Study of Two Bovine Bone Blocks (Sintered and Non-Sintered) Used for Bone Grafts: Physico-Chemical Characterization and In Vitro Bioactivity and Cellular Analysis
Abstract
1. Introduction
2. Materials and Methods
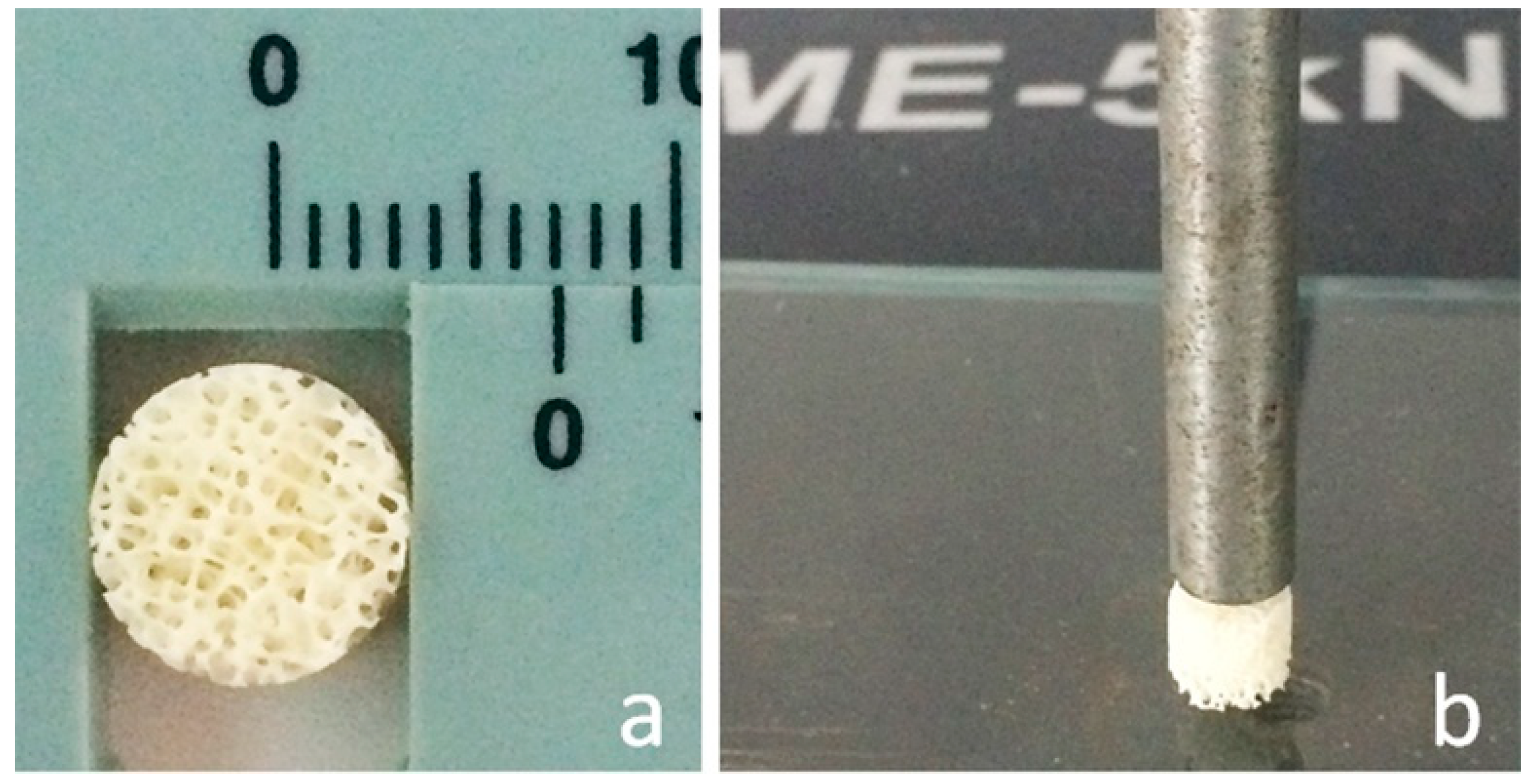
2.1. Materials
2.2. Materials Characterization.
2.3. In Vitro Assays
3. Results
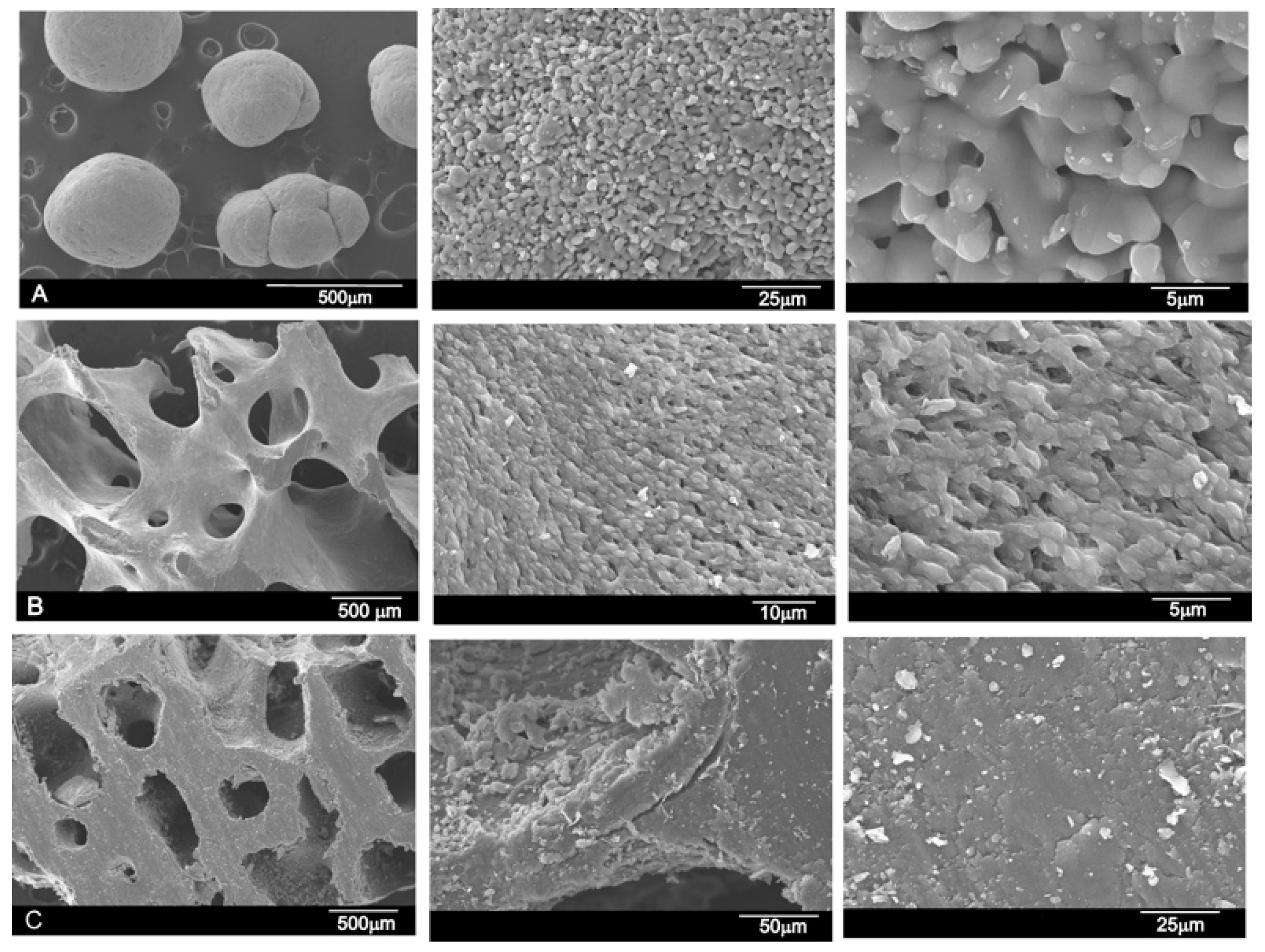
3.1. Results of the SEM and EDS Analyses
3.2. Results of the Fourier Transform Infrared Spectroscopy (FTIR) Analysis
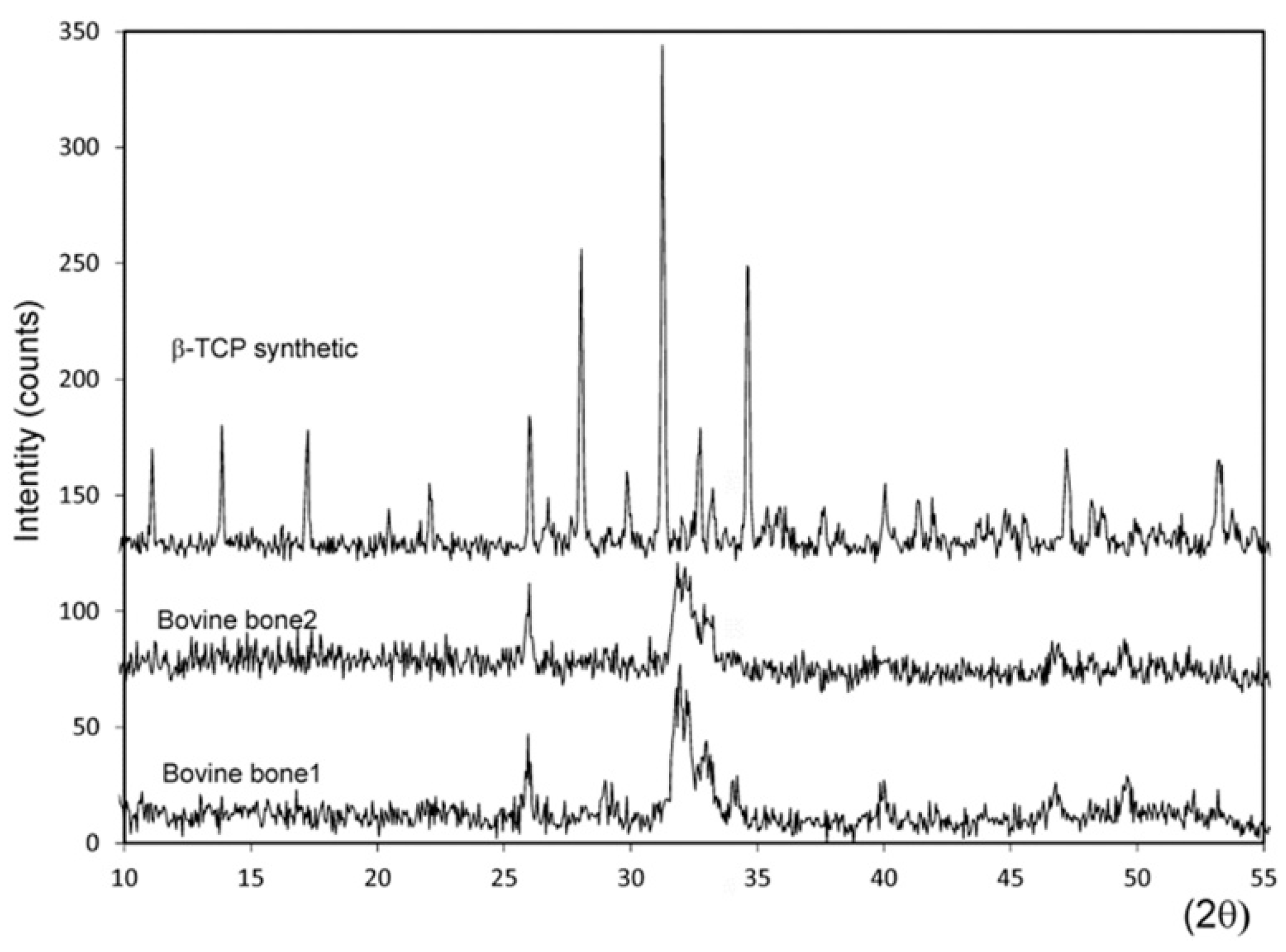
3.3. Results of the X-ray Diffraction (XRD) Analysis
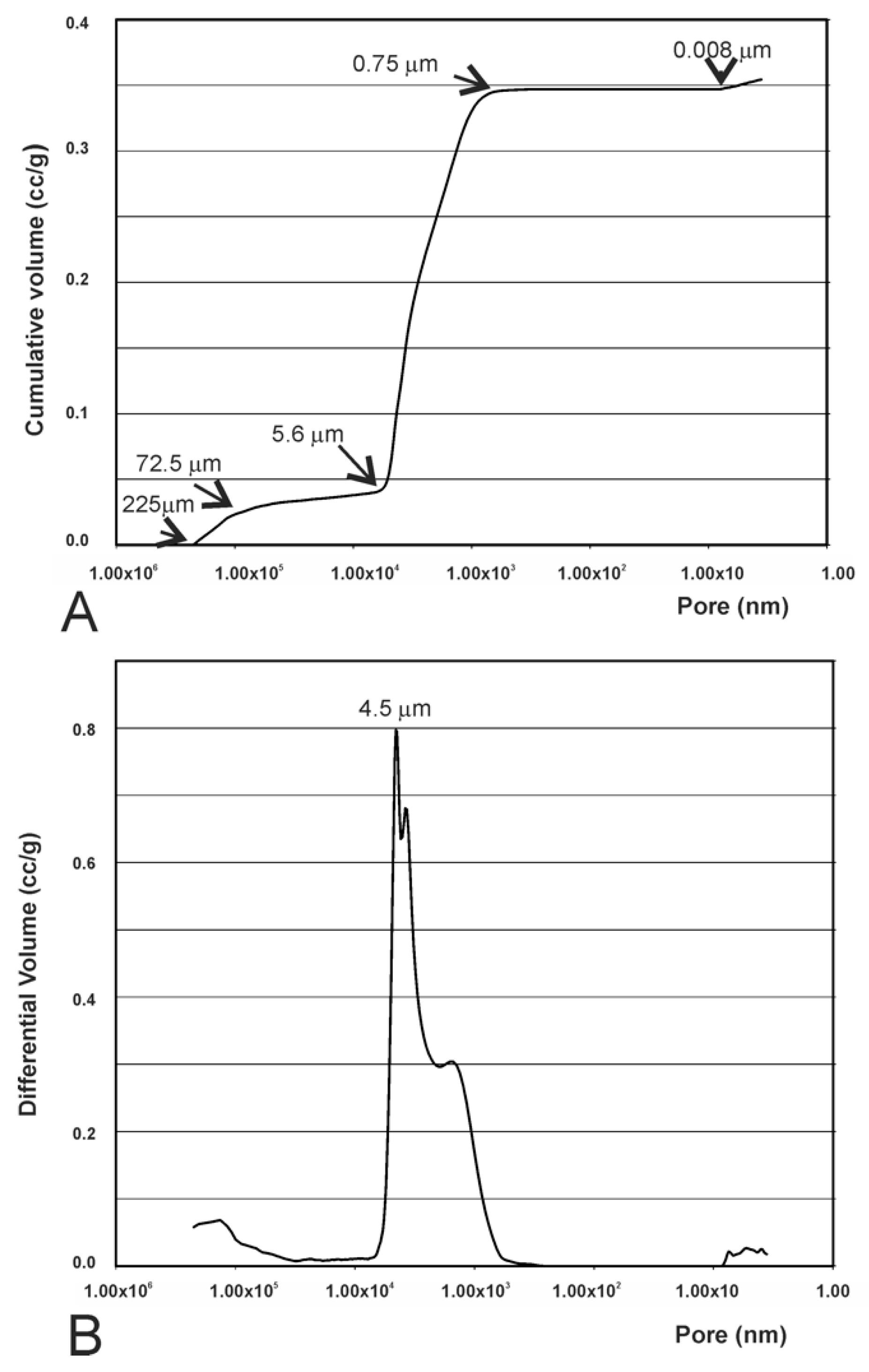
3.4. Results of the Mercury Intrusion Analysis
3.5. Results of the Helium Gas Pycnometry Analysis
3.6. Results of the Compressive Strength Analysis
3.7. Results of Bioactivity and Biodegradability Assays.
3.8. Results of the Viability and Proliferation of Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Barone, A.; Covani, U. Maxillary alveolar ridge reconstruction with nonvascularized autogenous block bone: Clinical results. J. Oral Maxillofac. Surg. 2007, 65, 2039–2046. [Google Scholar] [CrossRef] [PubMed]
- Mate Sanchez de Val, J.; Mazon, P.; Piattelli, A.; Calvo-Guirado, J.L.; Mareque Bueno, J.; Granero Marin, J.; De Aza, P. Comparison among the physical properties of calcium phosphate-based bone substitutes of natual or synthetic origin. Int. J. Appl. Ceram. Technol. 2018, 15, 930–937. [Google Scholar] [CrossRef]
- Khan, S.N.; Cammisa, F.P., Jr.; Sandhu, H.S.; Diwan, A.D.; Girardi, F.P.; Lane, J.M. The biology of bone grafting. J. Am. Acad. Orthop. Surg. 2005, 13, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Flynn, J.M. Fracture Repair and Bone Grafting. OKU 10: Orthopaedic Knowledge Update; American Academy of Orthopaedic Surgeons: Rosemont, IL, USA, 2011; pp. 11–21. [Google Scholar]
- Centers for Disease Control and Prevention (CDC). Update: Allograft-associated bacterial infections-United States, 2002. MMWR Morb. Mortal Wkly. Rep. 2002, 51, 207–210. [Google Scholar]
- Centers for Disease Control and Prevention (CDC). Septic arthritis following anterior cruciate ligament reconstruction using tendon allografts-Florida and Louisiana, 2000. MMWR Morb. Mortal Wkly. Rep. 2001, 50, 1081–1083. [Google Scholar]
- Ramírez Fernández, M.P.; Gehrke, S.A.; Mazón, P.; Calvo Guirado, J.L.; De Aza, P.N. Implant stability of biological hydroxyapatites used in dentistry. Materials 2017, 10, 644. [Google Scholar] [CrossRef] [PubMed]
- Szpalski, C.; Wetterau, M.; Barr, J.; Warren, S.M. Bone tissue engineering: Current strategies and techniques–part I: Scaffolds. Tissue Eng. Part B Rev. 2012, 18, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.T.; Rosenbaum, A.J. Bone grafts, bone substitutes and orthobiologics: The bridge between basic science and clinical advancements in fracture healing. Organogenesis 2012, 8, 114–124. [Google Scholar] [CrossRef]
- Ramírez Fernández, M.P.; Mazón, P.; Gehrke, S.A.; Calvo Guirado, J.L.; De Aza, P.N. Comparison of two xenograft materials used in sinus lift procedures. Material characterization and in vivo behavior. Materials 2017, 10, 623. [Google Scholar] [CrossRef]
- Danesh-Sani, S.A.; Engebretson, S.P.; Janal, M.N. Histomorphometric results of different grafting materials and effect of healing time on bone maturation after sinus floor augmentation: A systematic review and meta-analysis. J. Periodontal Res. 2017, 52, 301–312. [Google Scholar] [CrossRef]
- Meyer, U.; Joos, U.; Wiesmann, H.P. Biological and biophysical principles in extracorporal bone tissue engineering. Part I. Int. J. Oral Maxillofac. Surg. 2004, 33, 325–332. [Google Scholar] [CrossRef]
- Li, J.J.; Ebied, M.; Xu, J.; Zreiqat, H. Current Approaches to Bone Tissue Engineering: The Interface between Biology and Engineering. Adv. Healthc. Mater. 2018, 7, e1701061. [Google Scholar] [CrossRef] [PubMed]
- Akram, M.; Ahmed, R.; Shakir, I.; Ibrahim, W.A.W.; Hussain, R. Extracting hydroxyapatite and its precursors from natural resources. J. Mater. Sci. 2014, 49, 1461–1475. [Google Scholar] [CrossRef]
- Joschek, S.; Nies, B.; Krotz, R.; Goferich, A. Chemical and physicochemical characterization of porous hydroxyapatite ceramics made of natural bone. Biomaterials 2000, 21, 1645–1658. [Google Scholar] [CrossRef]
- Fernandes, H.R.; Gaddam, A.; Rebelo, A.; Brazete, D.; Stan, G.E.; Ferreira, J.M.F. Bioactive Glasses and Glass-Ceramics for Healthcare Applications in Bone Regeneration and Tissue Engineering. Materials 2018, 11, 2530. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, M.; Spiekermann, H.; Biesterfeld, S.; Edelhoff, D. Maxillary sinus augmentation using xenogenic bone substitute material bio-oss in combination with venous blood. A histologic and histomorphometric study in humans. Clin. Oral Implant. Res. 2000, 11, 217–229. [Google Scholar] [CrossRef]
- Calvo-Guirado, J.L.; Ramirez-Fernandez, M.P.; Mate-Sanchez de Val, J.E.; Negri, B.; Velasquez, P.; de Aza, P.N. Enhanced bone regeneration with a novel synthetic bone substitute in combination with a new natural cross-linked collagen membrane: Radiographic and histomorphometric study. Clin. Oral Implant. Res. 2015, 26, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Mate-Sanchez de Val, J.E.; Calvo-Guirado, J.L.; Gomez Moreno, G.; Perez Albacete-Martinez, C.; Mazón, P.; de Aza, P.N. Influence of hydroxyapatite granule size, porosity and crystallinity on tissue reaction in vivo. Part A: Synthesis, characterization of the materials and SEM analysis. Clin. Oral Implant. Res. 2016, 27, 1331–1338. [Google Scholar] [CrossRef]
- MatéSánchez de Val, J.E.; Calvo-Guirado, J.L.; Gomez Moreno, G.; Gherke, S.; Mazón, P.; De Aza, P.N. Influence of hydroxyapatite granule size, porosity and crystallinity on tissue reaction in vivo. Part B: A comparative study with biphasic synthetic biomaterials. Clin. Oral Implant. Res. 2017. [Google Scholar] [CrossRef]
- Lei, P.; Sun, R.; Wang, L.; Zhou, J.; Wan, L.; Zhou, T.; Hu, Y. A New Method for Xenogeneic Bone Graft Deproteinization: Comparative Study of Radius Defects in a Rabbit Model. PLoS ONE 2015, 31, e0146005. [Google Scholar] [CrossRef]
- Cestari, T.M.; Granjeiro, J.M.; de Assis, G.F.; Garlet, G.P.; Taga, R. Bone repair and augmentation using block of sintered bovine-derived anorganic bone graft in cranial bone defect model. Clin. Oral Implant. Res. 2009, 20, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Guirado, J.L.; Mate-Sanchez de Val, J.E.; Delgado Ruiz, R.A.; Romanos, G.; De Aza, P.N.; Vélasquez, P. Bone neo-formation and mineral degradation of 4Bone® Part II: Histological and histomophometric analysis in critical size defects in rabbits. Clin. Oral Implant. Res. 2015, 26, 1402–1406. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M.; Dohi, Y.; Ohgushi, H.; Shimaoka, H.; Ikeuchi, M.; Matsushima, A.; Yonemasu, K.; Hosoi, H. Influence of the porosity of hydroxyapatite ceramics on in vitro and in vivo bone formation by cultured rat bone marrow stromal cells. J. Mater. Sci. Mater. Med. 2006, 17, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Rocha, C.A.; Cestari, T.M.; Vidotti, H.A.; de Assis, G.F.; Garlet, G.P.; Taga, R. Sintered anorganic bone graft increases autocrine expression of VEGF, MMP-2 and MMP-9 during repair of critical-size bone defects. J. Mol. Histol. 2014, 45, 447–461. [Google Scholar] [CrossRef] [PubMed]
- De Aza, P.N.; Rodríguez, M.A.; Gehrke, S.A.; Maté-Sanchez de Val, J.E.; Calvo-Guirado, J.L. A Si-αTCP scaffold for biomedical applications: An experimental study using the rabbit tibia model. Appl. Sci. Basel 2017, 7, 706. [Google Scholar] [CrossRef]
- Ramírez Fernández, M.P.; Gehrke, S.A.; Pérez Albacete Martinez, C.; Calvo Guirado, J.L.; de Aza, P.N. SEM-EDX Study of the Degradation Process of Two Xenograft Materials Used in Sinus Lift Procedures. Materials 2017, 17, 542. [Google Scholar] [CrossRef] [PubMed]
- Ghanaati, S.; Barbeck, M.; Booms, P.; Lorenz, J.; Kirkpatrick, C.J.; Sader, R.A. Potential lack of “standardized” processing techniques for production of allogeneic and xenogeneic bone blocks for application in humans. Acta Biomater. 2014, 10, 3557–3562. [Google Scholar] [CrossRef] [PubMed]
- Meyer, S.; Floerkemeier, T.; Windhagen, H. Histological osseointegration of Tutobone: First results in human. Arch. Orthop. Trauma Surg. 2008, 128, 539–544. [Google Scholar] [CrossRef]
- Lorenz, J.; Kubesch, A.; Al-Maawi, S.; Schwarz, F.; Sader, R.A.; Schlee, M.; Ghanaati, S. Allogeneic bone block for challenging augmentation—A clinical, histological, and histomorphometrical investigation of tissue reaction and new bone formation. Clin. Oral Investig. 2018, 22, 3159–3169. [Google Scholar] [CrossRef]
- Prakash, S.K.; Mukerji, N.; Nath, F.P. Is tutobone an efficient alternative to other implants used in anterior cervical discectomy and fusion surgeries? Br. J. Neurosurg. 2017, 31, 40–344. [Google Scholar] [CrossRef]
- Makridis, K.G.; Ahmad, M.A.; Kanakaris, N.K.; Fragkakis, E.M.; Giannoudis, P.V. Reconstruction of iliac crest with bovine cancellous allograft after bone graft harvest for symphysis pubis arthrodesis. Int. Orthop. 2012, 36, 1701–1777. [Google Scholar] [CrossRef] [PubMed]
- Landi, E.; Tampieri, A.; Celotti, G.; Sprio, S. Densification behaviour and mechanisms of synthetic hydroxyapatites. J. Eur. Ceram. Soc. 2000, 20, 2377–2387. [Google Scholar] [CrossRef]
- García-Páez, I.H.; Carrodeguas, R.G.; Antonio, H.; Baudín, C.; Pena, P. Effect of Mg and Si co-substitution on microstructure and strength of tricalcium phosphate ceramics. J. Mech. Behav. Biomed. Mater. 2014, 30, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Advanced Technical Ceramics—Mechanical Properties of Monolithic Ceramics at Room Temperature—Part 5: Statistical Analysis; Endorsed by AENOR in January of 2007; British Standards Institution: London, UK, 2007.
- ASTM F1538-03(2017). Standard Specification for Glass and Glass Ceramic Biomaterials for Implantation; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- Kokubo, T.; Kim, H.M.; Kawashita, M.; Nakamura, T. What Kinds of Materials Exhibit Bone-Bonding. In Bone Engineering; Davies, J.E., Ed.; EM2: Toronto, ON, Canada, 2000; pp. 190–194. [Google Scholar]
- Velasquez, P.; Luklinska, Z.B.; Meseguer-Olmo, L.; Mate-Sanchez de Val, J.E.; Delgado-Ruiz, R.A.; Calvo-Guirado, J.L.; Ramirez-Fernandez, M.P.; De Aza, P.N. αTCP ceramic doped with Dicalcium Silicate for bone regeneration applications prepared by powder metallurgy method. In vitro and in vivo studies. J. Biomed. Mater. Res. A 2013, 101, 1943–1954. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, M.P.; Monteiro, F.J.; Manuel, C.M. Hydroxyapatite nanoparticles: A review of preparation methodologies. J. Appl. Biomater. Biomech. 2004, 2, 74–80. [Google Scholar] [PubMed]
- Mate-Sanchez de Val, J.E.; Calvo-Guirado, J.L.; Delgado-Ruiz, R.A.; Ramirez-Fernandez, M.P.; Martinez, I.M.; Granero-Marin, J.M.; Negri, B.; Chiva-Garcia, F.; Martinez-Gonzalez, J.M.; De Aza, P.N. New block graft of α-TCP with silicon in critical size defects in rabbits: Chemical characterization, histological, histomorphometric and micro-CT study. Ceram. Int. 2012, 38, 1563–1570. [Google Scholar] [CrossRef]
- Mate-Sanchez de Val, J.E.; Calvo-Guirado, J.L.; Delgado-Ruiz, R.A.; Ramirez-Fernandez, M.P.; Negri, B.; Abboud, M.; Martinez, I.M.; De Aza, P.N. Physical properties, mechanical behavior, and electron microscopy study of a new α-TCP block graft with silicon in an animal model. J. Biomed. Mater. Res. A 2012, 100, 3446–3454. [Google Scholar] [CrossRef] [PubMed]
- Tadic, D.; Epple, M. A thorough physicochemical characterization of 14 calcium phosphate-based bone substitution materials in comparison to natural bone. Biomaterials 2004, 25, 987–994. [Google Scholar] [CrossRef]
- Hazar Yoruça, A.B.; Karakaşb, A.; Koyunc, A.; Yildiza, T. Comparison of Properties of Hydroxyapatite Powders Synthesized by Chemical and Biomimetic Techniques. Acta Phys. Polonica A 2012, 121, 233–235. [Google Scholar] [CrossRef]
- Martínez, I.M.; Velasquez, P.A.; De Aza, P.N. Synthesis and stability of -Tricalcium Phosphate doped with Dicalcium silicate in the system Ca3(PO4)2–Ca2SiO4. Mater. Charact. 2010, 61, 761–767. [Google Scholar] [CrossRef]
- Martinez, I.M.; Meseguer-Olmo, L.; Bernabeu-Esclapez, A.; Velasquez, P.A.; De Aza, P.N. In vitro behavior of α-Tricalcium Phosphate doped with Dicalcium Silicate in the system Ca2SiO4–Ca3(PO4)2. Mater. Charact. 2012, 63, 47–55. [Google Scholar] [CrossRef]
- Ros-Tárraga, P.; Murciano, A.; Mazón, P.; Gehrke, S.A.; De Aza, P.N. New 3D stratified Si-Ca-P porous scaffolds obtained by sol-gel and polymer replica method: Microstructural, mineralogical and chemical characterization. Ceram. Int. 2017, 43, 6548–6553. [Google Scholar] [CrossRef]
- Antonakos, A.; Liarokapis, E.; Leventouri, T. Micro-Raman and FTIR studies of synthetic and natural apatites. Biomaterials 2007, 28, 3043–3054. [Google Scholar] [CrossRef]
- Ślósarczyk, A.; Paluszkiewicz, C.; Gawlicki, M.; Paszkiewicz, Z. The FTIR spectroscopy and QXRD studies of calcium phosphate based materials produced from the powder precursors with different CaP ratios. Ceram. Int. 1997, 23, 297–304. [Google Scholar] [CrossRef]
- Zuleta, F.; Murciano, A.; Gehrke, S.A.; Maté Sánchez de Val, J.E.; Calvo Guirado, J.L.; de Aza, P.N. A new biphasic dicalcium silicate bone cement implant. Materials 2017, 10, 758. [Google Scholar] [CrossRef]
- Smay, J. Dissolution of CaS Filled, HA:Beta-TCP Scaffolds with Hierarchical Pore Network; IADR: Miami, FL, USA, 2009; pp. 1–16. [Google Scholar]
- Rabadan-Ros Ruben Aznar-Cervantes, S.; Mazón, P.; Ros-Tarraga, P.; De Aza, P.N.; Meseguer-Olmo, L. Nurse’s A material enhance adhesion, growth and differentiation of human bone marrow-derived stromal mesenchymal stem cells. Materials 2017, 10, 347. [Google Scholar] [CrossRef] [PubMed]
- Meseguer-Olmo, L.; Aznar-Cervantes, S.; Mazón, P.; De Aza, P.N. In vitro behaviour of adult mesenchymal stem cells of human origin seeded on a novel bioactive ceramics in the Ca3(PO4)2–Ca2SiO4 system. J. Mater. Sci. Mater. Med. 2012, 23, 3003–3014. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Carinci, F.; Assenza, B.; Piattelli, M.; Murmura, G.; Piattelli, A. Vertical ridge augmentation of atrophic posterior mandible using an inlay technique with a xenograft without miniscrews and miniplates: Case series. Clin. Oral Implant. Res. 2011, 22, 1125–1130. [Google Scholar] [CrossRef] [PubMed]
- Bettinger, C.J.; Langer, R.; Borenstein, J.T. Engineering substrate topography at the micro- and nanoscale to control cell function. Angew. Chem. Int. Ed. Engl. 2009, 48, 5406–5415. [Google Scholar] [CrossRef] [PubMed]
- Mazón PGarcía-Bernal, D.; Meseguer-Olmo, L.; Cragnolini, F.; De Aza, P.N. Human mesenchymal stem cell proliferation, differentiation and apoptosis in response to ceramic chemistry and surface roughness. Ceram. Int. 2015, 41, 6631–6644. [Google Scholar] [CrossRef]
- Witek, L.; Smay, J.; Silva Nelson, R.F.A.; Guda, T.; Ong, J.L.; Coelho, P.G. Sintering effects on chemical and physical properties of bioactive ceramics. J. Adv. Ceram. 2013, 2, 274–284. [Google Scholar] [CrossRef]
- Mkukuma, L.D.; Skakle, J.M.S.; Gibson, I.R.; Imrie, C.T.; Aspden, R.M.; Hukins, D.W.L. Effect of the proportion of organic mate- rial in bone on thermal decomposition of bone mineral: An investigation of a variety of bones from different species using thermogravimetric analysis coupled to mass spectrometry, high temperature x-ray diffraction and Fourier transform infrared spectroscopy. Calcif. Tissue Int. 2004, 75, 321–328. [Google Scholar] [PubMed]

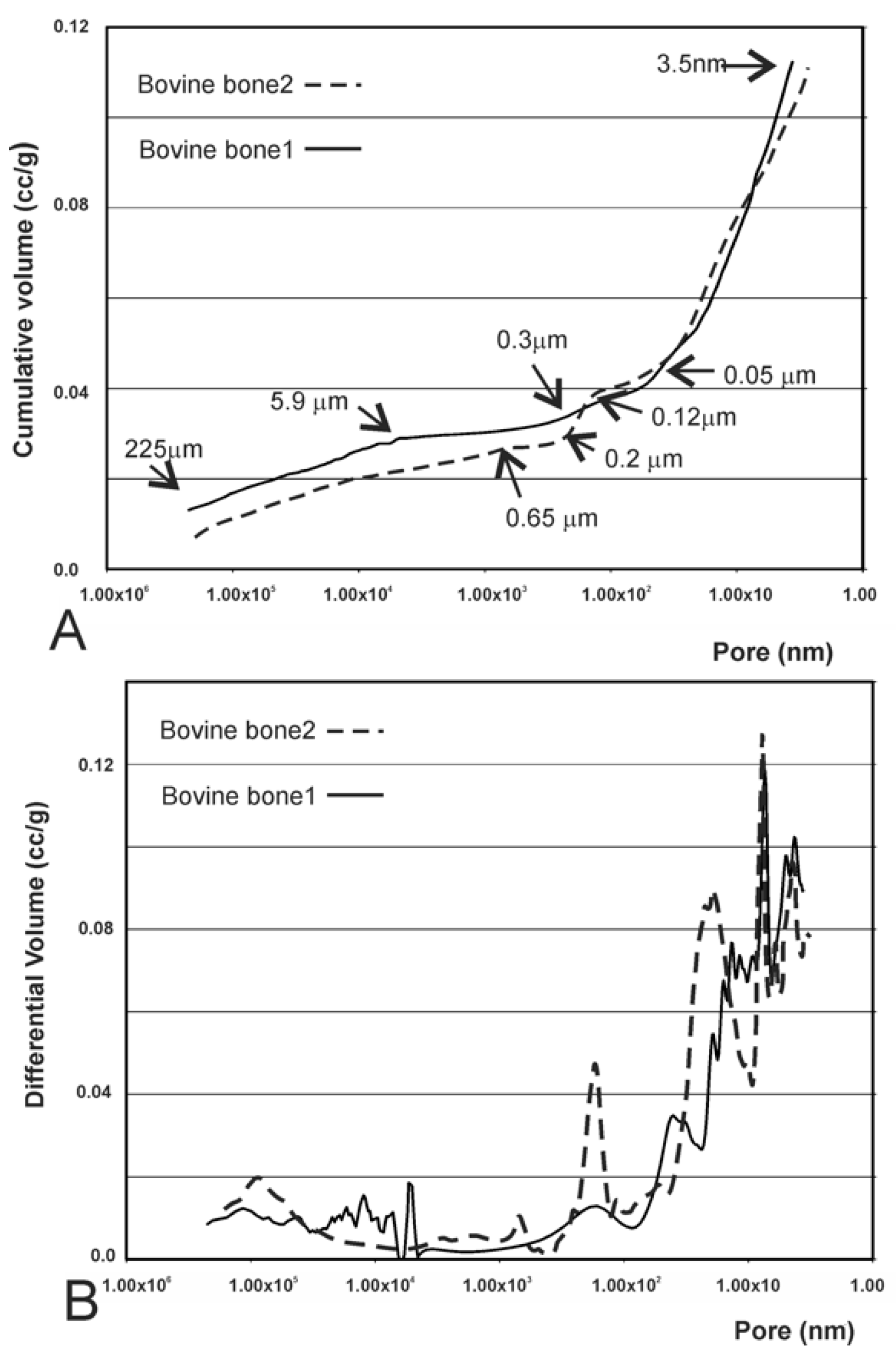
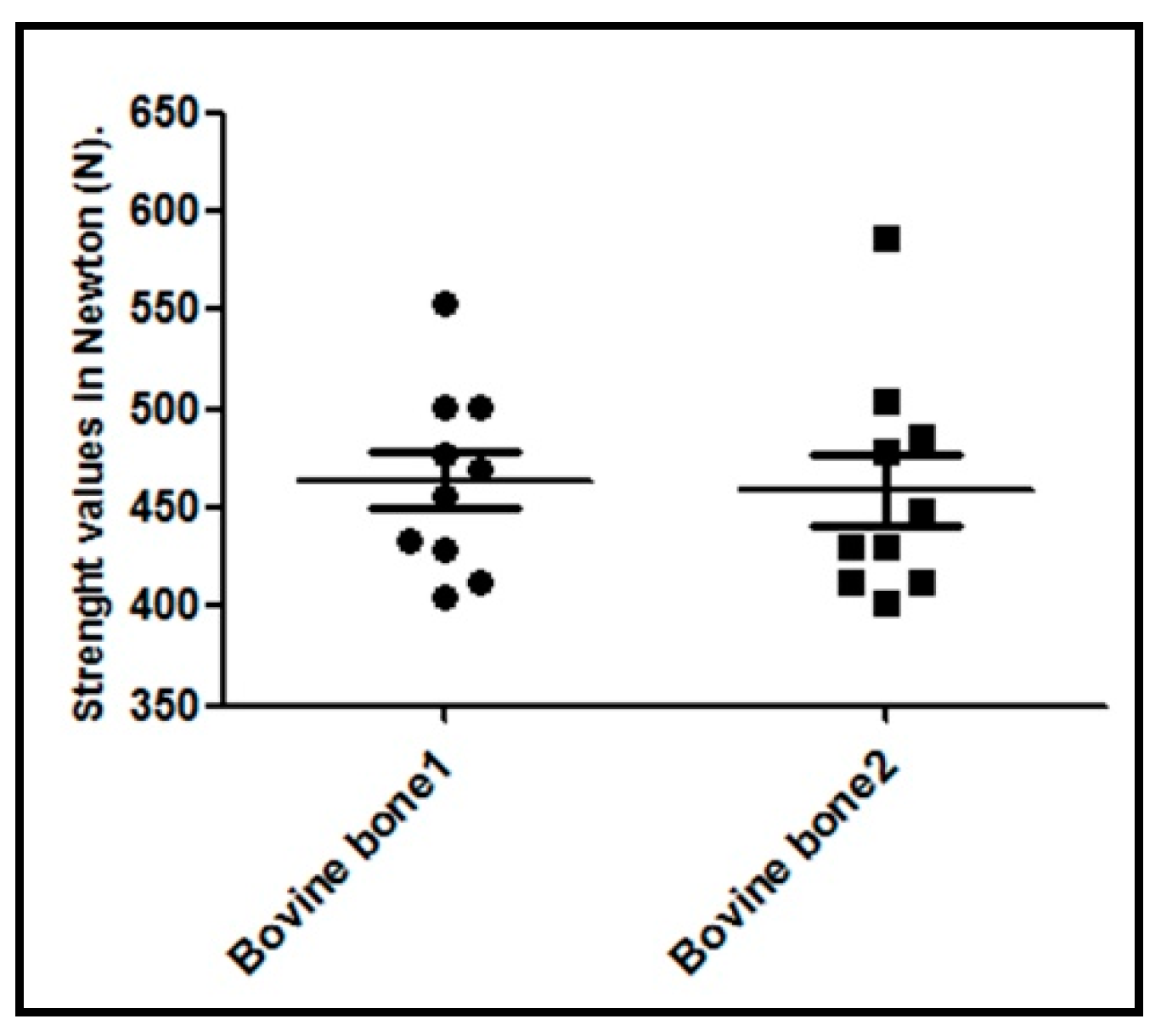
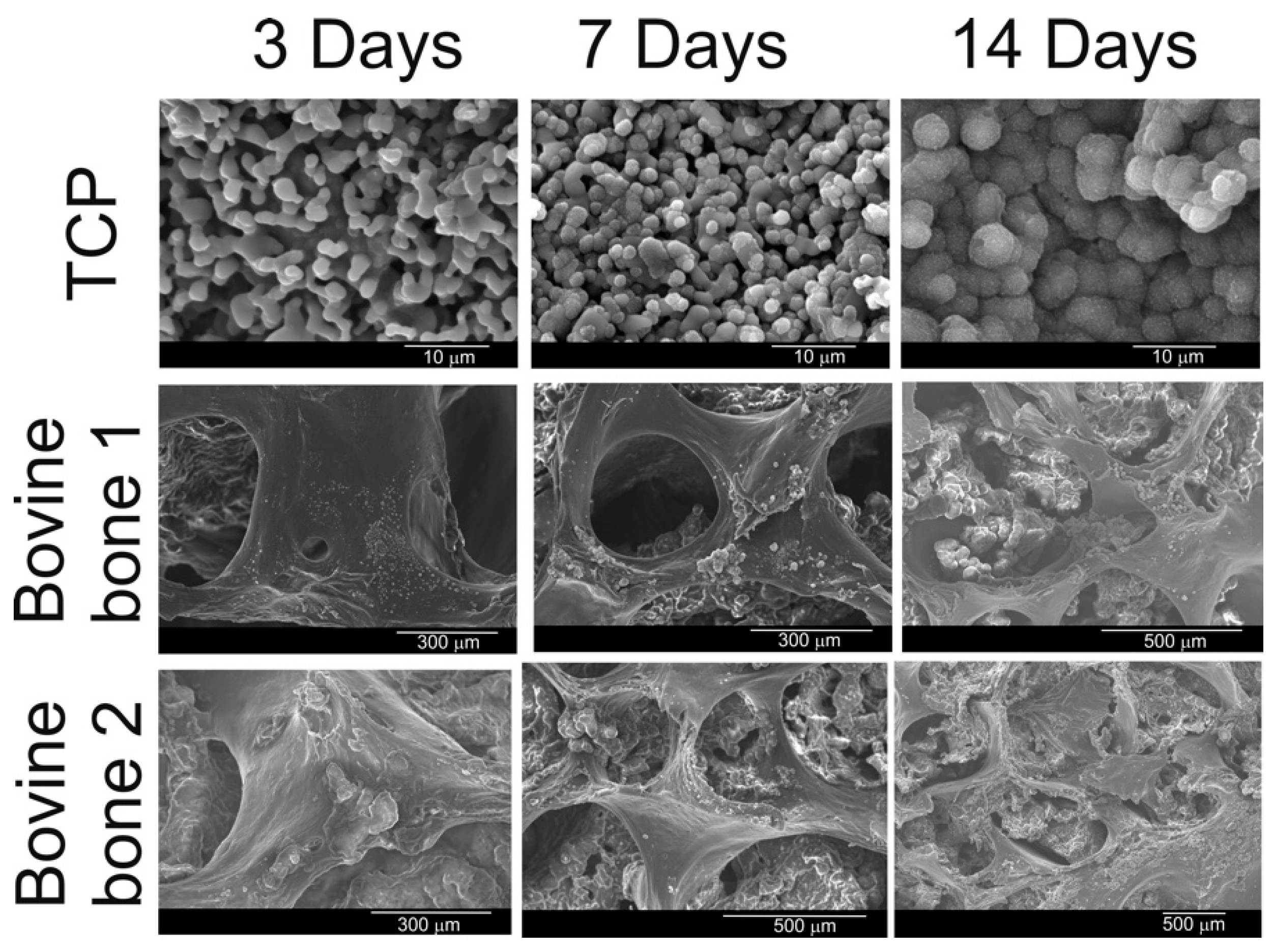

| Biomaterial | Intruded Volume (cc/g) | Total Porosity (%) | Intraparticle Porosity (%) a | Interparticle Porosity (%) b |
|---|---|---|---|---|
| Bovine bone 1 | 0.1127 | 20.21 | 15.013 | 5.1969 |
| Bovine bone 2 | 0.1119 | 19.28 | 16.29 | 2.9902 |
| Synthetic β-TCP | 0.3544 | 53.24 | 37.45 | 15.79 |
| Biomaterial | Real Density (g/cm3) | Apparent Density (g/cm3) |
|---|---|---|
| Bovine bone 1 | 2.25 | 1.79 |
| Bovine bone 2 | 2.13 | 1.72 |
| Synthetic TCP | 3.22 | 1.50 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gehrke, S.A.; Mazón, P.; Pérez-Díaz, L.; Calvo-Guirado, J.L.; Velásquez, P.; Aragoneses, J.M.; Fernández-Domínguez, M.; De Aza, P.N. Study of Two Bovine Bone Blocks (Sintered and Non-Sintered) Used for Bone Grafts: Physico-Chemical Characterization and In Vitro Bioactivity and Cellular Analysis. Materials 2019, 12, 452. https://doi.org/10.3390/ma12030452
Gehrke SA, Mazón P, Pérez-Díaz L, Calvo-Guirado JL, Velásquez P, Aragoneses JM, Fernández-Domínguez M, De Aza PN. Study of Two Bovine Bone Blocks (Sintered and Non-Sintered) Used for Bone Grafts: Physico-Chemical Characterization and In Vitro Bioactivity and Cellular Analysis. Materials. 2019; 12(3):452. https://doi.org/10.3390/ma12030452
Chicago/Turabian StyleGehrke, Sergio Alexandre, Patricia Mazón, Leticia Pérez-Díaz, José Luis Calvo-Guirado, Pablo Velásquez, Juan Manuel Aragoneses, Manuel Fernández-Domínguez, and Piedad N. De Aza. 2019. "Study of Two Bovine Bone Blocks (Sintered and Non-Sintered) Used for Bone Grafts: Physico-Chemical Characterization and In Vitro Bioactivity and Cellular Analysis" Materials 12, no. 3: 452. https://doi.org/10.3390/ma12030452
APA StyleGehrke, S. A., Mazón, P., Pérez-Díaz, L., Calvo-Guirado, J. L., Velásquez, P., Aragoneses, J. M., Fernández-Domínguez, M., & De Aza, P. N. (2019). Study of Two Bovine Bone Blocks (Sintered and Non-Sintered) Used for Bone Grafts: Physico-Chemical Characterization and In Vitro Bioactivity and Cellular Analysis. Materials, 12(3), 452. https://doi.org/10.3390/ma12030452










