Study of Catalytic Combustion of Chlorobenzene and Temperature Programmed Reactions over CrCeOx/AlFe Pillared Clay Catalysts
Abstract
:1. Introduction
2. Experimental Section
2.1. AlFe-PILC and Catalyst Preparation
2.2. Characterization
2.3. Catalytic Performance Tests and Temperature-Programmed Reactions
3. Results and Discussion
3.1. Material Textural Properties
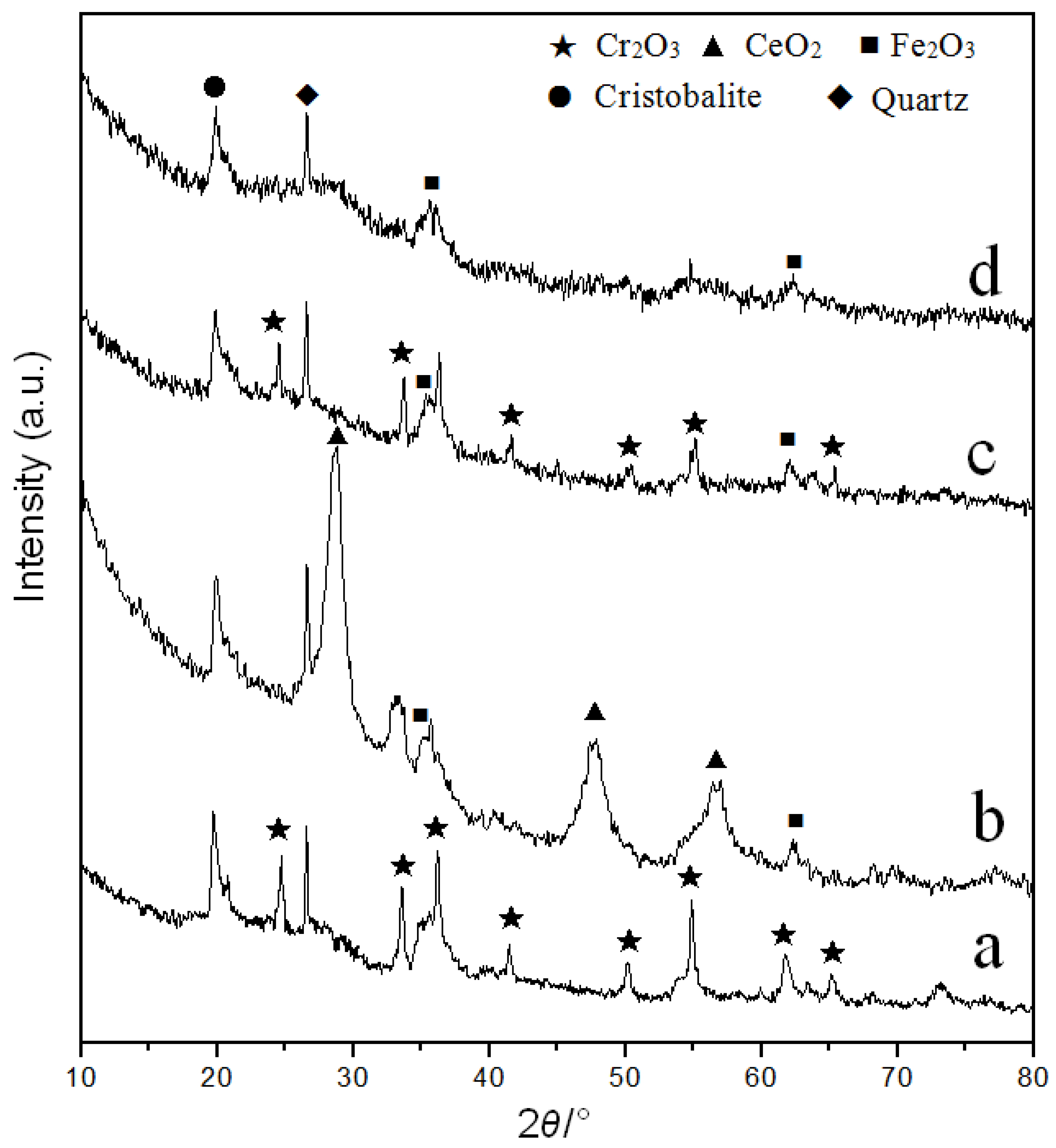
3.1.1. XRD Analysis
3.1.2. N2 Adsorption/Desorption
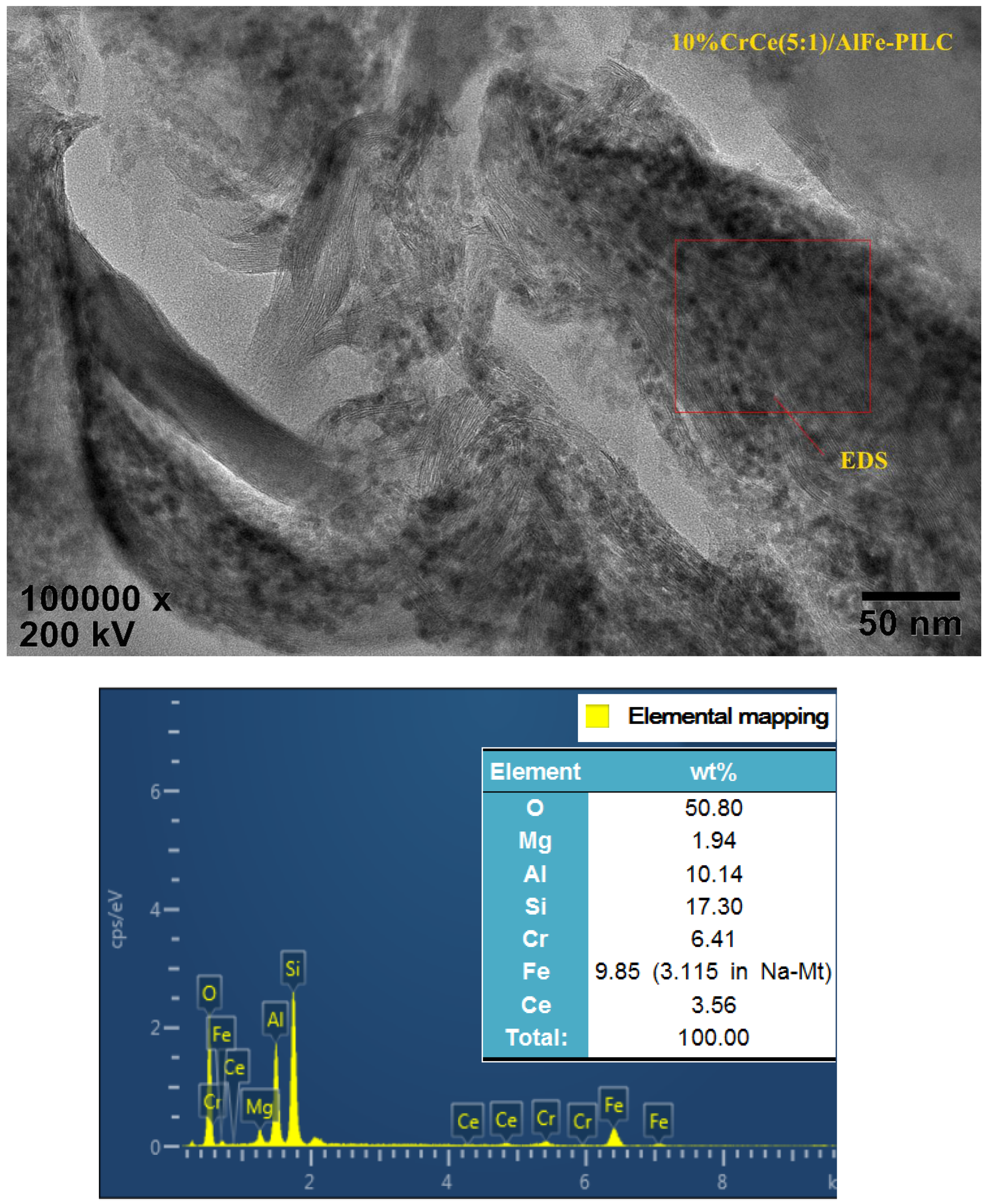
3.1.3. HRTEM Analysis
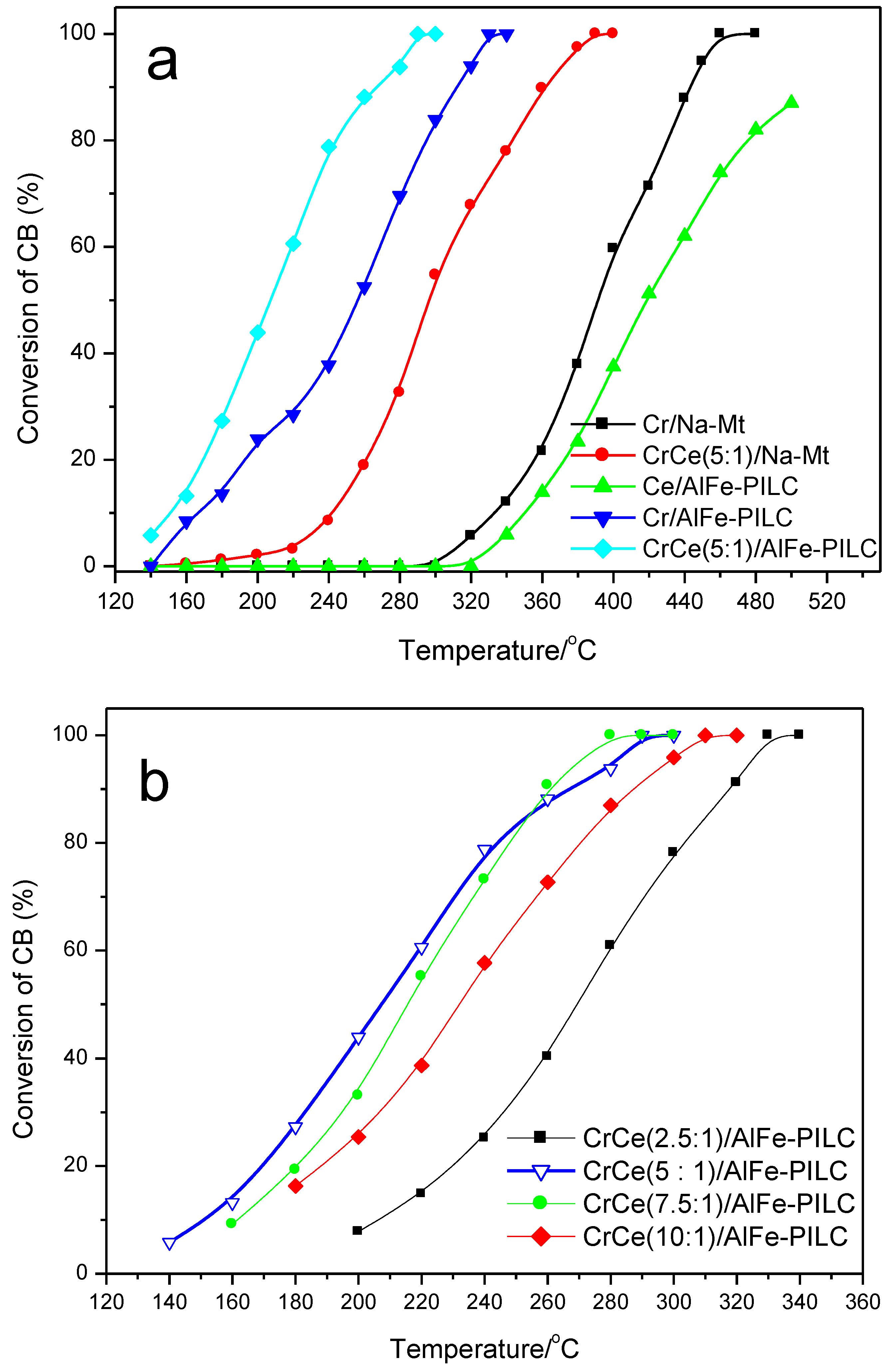
3.2. Catalytic Performance Test
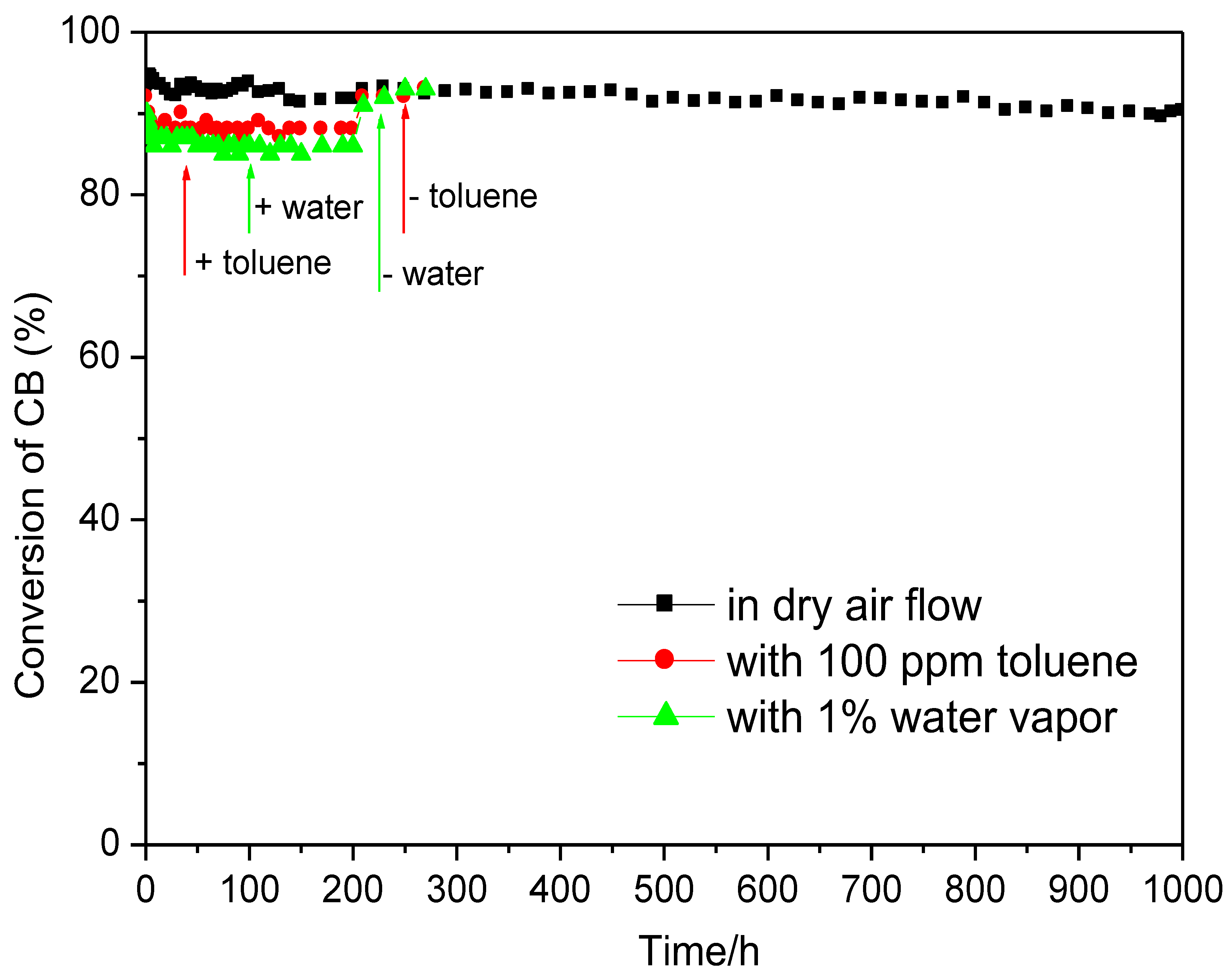
3.2.1. CB Combustion and Durability Test
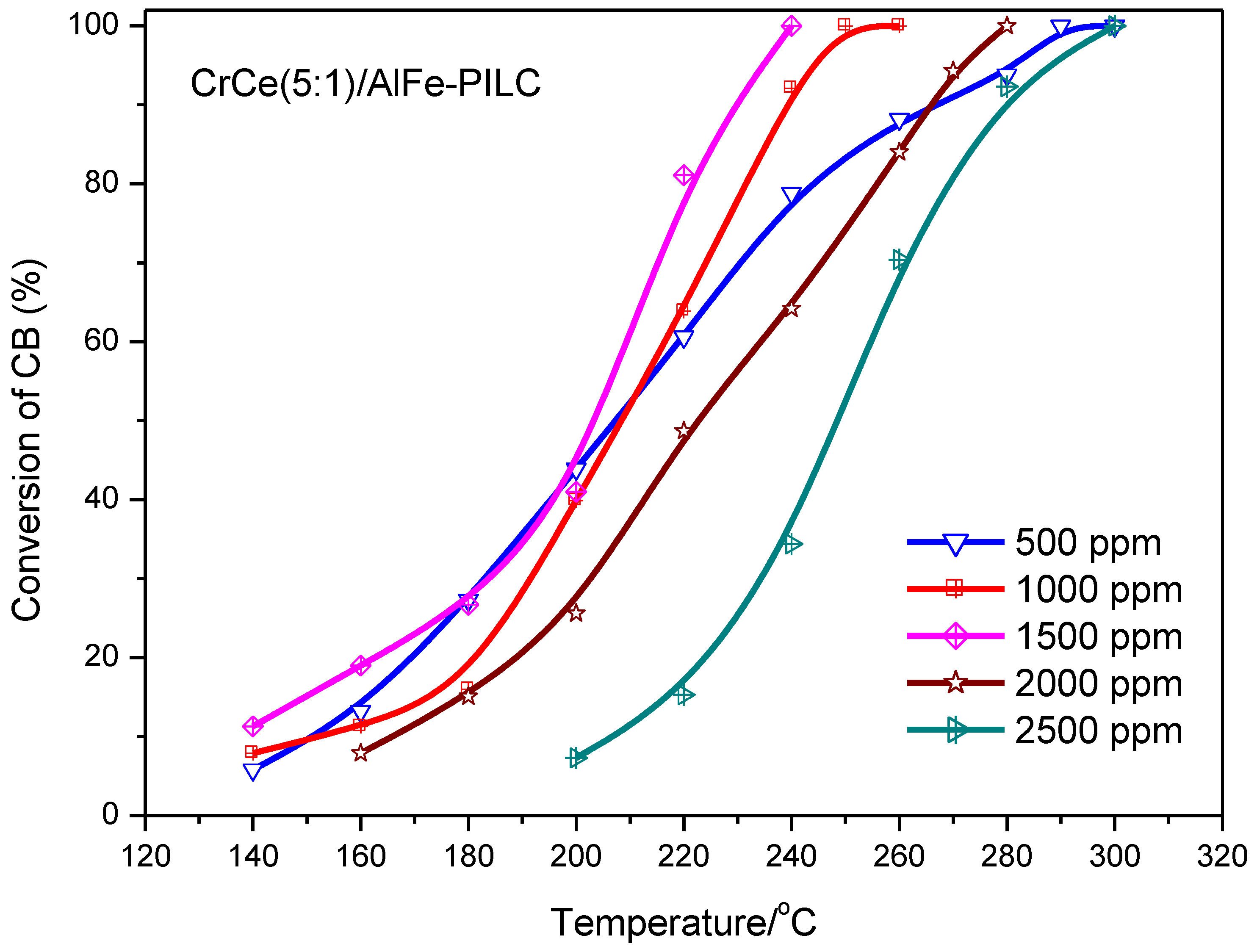
3.2.2. Effect of CB Concentration and Gas Hourly Space Velocity
3.3. Temperature-Programmed Reaction Studies
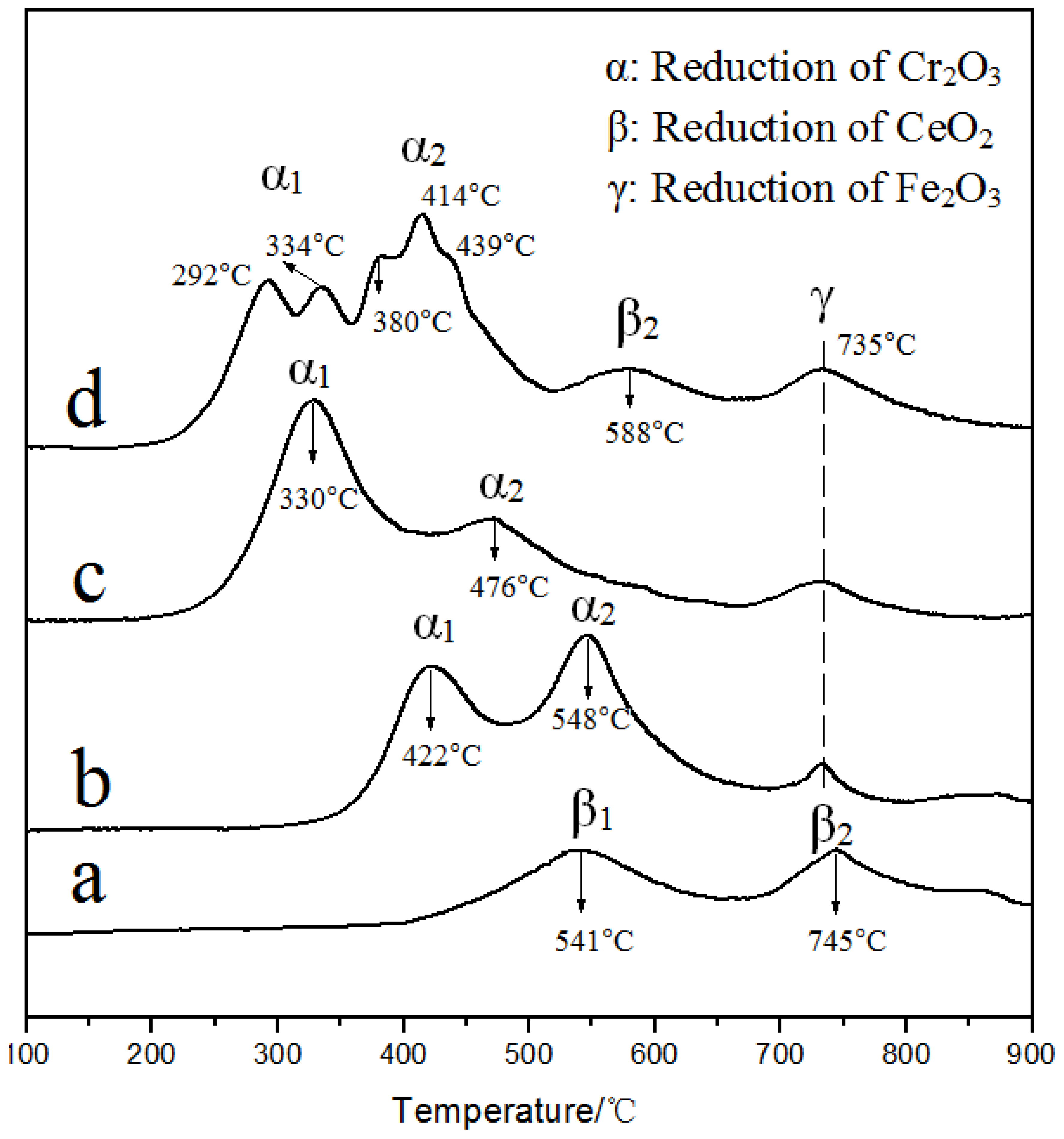
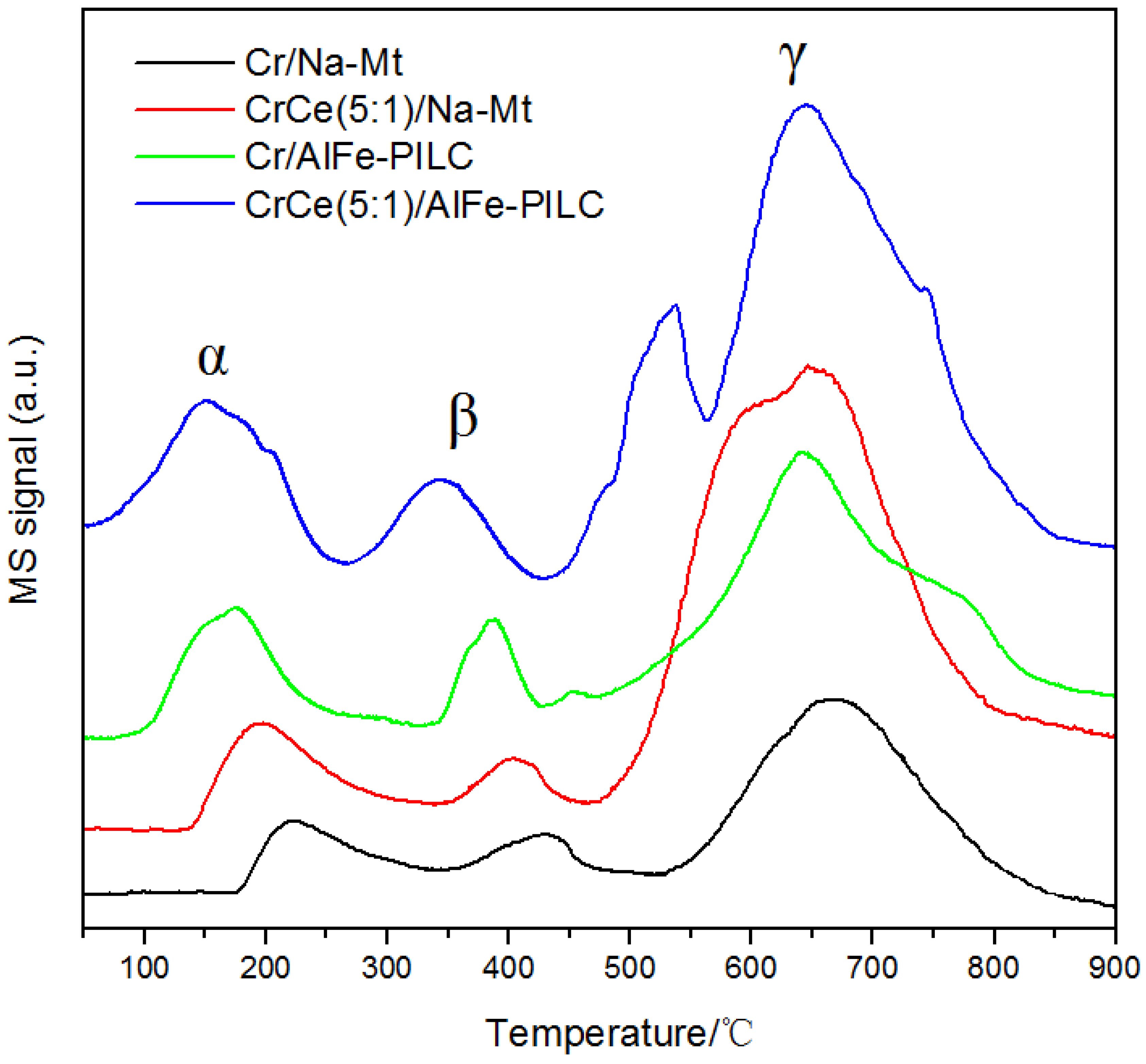
3.3.1. H2-TPR Analysis
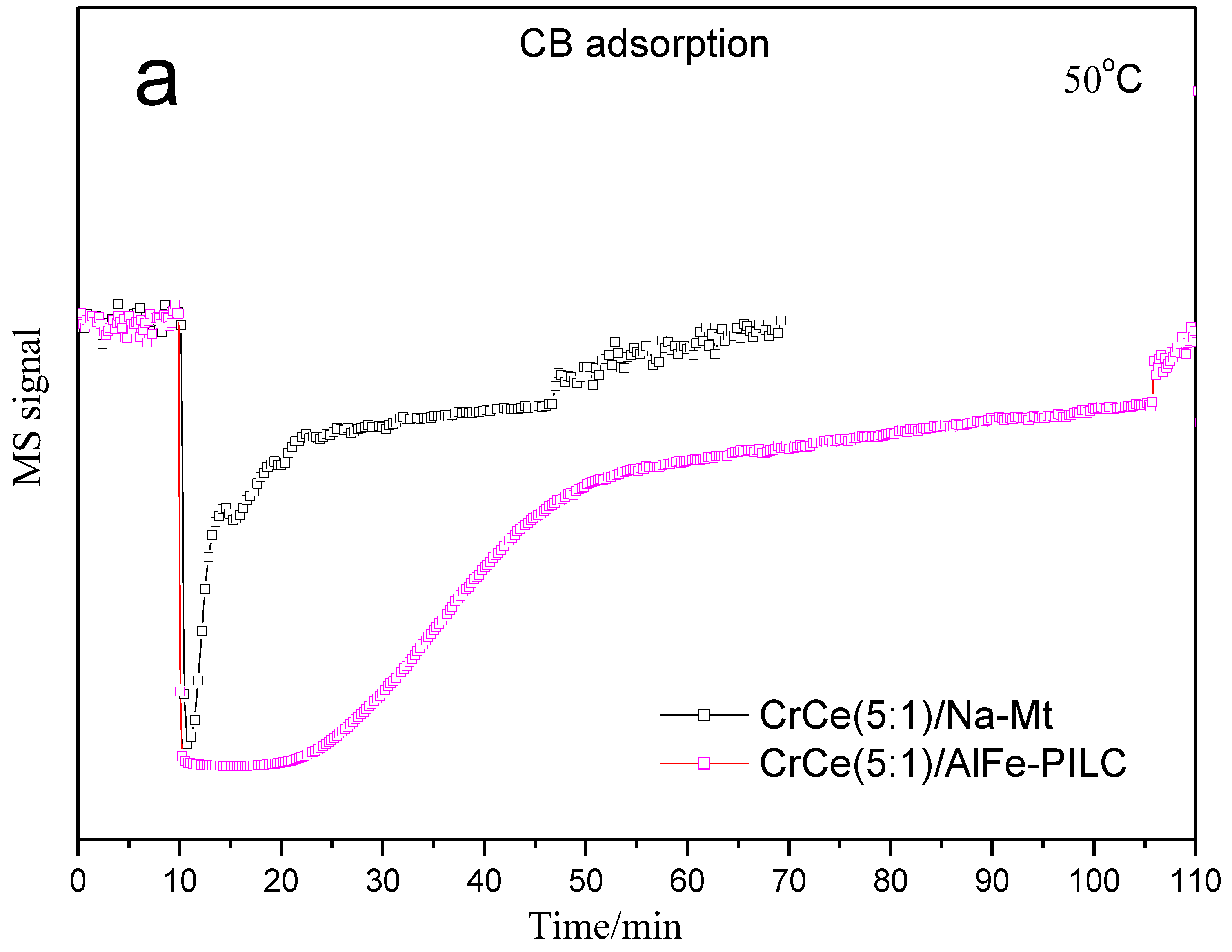
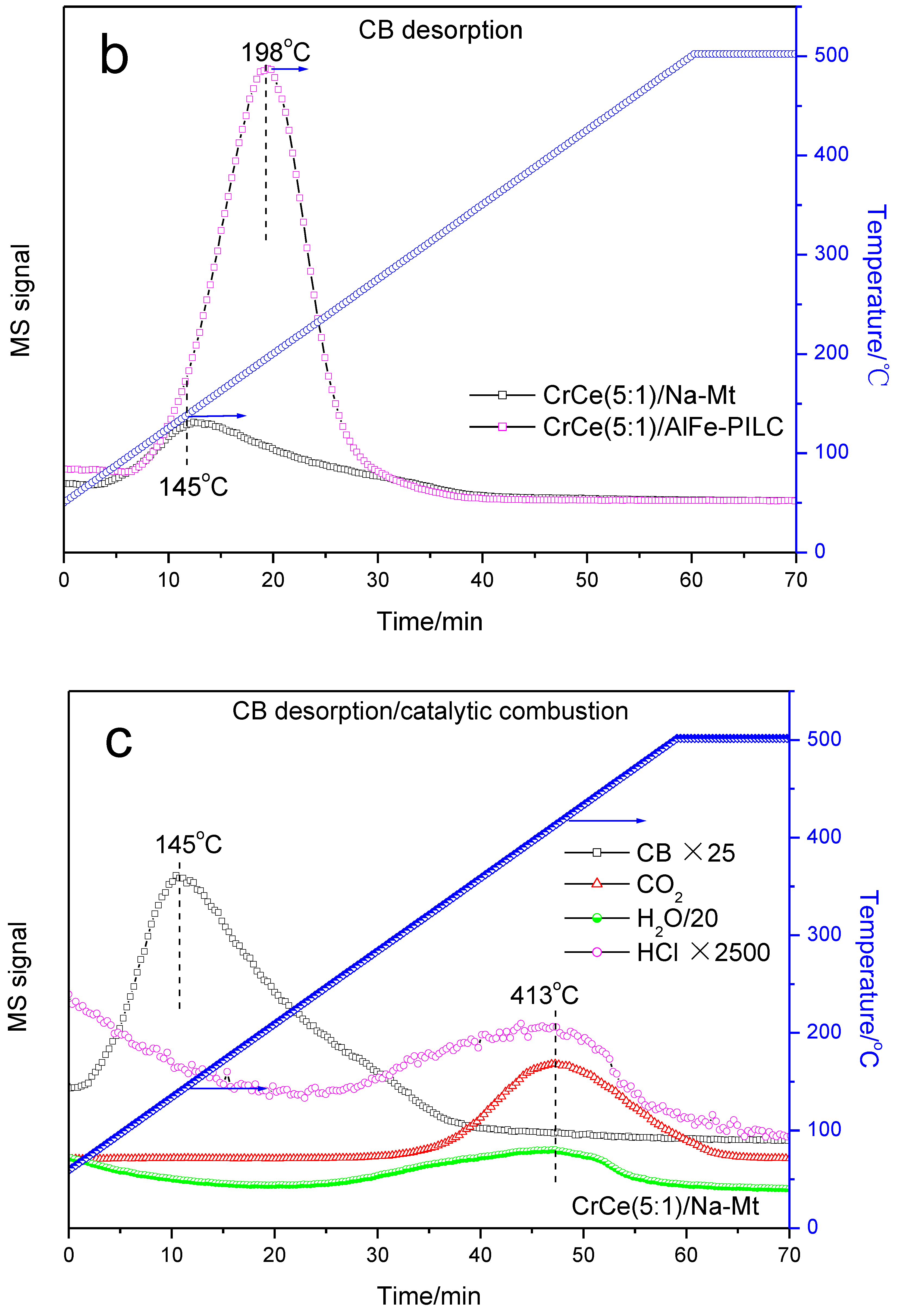
3.3.2. TPD and TPSR Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Rivas, B.; Guillén-Hurtado, N.; López-Fonseca, R.; Coloma-Pascual, F.; García-García, A.; Gutiérrez-Ortiz, J.I.; Bueno-López, A. Activity, selectivity and stability of praseodymium-doped CeO2 for chlorinated VOCs catalytic combustion. Appl. Catal. B Environ. 2012, 121, 162–170. [Google Scholar] [CrossRef]
- Weng, X.L.; Sun, P.F.; Long, Y.; Meng, Q.J.; Wu, Z.B. Catalytic Oxidation of chlorobenzene over MnxCe1-xO2/HZSM-5 catalysts: A study with practical implications. Environ. Sci. Technol. 2017, 51, 8057–8066. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.B.; Lei, C.; Wei, C.H.; Zeng, G.M. Chlorinated volatile organic compounds (Cl-VOCs) in environment—Sources, potential human health impacts, and current remediation technologies. Environ. Int. 2014, 71, 118–138. [Google Scholar] [CrossRef] [PubMed]
- Giraudon, J.M.; Nguyen, T.B.; Leclercq, G.; Siffert, S.; Lamonier, J.F.; Aboukais, A.; Vantomme, A.; Su, B.L. Chlorobenzene total oxidation over palladium supported on ZrO2, TiO2 nanostructured supports. Catal. Today 2008, 137, 379–384. [Google Scholar] [CrossRef]
- Cai, T.; Huang, H.; Deng, W.; Dai, Q.G.; Liu, W.; Wang, X.Y. Catalytic combustion of 1,2-dichlorobenzene at low temperature over Mn-modified Co3O4 catalysts. Appl. Catal. B Environ. 2015, 166, 393–405. [Google Scholar] [CrossRef]
- Cao, S.; Fei, X.Q.; Wen, Y.X.; Sun, Z.X.; Wang, H.Q.; Wu, Z.B. Bimodal mesoporous TiO2 supported Pt, Pd and Ru catalysts and their catalytic performance and deactivation mechanism for catalytic combustion of Dichloromethane (CH2Cl2). Appl. Catal. A Gen. 2018, 550, 20–27. [Google Scholar] [CrossRef]
- Huang, H.; Gu, Y.F.; Zhao, J.; Wang, X.Y. Catalytic combustion of chlorobenzene over VOx/CeO2 catalysts. J. Catal. 2015, 326, 54–68. [Google Scholar] [CrossRef]
- He, F.; Luo, J.Q.; Liu, S.T. Novel metal loaded KIT-6 catalysts and their applications in the catalytic combustion of chlorobenzene. Chem. Eng. J. 2016, 294, 362–370. [Google Scholar] [CrossRef]
- Huang, H.; Dai, Q.G.; Wang, X.Y. Morphology effect of Ru/CeO2 catalysts for the catalytic combustion of chlorobenzene. Appl. Catal. B Environ. 2014, 158, 96–105. [Google Scholar] [CrossRef]
- Gannoun, C.; Turki, A.; Kochkar, H.; Delaigle, R.; Eloy, P.; Ghorbel, A.; Gaigneaux, E.M. Elaboration and characterization of sulfated and unsulfated V2O5/TiO2 nanotubes catalysts for chlorobenzene total oxidation. Appl. Catal. B Environ. 2014, 147, 58–64. [Google Scholar] [CrossRef]
- Scirè, S.; Minicò, S.; Crisafulli, C. Pt catalysts supported on H-type zeolites for the catalytic combustion of chlorobenzene. Appl. Catal. B Environ. 2003, 45, 117–125. [Google Scholar] [CrossRef]
- Zhao, W.; Cheng, J.; Wang, L.; Chu, J.L.; Qu, J.K.; Liu, Y.H.; Li, S.H.; Zhang, H.; Wang, J.C.; Hao, Z.P.; Qi, T. Catalytic combustion of chlorobenzene on the Ln modified Co/HMS. Appl. Catal. B Environ. 2012, 127, 246–254. [Google Scholar] [CrossRef]
- Shao, Y.; Xu, Z.Y.; Wan, H.Q.; Chen, H.; Liu, F.L.; Li, L.Y.; Zheng, S.R. Influence of ZrO2 properties on catalytic hydrodechlorination of chlorobenzene over Pd/ZrO2 catalysts. J. Hazard. Mater. 2010, 179, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.G.; Bai, S.X.; Wang, J.W.; Li, M.; Wang, X.Y.; Lu, G.Z. The effect of TiO2 doping on catalytic performances of Ru/CeO2 catalysts during catalytic combustion of chlorobenzene. Appl. Catal. B Environ. 2013, 142, 222–233. [Google Scholar] [CrossRef]
- Gu, Y.L.; Yang, Y.X.; Qiu, Y.M.; Sun, K.P.; Xu, X.L. Combustion of dichloromethane using copper–manganese oxides supported on zirconium modified titanium-aluminum catalysts. Catal. Commun. 2010, 12, 277–281. [Google Scholar] [CrossRef]
- Sun, P.F.; Wang, W.L.; Dai, X.X.; Weng, X.L.; Wu, Z.B. Mechanism study on catalytic oxidation of chlorobenzene over MnxCe1-xO2/H-ZSM5 catalysts under dry and humid conditions. Appl. Catal. B Environ. 2016, 198, 389–397. [Google Scholar] [CrossRef]
- He, C.; Xu, B.T.; Shi, J.W.; Qiao, N.L.; Hao, Z.P.; Zhao, J.L. Catalytic destruction of chlorobenzene over mesoporous ACeOx (A = Co, Cu, Fe, Mn, or Zr) composites prepared by inorganic metal precursor spontaneous precipitation. Fuel Process. Technol. 2015, 130, 179–187. [Google Scholar] [CrossRef]
- Debecker, D.P.; Bertinchamps, F.; Blangenois, N.; Eloy, P.; Gaigneaux, E.M. On the impact of the choice of model VOC in the evaluation of V-based catalysts for the total oxidation of dioxins: Furan vs. chlorobenzene. Appl. Catal. B Environ. 2007, 74, 223–232. [Google Scholar] [CrossRef]
- Hetrick, C.E.; Lichtenberger, J.; Amiridis, M.D. Catalytic oxidation of chlorophenol over V2O5/TiO2 catalysts. Appl. Catal. B Environ. 2008, 77, 255–263. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Liu, X.L.; Zeng, J.L.; Guo, Y.Y.; Zhu, T.Y. Kinetics and mechanism study on catalytic oxidation of chlorobenzene over V2O5/TiO2 catalysts. J. Mol. Catal. A Chem. 2015, 402, 1–9. [Google Scholar] [CrossRef]
- Choi, K.H.; Shokouhimehr, M.; Sung, Y.E. Heterogeneous Suzuki cross-coupling reaction catalyzed by magnetically recyclable nanocatalyst. Bull. Korean Chem. Soc. 2013, 34, 1477–1480. [Google Scholar] [CrossRef]
- Shokouhimehr, M.; Asl, M.S.; Mazinani, B. Modulated large-pore mesoporous silica as an efficient base catalyst for the Henry reaction. Res. Chem. Intermed. 2018, 44, 1617–1626. [Google Scholar] [CrossRef]
- Shokouhimehr, M.; Hong, K.; Lee, T.H.; Moon, C.W.; Hong, S.P.; Zhang, K.Q.; Suh, J.M.; Choi, K.S.; Varma, R.S.; Jang, H.W. Magnetically retrievable nanocomposite adorned with Pd nanocatalysts: Efficient reduction of nitroaromatics in aqueous media. Green Chem. 2018, 20, 3809–3817. [Google Scholar] [CrossRef]
- Zhang, K.Q.; Suh, J.M.; Choi, J.W.; Jang, H.W.; Shokouhimehr, M.; Varma, R.S. Recent advances in the nanocatalyst-assisted NaBH4 reduction of nitroaromatics in Water. ACS Omega 2019, 4, 483–495. [Google Scholar] [CrossRef]
- Shokouhimehr, M. Magnetically separable and sustainable nanostructured catalysts for heterogeneous reduction of nitroaromatics. Catalysts 2015, 5, 534–560. [Google Scholar] [CrossRef]
- Li, D.; Li, C.S.; Suzuki, K.Z. Catalytic oxidation of VOCs over Al- and Fe-pillared montmorillonite. Appl. Clay Sci. 2013, 77, 56–60. [Google Scholar] [CrossRef]
- Aznárez, A.; Korili, S.A.; Gil, A. The promoting effect of cerium on the characteristics and catalytic performance of palladium supported on alumina pillared clays for the combustion of propene. Appl. Catal. A Gen. 2014, 474, 95–99. [Google Scholar] [CrossRef]
- Aznárez, A.; Delaigle, R.; Eloy, P.; Gaigneaux, E.M.; Korili, S.A.; Gil, A. Catalysts based on pillared clays for the oxidation of chlorobenzene. Catal. Today 2015, 246, 15–27. [Google Scholar] [CrossRef]
- Vicente, M.A.; Gil, A.; Bergaya, F. Pillared Clays and Clay Minerals. In Developments in Clay Science 5A; Elsevier Science: Amsterdam, The Netherlands, 2013; Chapter 10.5; pp. 523–557. [Google Scholar]
- Zuo, S.F.; Yang, P.; Wang, X.Q. Efficient and environmentally friendly synthesis of AlFe-PILC-supported MnCe catalysts for benzene combustion. ACS Omega 2017, 2, 5179–5186. [Google Scholar] [CrossRef]
- Zuo, S.F.; Ding, M.L.; Tong, J.; Feng, L.C.; Qi, C.Z. Study on the preparation and characterization of a titanium-pillared clay-supported CrCe catalyst and its application to the degradation of a low concentration of chlorobenzene. Appl. Clay Sci. 2015, 105, 118–123. [Google Scholar] [CrossRef]
- Cheng, Z.; Chen, Z.; Li, J.R.; Zuo, S.F.; Yang, P. Mesoporous silica-pillared clays supported nanosized Co3O4-CeO2 for catalytic combustion of toluene. Appl. Surf. Sci. 2018, 459, 32–39. [Google Scholar] [CrossRef]
- Booij, E.; Kloprogge, J.T.; van Veen, J.R. Large pore REE/Al pillared bentonites: Preparation, structural aspects and catalytic properties. Appl. Clay Sci. 1996, 11, 155–162. [Google Scholar] [CrossRef]
- He, C.; Yu, Y.K.; Shen, Q.; Chen, J.S.; Qiao, N.L. Catalytic behavior and synergistic effect of nanostructured mesoporous CuO-MnOx-CeO2 catalysts for chlorobenzene destruction. Appl. Surf. Sci. 2014, 297, 59–69. [Google Scholar] [CrossRef]
- Basińska, A.; Jóźwiak, W.K.; Góralski, J.; Domka, F. The behaviour of Ru/Fe2O3 catalysts and Fe2O3 supports in the TPR and TPO conditions. Appl. Catal. A Gen. 2000, 190, 107–115. [Google Scholar] [CrossRef]
- Durán, F.G.; Barbero, B.P.; Cadús, L.E.; Rojas, C.; Centeno, M.A.; Odriozola, J.A. Manganese and iron oxides as combustion catalysts of volatile organic compounds. Appl. Catal. B Environ. 2009, 92, 194–201. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, R.J.; Yang, X.X. Simultaneous catalytic removal of NOx and diesel soot particulates over La1-xCexNiO3 perovskite oxide catalysts. Catal. Commun. 2009, 10, 1029–1033. [Google Scholar] [CrossRef]
- Wang, X.Y.; Zuo, J.C.; Luo, Y.J.; Jiang, L.L. New route to CeO2/LaCoO3 with high oxygen mobility for total benzene oxidation. Appl. Surf. Sci. 2017, 396, 95–101. [Google Scholar] [CrossRef]
- Lambrou, P.S.; Efstathiou, A.M. The effects of Fe on the oxygen storage and release properties of model Pd–Rh/CeO2–Al2O3 three-way catalyst. J. Catal. 2006, 240, 182–193. [Google Scholar] [CrossRef]
- Feng, Y.J.; Li, L.; Niu, S.F.; Qu, Y.; Zhang, Q.; Li, Y.S.; Zhao, W.R.; Li, H.; Shi, J.L. Controlled synthesis of highly active mesoporous Co3O4 polycrystals for low temperature CO oxidation. Appl. Catal. B Environ. 2012, 111, 461–466. [Google Scholar] [CrossRef]




| Samples | SBET (m2/g) | Amesa (m2/g) | Vpb (cm3/g) | Vmicc (cm3/g) |
|---|---|---|---|---|
| Na-Mt | 51 | 41 | 0.076 | 0.0043 |
| CrCe(5:1)/Na-Mt | 22 | 22 | 0.058 | - |
| AlFe-PILC | 318 | 168 | 0.195 | 0.077 |
| Cr/AlFe-PILC | 221 | 71 | 0.165 | 0.066 |
| CrCe(5:1)/AlFe-PILC | 13 | 82 | 0.156 | 0.026 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, Y.; Ye, N.; Situ, D.; Zuo, S.; Wang, X. Study of Catalytic Combustion of Chlorobenzene and Temperature Programmed Reactions over CrCeOx/AlFe Pillared Clay Catalysts. Materials 2019, 12, 728. https://doi.org/10.3390/ma12050728
Qiu Y, Ye N, Situ D, Zuo S, Wang X. Study of Catalytic Combustion of Chlorobenzene and Temperature Programmed Reactions over CrCeOx/AlFe Pillared Clay Catalysts. Materials. 2019; 12(5):728. https://doi.org/10.3390/ma12050728
Chicago/Turabian StyleQiu, Yingnan, Na Ye, Danna Situ, Shufeng Zuo, and Xianqin Wang. 2019. "Study of Catalytic Combustion of Chlorobenzene and Temperature Programmed Reactions over CrCeOx/AlFe Pillared Clay Catalysts" Materials 12, no. 5: 728. https://doi.org/10.3390/ma12050728
APA StyleQiu, Y., Ye, N., Situ, D., Zuo, S., & Wang, X. (2019). Study of Catalytic Combustion of Chlorobenzene and Temperature Programmed Reactions over CrCeOx/AlFe Pillared Clay Catalysts. Materials, 12(5), 728. https://doi.org/10.3390/ma12050728





