Phase Transition and Metallization of Orpiment by Raman Spectroscopy, Electrical Conductivity and Theoretical Calculation under High Pressure
Abstract
1. Introduction
2. Experimental and Computational Details
2.1. Sample Description
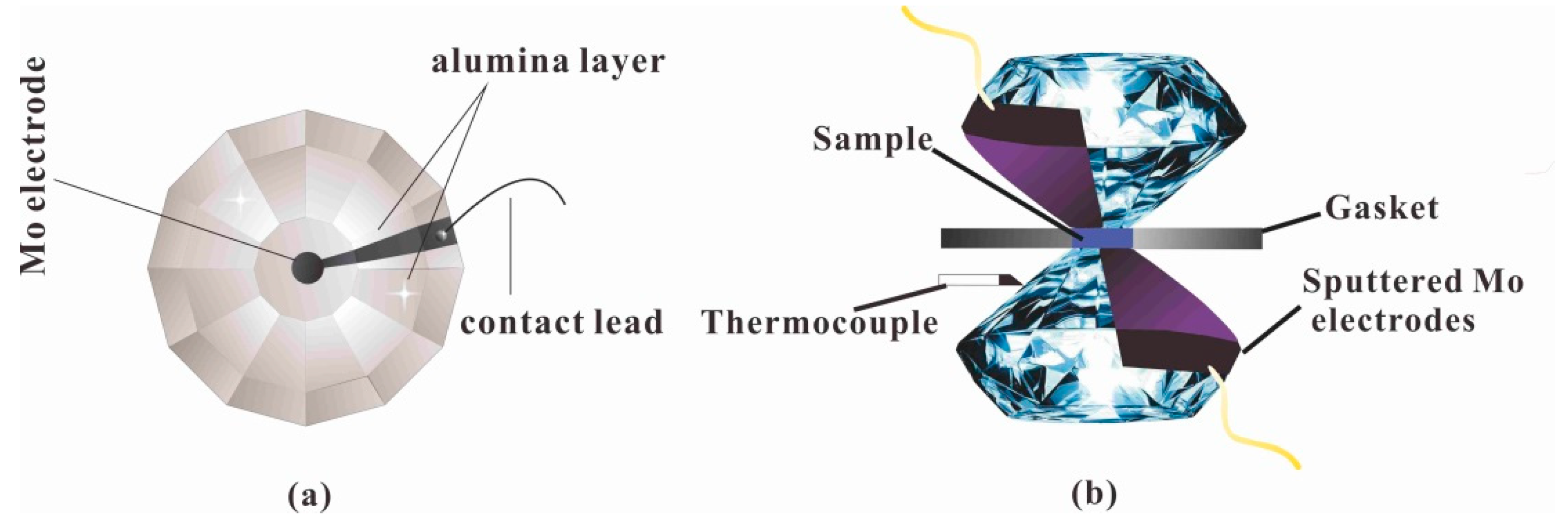
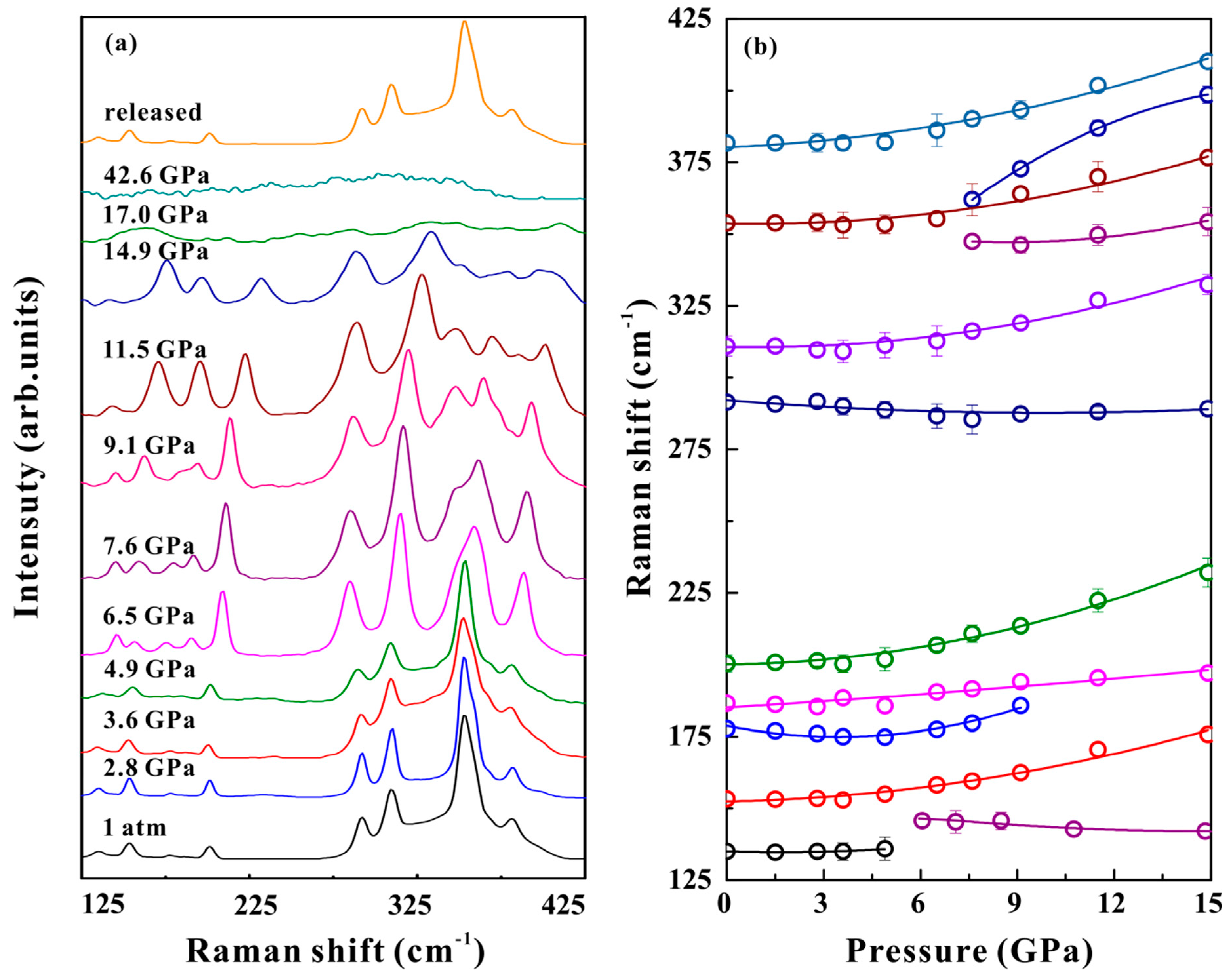
2.2. High-Pressure Raman Scattering Measurements
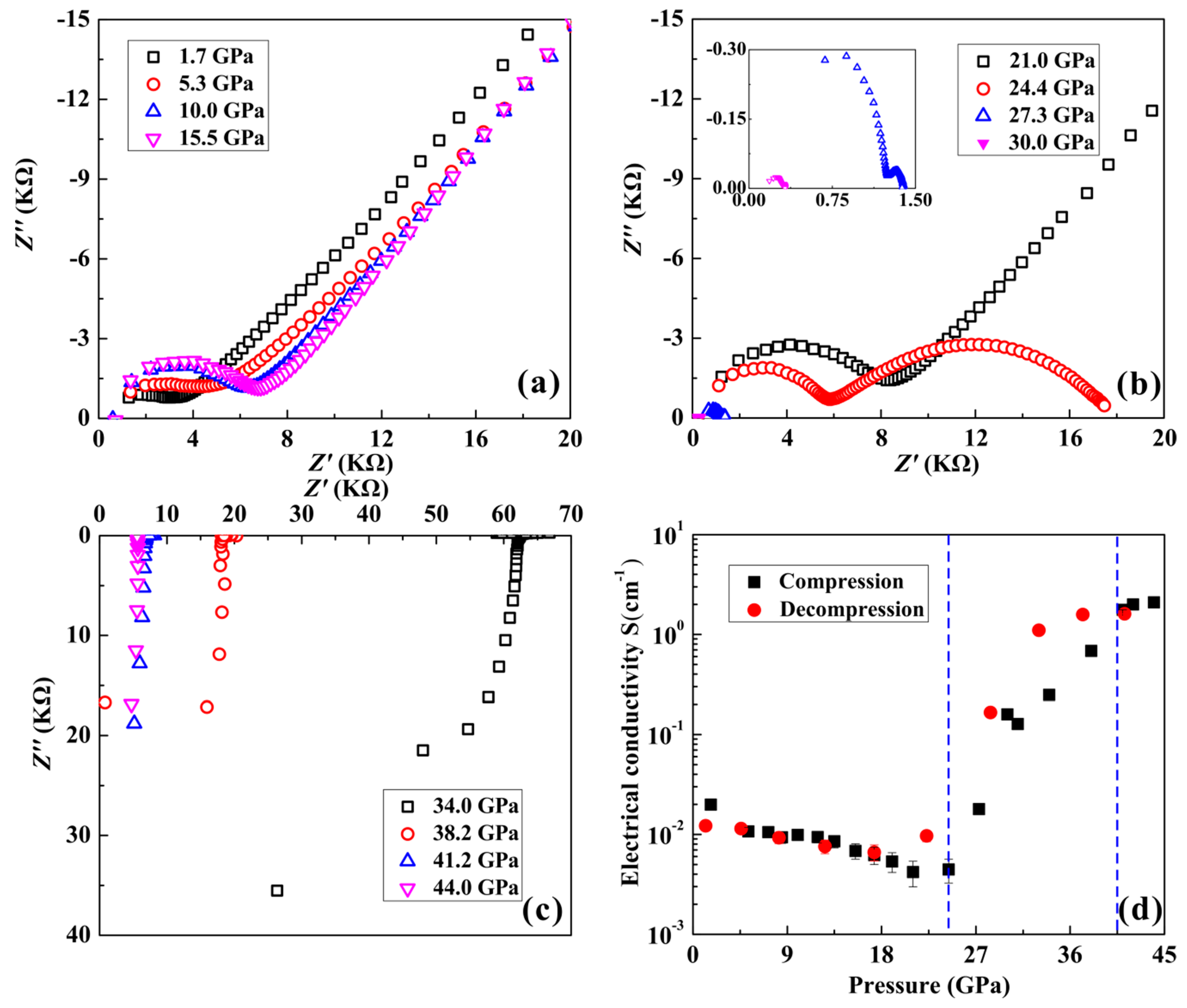
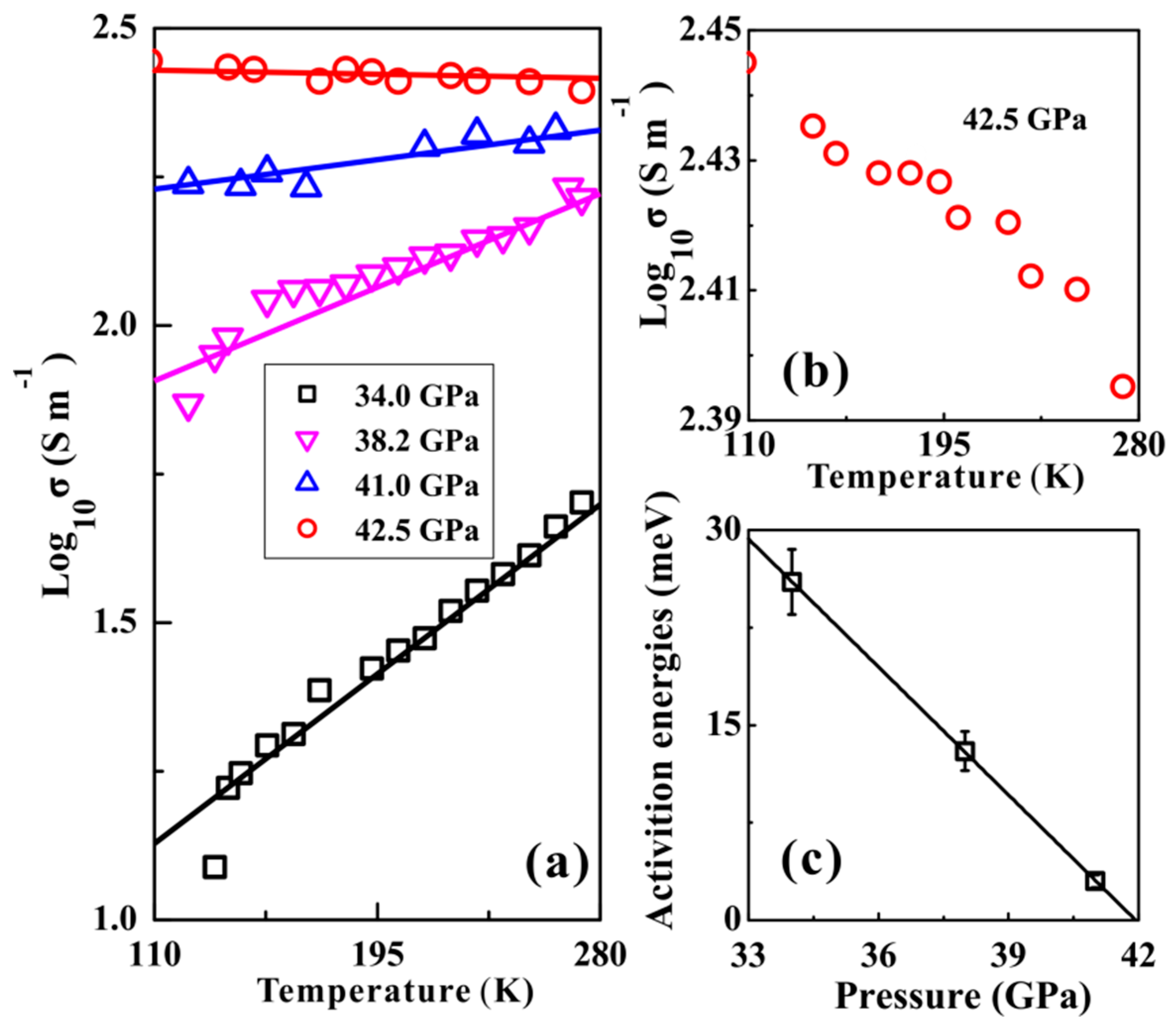
2.3. High-Pressure Conductivity Measurements
2.4. Computational Details
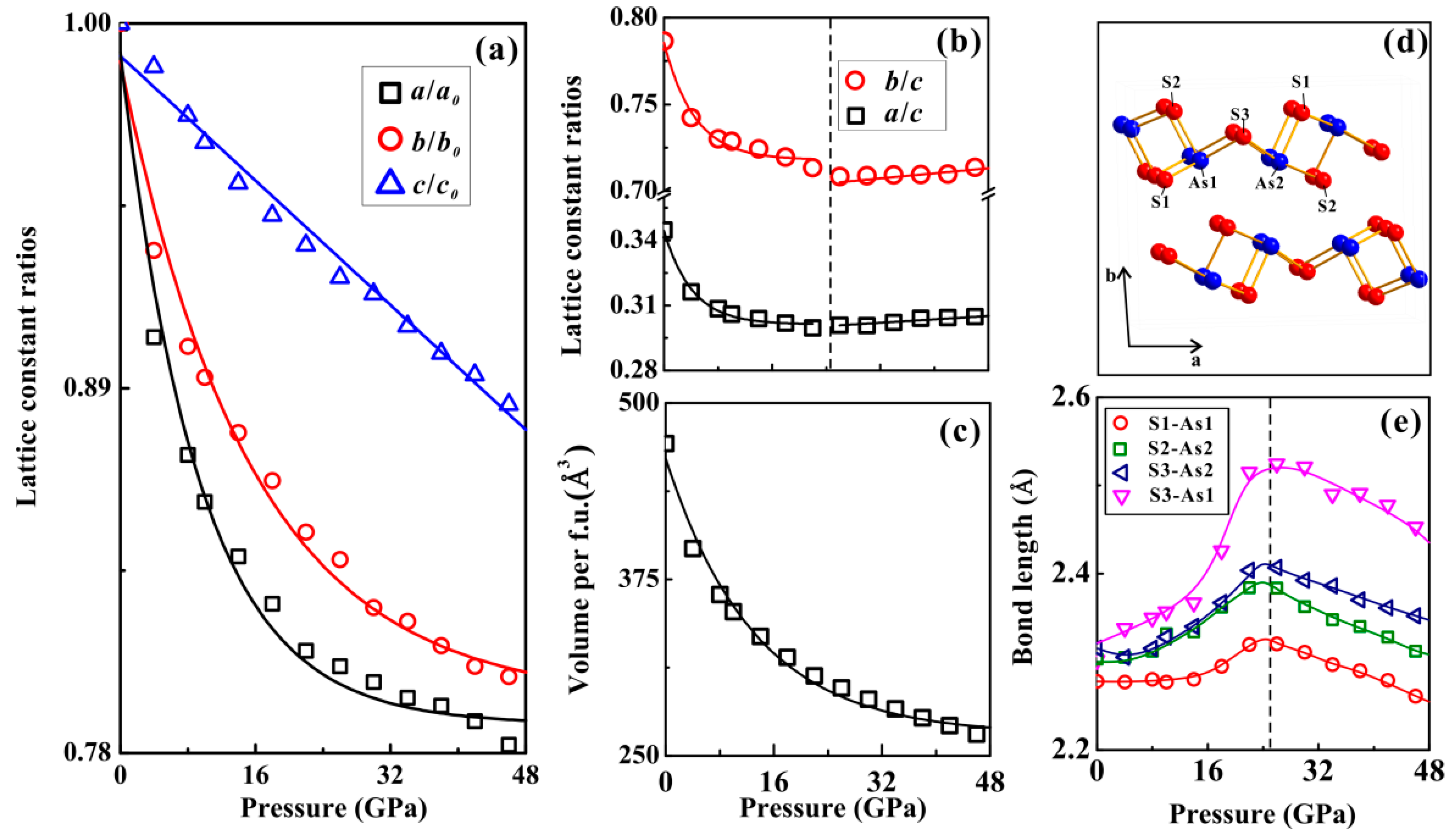
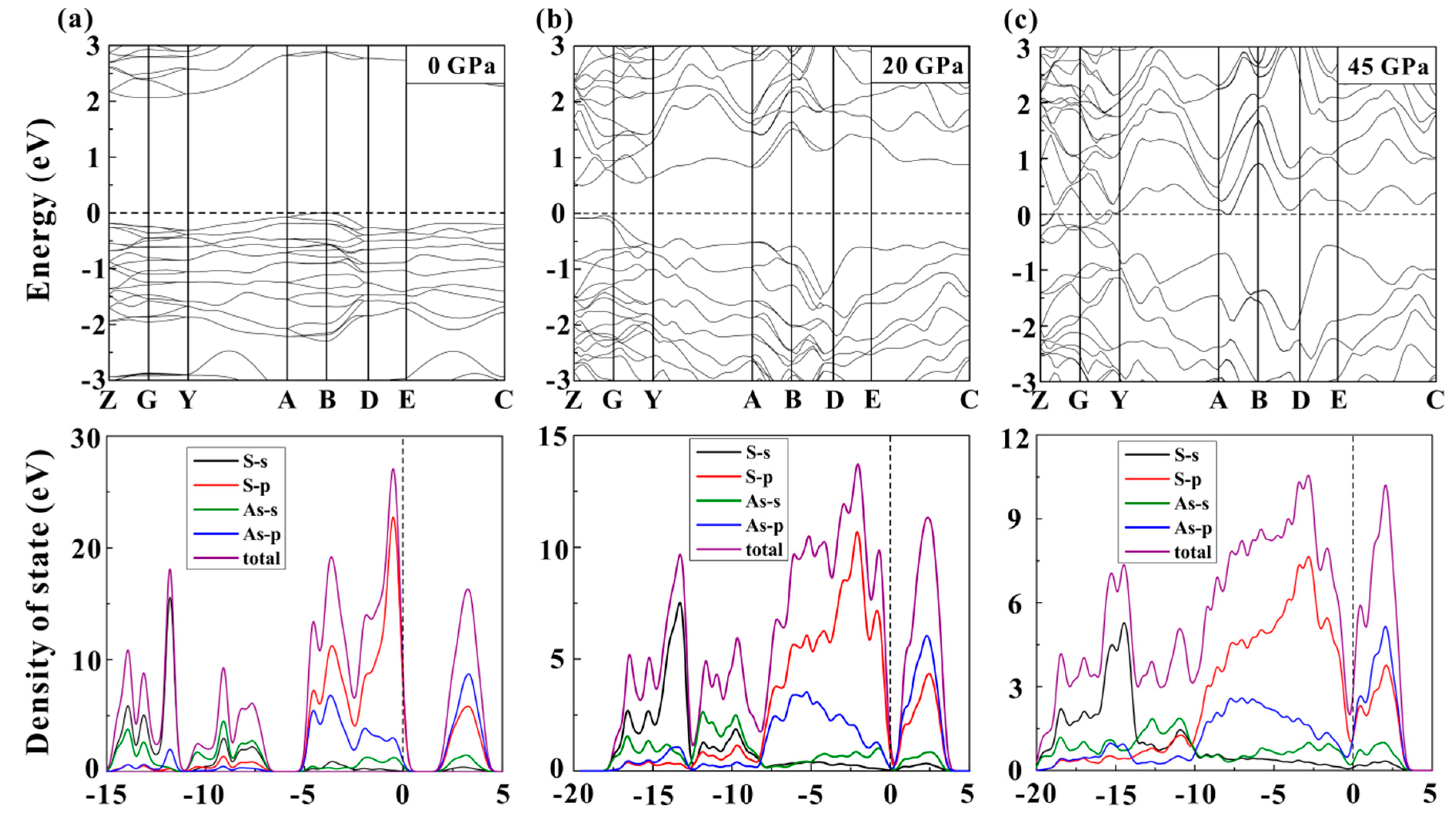
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Snyder, G.J.; Toberer, E.S. Complex thermoelectric materials. Nat. Mater. 2008, 7, 105. [Google Scholar] [CrossRef] [PubMed]
- Pawar, S.H.; Bhosale, P.N.; Uplane, M.D.; Tamhankar, S. Growth of Bi2S3 film using a solution-gas interface technique. Thin Solid Films 1983, 110, 165–170. [Google Scholar] [CrossRef]
- Hasan, M.Z.; Kane, C.L. Colloquium: Topological insulators. Rev. Mod. Phys. 2010, 82, 3045–3067. [Google Scholar] [CrossRef]
- Moore, J.E. The birth of topological insulators. Nature 2010, 464, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Debbichi, L.; Kim, H.; Bjorkman, T.; Eriksson, O.; Lebegue, S. First-principles investigation of two-dimensional trichalcogenide and sesquichalcogenide monolayers. Phys. Rev. B: Condens. Matter 2016, 93, 245307. [Google Scholar] [CrossRef]
- Asobe, M. Nonlinear optical properties of chalcogenide glass fibers and their application to all-optical switching. Opt. Fiber Technol. 1997, 3, 142–148. [Google Scholar] [CrossRef]
- Zallen, R. Effect of pressure on optical properties of crystalline As2S3. High Pressure Res. 2004, 24, 117–118. [Google Scholar] [CrossRef]
- Lavrentiev, A.A.; Gabrel’yan, B.V.; Nikiforov, I.Y.; Vorzhev, V.B. Electronic energy structure of As2S3, AsSI, AgAsS2, and TiS2 semiconductors. J. Struct. Chem. 2005, 46, 805–812. [Google Scholar] [CrossRef]
- Pu, C.; Dai, L.D.; Li, H.P.; Hu, H.Y.; Liu, K.X.; Yang, L.F.; Hong, M.L. Pressure-induced phase transitions of ZnSe under different pressure environments. AIP Adv. 2019, 9, 025004. [Google Scholar] [CrossRef]
- Yang, L.F.; Dai, L.D.; Li, H.P.; Hu, H.Y.; Liu, K.X.; Pu, C.; Hong, M.L. Pressure-induced metallization in MoSe2 under different pressure conditions. RSC Adv. 2019, 9, 5794–5803. [Google Scholar] [CrossRef]
- Dai, L.D.; Zhuang, Y.K.; Li, H.P.; Wu, L.; Hu, H.Y.; Liu, K.X.; Yang, L.F.; Pu, C. Pressure-induced irreversible amorphization and metallization with a structural phase transition in arsenic telluride. J. Mater. Chem. 2017, 5, 12157–12162. [Google Scholar] [CrossRef]
- Dai, L.D.; Liu, K.X.; Li, H.P.; Wu, L.; Hu, H.Y.; Zhuang, Y.K.; Yang, L.F.; Pu, C.; Liu, P.F. Pressure-induced irreversible metallization accompanying the phase transitions in Sb2S3. Phys. Rev. B: Condens. Matter 2018, 97, 024103. [Google Scholar] [CrossRef]
- Vilaplana, R.; Gomis, O.; Manjón, F.J.; Segura, A.; Pérez-González, E.; Rodríguez-Hernández, P.; Muñoz, A.; González, J.; Marín-Borrás, V.; Muñoz-Sanjosé, V.; et al. High-pressure vibrational and optical study of Bi2Te3. Phys. Rev. B: Condens. Matter 2011, 84, 104112. [Google Scholar] [CrossRef]
- Zhang, J.L.; Zhang, S.J.; Weng, H.M.; Zhang, W.; Yang, L.X.; Liu, Q.Q.; Feng, S.M.; Wang, X.C.; Yu, R.C.; Cao, L.Z.; et al. Pressure-induced superconductivity in topological parent compound Bi2Te3. Proc. Natl. Acad. Sci. U.S.A. 2011, 108, 24. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.K.; Liu, C.L.; Zhang, X.; Ke, F.; Han, Y.H.; Peng, G.; Ma, Y.Z.; Gao, C.X. Electronic topological transition and semiconductor-to-metal conversion of Bi2Te3 under high pressure. Appl. Phys. Lett. 2013, 103, 052102. [Google Scholar] [CrossRef]
- Kong, P.P.; Sun, F.; Xing, L.Y.; Zhu, J.; Zhang, S.J.; Li, W.M.; Liu, Q.Q.; Wang, X.C.; Feng, S.M.; Yu, X.H.; et al. Superconductivity in strong spin orbital coupling compound Sb2Se3. Sci. Rep. 2014, 4, 6679. [Google Scholar] [CrossRef] [PubMed]
- Bolotina, N.B.; Brazhkin, V.V.; Dyuzheva, T.I.; Katayama, Y.; Kulikova, L.F.; Lityagina, L.V.; Nikolaev, N.A. High-pressure polymorphism of As2S3 and new AsS2 modification with layered structure. JETP. Lett. 2013, 98, 539–543. [Google Scholar] [CrossRef]
- Besson, J.M.; Cernogora, J.; Zallen, R. Effect of pressure on optical properties of crystalline As2S3. Phys. Rev. B: Condens. Matter 1980, 22, 3866. [Google Scholar] [CrossRef]
- Andrikopoulos, K.S.; Christofilos, D.; Kourouklis, G.A.; Yannopoulos, S.N. Pressure Raman study of vibrational modes of glassy As2X3 (X:O, S). High Pressure Res. 2006, 26, 401–406. [Google Scholar] [CrossRef]
- Andrikopoulos, K.S.; Christofilos, D.; Kourouklis, G.A.; Yannopoulos, S.N. Pressure dependence of the boson peak in glassy As2S3 studied by Raman scattering. J. Non-Cryst. Solids 2006, 352, 4594–4600. [Google Scholar] [CrossRef]
- Struzhkin, V.V.; Goncharov, A.F.; Caracas, R.; Mao, H.K.; Hemley, R.J. Synchrotron infrared spectroscopy of the pressure-induced insulator-metal transitions in glassy As2S3 and As2Se3. Phys. Rev. B: Condens. Matter 2008, 77, 165133. [Google Scholar] [CrossRef]
- Efthimiopoulos, I.; Kemichick, J.; Zhou, X.; Khare, S.V.; Ikuta, D.; Wang, Y.J. High-pressure studies of Bi2S3. J. Phys. Chem. A 2014, 118, 1713–1720. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.G.; Yang, L.X.; Yu, Z.H.; Wang, Y.C.; Li, Y.; Yang, K.; Liu, Z.G.; Wang, Y. Structural phase transitions and metallized phenomena in arsenic telluride under High Pressure. Inorg. Chem. 2016, 55, 3907–3914. [Google Scholar] [CrossRef] [PubMed]
- Radescu, S.; Mujica1, A.; Rodrguez Hernandez, P.; Munoz, A.; Ibanez, J.; Sans, J.A.; Cuenca-Gotor, V.P.; Manjon, F.J. Study of the orpiment and anorpiment phases of As2S3 under pressure. J. Phys. Conf. Ser. 2017, 950, 042018. [Google Scholar] [CrossRef]
- Liu, K.X.; Dai, L.D.; Li, H.P.; Wu, L.; Hu, H.Y.; Zhuang, Y.K.; Yang, L.F.; Pu, C. Migration of impurity level reflected in the electrical conductivity variation for natural pyrite at high temperature and high pressure. Phys. Chem. Miner. 2018, 45, 85–92. [Google Scholar] [CrossRef]
- Liu, K.X.; Dai, L.D.; Li, H.P.; Wu, L.; Hu, H.Y.; Zhuang, Y.K.; Yang, L.F.; Pu, C.; Hong, M.L. Pressure-induced phase transitions for goethite investigated by Raman spectroscopy and electrical conductivity. High Pressure Res. 2019, 38, 106–116. [Google Scholar] [CrossRef]
- Yang, L.F.; Dai, L.D.; Li, H.P.; Hu, H.Y.; Zhuang, Y.K.; Liu, K.X.; Pu, C.; Hong, M.L. Pressure-induced structural phase transition and dehydration for gypsum investigated by Raman spectroscopy and electrical conductivity. Chem. Phys. Lett. 2018, 706, 151–157. [Google Scholar] [CrossRef]
- Gibbs, G.V.; Wallace, A.F.; Zallen, R.R.; Downs, T.; Ross, N.L.; Cox, D.F.; Rosso, K.M. Bond paths and van der Waals interactions in orpiment, As2S3. J. Phys. Chem. 2010, 114, 6550–6557. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.F.; Zhou, Y.; Frost, R.L. Structure comparison of orpiment and realgar by Raman spectroscopy. Spectrosc. Lett. 2017, 50, 23–29. [Google Scholar] [CrossRef]
- Roberto, F. The infrared and Raman spectra of realgar and orpiment. Am. Mineral. 1969, 54, 1062–1074. [Google Scholar]
- Mamedov, S.; Drichko, N. Characterization of 2D As2S3 crystal by Raman spectroscopy. MRS. Adv. 2018, 1, 385–390. [Google Scholar] [CrossRef]
- Dai, L.D.; Hu, H.Y.; Li, H.P.; Wu, L.; Hui, K.S.; Jiang, J.J.; Sun, W.Q. Influence of temperature, pressure, and oxygen fugacity on the electrical conductivity of dry eclogite, and geophysical implications. Geochem. Geophys. Geosyst. 2016, 17, 2394–2407. [Google Scholar] [CrossRef]
- Hu, H.Y.; Dai, L.D.; Li, H.P.; Hui, K.S.; Sun, W.Q. Influence of dehydration on the electrical conductivity of epidote and implications for high-conductivity anomalies in subduction zones. J. Geophys. Res. Solid Earth 2017, 122, 2751–2762. [Google Scholar] [CrossRef]


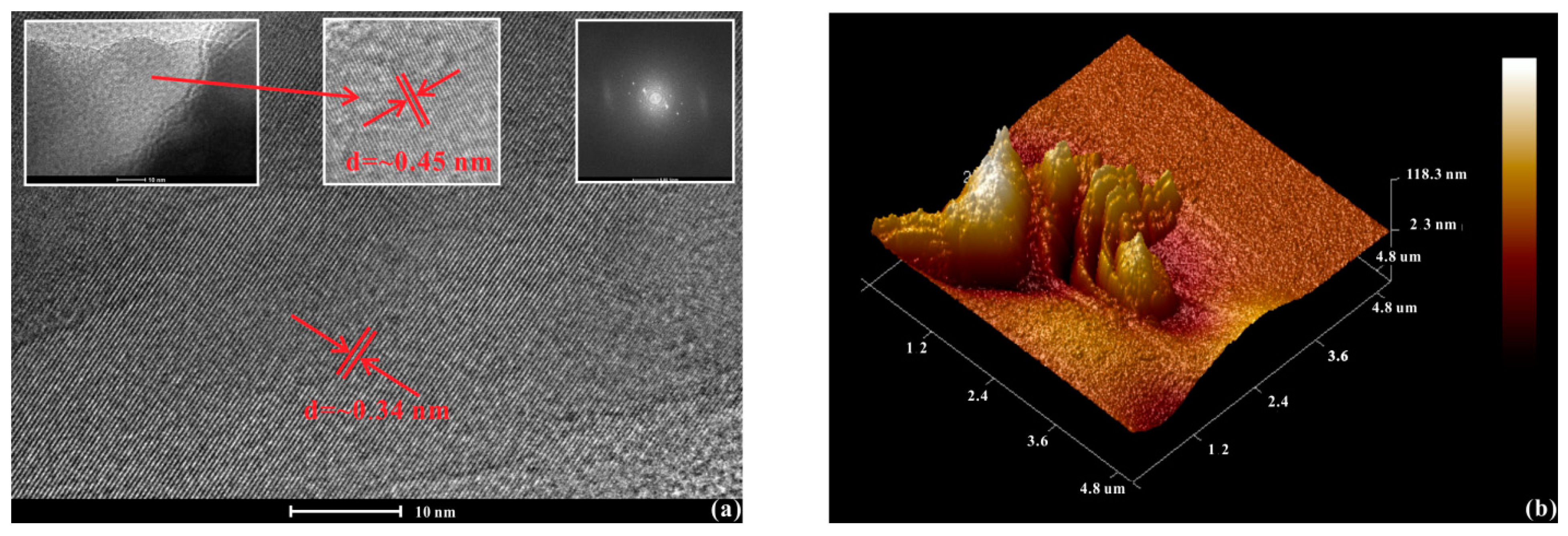
| Mode Number | ω0 (cm−1) | α (cm−1GPa−1) | β (cm−1GPa‒2) |
|---|---|---|---|
| 1 | 135 | −0.420 | 0.120 |
| 2 | 153 | 0.240 | 0.095 |
| 3 | 177 | −2.220 | 0.320 |
| 4 | 187 | 0.683 | 0.013 |
| 5 | 201 | 0.125 | 0.147 |
| 6 | 290 | −0.891 | 0.046 |
| 7 | 308 | −0.204 | 0.123 |
| 8 | 353 | −0.193 | 0.118 |
| 9 | 380 | 0.474 | 0.107 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, K.; Dai, L.; Li, H.; Hu, H.; Yang, L.; Pu, C.; Hong, M.; Liu, P. Phase Transition and Metallization of Orpiment by Raman Spectroscopy, Electrical Conductivity and Theoretical Calculation under High Pressure. Materials 2019, 12, 784. https://doi.org/10.3390/ma12050784
Liu K, Dai L, Li H, Hu H, Yang L, Pu C, Hong M, Liu P. Phase Transition and Metallization of Orpiment by Raman Spectroscopy, Electrical Conductivity and Theoretical Calculation under High Pressure. Materials. 2019; 12(5):784. https://doi.org/10.3390/ma12050784
Chicago/Turabian StyleLiu, Kaixiang, Lidong Dai, Heping Li, Haiying Hu, Linfei Yang, Chang Pu, Meiling Hong, and Pengfei Liu. 2019. "Phase Transition and Metallization of Orpiment by Raman Spectroscopy, Electrical Conductivity and Theoretical Calculation under High Pressure" Materials 12, no. 5: 784. https://doi.org/10.3390/ma12050784
APA StyleLiu, K., Dai, L., Li, H., Hu, H., Yang, L., Pu, C., Hong, M., & Liu, P. (2019). Phase Transition and Metallization of Orpiment by Raman Spectroscopy, Electrical Conductivity and Theoretical Calculation under High Pressure. Materials, 12(5), 784. https://doi.org/10.3390/ma12050784





