. J. Esthet. Dent. 2010, 5, 78–100. [Google Scholar] [PubMed]
- Reich, S.; Petschelt, A.; Lohbauer, U. The effect of finish line preparation and layer thickness on the failure load and fractography of ZrO2 copings. J. Prosthet. Dent. 2008, 99, 369–376. [Google Scholar] [CrossRef]
- Tinschert, J.; Schulze, K.A.; Natt, G.; Latzke, P.; Heussen, N.; Spiekermann, H. Clinical behavior of zirconia-based fixed partial dentures made of DC-Zirkon: 3-year results. Int. J. Prosthodont. 2008, 21, 217–222. [Google Scholar]
- Balkaya, M.C.; Cinar, A.; Pamuk, S. Influence of firing cycles on the margin distortion of 3 all-ceramic crown systems. J. Prosthet. Dent. 2005, 93, 346–355. [Google Scholar] [CrossRef]
- Poggio, C.E.; Dosoli, R.; Ercoli, C. A retrospective analysis of 102 zirconia single crowns with knife-edge margins. J. Prosthet. Dent. 2012, 107, 316–321. [Google Scholar] [CrossRef]
- Heintze, S.D.; Rousson, V. Survival of zirconia- and metal-supported fixed dental prostheses: A systematic review. J. Prosthet. Dent. 2010, 23, 493–502. [Google Scholar]
- Guess, P.C.; Bonfante, E.A.; Silva, N.R.; Coelho, P.G.; Thompson, V.P. Effect of core design and veneering technique on damage and reliability of Y-TZP-supported crowns. Dent. Mater. 2013, 29, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Scherrer, S.S.; Quinn, J.B.; Quinn, G.D.; Wiskott, H.W. Fractographic ceramic failure analysis using the replica technique. Dent. Mater. 2007, 23, 1397–1404. [Google Scholar] [CrossRef] [PubMed]
- Lohbauer, U.; Amberger, G.; Quinn, G.D.; Scherrer, S.S. Fractographic analysis of a dental zirconia framework: A case study on design issues. J. Mech. Behav. Biomed. Mater. 2010, 3, 623–629. [Google Scholar] [CrossRef]
- Potiket, N.; Chiche, G.; Finger, I.M. In vitro fracture strength of teeth restored with different all-ceramic crown systems. J. Prosthet. Dent. 2004, 92, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Bindl, A.; Lüthy, H.; Mörmann, W.H. Thin-wall ceramic CAD/CAM crown copings: Strength and fracture pattern. J. Oral Rehabil. 2006, 33, 520–528. [Google Scholar] [CrossRef]
- Rekow, E.D.; Silva, N.R.; Coelho, P.G.; Zhang, Y.; Guess, P.; Thompson, V.P. Performance of dental ceramics: Challenges for improvements. J. Dent. Res. 2011, 90, 937–952. [Google Scholar] [CrossRef]
- Aboushelib, M.N. Fatigue and fracture resistance of zirconia crowns prepared with different finish line designs. J. Prosthodont. 2012, 21, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Sergo, V.; Lughi, V.; Pezzotti, G.; Lucchini, E.; Meriani, S.; Muraki, N.; Katagiri, G.; Lo Casto, S.; Nishida, T. The effect of wear on the tetragonal-to-monoclinic transformation and the residual stress distribution in zirconia-toughened alumina cutting tools. Wear 1998, 214, 264–270. [Google Scholar] [CrossRef]
- Dauskardt, R.H.; Marshall, D.B.; Ritchie, R.O. Cyclic fatigue-crack propagation in magnesia-partially stabilized zirconia ceramics. J. Am. Ceram. Soc. 1990, 73, 893–903. [Google Scholar] [CrossRef]
- Pezzotti, G.; Porporati, A.A. Raman spectroscopic analysis of phase-transformation and stress patterns in zirconia hip joints. J. Biomed. Opt. 2004, 9, 372–384. [Google Scholar] [CrossRef] [PubMed]
- Naumenko, A.P.; Berezovska, N.I.; Biliy, M.M.; Shevchenko, O.V. Vibrational Analysis and Raman Spectra of Tetragonal Zirconia. Phys. Chem. Solid State 2008, 9, 121–125. [Google Scholar]
- Lughi, V.; Sergo, V. Low temperature degradation -aging- of zirconia: A critical review of the relevant aspects in dentistry. Dent. Mater. 2010, 26, 807–820. [Google Scholar] [CrossRef]
- Ramos, C.M.; Tabata, A.S.; Cesar, P.F.; Rubo, J.H.; Fracisconi, P.A.; Sanches Borges, A.F. Application of Micro-Raman Spectroscopy to the Study of Yttria-Stabilized Tetragonal Zirconia Polycrystal (Y-TZP) Phase Transformation. Appl. Spectrosc. 2015, 69, 810–814. [Google Scholar] [CrossRef] [PubMed]
- Cadenaro, M.; Codan, B.; Navarra, C.O.; Marchesi, G.; Turco, G.; Di Lenarda, R.; Breschi, L. Contraction stress, elastic modulus, and degree of conversion of three flowable composites. Eur. J. Oral Sci. 2011, 119, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Navarra, C.O.; Cadenaro, M.; Armstrong, S.R.; Jessop, J.; Antoniolli, F.; Sergo, V.; Di Lenarda, R.; Breschi, L. Degree of conversion of Filtek Silorane Adhesive System and Clearfil SE Bond within the hybrid and adhesive layer: An in situ Raman analysis. Dent. Mater. 2009, 25, 1178–1185. [Google Scholar] [CrossRef] [PubMed]
- Beuer, F.; Stimmelmayr, M.; Gueth, J.F.; Edelhoff, D.; Naumann, M. In vitro performance of full-contour zirconia single crowns. Dent. Mater. 2012, 28, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Schmitter, M.; Mueller, D.; Rues, S. Chipping behaviour of all-ceramic crowns with zirconia framework and CAD/CAM manufactured veneer. J. Dent. 2012, 40, 154–162. [Google Scholar] [CrossRef]
- Stawarczyk, B.; Ozcan, M.; Hallmann, L.; Roos, M.; Trottmann, A.; Hämmerle, C.H. Impact of air-abrasion on fracture load and failure type of veneered anterior Y-TZP crowns before and after chewing simulation. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 1683–1690. [Google Scholar] [CrossRef]
- Nelson, J. Wheeler’s Dental Anatomy, Physiology and Occlusion, 10th ed.; Saunders: Philadelphia, PA, USA, 2014. [Google Scholar]
- Trier, A.C.; Parker, M.H.; Cameron, S.M.; Brousseau, J.S. Evaluation of resistance form of dislodged crowns and retainers. J. Prosthet. Dent. 1998, 80, 405–459. [Google Scholar] [CrossRef]
- Kokubo, Y.; Tsumita, M.; Kano, T.; Fukushima, S. The influence of zirconia coping designs on the fracture load of all-ceramic molar crowns. Dent. Mater. J. 2011, 30, 281–285. [Google Scholar] [CrossRef]
- Rosentritt, M.; Siavikis, G.; Behr, M.; Kolbeck, C.; Handel, G. Approach for valuating the significance of laboratory simulation. J. Dent. 2008, 36, 1048–1053. [Google Scholar] [CrossRef]
- Rosentritt, M.; Behr, M.; van der Zel, J.M.; Feilzer, A.J. Approach for valuating the influence of laboratory simulation. Dent. Mater. 2009, 25, 348–352. [Google Scholar] [CrossRef]
- Lutz, F.; Krejci, I.; Barbakow, F. Chewing pressure vs. wear of composites and opposing enamel cusps. J. Dent. Res. 1992, 71, 1525–1529. [Google Scholar] [CrossRef]
- Steiner, M.; Mitsias, M.E.; Ludwig, K.; Kern, M. In vitro evaluation of a mechanical testing chewing simulator. Dent. Mater. 2009, 25, 494–499. [Google Scholar] [CrossRef]
- Inokoshi, M.; Shimizu, H.; Nozaki, K.; Takagaki, T.; Yoshihara, K.; Nagaoka, N.; Zhang, F.; Vleugels, J.; Van Meerbeek, B.; Minakuchi, S. Crystallographic and morphological analysis of sandblasted highly translucent dental zirconia. Dent. Mater. 2018, 34, 508–518. [Google Scholar] [CrossRef]
- Roitero, E.; Anglada, M.; Mücklich, F.; Jiménez-Piqué, E. Mechanical reliability of dental grade zirconia after laser patterning. J. Mech. Behav. Biomed. Mater. 2018, 86, 257–263. [Google Scholar] [CrossRef]
- Rohr, N.; Märtin, S.; Fischer, J. Correlations between fracture load of zirconia implant supported single crowns and mechanical properties of restorative material and cement. Dent. Mater. J. 2018, 37, 222–228. [Google Scholar] [CrossRef]
- Poggio, C.; Pigozzo, M.; Ceci, M.; Scribante, A.; Beltrami, R.; Chiesa, M. Influence of different luting protocols on shear bond strength of computer aided design/computer aided manufacturing resin nanoceramic material to dentin. Dent. Res. J. 2016, 13, 91–97. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Lin, Y.; Deng, X.; Li, M.; Nan, C. In Vitro Cell Proliferation and Mechanical Behaviors Observed in Porous Zirconia Ceramics. Materials 2016, 9, 218. [Google Scholar] [CrossRef]
- Volpato, C.A.M.; Carvalho, Ó.S.N.; Pereira, M.R.D.C.; Correia Pereira da Silva, F.S. Evaluation of the color and translucency of glass-infiltrated zirconia based on the concept of functionally graded materials. J. Prosthet. Dent. 2019. [Google Scholar] [CrossRef]
- Kelly, J.R.; Denry, I. Stabilized zirconia as a structural ceramic: An overview. Dent. Mater. 2008, 24, 289–298. [Google Scholar] [CrossRef]
- Kosmac, T.; Oblak, C.; Jevnikar, P.; Funduk, N.; Marion, L. Strength and reliability of surface treated Y-TZP dental ceramics. J. Biomed. Mater. Res. 2000, 53, 304–313. [Google Scholar] [CrossRef]
- Bal, B.S.; Zhu, W.; Zanocco, M.; Marin, E.; Sugano, N.; McEntire, B.J.; Pezzotti, G. Reconciling in vivo and in vitro kinetics of the polymorphic transformation in zirconia-toughened alumina for hip joints: I. Phenomenology. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 72, 252–258. [Google Scholar] [CrossRef]
- Sundh, A.; Molin, M.; Sjögren, G. Fracture resistance of yttrium oxide partially-stabilized zirconia all-ceramic bridges after veneering and mechanical fatigue testing. Dent. Mater. 2005, 21, 476–482. [Google Scholar] [CrossRef]
- Swab, J.J. Low temperature degradation of Y-TZP materials. J. Mater. Sci. 1991, 26, 6706–6714. [Google Scholar] [CrossRef]
- Cotes, C.; Arata, A.; Melo, R.M.; Bottino, M.A.; Machado, J.P.B.; Souza, R.O.A. Effects of aging procedures on the topographic surface, structural stability, and mechanical strength of a ZrO2-based dental ceramic. Dent. Mater. 2014, 30, e396–e404. [Google Scholar] [CrossRef]
- Chevalier, J.; Gremillard, L.; Deville, S. Low-temperature degradation of zirconia and implications for biomedical implants. Annu. Rev. Mater. Res. 2007, 37, 1–32. [Google Scholar] [CrossRef]
- Turrell, G. The Raman effect. In Raman Microscopy; Turrell, G., Corset, J., Eds.; Academic Press: London, UK, 1996; pp. 1–23. [Google Scholar]
- Pereira, G.K.; Venturini, A.B.; Silvestri, T.; Dapieve, K.S.; Montagner, A.F.; Soares, F.Z.; Valandro, L.F. Low-temperature degradation of Y-TZP ceramics: A systematic review and meta-analysis. J. Mech. Behav. Biomed. Mater. 2015, 55, 151–163. [Google Scholar] [CrossRef]
- Komine, F.; Iwai, T.; Kobayashi, K.; Matsumura, H. Marginal and internal adaptation of zirconium dioxide ceramic copings and crowns with different finish line designs. Dent. Mater. J. 2007, 26, 659–664. [Google Scholar] [CrossRef]
- Beuer, F.; Aggstaller, H.; Richter, J.; Edelhoff, D.; Gernet, W. Influence of preparation angle on marginal and internal fit of CAD/CAM-fabricated zirconia crown copings. Quintessence Int. 2009, 40, 243–250. [Google Scholar]
- Lohbauer, U.; Petschelt, A.; Greil, P. Lifetime prediction of CAD/CAM dental ceramics. J. Biomed. Mater. Res. 2002, 63, 780–785. [Google Scholar] [CrossRef]
- Di Febo, G.; Carnevale, G.; Sterrantino, S.F. Treatment of a case of advanced periodontitis: Clinical procedures utilizing the “combined preparation” technique. Int. J. Periodontics Restor. Dent. 1985, 5, 52–62. [Google Scholar]
- Beuer, F.; Aggstaller, H.; Edelhoff, D.; Gernet, W. Effect of preparation design on the fracture resistance of zirconia crown copings. Dent. Mater. J. 2008, 27, 362–367. [Google Scholar] [CrossRef]
- Proos, K.A.; Swain, M.V.; Ironside, J.; Steven, G.P. Influence of core thickness on a restored crown of a first premolar using finite element analysis. Int. J. Prosthodont. 2003, 16, 474–480. [Google Scholar]
- Cho, L.R.; Choi, J.; Yi, Y.J.; Park, C.J. Effect of finish line variants on marginal accuracy and fracture strength of ceramic optimized polymer/fiber-reinforced composite crowns. J. Prosthet. Dent. 2004, 9, 554–560. [Google Scholar] [CrossRef]
- Ramos, G.F.; Monteiro, E.B.; Bottino, M.A.; Zhang, Y.; de Melo, R.M. Failure Probability of Three Designs of Zirconia Crowns. Int. J. Periodontics Restor. Dent. 2015, 35, 843–849. [Google Scholar] [CrossRef]

| Group | Material | Composition | Preparation |
|---|---|---|---|
| G1a (n = 10) | NobelProcera Zirconia (Nobel Biocare) | ZrO2 + Y2O3 + HfO2 > 99%, Y2O3 4.5-5.4%, HfO2 < 5%, Al2O3 < 0.5% | Feather-edge |
| G1b (n = 10) | Slight-chamfer | ||
| G1c (n = 10) | Deep-chamfer | ||
| G2a (n = 10) | LAVA Classic (3M ESPE) | 3 mol% Y-TZP + Al2O3 | Feather-edge |
| G2b (n = 10) | Slight-chamfer | ||
| G2c (n = 10) | Deep-chamfer | ||
| G3a (n = 10) | LAVA Plus (3M ESPE) | 3 mol% Y-TZP + Al2O3 0.1% + ionic staining components | Feather-edge |
| G3b (n = 10) | Slight-chamfer | ||
| G3c (n = 10) | Deep-chamfer |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sorrentino, R.; Navarra, C.O.; Di Lenarda, R.; Breschi, L.; Zarone, F.; Cadenaro, M.; Spagnuolo, G. Effects of Finish Line Design and Fatigue Cyclic Loading on Phase Transformation of Zirconia Dental Ceramics: A Qualitative Micro-Raman Spectroscopic Analysis. Materials 2019, 12, 863. https://doi.org/10.3390/ma12060863
Sorrentino R, Navarra CO, Di Lenarda R, Breschi L, Zarone F, Cadenaro M, Spagnuolo G. Effects of Finish Line Design and Fatigue Cyclic Loading on Phase Transformation of Zirconia Dental Ceramics: A Qualitative Micro-Raman Spectroscopic Analysis. Materials. 2019; 12(6):863. https://doi.org/10.3390/ma12060863
Chicago/Turabian StyleSorrentino, Roberto, Chiara Ottavia Navarra, Roberto Di Lenarda, Lorenzo Breschi, Fernando Zarone, Milena Cadenaro, and Gianrico Spagnuolo. 2019. "Effects of Finish Line Design and Fatigue Cyclic Loading on Phase Transformation of Zirconia Dental Ceramics: A Qualitative Micro-Raman Spectroscopic Analysis" Materials 12, no. 6: 863. https://doi.org/10.3390/ma12060863
APA StyleSorrentino, R., Navarra, C. O., Di Lenarda, R., Breschi, L., Zarone, F., Cadenaro, M., & Spagnuolo, G. (2019). Effects of Finish Line Design and Fatigue Cyclic Loading on Phase Transformation of Zirconia Dental Ceramics: A Qualitative Micro-Raman Spectroscopic Analysis. Materials, 12(6), 863. https://doi.org/10.3390/ma12060863










