Corrosion Characteristics of Copper-Added Austempered Gray Cast Iron (AGCI)
Abstract
:1. Introduction
2. Materials and Methods
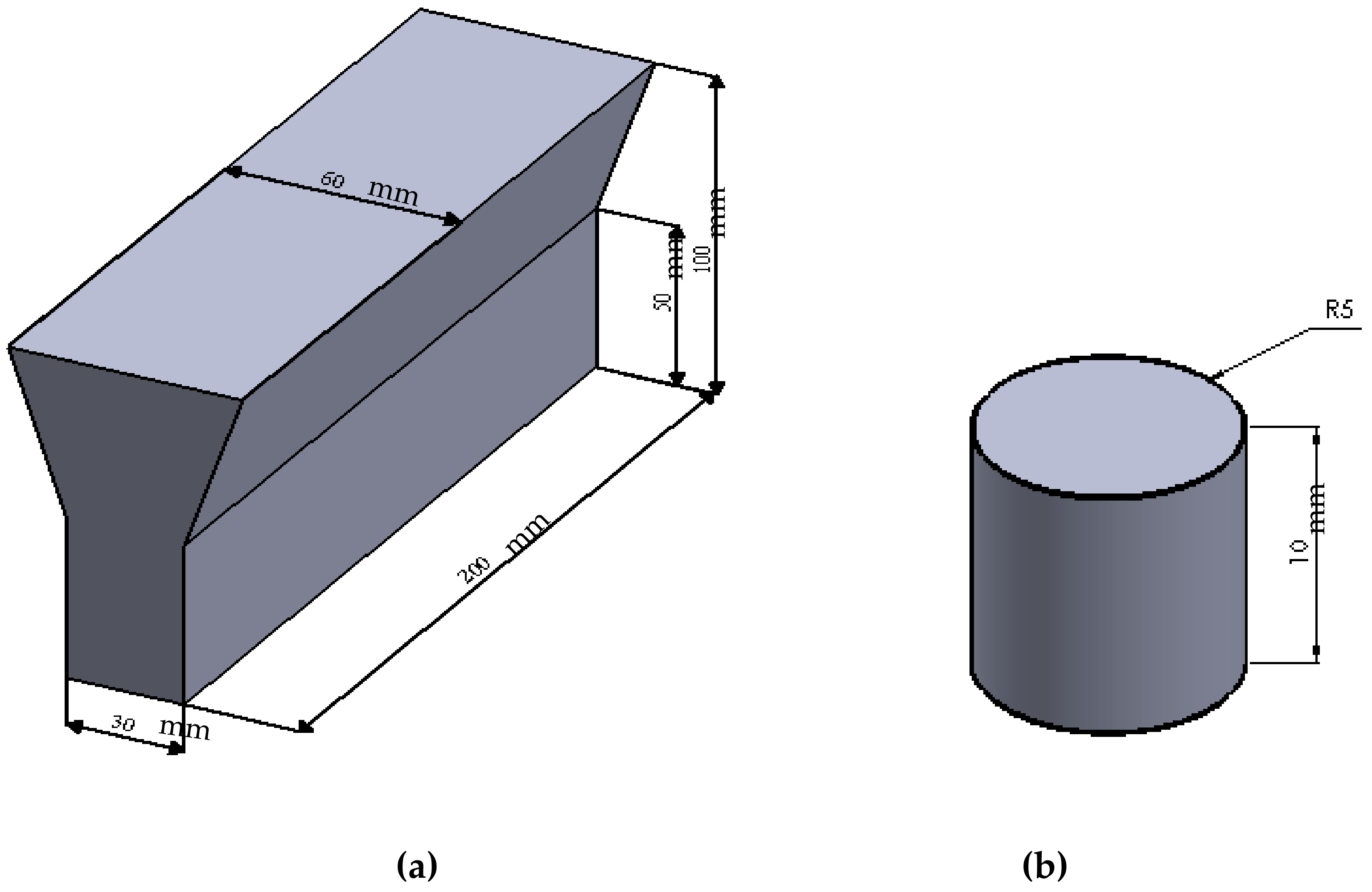
2.1. Sample Preparation
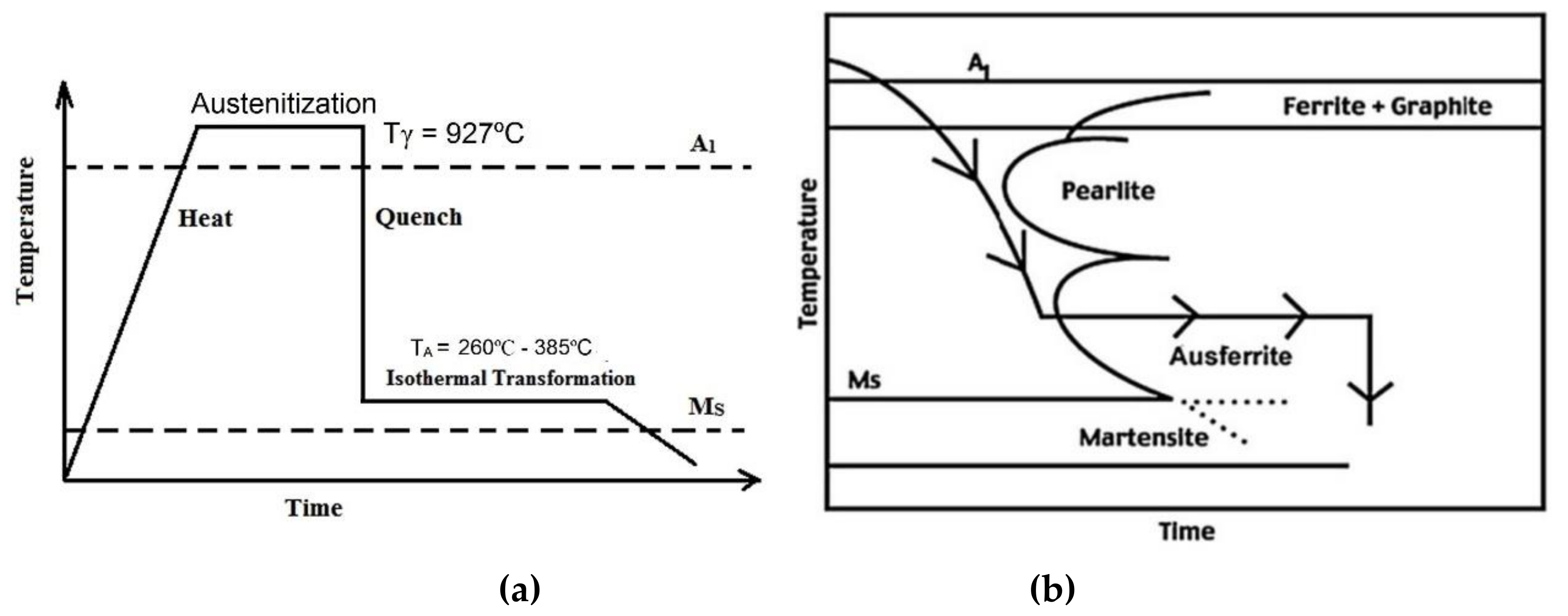
2.2. Heat Treatment of Samples
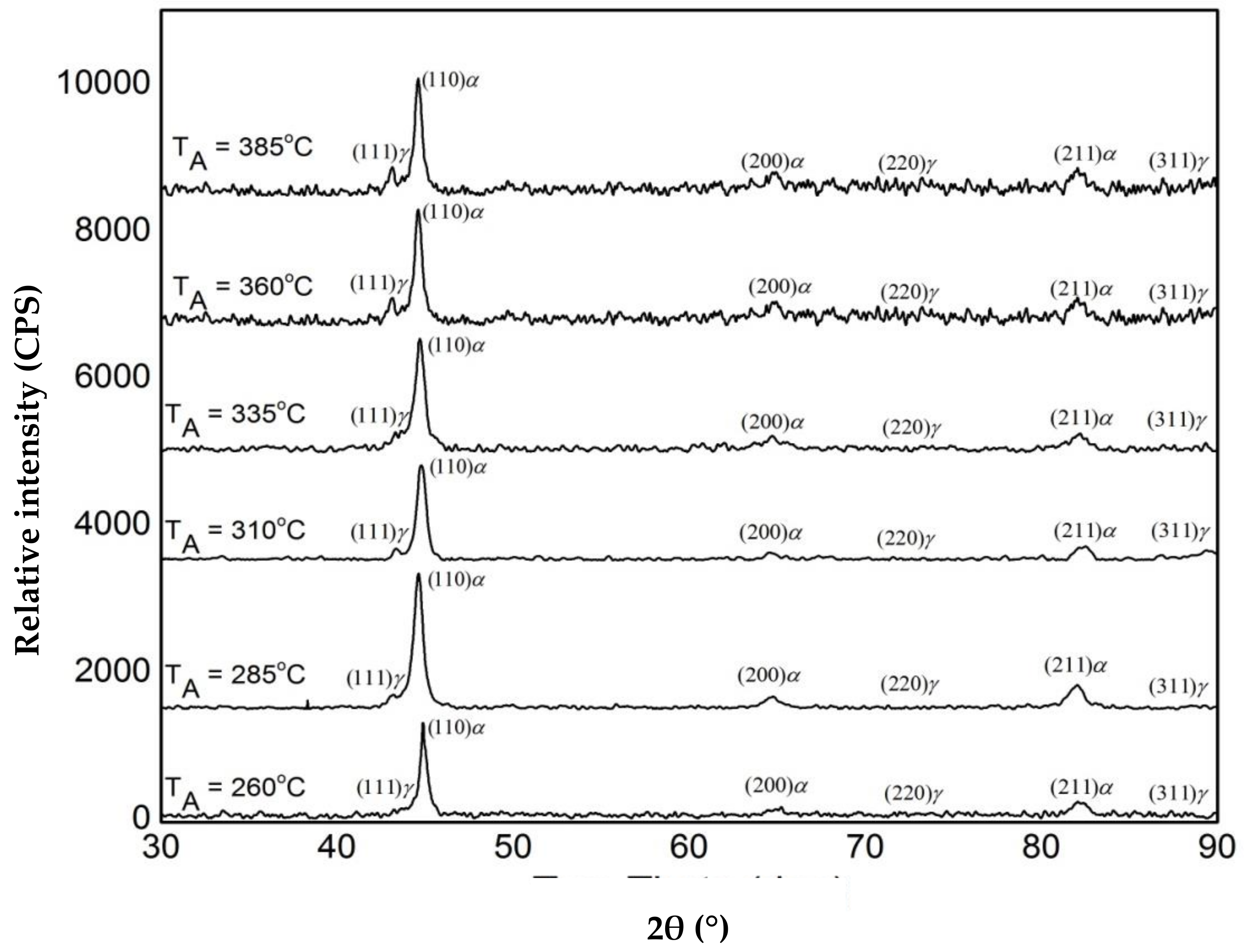
2.3. Metallography and X-ray Diffraction (XRD)
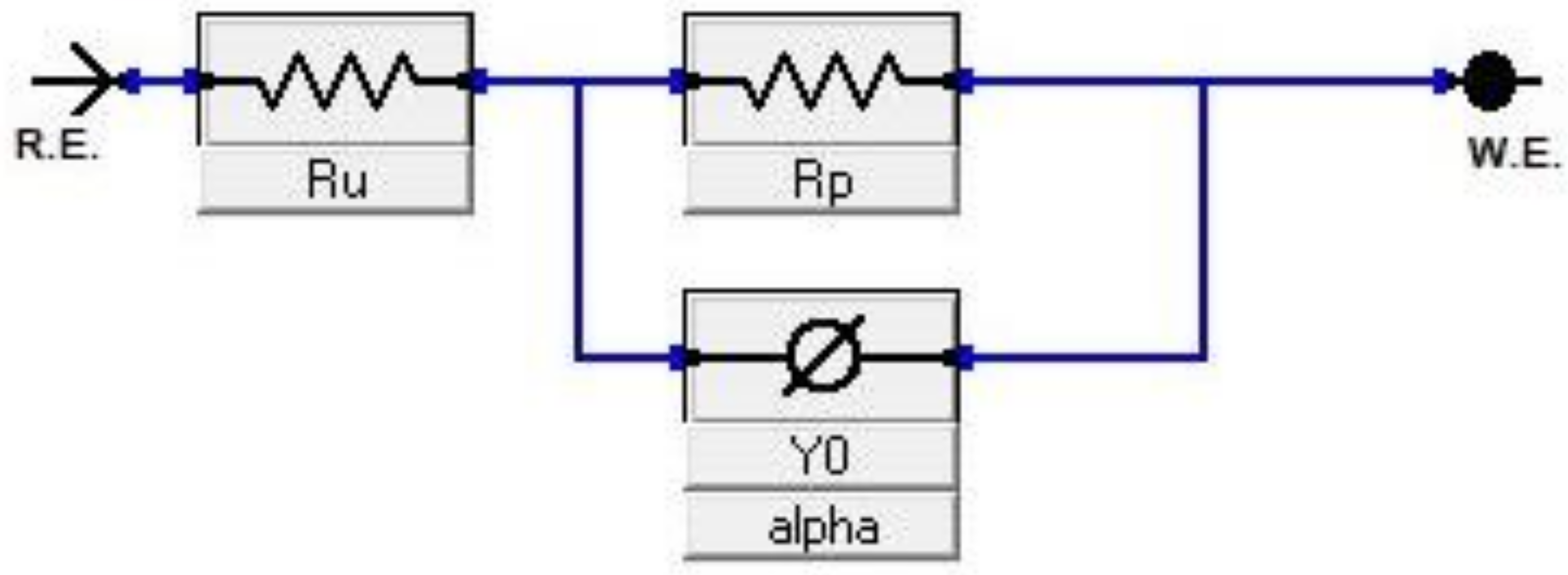
2.4. Electrochemical (Corrosion) Test
3. Results and Discussion
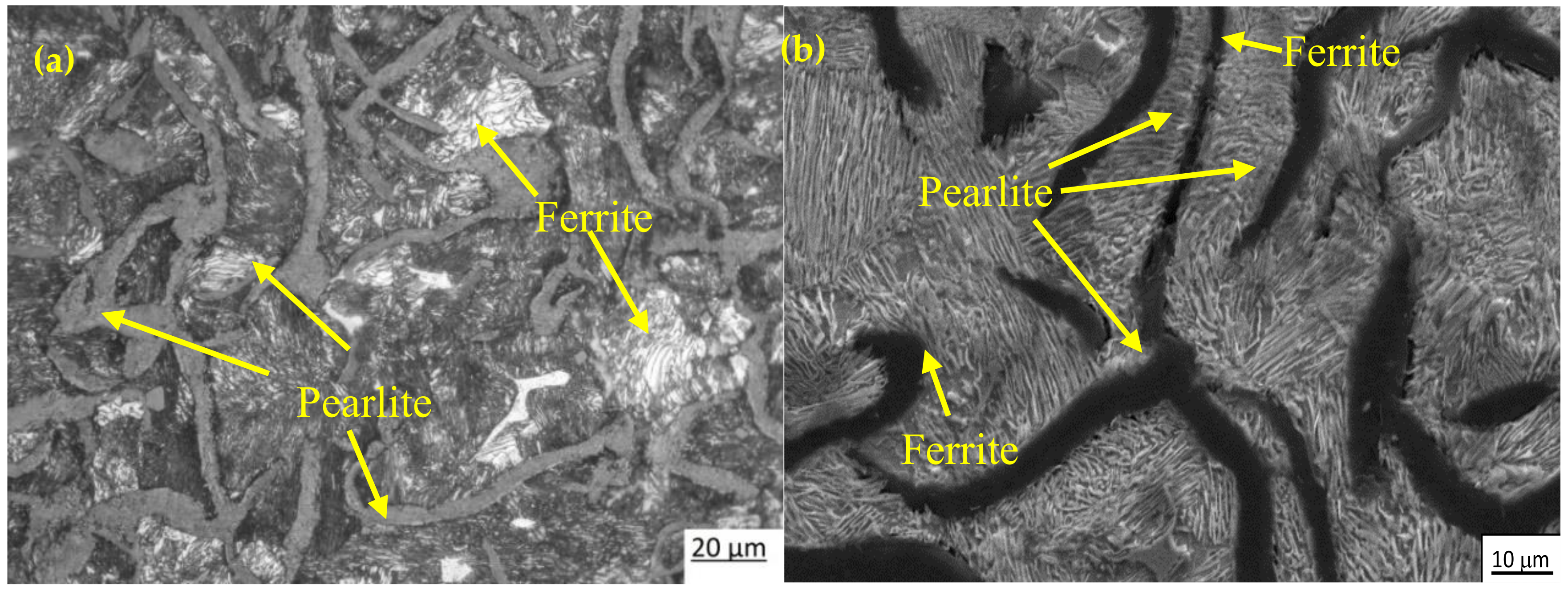
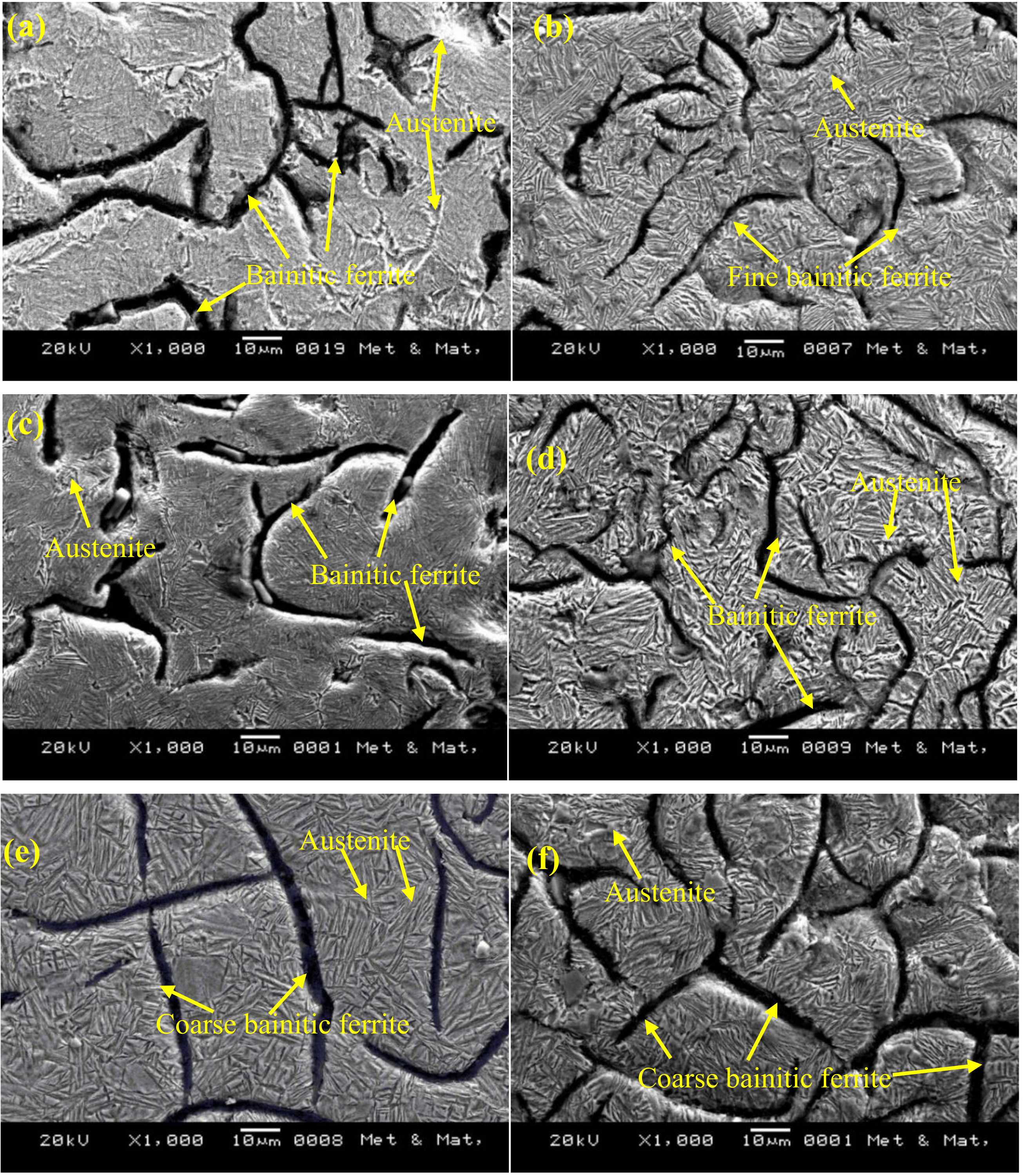
3.1. Microstructure and XRD Analysis
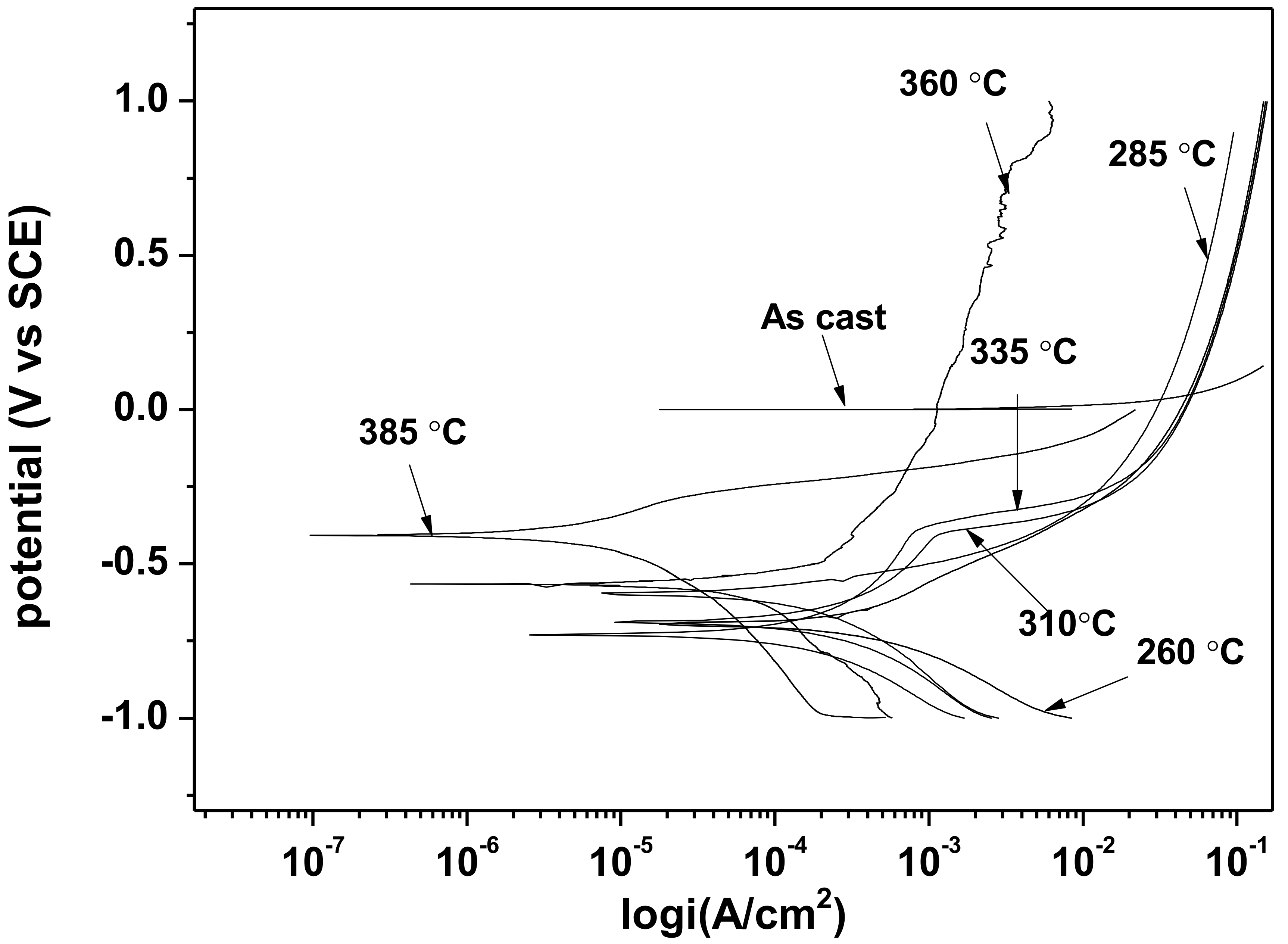
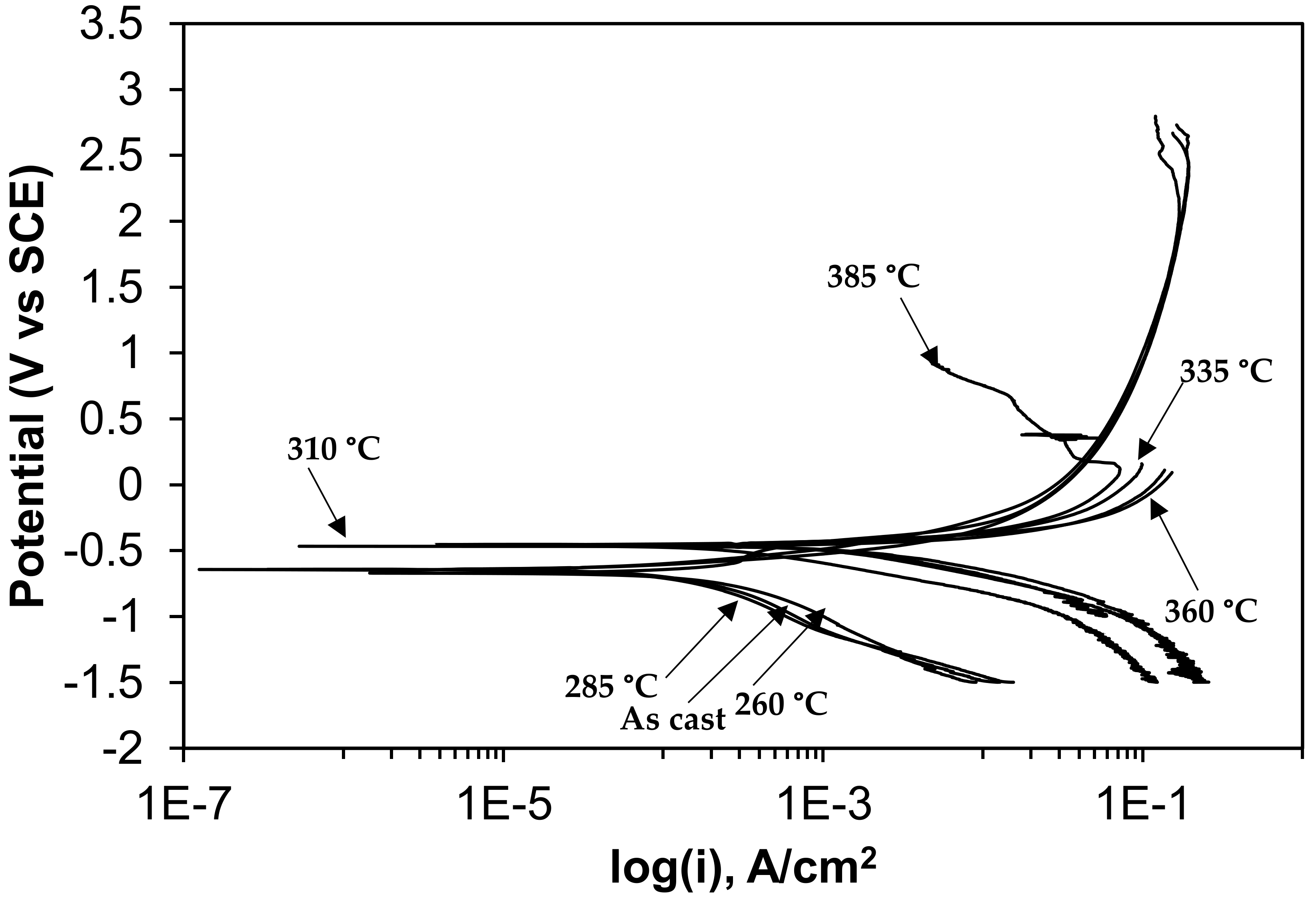
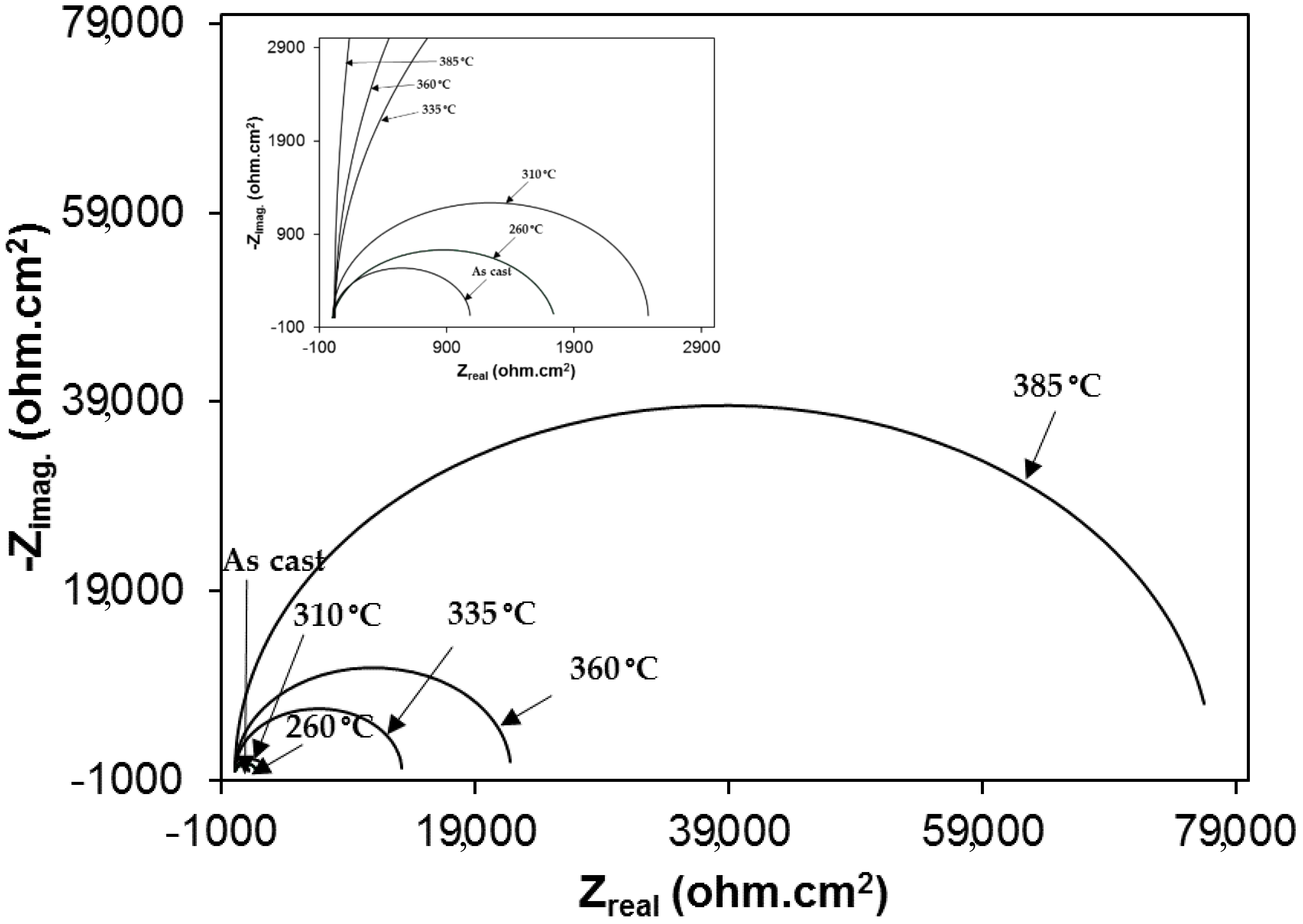
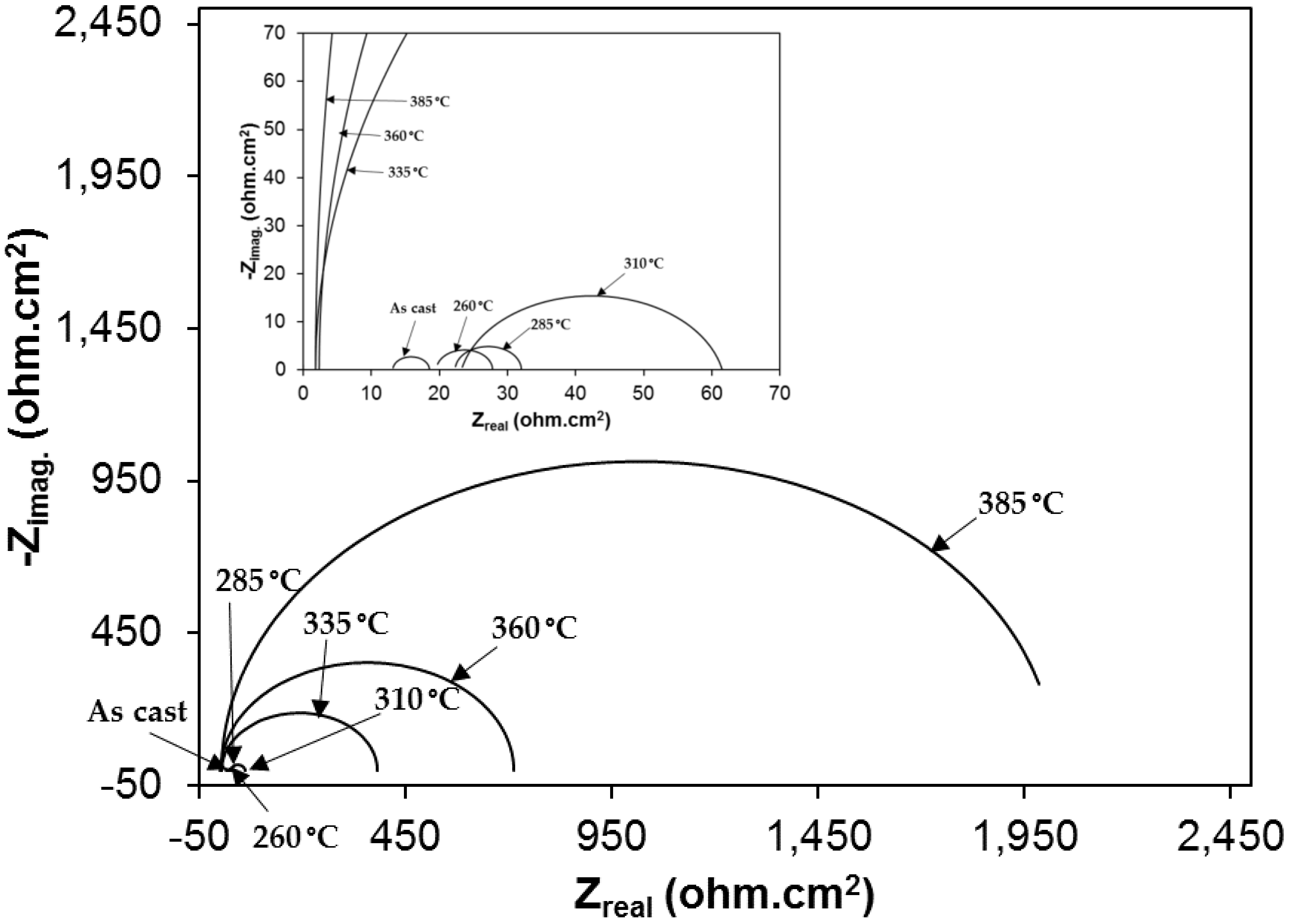
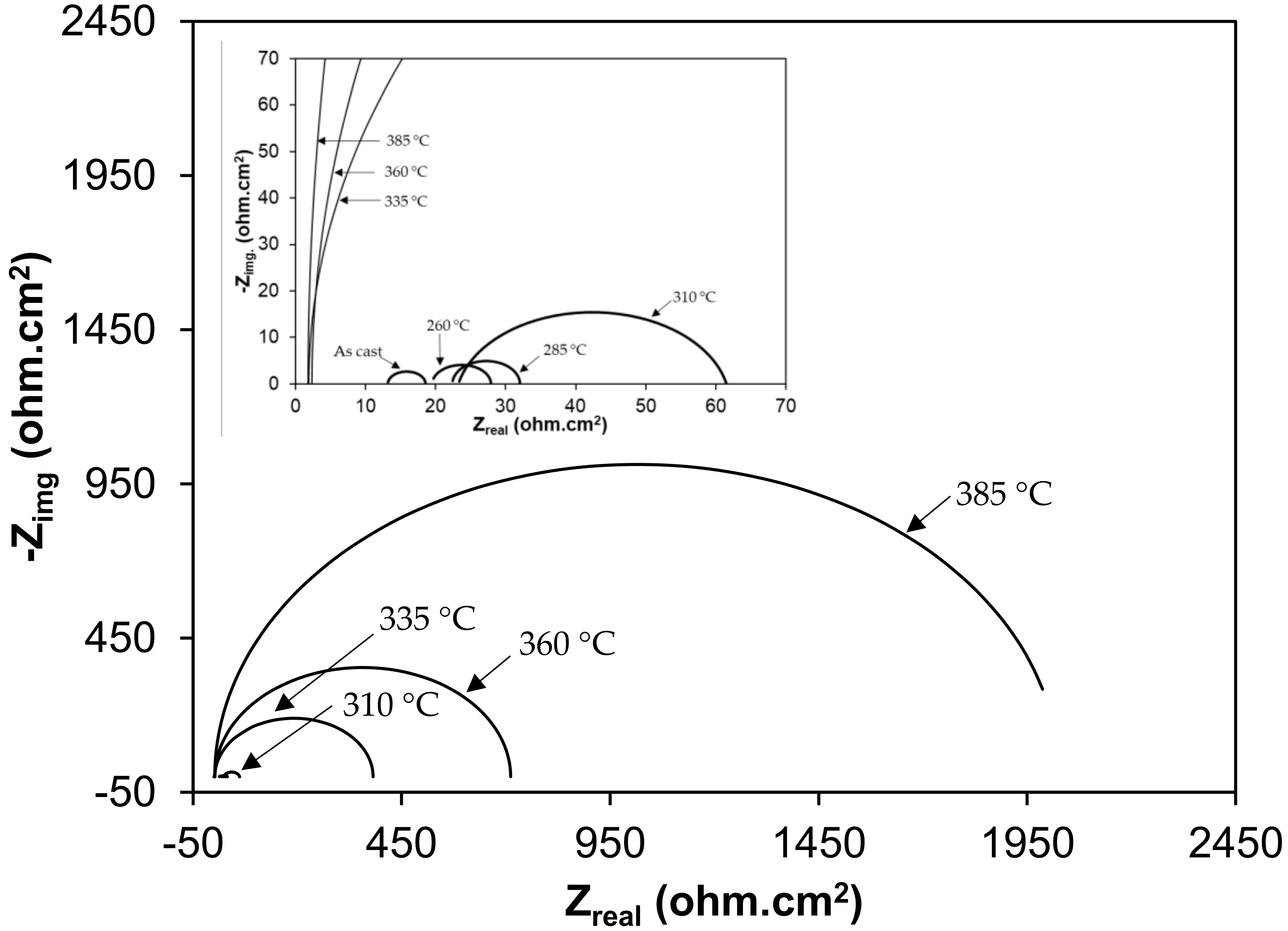
3.2. Electrochemical Behavior of As-Cast Gray Iron and AGCI in Different Solutions
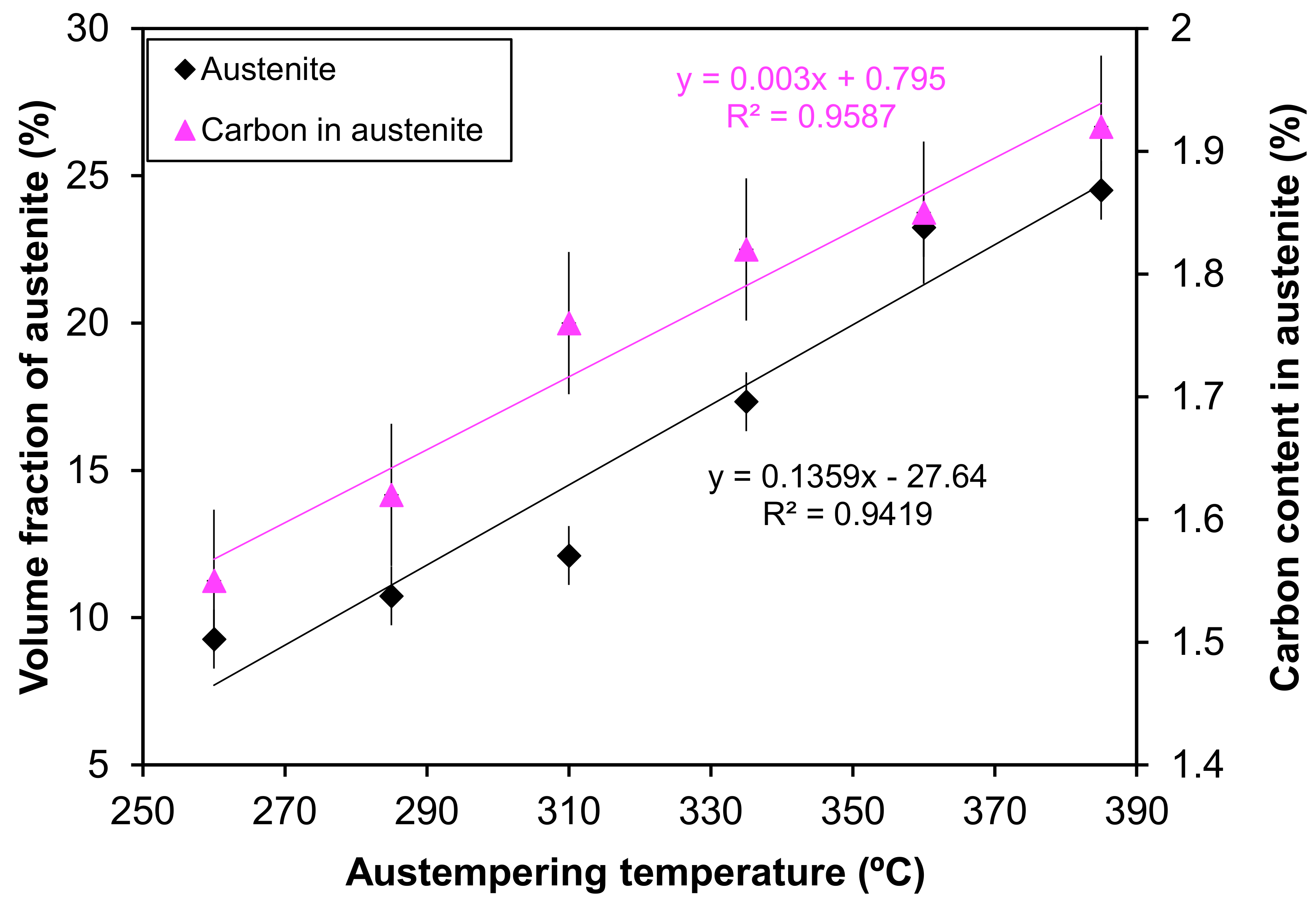
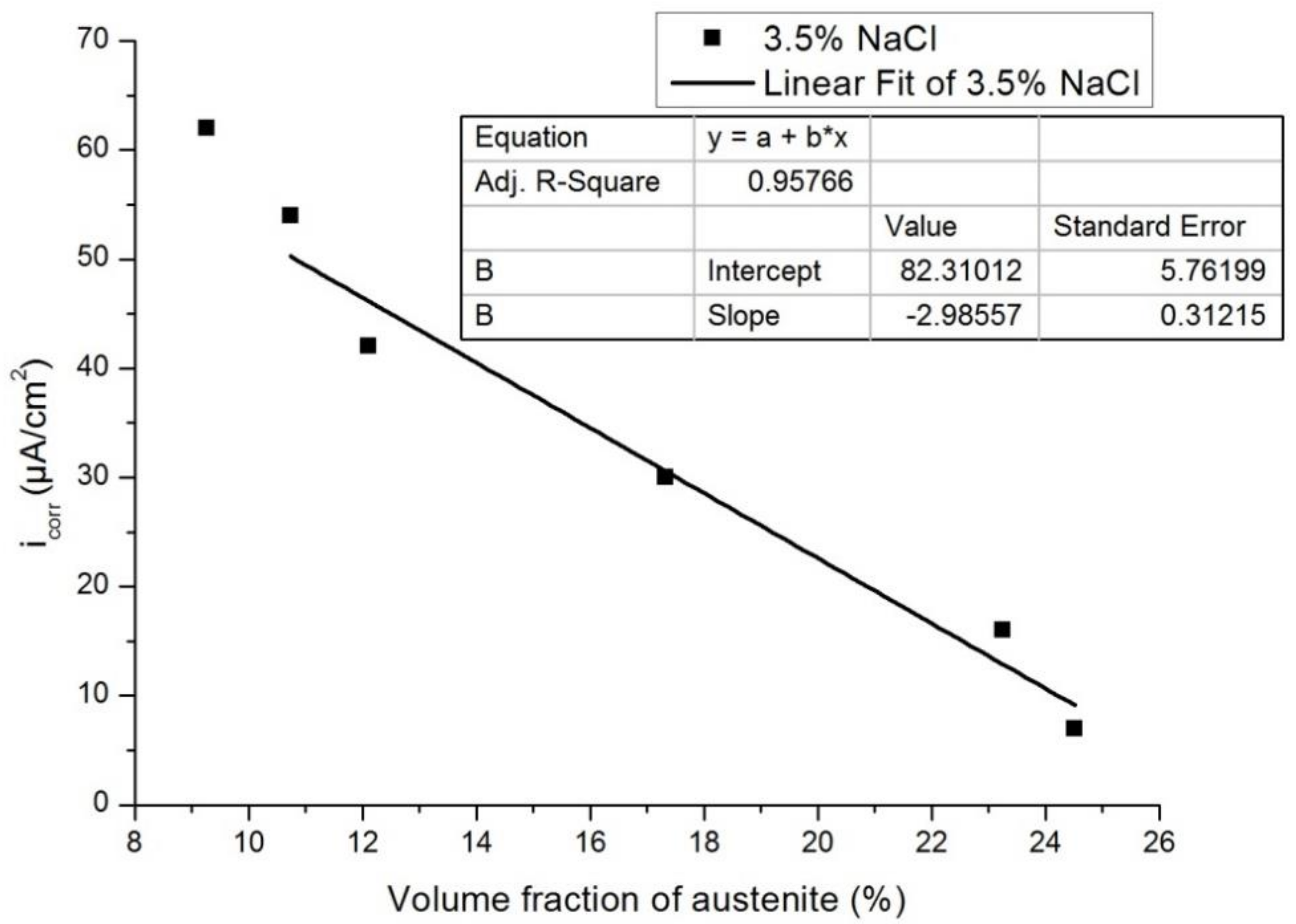
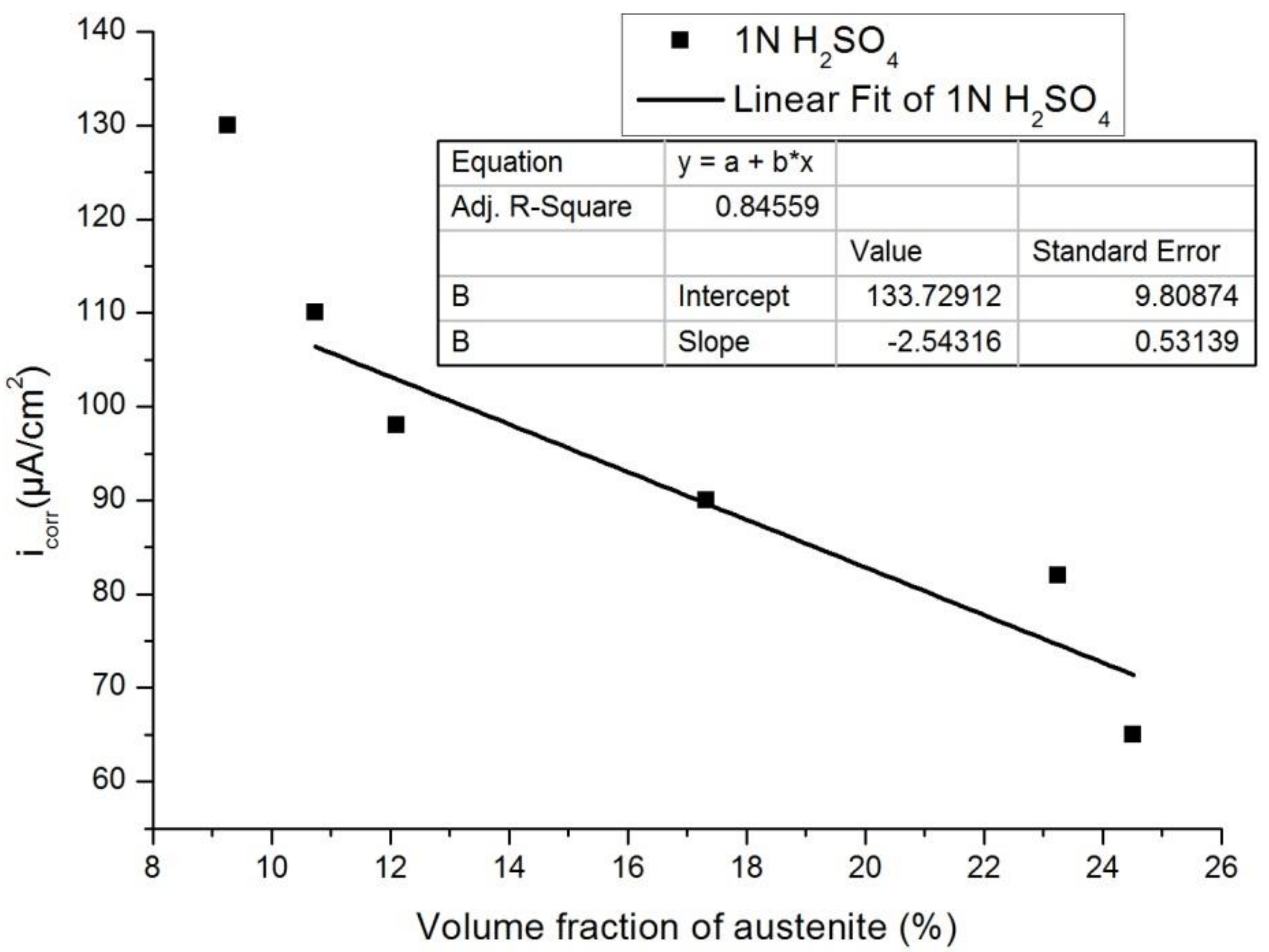
3.3. Effect of Austenite Content on Corrosion Behavior
3.4. Microstructure after Corrosion
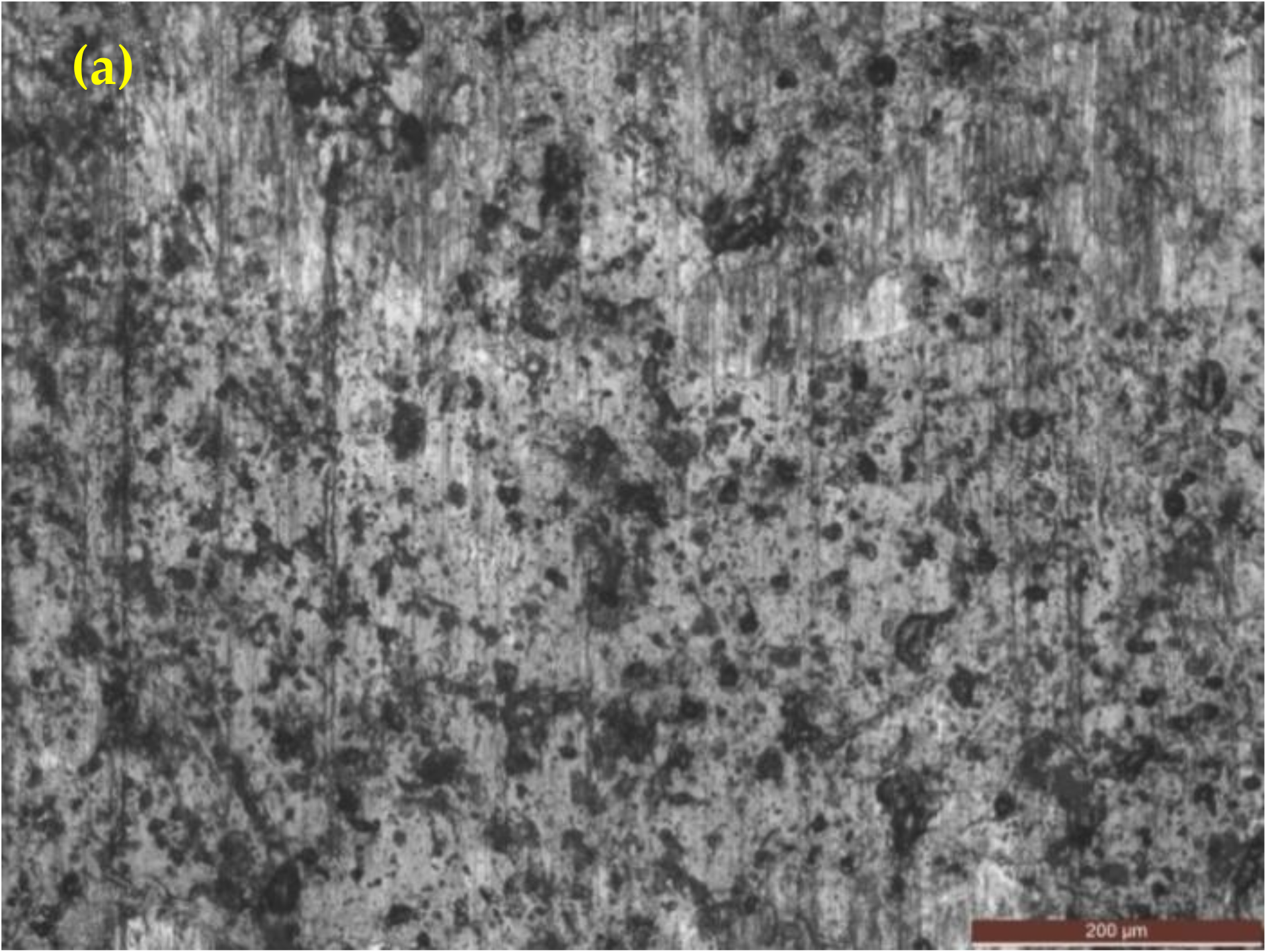
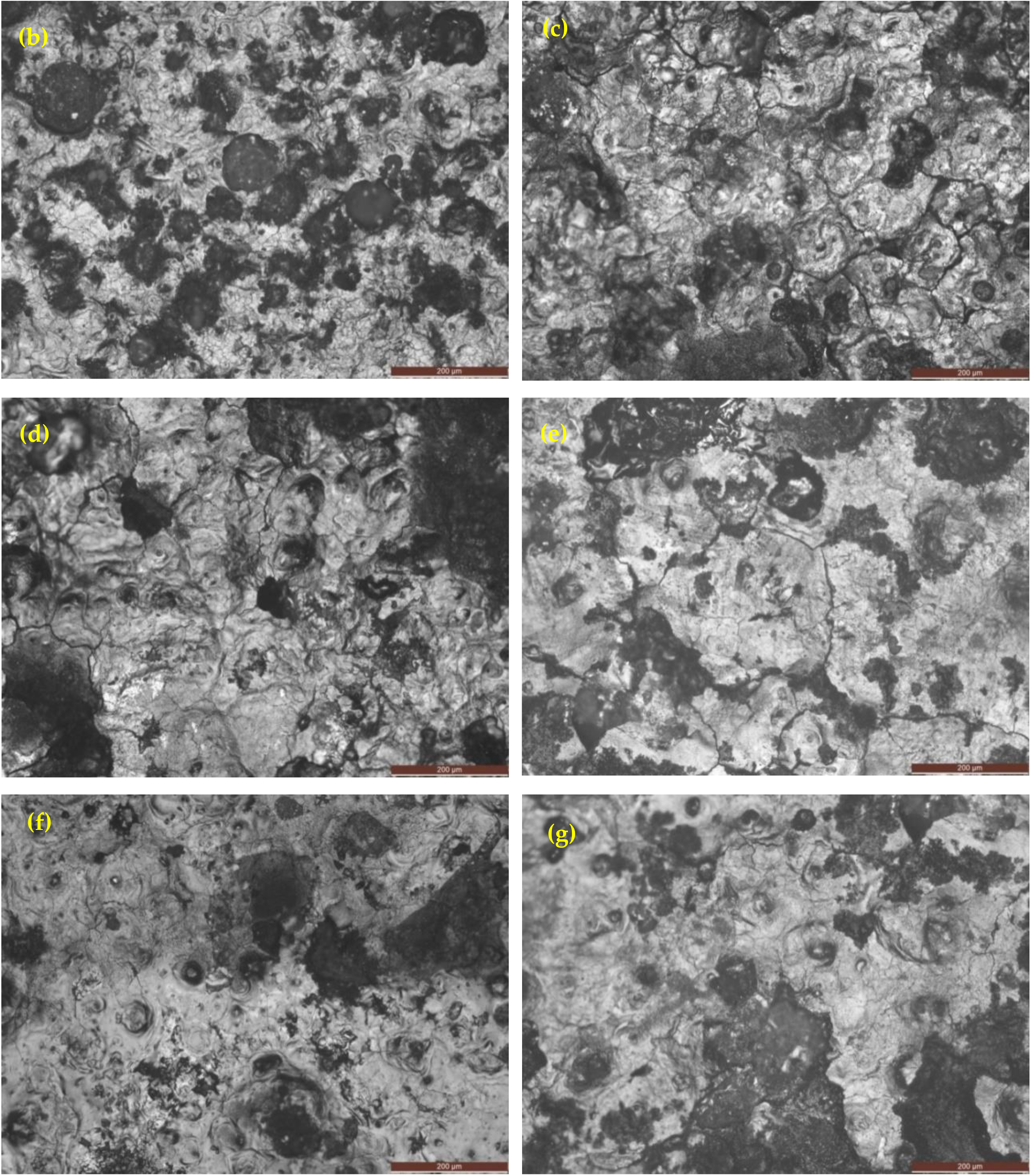
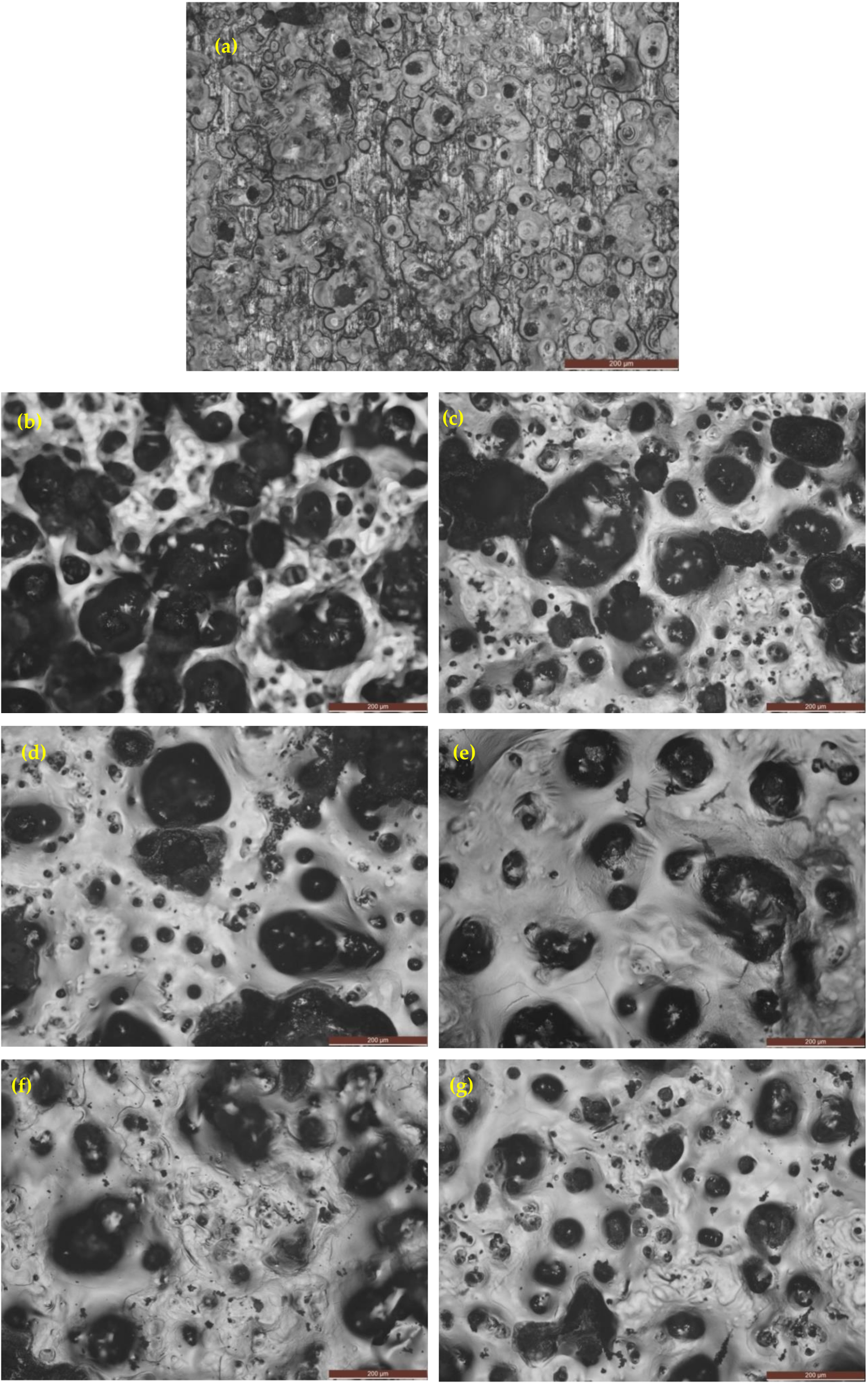
3.4.1. Optical Images after Corrosion in 3.5% NaCl Solution
3.4.2. Optical Images after Corrosion in 0.5 M H2SO4 Solution
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khanna, O.P. Foundry Technology; Dhanpath Rai Publishers: New Delhi, India, 2011. [Google Scholar]
- ASM Hand Book, Properties and Selection: Irons, Steels, and High Performance Alloys; ASM International: Geauga County, OH, USA, 1993.
- Asadi, Z.S.; Melchers, R.E. Pitting corrosion of older underground cast iron pipes. Corros. Eng. Sci. Technol. 2017, 52, 459–469. [Google Scholar] [CrossRef]
- Sami, A.A.; Saffa, M.H. Ductile and gray cast iron deterioration with time in various NaCl salt concentrations. J. Eng. Technol. 2008, 26, 1–15. [Google Scholar]
- Rana, A.M.; Khan, A.; Amjad, S. Microstructure evaluation in heat treated cast irons. J. Res. Sci. 2001, 12, 65–71. [Google Scholar]
- Muthukumarasamy, S.; Sadiq, B.A.; Sesham, S. High strength ductile irons-as cast bainitic ductile iron andaustempered ductile iron. Indian Foundry J. 1992, 8, 23–29. [Google Scholar]
- Prasanna, N.D.; Muralidhara, M.K.; Mohit, K.A. Mechanical properties and corrosioncharacteristics of IS400/12 grade ductile iron. In Proceedings of the Transactions of 57th International Foundrymen Congress, Kolkata, India, 13–15 February 2009; pp. 89–95. [Google Scholar]
- Banerjee, A.; Mitra, P.K.; Chattopadhyay, D.P. Effect of section thickness, 0.5% Cu additionand different austempering temperatures on the microstructure and corrosion properties of ADI. In Proceedings of the Transactions of 61st Indian Foundry Congress, Kolkata, India, 27–29 January 2013; pp. 1–3. [Google Scholar]
- Afolabi, A.S. Effect of austempering temperature and time on corrosion behaviour of ductile iron in chloride and acidic media. Anti-Corros. Methods Mater. 2011, 58, 190–195. [Google Scholar] [CrossRef]
- Hsu, C.-H.; Chen, M.-L. Corrosion behavior of nickel alloyed and austempered ductile irons in 3.5% sodium chloride. Corros. Sci. 2010, 58, 2945–2949. [Google Scholar] [CrossRef]
- Kovacs, B.V.; Keough, J.R. Physical properties and application of austempered gray iron. AFS Trans 1993, 93–141, 283–291. [Google Scholar]
- Hsu, C.H.; Shy, Y.H.; Yu, Y.H. Effect of austempering heat treatment on fracture toughness of copper alloyed gray iron. Mater. Chem. Phys. 2000, 63, 75–81. [Google Scholar] [CrossRef]
- Rundman, K.B.; Parolini, J.R.; Moore, D.J. Relationship between tensile properties and matrix microstructure in austempered gray iron. AFS Trans. 2005, 145, 51–55. [Google Scholar]
- Olawale, J.O.; Oluwaseguun, K.M.; Ezemenaka, D.I. Production of austempered gray iron (AGI) using forced air cooling. Mater. Perform. Charact. 2014, 3, 355–370. [Google Scholar] [CrossRef]
- Sarkar, T.; Bose, P.K.; Sutradhar, G. Mechanical and Tribological Characteristics of Copper Alloyed Austempered Gray Cast Iron (AGI). Mater. Today Proc. 2018, 5, 3664–3673. [Google Scholar] [CrossRef]
- Vadiraj, A.; Balachandran, G.; Kamaraj, M. Structure–property correlation in austempered alloyed hypereutectic gray cast irons. Mater. Sci. Eng. A 2010, 527, 782–788. [Google Scholar] [CrossRef]
- Vadiraj, A.; Balachandran, G.; Kamaraj, M. Studies on mechanical and wear properties of alloyed hypereutectic gray cast irons in the as-cast pearlitic and austempered conditions. Mater. Des. 2010, 31, 951–955. [Google Scholar] [CrossRef]
- Harvey, J.N.; Noble, G.A. Inoculation of cast irons—An overview. In Proceedings of the 55th Indian Foundry Congress, Agra, India, 2–4 February 2007; pp. 343–360. [Google Scholar]
- Gorny, M.; Tyrala, E.; Lopez, H. Effect of copper and nickel on the transformation kinetics of austempered ductile iron. J. Mater. Eng. Perform. 2014, 23, 3505–3510. [Google Scholar] [CrossRef]
- Bayati, H.; Elliott, R. The concept of an austempered heat treatment processing window. Int. J. Cast Met. Res. 1999, 11, 413–417. [Google Scholar] [CrossRef]
- Dasgupta, R.K.; Mondal, D.K.; Chakrabarti, A.K. Evolution of microstructures during austempering of ductile irons alloyed with manganese and copper. Metall. Mater. Trans. A 2013, 44, 1376–1387. [Google Scholar] [CrossRef]
- Bayati, H.; Elliott, R. Relationship between structure and mechanical properties in high manganese alloyed ductile iron. Mater. Sci. Technol. 1995, 11, 284–293. [Google Scholar] [CrossRef]
- Patutunda, S.K. Development of austempered ductile iron (ADI) with simultaneous high yield strength and fracture toughness by a novel two-step austempering process. Mater. Sci. Eng. A 2001, 315, 70–80. [Google Scholar] [CrossRef]
- Yang, J.; Putatunda, S.K. Improvement in strength and toughness of austempered ductile cast iron by novel two-step austempering process. Mater. Des. 2004, 25, 219–230. [Google Scholar] [CrossRef]

| Element | C | Si | Mn | P | S | Cu | Fe |
|---|---|---|---|---|---|---|---|
| Composition | 3.46 | 2.27 | 0.53 | 0.019 | 0.01 | 0.50 | Bal. |
| Sample Condition | Icorr (μA/cm2) | Ecorr (V) |
|---|---|---|
| Austempering Temperature (°C) | - | - |
| As-cast gray iron | 70 | –0.80 |
| 927 °C–260 °C | 62 | –0.75 |
| 927 °C–285 °C | 54 | –0.70 |
| 927 °C–310 °C | 42 | –0.62 |
| 927 °C–335 °C | 30 | –0.50 |
| 927 °C–360 °C | 16 | –0.45 |
| 927 °C–385 °C | 7 | –0.40 |
| Sample Condition | Icorr (µA/cm2) | Ecorr (V) |
|---|---|---|
| Austempering Temperature (°C) | - | - |
| As-cast gray iron | 150 | –0.60 |
| 927 °C–260 °C | 130 | –0.55 |
| 927 °C–285 °C | 110 | –0.50 |
| 927 °C–310 °C | 98 | –0.48 |
| 927 °C–335 °C | 90 | –0.42 |
| 927 °C–360 °C | 82 | –0.38 |
| 927 °C–385 °C | 65 | –0.36 |
| Sample Condition | Rp (ohm.cm2) |
|---|---|
| Austempering Temperature (°C) | - |
| As-cast gray iron | 930 (± 46) |
| 927 °C–260 °C | 1850 (± 91) |
| 927 °C–310 °C | 2550 (± 102) |
| 927 °C–335 °C | 15,000 (± 450) |
| 927 °C–360 °C | 20,000 (± 1100) |
| 927 °C–385 °C | 80,000 (± 1600) |
| Sample Condition | Rp (ohm.cm2) |
|---|---|
| Austempering Temperature (°C) | - |
| As-cast gray iron | 20 (± 1) |
| 927 °C–260 °C | 28 (± 1) |
| 927 °C–285 °C | 32 (± 2) |
| 927 °C–310 °C | 62 (± 2) |
| 927 °C–335 °C | 450 (± 13) |
| 927 °C–360 °C | 700 (± 28) |
| 927 °C–385 °C | 2000 (± 85) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seikh, A.H.; Sarkar, A.; Singh, J.K.; Mohammed, S.M.A.K.; Alharthi, N.; Ghosh, M. Corrosion Characteristics of Copper-Added Austempered Gray Cast Iron (AGCI). Materials 2019, 12, 503. https://doi.org/10.3390/ma12030503
Seikh AH, Sarkar A, Singh JK, Mohammed SMAK, Alharthi N, Ghosh M. Corrosion Characteristics of Copper-Added Austempered Gray Cast Iron (AGCI). Materials. 2019; 12(3):503. https://doi.org/10.3390/ma12030503
Chicago/Turabian StyleSeikh, Asiful H., Amit Sarkar, Jitendra Kumar Singh, Sohail M. A. Khan Mohammed, Nabeel Alharthi, and Manojit Ghosh. 2019. "Corrosion Characteristics of Copper-Added Austempered Gray Cast Iron (AGCI)" Materials 12, no. 3: 503. https://doi.org/10.3390/ma12030503
APA StyleSeikh, A. H., Sarkar, A., Singh, J. K., Mohammed, S. M. A. K., Alharthi, N., & Ghosh, M. (2019). Corrosion Characteristics of Copper-Added Austempered Gray Cast Iron (AGCI). Materials, 12(3), 503. https://doi.org/10.3390/ma12030503






